Abstract
A device is presented for efficiently enriching parahydrogen by pulsed injection of ambient hydrogen gas. Hydrogen input to the generator is pulsed at high pressure to a catalyst chamber making thermal contact with the cold head of a closed cycle cryostat maintained between 15 and 20 K. The system enables fast production (0.9 standard liters per minute) and allows for a wide range of production targets. Production rates can be systematically adjusted by varying the actuation sequence of high-pressure solenoid valves, which are controlled via an open source microcontroller to sample all combinations between fast and thorough enrichment by varying duration of hydrogen contact in the catalyst chamber. The entire enrichment cycle from optimization to quantification and storage kinetics are also described. Conversion of the para spin-isomer to orthohydrogen in borosilicate tubes was measured at 8 minute intervals over a period of 64 hours with a 12 Tesla NMR spectrometer. These relaxation curves were then used to extract initial enrichment by exploiting the known equilibrium (relaxed) distribution of spin isomers with linear least squares fitting to a single exponential decay curve with an estimated error less than or equal to 1 %. This procedure is time-consuming, but requires only one sample pressurized to atmosphere. Given that tedious matching to external references are unnecessary with this procedure, we find it to be useful for periodic inspection of generator performance. The equipment and procedures offer a variation in generator design that eliminate the need to meter flow while enabling access to increased rates of production. These tools for enriching and quantifying parahydrogen have been in steady use for 3 years and should be helpful as a template or as reference material for building and operating a parahydrogen production facility.
Keywords: PASADENA, parahydrogen, orthohydrogen, hyperpolarization, SABRE, PHIP
I. Introduction
Technologies for increasing the polarization in ensembles of magnetic moments are now enabling rapid assays of metabolism and structure with increased resolution in living tissue [1; 2]. These methods are collectively referred to as hyperpolarization and are being used more frequently as tools for probing cellular metabolism on a time-scale of seconds [3; 4]. When infused into organisms harboring tumor cells and other metabolic disorders, pyruvate and lactate penetrate metabolic cycles and the polarized states have been shown to be sufficiently long-lived to exhibit differential conversion in cancer compared to normal tissue [5]. Dynamic nuclear polarization (DNP) has been most common in these early studies, stemming from the availability of a commercial instrument and the flexibility to polarize small molecules. Techniques based on chemical addition (PASADENA - Parahydrogen And Synthesis Allow Dramatically Enhanced Nuclear Alignment) [6] or exchange (SABRE - signal amplification by reversible exchange) [7] of parahydrogen give similar enhancements with less elaborate equipment, and therefore could emerge as leading sensors for probing metabolism [8; 9].
A basic requirement for PASADENA and SABRE is the enrichment of parahydrogen gas from equilibrium mixtures of ortho and parahydrogen. The para isomer is energetically favored, and when molecular symmetry is broken with a paramagnetic catalyst, triplet (ortho) states are rapidly converted to singlet (para) states. The efficiency of this conversion to parahydrogen depends on the degree of cooling during exposure to suitable catalysts, which are used to increase the otherwise slow rate of conversion. Diverse solutions to this problem have been reported, and these are differentiated by cold source, degree of automation, and manner of gas flow.
The simplest method to produce significant enrichment is by flowing hydrogen through a catalyst chamber that makes thermal contact with liquid nitrogen. Transitioning from the 50 % enrichment levels attainable with this arrangement though, towards the theoretical limit of enrichment requires much lower temperatures (~20 K). Helium cryo-coolers can provide this cooling with minimal maintenance overhead. Aside from operational temperature, a parahydrogen generator design should enable ambient hydrogen to flow into a cold chamber that contains catalyst, where it then remains in contact for an duration that is sufficiently long to be enable uniform conversion to the singlet state. Finally, enriched hydrogen is ejected to a storage tank or interfaced directly to a parahydrogen polarizer. The solution to this fluid-flow problem distinguishes most of the equipment when temperature of operation is held constant.
