Abstract
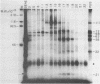
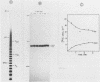
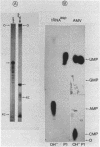
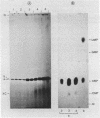
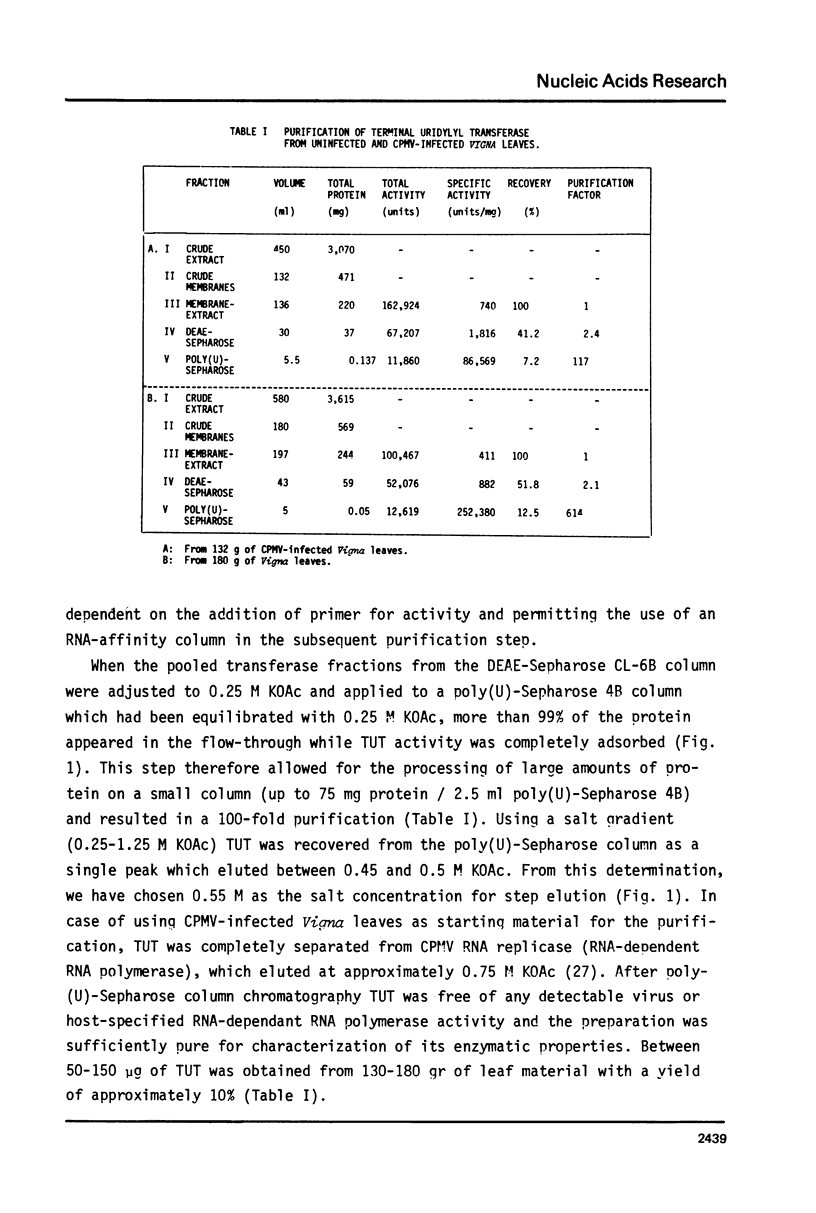
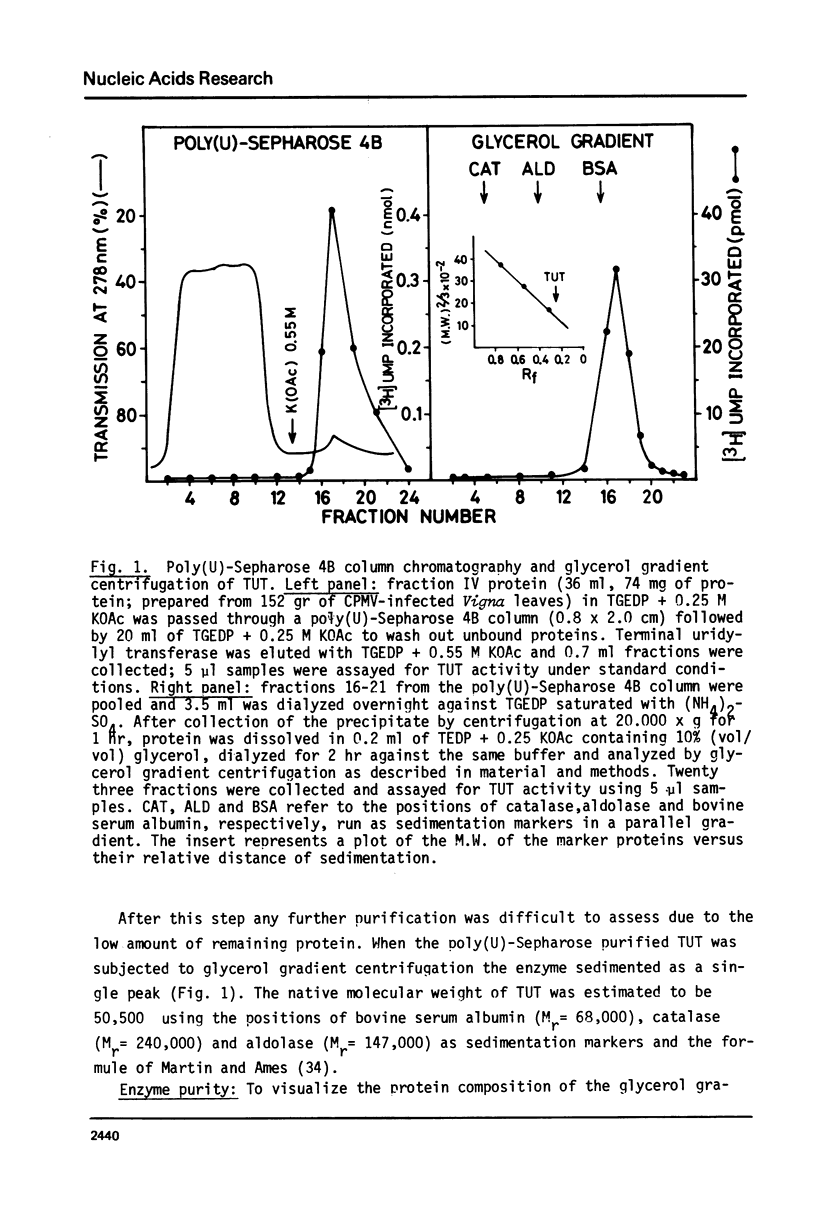
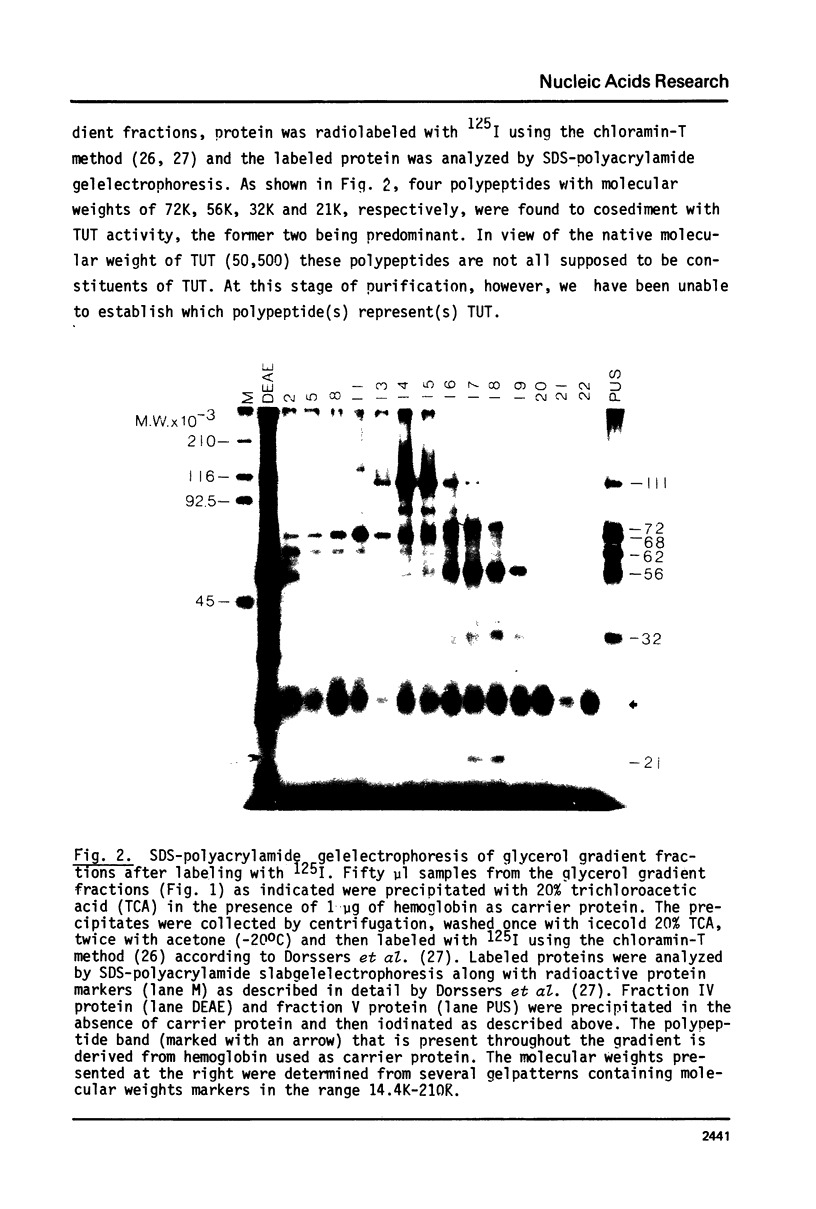
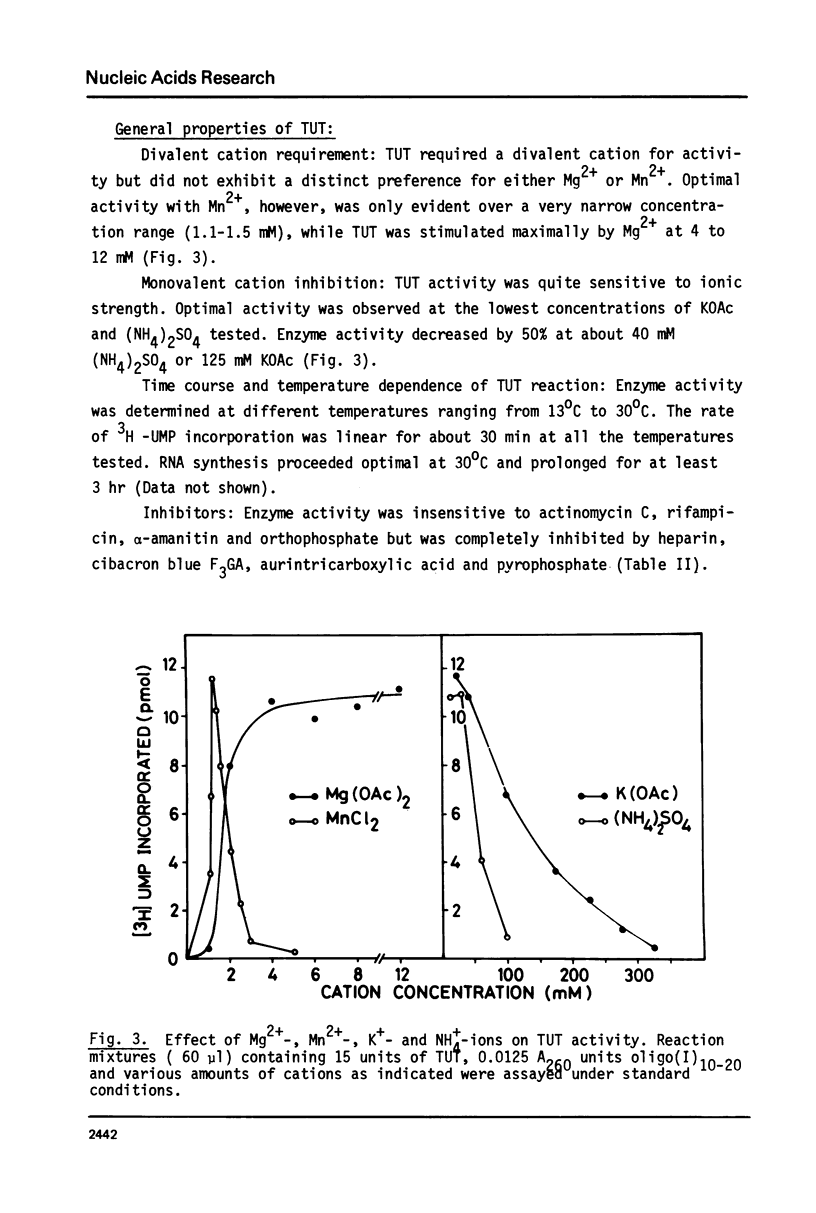
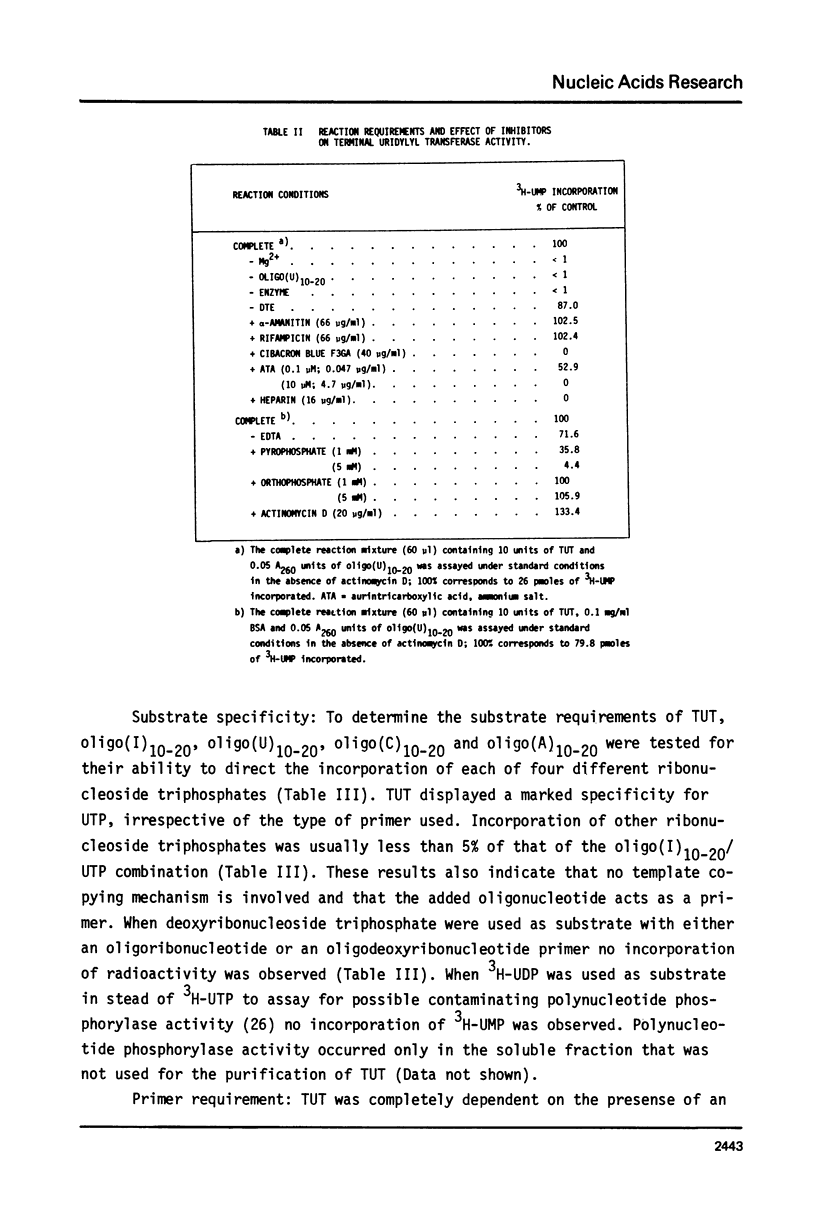
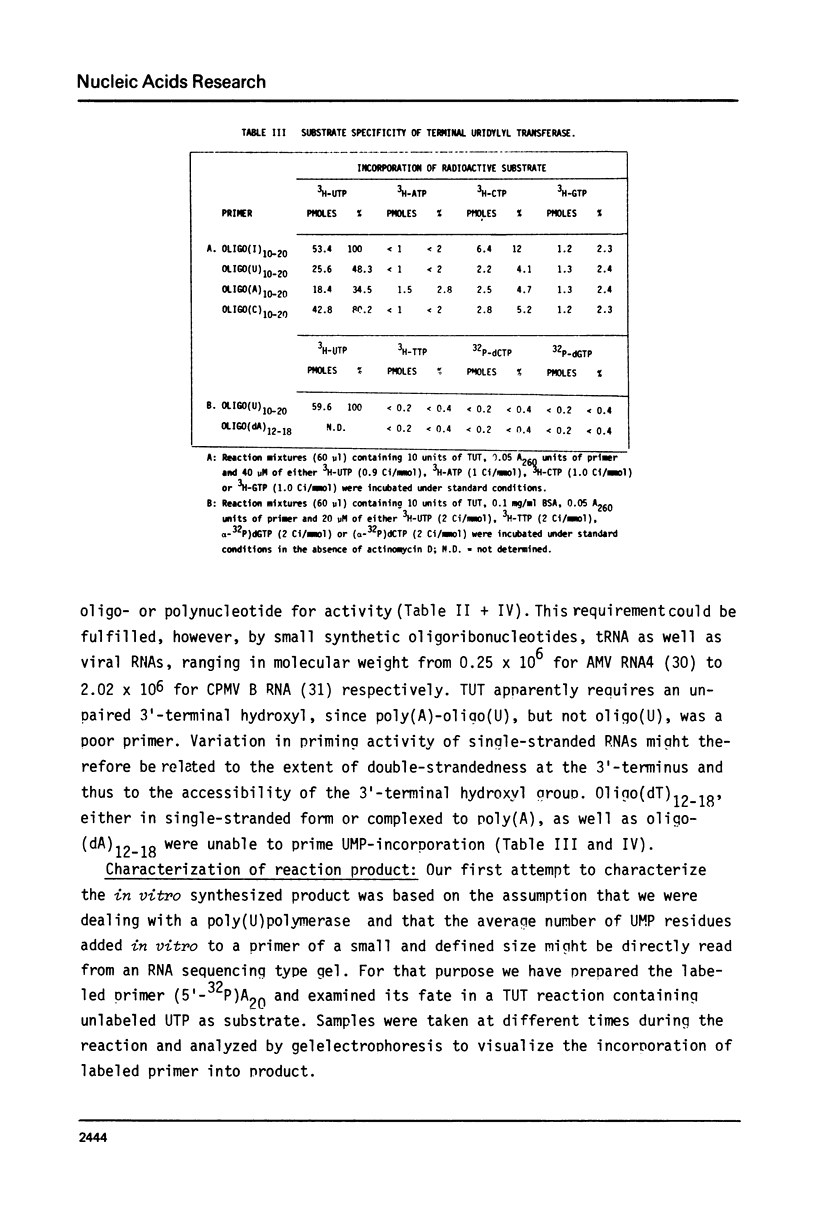
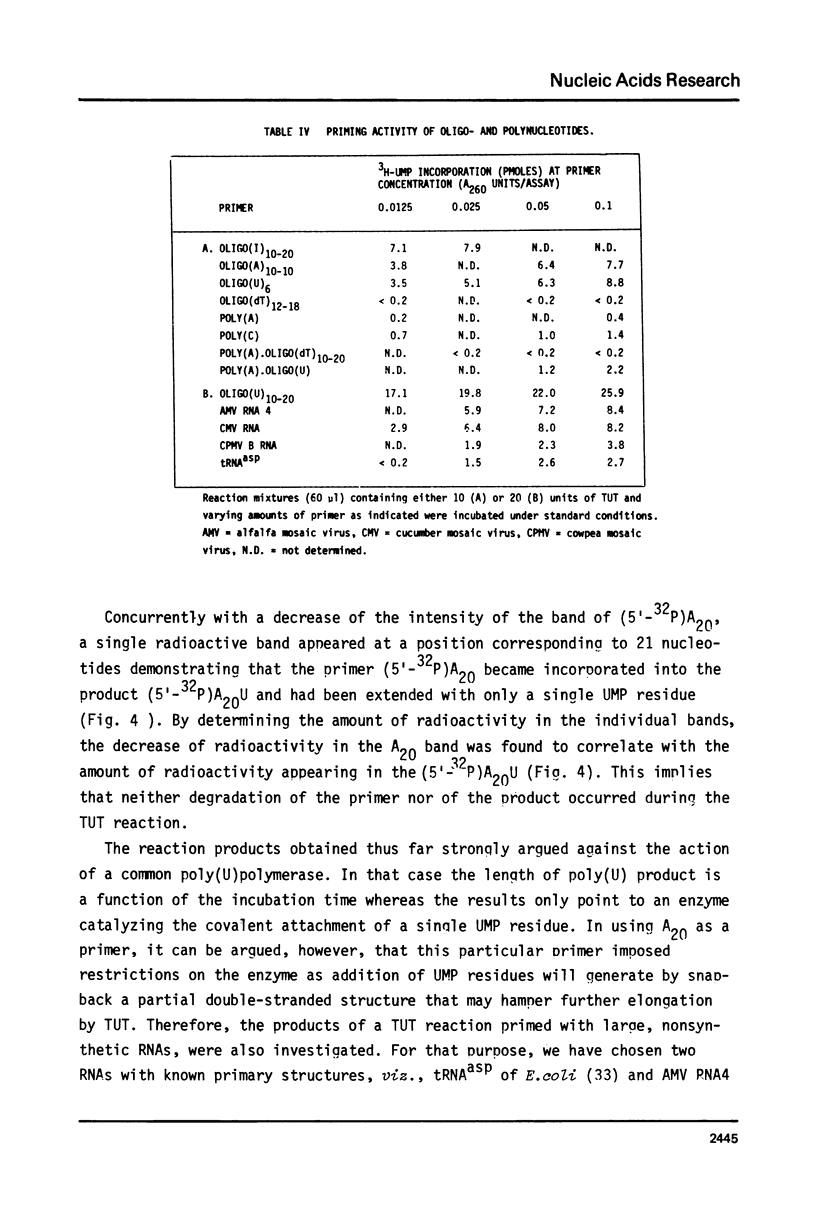
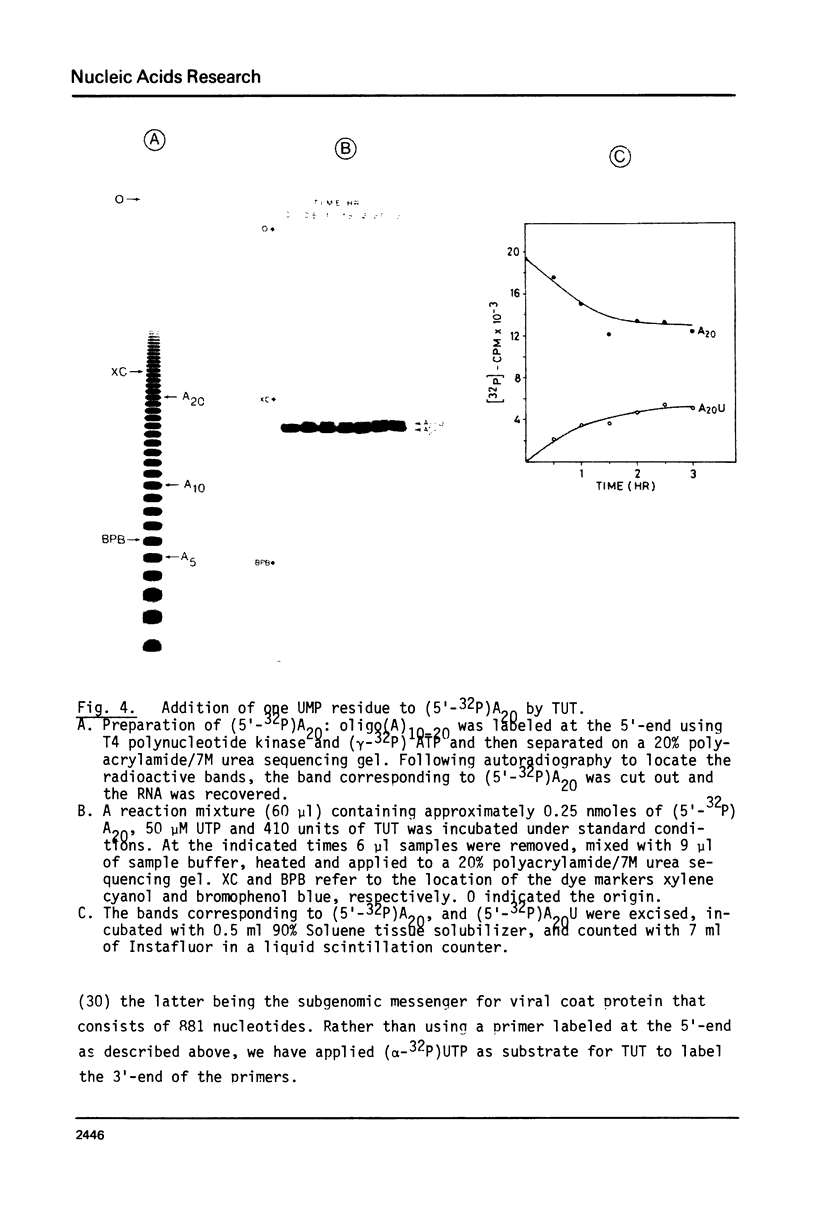
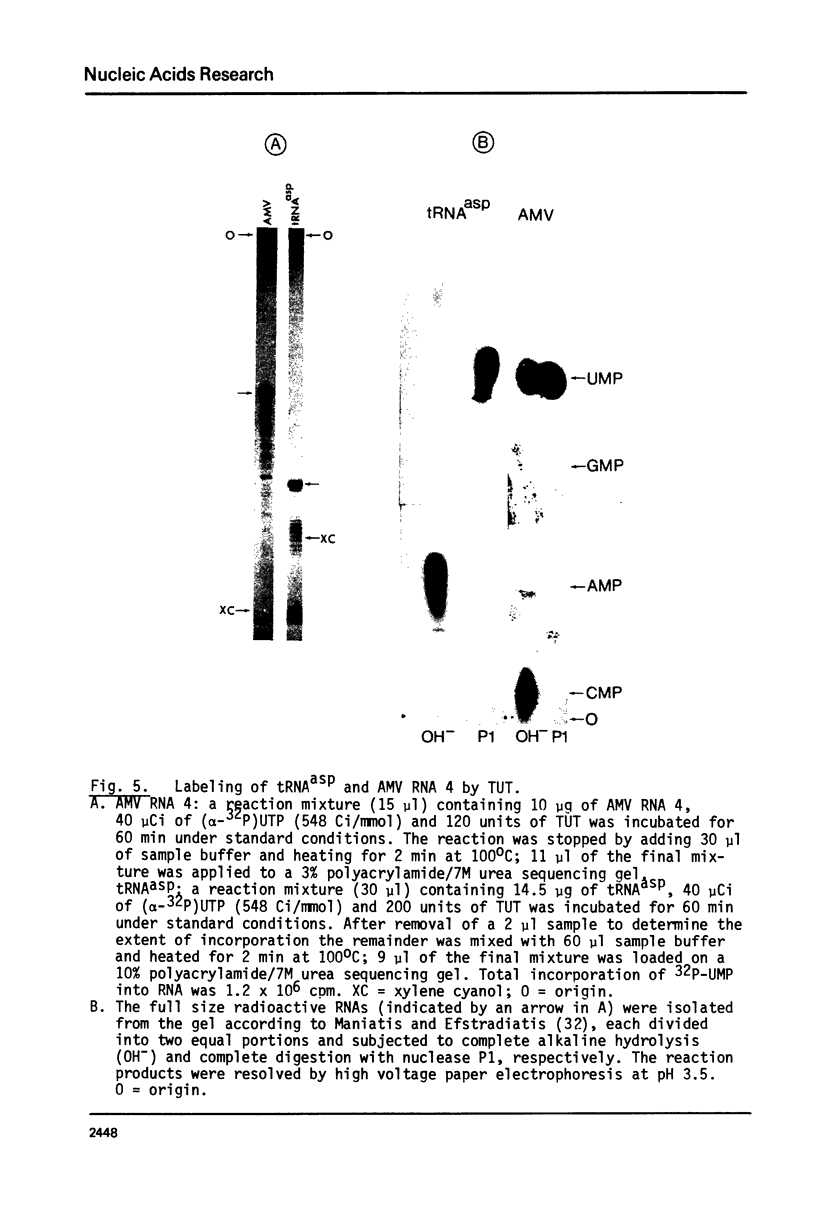
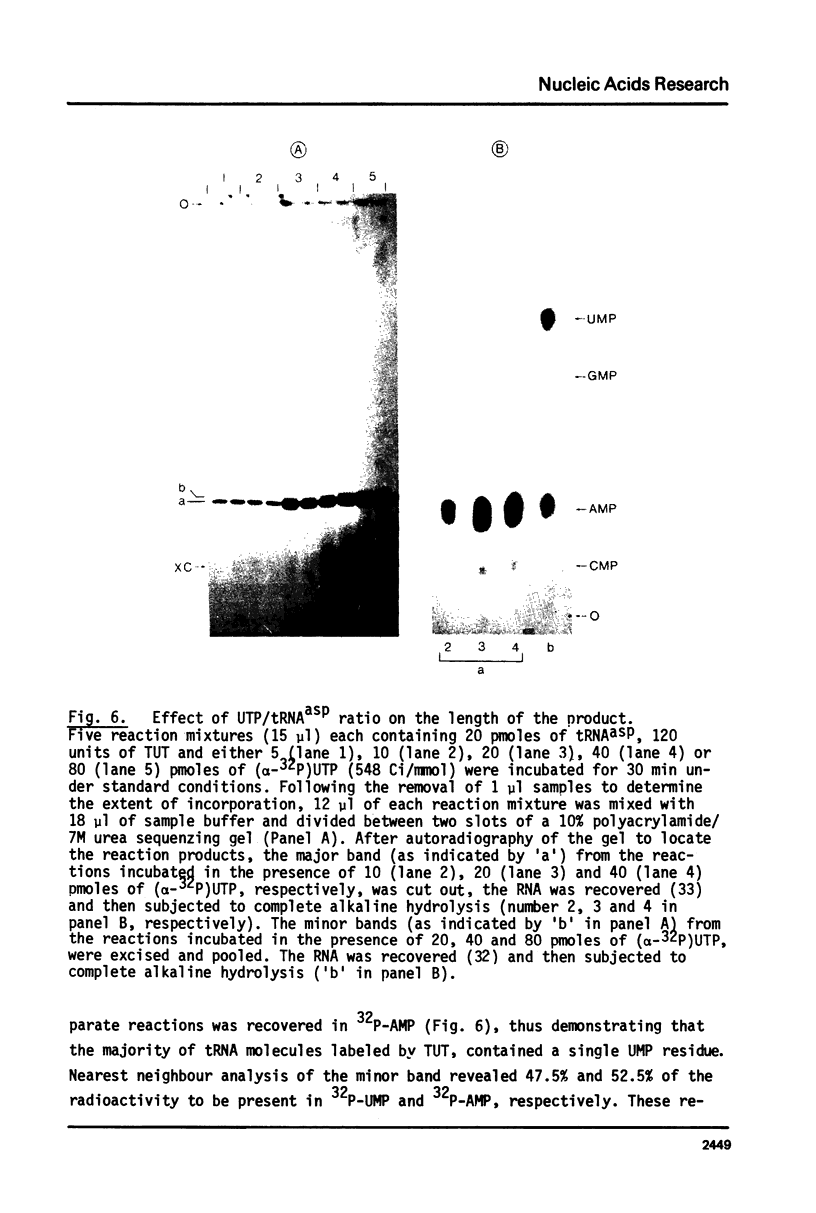
An enzyme which catalyzes the addition of a single UMP residue from UTP to the 3'-end of an RNA primer and which is referred to as terminal uridylyl transferase (TUT) has been extensively purified from the membrane fraction of vigna unguiculata leaves. The purification procedure involved (i) solubilization by cation depletion (ii) DEAE-Sepharose CL-6B column chromatography (iii) affinity chromatography of poly(U)-Sepharose 4B and (iv) glycerol gradient centrifugation. The molecular weight of the