The device presented here operates with a closed-cycle cryostat which is maintained at a nominal set-point of 15 K (with operation between 15-20 K) and enriches parahydrogen with high pressure, pulsed injections of ambient hydrogen. In this design, restricted or metered flow are replaced by discrete pulses. By minimizing the dependence of production rate on system resistance, contact time (interaction of hydrogen with catalyst chamber) can be adjusted arbitrarily. Automation is straightforward and amounts to programming a controller to actuate high pressure solenoid valves with the desired timings. When operated to achieve a final fill pressure of 240 psi, this system generates highly enriched parahydrogen (> 98 %) at 0.9 SLM (standard liters per minute). Procedures for quantifying enrichment in sealed tubes without the necessity of external concentration references are also described. Finally, relaxation kinetics are investigated in commercially available aluminum storage tanks, thus encompassing all aspects of building and operating a parahydrogen production facility.
II. Methods
II.a. System schematic
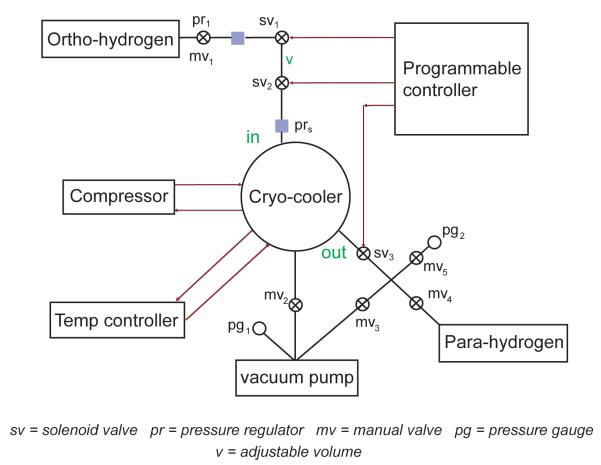
A custom controller module based on an open source microcontroller (Arduino Mega, SparkFun, Boulder, CO) was used to inject hydrogen gas at high pressure from bulk storage to a cryo-cooler cold head and finally to storage tanks (Figure 1). These injections were used to tailor enrichment and preparation time, with longer preparation times corresponding to higher enrichments, by actuating an array of explosion proof solenoid valves rated at 500 psi (Series 20, 2-way NC, Peter Paul Electronics, New Britain CT). Manual butterfly valves (part number 9798K31, McMaster-Carr, Aurora, OH) were used in various places to expand the utility of the generator equipment. The cryo-cooler was selectively evacuated by closing mv3 and mv1 (solenoids sv1 and sv2 closed). Parahydrogen tanks and the quantification module (Figure 2) were selectively evacuated by closing mv2 with sv3 and opening mv4 (Figure 1). Relief valves (Prs, Figure 1) (part number 9798K31 @ 325 psi, McMaster Carr, Aurora, OH) insulate the cryo-cooler from extreme pressures that would arise from an improbable, upstream regulator failure or a sudden loss of cooler power. Pulses of ambient hydrogen were delivered from the volume between the bulk hydrogen storage and the cryo-cooler input port (v, Figure 1) to the cryo-cooler cold head (DE-204, Advanced Research Systems, Allentown, PA). The temperature controller (Model 9700, Scientific Instruments, West Palm Beach, FL) operates in a closed loop with the compressor (ARS-4HW, Advanced Research Systems, Allentown, PA) to maintain the cold head temperature set point. A Pirani 945 controller with a series 345 vacuum gauge (Kurt J. Lesker, Pittsburgh, PA) was used to monitor the vacuum quality. Hydrogen gas makes thermal contact with the first stage of the cryo-cooler via stainless steel tubing (316 grade, 1/8" OD), and to the second stage via a custom-built copper chamber. This chamber housed a scaffold assembly, consisting of 5 discs threaded onto a center axle with uniform separation. Each disc contained holes for gas passage and these openings were distributed on an approximate helix to increase path length through the catalyst chamber. This chamber was filled with iron III oxide catalyst (371254, Sigma Aldrich Corp, St. Louis, MO) and secured to the second stage cold head with an indium seal. A solenoid on the cryo-cooler output, sv3, optionally reduced dead-volume between the output port and storage tank. Upon ejection from the catalyst chamber, enriched parahydrogen was collected in 10 L aluminum storage containers (model 14740NOS, Nitrous Oxides Systems (NOS) tank, Holley Performance Products, Bowling Green, KY).
Figure 1.
System schematic for production of parahydrogen by pulsed injection of hydrogen gas. Solenoid (svi) operation is under programmable control to tailor enrichment and preparation time. Aliquots are delivered from an adjustable volume v. The cryo-cooler, temperature controller, and compressor operate in a closed loop. Hydrogen makes thermal contact with the first stage of the cryo-cooler via stainless steel tubing and is routed through a diffusion restricted copper chamber that contains a suitable catalyst in thermal equilibrium with the second stage cold head.
Figure 2.
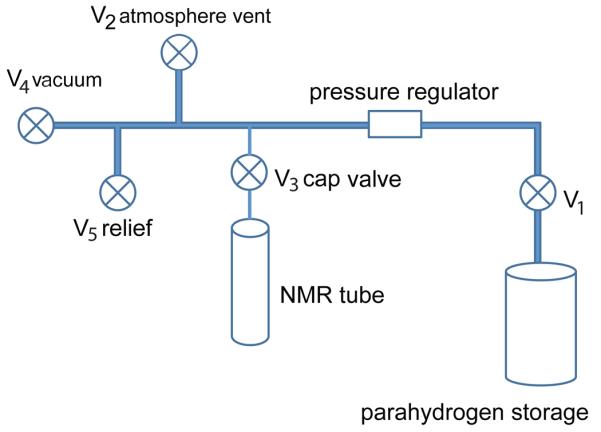
Apparatus for filling NMR tubes at low pressure with parahydrogen gas. V1 through V5 correspond to manual butterfly valves: V1 is an aluminum tank valve, V2 is used to normalize the apparatus to atmospheric pressure, V3 is a valve built into the cap of a low-pressure NMR tube, V4 allows evacuation of the assembly, and V5 is a relief valve for preventing inadvertent explosion of the glass components.
II.b. Filling borosilicate tubes with parahydrogen gas
97% parahydrogen gas was produced from ultra-high purity hydrogen gas (A-L Compressed Gases, Nashville, TN) using the custom generator described above (see section II.a). Production temperature was maintained between 15 and 20 K with the temperature controller set to 15 K. This is just above the freezing point of hydrogen and within the range required to maintain liquid hydrogen. The manufacturer quoted hydrogen purity was 99.9995% with a residual oxygen content < 1 ppm, residual moisture content < 1 ppm, and residual hydrocarbon content < 0.5 ppm. Low-pressure/vacuum Pyrex® NMR tubes (borosilicate glass, 5 mm outer diameter, 8 inches long) (product number 507-LPV-8, Wilmad-Labglass, Vineland, NJ, USA) were used for all NMR spectroscopic studies. The NMR tubes were filled from the aluminum parahydrogen storage tank using a custom filling apparatus (Figure 2). The components of this setup were connected using PTFE tubing (1/8” OD, 1/16” ID) with 1/8” push-to-connect tube fittings (part number 5779K31, McMaster-Carr, Aurora, OH). Catastrophic pressurization of the NMR tube was prevented by the safety valve (V5) (part number 9024K11 @ 10 psi, McMaster-Carr, Aurora, OH). For loading the NMR tube with parahydrogen, first the filling setup was interfaced to the parahydrogen generator in a valve configuration (mv1-mv2 closed, sv1-sv3 closed) that isolated the parahydrogen storage tank, vacuum pump, and filling apparatus. The custom filling apparatus was then evacuated to < 2 × 10−2 Torr by opening mv3 (Figure 1) with valves 3 and 4 open. After vacuum was achieved, V4 was closed and the parahydrogen tank opened (V1) to a regulated pressure of slightly greater than 1 atm. Parahydrogen gas then flowed from the parahydrogen tank to the NMR tube, which was filled to a nominal pressure of 1 atm by slowly venting to atmosphere (Figure 2, V2). After filling, the NMR tube was then sealed by closing the tube cap valve (Figure 2, V3), followed by shutting off the gas flow (V1). The system was re-evacuated immediately prior to collecting additional parahydrogen samples using the roughing pump attached to the parahydrogen generator.