native enzyme was approximately 50,000 as determined by velocity sedimentation. Under conditions that were optimal for UMP-incorporation (5 mM Mg2+, low salt, 30 degrees C) TUT displayed a marked specificity for UTP as substrate, was unable to incorporate deoxyribonucleoside triphosphates and required a single-stranded oligo- or polyribonucleotide as primer. When oligoA20, tRNAasp of E. coli or alfalfa mosaic virus RNA 4 were used as primers at various substrate to primer ratio's, the vast majority of the product appeared to consist of primer molecules elongated with a single UMP residue as shown by polyacrylamide gelelectrophoresis and nearest neighbour analysis. We believe TUT to be a novel enzyme which has not been reported before and which may be a feasible tool in RNA sequencing as it enables the specific 3'-terminal labeling of RNA molecules.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brederode F. T., Koper-Zwarthoff E. C., Bol J. F. Complete nucleotide sequence of alfalfa mosaic virus RNA 4. Nucleic Acids Res. 1980 May 24;8(10):2213–2223. doi: 10.1093/nar/8.10.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brishammar S., Juntti N. A poly(U) polymerase in tobacco leaves. Biochim Biophys Acta. 1975 Apr 2;383(4):351–358. doi: 10.1016/0005-2787(75)90304-4. [DOI] [PubMed] [Google Scholar]
- Brishammar S., Juntti N. RNA-synthesizing enzymes in healthy and TMV-infected tobacco leaves. Partial purification and characterization to tobacco polynucleotide phosphorylase. Arch Biochem Biophys. 1974 Sep;164(1):224–232. doi: 10.1016/0003-9861(74)90026-5. [DOI] [PubMed] [Google Scholar]
- Burkard G., Keller E. B. Poly(A) polymerase and poly(g) polymerase in wheat chloroplasts. Proc Natl Acad Sci U S A. 1974 Feb;71(2):389–393. doi: 10.1073/pnas.71.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. S. A critical comparison of commonly used procedures for the assay of terminal deoxynucleotidyl transferase in crude tissue extracts. Nucleic Acids Res. 1977 Dec;4(12):4305–4312. doi: 10.1093/nar/4.12.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta A., Baron M. H., Baltimore D. Poliovirus replicase: a soluble enzyme able to initiate copying of poliovirus RNA. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2679–2683. doi: 10.1073/pnas.76.6.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta A., Zabel P., Baltimore D. Dependence of the activity of the poliovirus replicase on the host cell protein. Cell. 1980 Feb;19(2):423–429. doi: 10.1016/0092-8674(80)90516-4. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Jendrisak J. J., Burgess R. R. Templates for eukaryotic RNA polymerase II: artefacts can produce an apparent preference for denatured DNA over native DNA. Anal Biochem. 1977 May 1;79(1-2):181–189. doi: 10.1016/0003-2697(77)90392-x. [DOI] [PubMed] [Google Scholar]
- Edmonds M. A cytidine triphosphate polymerase from thymus nuclei. 1. Purification and properties of the enzyme and its polynucleotide primer. J Biol Chem. 1965 Dec;240(12):4621–4628. [PubMed] [Google Scholar]
- Edmonds M., Winters M. A. Polyadenylate polymerases. Prog Nucleic Acid Res Mol Biol. 1976;17:149–179. doi: 10.1016/s0079-6603(08)60069-0. [DOI] [PubMed] [Google Scholar]
- Flanegan J. B., Baltimore D. Poliovirus polyuridylic acid polymerase and RNA replicase have the same viral polypeptide. J Virol. 1979 Jan;29(1):352–360. doi: 10.1128/jvi.29.1.352-360.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanegan J. B., Baltimore D. Poliovirus-specific primer-dependent RNA polymerase able to copy poly(A). Proc Natl Acad Sci U S A. 1977 Sep;74(9):3677–3680. doi: 10.1073/pnas.74.9.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukasawa H., Mizuochi T. DNA-dependent poly (G) synthesizing activities in DNA-dependent RNA polymerase fractions from cauliflower inflorescence. Biochem Biophys Res Commun. 1974 May 20;58(2):405–411. doi: 10.1016/0006-291x(74)90379-9. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Yamaizumi K., Nishimura S. Oligonucleotide sequences of RNase T 1 and pancreatic RNase digests of E. coli aspartic acid tRNA. Biochem Biophys Res Commun. 1972 Dec 18;49(6):1605–1609. doi: 10.1016/0006-291x(72)90525-6. [DOI] [PubMed] [Google Scholar]
- Hibi T., Rezelman G., Van Kammen A. Infection of cowpea mesophyll protoplasts with cowpea mosaic virus. Virology. 1975 Apr;64(2):308–318. doi: 10.1016/0042-6822(75)90107-5. [DOI] [PubMed] [Google Scholar]