II.c. NMR spectroscopy of hydrogen gas
The parahydrogen samples were analyzed within 10 minutes of collection with 1H spectroscopy at 12 Tesla (Bruker spectrometer, Bruker, Billerica, MA). Proton spectra were acquired at 298 K (Figure 3) with a pulse-acquire sequence using 30° RF (11 us) excitation pulses, pre-acquisition delays of 6 us, and 10.31 ms acquisition times. 256 transients were signal averaged with a repetition time of 70 ms, chosen to be much greater than the T1 of orthohydrogen (~20 times) [10]. The spectral width was 25 kHz (50 ppm), and all spectra were processed with 10 Hz line broadening polynomial baseline corrections. Proton resonances at 7.4 ppm [11] originating from orthohydrogen were integrated from 3 to 15 ppm. While the conversion between the two spin-isomers of hydrogen is slow, by comparison the spin lattice relaxation time, T1, of orthohydrogen in the gas phase is very short 3.7 ms [10]. Moreover, the T2 of ortho-H2 gas is less than 0.5 ms [10], with the result that NMR line widths are on the order of 1 kHz [10; 12].
Figure 3.
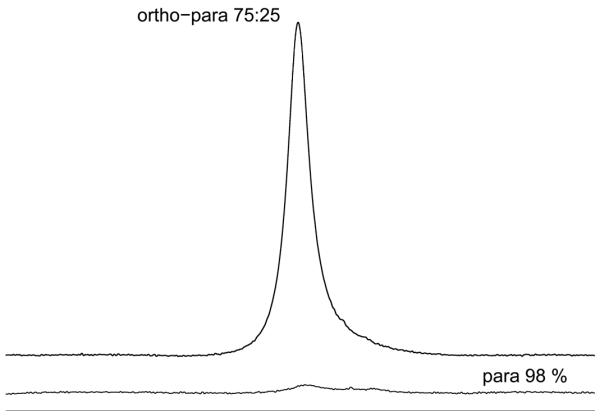
Proton spectrum acquired from parahydrogen immediately after enrichment and after 4 days (ortho-para 75:25) in contact with borosilicate NMR tubes. The proton signal grows over time as parahydrogen relaxes to orthohydrogen with broad lines arising from efficient T2 relaxation.
III. Results and Discussion
III.a. System operation
Orthohydrogen was pulsed at high pressure into a cold copper chamber filled with an iron oxide catalyst to achieve enrichment with this apparatus (Figure 1). Prior to operation, the overall system was evacuated to 1.5 × 10−2 Torr by opening solenoid valves in tandem with manual valves mv3 and mv4. The closed-cycle helium cryo-cooler is then operated under this vacuum with all valves closed except mv3 until the temperature set-point is reached. With the cold head stabilized at 15 K, the solenoid valve controller is activated to deliver timed bursts to the copper conversion chamber. The controller can be operated in manual mode as well, with the solenoids actuated by toggle switches. The volume (v, Figure 1) serves as a reservoir which is adjusted to deliver the most dense pulse of hydrogen that enables cold head temperature to be maintained with +/− 1 K. With sv2 and sv3 closed along with sv1 open, v was filled to the input pressure set by the regulator pr1. The conversion chamber was charged by opening sv2 with sv1 and sv3 closed. Isolating a reservoir, v, in this way provides an added layer of safety against inadvertent overcharging and consequent disruptive temperature fluctuations in the cold head. The exothermic conversion of ortho to parahydrogen can potentially lead to failure of the conversion chamber seal if the cooling power is exceeded long enough to vaporize liquid hydrogen and if the relief valve is at a higher rating. The path lengths from sv2 to cold head and from cold head to sv3 are minimized to reduce dead-volume. This valve sequence therefore approximates a high-pressure injection to the cold head which can be tuned to achieve either long contact time and fill cycles, or short contacts with corresponding reductions in fill times. Long contact times lead to more thorough enrichments and should be adjusted based on the kinetics of the ortho-para conversion in the catalyst chamber. After exposure to the conversion chamber at 15 K, sv3 can be opened to charge parahydrogen to a holding tank. During the contact time, sv1 is opened to fill v, and when sv3 is opened, sv2 is opened to simultaneously expunge the conversion chamber contents. This procedure is completed in the constant fill cycle program until the desired pressure in the holding tank is reached (typically 240 psi). For practical reference, a 10 L storage tank with quick-connects filled at 240 psi translates to 100 PASADENA experiments carried out at 100 psi in a 60 mL reactor.