- Hozumi N., Haruna I., Watanabe I., Mikoshiba K., Tsukada Y. Poly(U) polymerase in rat brain. Nature. 1975 Jul 24;256(5515):337–339. doi: 10.1038/256337a0. [DOI] [PubMed] [Google Scholar]
- KLEMPERER H. G. The incorporation of uridine 5'-phosphate into ribonucleic acid by an enzyme preparation from rat liver. Biochim Biophys Acta. 1963 Jul 30;72:403–415. [PubMed] [Google Scholar]
- Leung W. C., Leung M. F., Rawls W. E. Distinctive RNA transcriptase, polyadenylic acid polymerase, and polyuridylic acid polymerase activities associated with Pichinde virus. J Virol. 1979 Apr;30(1):98–107. doi: 10.1128/jvi.30.1.98-107.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Maniatis T., Efstratiadis A. Fractionation of low molecular weight DNA or RNA in polyacrylamide gels containing 98% formamide or 7 M urea. Methods Enzymol. 1980;65(1):299–305. doi: 10.1016/s0076-6879(80)65040-x. [DOI] [PubMed] [Google Scholar]
- Milchev G. I., Hadjiolov A. A. Association of poly(A) and poly(U) polymerases with cytoplasmic ribosomes. Eur J Biochem. 1978 Mar;84(1):113–121. doi: 10.1111/j.1432-1033.1978.tb12147.x. [DOI] [PubMed] [Google Scholar]
- Reijnders L., Aalbers A. M., van Kammen A., Thuring R. W. Molecular weights of plant viral RNAs determined by gel electrophoresis under denaturing conditions. Virology. 1974 Aug;60(2):515–521. doi: 10.1016/0042-6822(74)90345-6. [DOI] [PubMed] [Google Scholar]
- Rose K. M., Jacob S. T. Nuclear poly(A) polymerase from rat liver and a hepatoma. Comparison of properties, molecular weights and amino acid compositions. Eur J Biochem. 1976 Aug 1;67(1):11–21. doi: 10.1111/j.1432-1033.1976.tb10626.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Wilkie N. M., Smellie R. M. Chain extension of ribonucleic acid by enzymes from rat liver cytoplasm. Biochem J. 1968 Oct;109(4):485–494. doi: 10.1042/bj1090485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie N. M., Smellie R. M. Polyribonucleotide synthesis by subfractions of microsomes from rat liver. Biochem J. 1968 Sep;109(2):229–238. doi: 10.1042/bj1090229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel P., Jongen-Neven I., Van Krammen A. In vitro replication of cowpea mosaic virus RNA. II. Solubilization of membrane-bound replicase and the partial purification of the solubilized enzyme. J Virol. 1976 Mar;17(3):679–685. doi: 10.1128/jvi.17.3.679-685.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel P., Jongen-Neven I., van Kammen A. In Vitro Replication of Cowpea Mosaic Virus RNA III. Template Recognition by Cowpea Mosaic Virus RNA Replicase. J Virol. 1979 Jan;29(1):21–33. doi: 10.1128/jvi.29.1.21-33.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel P., Weenen-Swaans H., van Kammen A. In vitro replication of cowpea mosaic virus RNA: I. Isolation and properties of the membrane-bound replicase. J Virol. 1974 Nov;14(5):1049–1055. doi: 10.1128/jvi.14.5.1049-1055.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos R. C., de Regt V. C., Brand R. C. A rapid method for desalting and concentrating oligonucleotide solutions. Anal Biochem. 1971 Jun;41(2):293–296. doi: 10.1016/0003-2697(71)90145-x. [DOI] [PubMed] [Google Scholar]