A discussion of equipment advantage is necessarily biased by variations in component availability and specialty expertise available to individual labs. Effective designs based on continuous flow have been reported [13; 14] that achieve high enrichment and production rates of up to 0.4 SLM [14]. When compared to restricted flow, pulsed-injections are inherently fast because flow rates and contact time are decoupled. Three high-pressure, explosion-proof solenoid valves (sv1-sv3) are combined with standard pressure regulators. Manual valves enable the apparatus to serve additionally as a general use filling station. The process of enrichment is distilled to a small number of events, amounting to the repetitive application of a sequence of two sets of solenoid valve states. We have found this useful in comparison to adjustment of flow, which can take a continuum of values and shift subtly from day to day [14] depending on extraneous factors such as ambient temperature. In addition, with continuous flow, contact times are limited to the minimal flow rate and charge density depends on resistance, or input pressure. Considered in terms of production velocity (e.g. SLM), pulsed injection allows the volume to increase without concomitant increases in resistance and decreases in flow. This allows the equipment to operate near the theoretical rate of parahydrogen production, which is in turn dominated by the cryostat cooling capacity. At a final fill pressure of 240 psi, this system generates 0.9 SLM, in proximity to the theoretical maximum based on a cryostat with 6 watts cooling capacity at 15 K. In summary, high production rates are possible with fully adjustable contact times in a design that requires only two distinct sets of solenoid valve actuations for an entire fill cycle.
III.b Quantification of parahydrogen enrichment
Parahydrogen is nonmagnetic and therefore poses challenges for direct quantification by NMR. The otherwise slow back-conversion from para to orthohydrogen occurs on a much shorter time scale in borosilicate tubing, however, and this serendipitous attribute can be leveraged to quantify enrichment without an external reference. We measured the kinetics of para to orthohydrogen conversion in low-pressure borosilicate NMR tubes by averaging 256 free-induction decays at a 1.8 s recycle delay at 8 minute intervals during the course of 64 hours. Signal intensity was fit to an exponential decay function with a time constant, t1 = 846 ± 5 minutes, and R2 = 0.998 (red trace, Figure 4).
Figure 4.
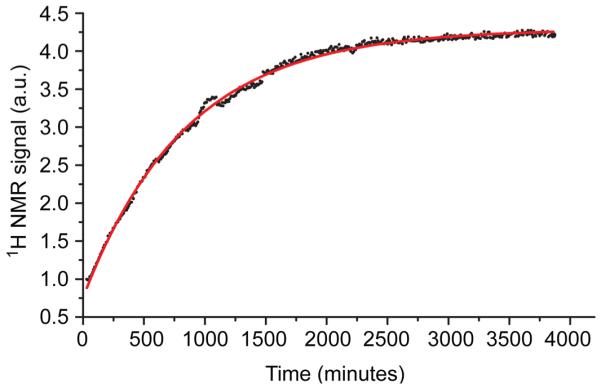
Decay of parahydrogen in a borosilicate NMR tube monitored by the recovery of orthohydrogen NMR signal.
1H NMR spectra were acquired within 10 minutes of filling the apparatus (Figure 2) with parahydrogen and again after 72 hours had elapsed (approximately 5 t1 periods). Percent enrichment was calculated as follows:
| (1) |
where S0 is the NMR signal recorded within 10 minutes after filling the NMR tube and Seq is the NMR signal from the sample after 5 t1 periods, corresponding to thermodynamic equilibration to 75% orthohydrogen. The utility of this procedure versus comparing the signal to equilibrium hydrogen gas is that the sample is quantified internally, over several points as it approaches equilibrium. If an external sample is used for quantification by comparison, differential pressure, temperature, or chemical environment could be different, and although the effects of either may amount to minimal or improbable loss in accuracy, the residual signal (from orthohydrogen) in the enriched sample is quite noisy (Figure 3), impeding precise integration.
Several physical attributes of parahydrogen differ in comparison to orthohydrogen and have been used to quantify enrichment to accuracies on the order of one percent. Enrichment has been quantified by thermal conductivity, acoustical properties, and Raman spectra. A review of techniques to quantify enrichment is outside the scope of this manuscript. Our approach is rather to report a simple NMR method suited to our equipment and expertise. The protocol is time-consuming but the measurements are needed only periodically. The intent is to capture data where the integrity can be inferred from the data itself, or in the case of a leaky sample, where the mechanism of failure can be deduced. A similarly straightforward alternative is to leverage the differences in thermal properties. Thermal conductivity differs by ~20 % in para versus orthohydrogen at 150 K [13; 14]. This approach has been used to quantify enrichment in gaseous mixtures [13; 14; 15] and appears to be effective in the temperature range of 80-250 K. Use of this approach requires that the thermal conductivity cell be chilled. This cell typically has a volume ~ 200 mL and with recurring calibration [16] is enrichment can be determined to a precision of 1-3 % using this method [15].
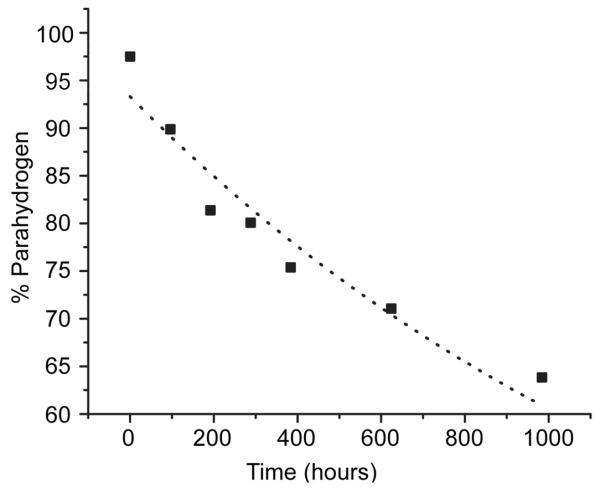
III.c. Kinetics of para- to orthohydrogen relaxation in aluminum storage tanks
The kinetics of para- to orthohydrogen relaxation in aluminum storage tanks were measured by sampling a small amount of hydrogen gas over a period commensurate with the slow relaxation kinetics in this system. NMR tubes were filled at low pressure with the method described above, and spectra were acquired over a period of 41 days (see Section II.b and II.c for a description of methods). The decay of parahydrogen to equilibrium in these aluminum storage tanks was fit to a mono-exponential decay with a time constant, t1 = 63.7 ± 8.3 days, R2 = 0.92. (dotted line, Figure 5). Techniques for customizing storage tanks to nearly eliminate back-conversion have been reported [14]. For the purposes of a parahydrogen polarization laboratory, the loss of 2 % enrichment per week is innocuous though, and affords the use of mass-produced tanks made available for storing nitrous oxide on race cars.
Figure 5.
The relaxation of para to orthohydrogen gas in an aluminum storage tank.
III.d. 13C PIP demonstration using PASADENA with in situ detection at 48 mT
To demonstrate the utility of parahydrogen for hyperpolarization, 13C spectra enhanced by the PASADENA effect [6; 17] were acquired in situ with a previously described polarizer [18], equipped with more sensitive 13C/1H MR coils (Coffey, A. M. et al. manuscript in preparation). 2-hydroxyethyl 1-13C-acrylate-d2,3,3 was used as the unsaturated PASADENA precursor to yield 13C hyperpolarized 2-hydroxyethyl 1-13C-propionate-d2,3,3. Polarization measurements were conducted using the method described earlier [18] and yielded approximately 20 % polarization (Figure 6).
Figure 6.
The hydrogenation of 2-hydroxyethyl 1-13C-acrylate-d2,3,3 with parahydrogen to produce hyperpolarized 2-hydroxyethyl 1-13C-propionate-d2,3,3. The reference 13C spectrum was obtained from 170 millimoles of saturated solution of sodium 1-13C-acetate dissolved in D2O at 13C Boltzmann polarization, 64 signal averages, and a recycle delay of 200 seconds. The 13C spectrum was recorded immediately after the parahydrogen-induced polarization procedure using a single scan acquisition from 15 micromoles of 2-hydroxyethyl 1-13C-propionate-d2,3,3 in approximately 4 mL 99.8% D2O. A slight shift in the static field evident from the left panel occurred between calibration and acquisition.
IV. Conclusions
The design and operation of an efficient parahydrogen generator as well as protocols for quantifying performance (enrichment) have been described. The device operates by injecting discrete aliquots of hydrogen gas at high pressure (300 psi) to a catalyst chamber maintained between 15 and 20 K and has routinely produced 98 % enrichment at a production rate of 0.9 SLM. When compared to metered continuous flow or restricted diffusion systems, this generator compares favorably in terms of dynamic range due to the separation of input charge density from flow rate. Arbitrarily long contact between input hydrogen and the catalyst chamber ensures thorough enrichment across a range of catalyst performances. Alternately, high input pressures are achieved without concomitant path resistance and can be used to drive production rates up to the limits of cryocooler heating capacity and catalyst conversion rates. The equipment is readily automated due to its reliance on the repeated application of small sets of solenoid valve actuations. It is durable, uses standard components with widespread availability, and has proven capable of fast, reliable production for more than three years in our laboratory. We anticipate that the equipment, along with the practical aspects of quantification and storage kinetics will be useful for new investigators planning to use parahydrogen as a polarization reservoir.
Highlights.
>We present the design and performance of a pulsed injection parahydrogen generator.
> We present a simple protocol for quantifying enrichment.
> We investigate relaxation kinetics in mass-produced aluminum storage tanks.
Acknowledgments
We gratefully acknowledge support from the National Institutes of Health R00 (1R00CA134749, 3R00CA134749-02S1), the National Cancer Institute (R25 CA136440, ICMIC 5P50 CA128323-03) and Prevent Cancer Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
V. References
- [1].Lupo JM, Chen AP, Zierhut ML, Bok RA, Cunningham CH, Kurhanewicz J, Vigneron DB, Nelson SJ. Analysis of hyperpolarized dynamic C-13 lactate imaging in a transgenic mouse model of prostate cancer. Magnetic Resonance Imaging. 2010;28:153–162. doi: 10.1016/j.mri.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Day SE, Kettunen MI, Gallagher FA, Hu DE, Lerche M, Wolber J, Golman K, Ardenkjaer-Larsen JH, Brindle KM. Detecting tumor response to treatment using hyperpolarized C-13 magnetic resonance imaging and spectroscopy. Nature Medicine. 2007;13:1382–1387. doi: 10.1038/nm1650. [DOI] [PubMed] [Google Scholar]
- [3].Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Golman K, Axelsson O, Johannesson H, Mansson S, Olofsson C, Petersson JS. Parahydrogen-induced polarization in imaging: Subsecond C-13 angiography. Magnetic Resonance in Medicine. 2001;46:1–5. doi: 10.1002/mrm.1152. [DOI] [PubMed] [Google Scholar]
- [5].Kurhanewicz J, Vigneron DB, Brindle K, Chekmenev EY, Comment A, Cunningham CH, DeBerardinis RJ, Green GG, Leach MO, Rajan SS, Rizi RR, Ross BD, Warren WS, Malloy CR. Analysis of Cancer Metabolism by Imaging Hyperpolarized Nuclei: Prospects for Translation to Clinical Research Neoplasia. 2011;13:81–97. doi: 10.1593/neo.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bowers CR, Weitekamp DP. Para-Hydrogen and Synthesis Allow Dramatically Enhanced Nuclear Alignment. Journal of the American Chemical Society. 1987;109:5541–5542. [Google Scholar]
- [7].Adams RW, Aguilar JA, Atkinson KD, Cowley MJ, Elliott PIP, Duckett SB, Green GGR, Khazal IG, Lopez-Serrano J, Williamson DC. Reversible Interactions with para-Hydrogen Enhance NMR Sensitivity by Polarization Transfer. Science. 2009;323:1708–1711. doi: 10.1126/science.1168877. [DOI] [PubMed] [Google Scholar]
- [8].Bhattacharya P, Chekmenev EY, Perman WH, Harris KC, Lin AP, Norton VA, Tan CT, Ross BD, Weitekamp DP. Towards hyperpolarized 13C-succinate imaging of brain cancer. Journal of Magnetic Resonance. 2007;186:150–155. doi: 10.1016/j.jmr.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chekmenev EY, Hovener J, Norton VA, Harris K, Batchelder LS, Bhattacharya P, Ross BD, Weitekamp DP. PASADENA hyperpolarization of succinic acid for MRI and NMR spectroscopy. Journal of the American Chemical Society. 2008;130:4212–4213. doi: 10.1021/ja7101218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gamliel A, Allouche-Arnon H, Nalbandian R, Barzilay CM, Gomori JM, Katz-Brull R. An Apparatus for Production of Isotopically and Spin-Enriched Hydrogen for Induced Polarization Studies. Applied Magnetic Resonance. 2010;39:329–345. [Google Scholar]
- [11].Fujiwara H, Yamabe J, Nishimura S. Determination of chemical shift of gas-phase hydrogen molecules by H-1 nuclear magnetic resonance. Chemical Physics Letters. 2010;498:42–44. [Google Scholar]
- [12].Bhattacharya P, Harris K, Lin AP, Mansson M, Norton VA, Perman WH, Weitekamp DP, Ross BD. Ultra-fast three dimensional imaging of hyperpolarized C-13 in vivo. Magnetic Resonance Materials in Physics Biology and Medicine. 2005;18:245–256. doi: 10.1007/s10334-005-0007-x. [DOI] [PubMed] [Google Scholar]
- [13].Bradshaw TW, Norris JOW. Observations On The Use of A Thermal-Conductivity Cell to Measure The Para Hydrogen Cconcentration In A Mixture of Para And Ortho Hydrogen Gas. Review of Scientific Instruments. 1987;58:83–85. [Google Scholar]
- [14].Stewart AT, Squires GL. Analysis of ortho- and para-hydrogen mixtures by the thermal conductivity method. Journal of Scientific Instruments. 1955;32:26. [Google Scholar]
- [15].Tam S, Fajardo ME. Ortho/para hydrogen converter for rapid deposition matrix isolation spectroscopy. Review of Scientific Instruments. 1999;70:1926–1932. [Google Scholar]
- [16].Tom BA, Bhasker S, Miyamoto Y, Momose T, McCall BJ. Producing and quantifying enriched para-H-2. Review of Scientific Instruments. 2009;80:3. doi: 10.1063/1.3072881. [DOI] [PubMed] [Google Scholar]
- [17].Bowers CR, Weitekamp DP. Transformation of Symmetrization Order to Nuclear-Spin Magnetization by Chemical-Reaction and Nuclear-Magnetic-Resonance. Physical Review Letters. 1986;57:2645–2648. doi: 10.1103/PhysRevLett.57.2645. [DOI] [PubMed] [Google Scholar]
- [18].Waddell KW, Coffey AM, Chekmenev EY. In situ Detection of PHIP at 48 mT: Demonstration using a Centrally Controlled Polarizer. Journal of the American Chemical Society. 2011;133:97–101. doi: 10.1021/ja108529m. [DOI] [PMC free article] [PubMed] [Google Scholar]