Abstract
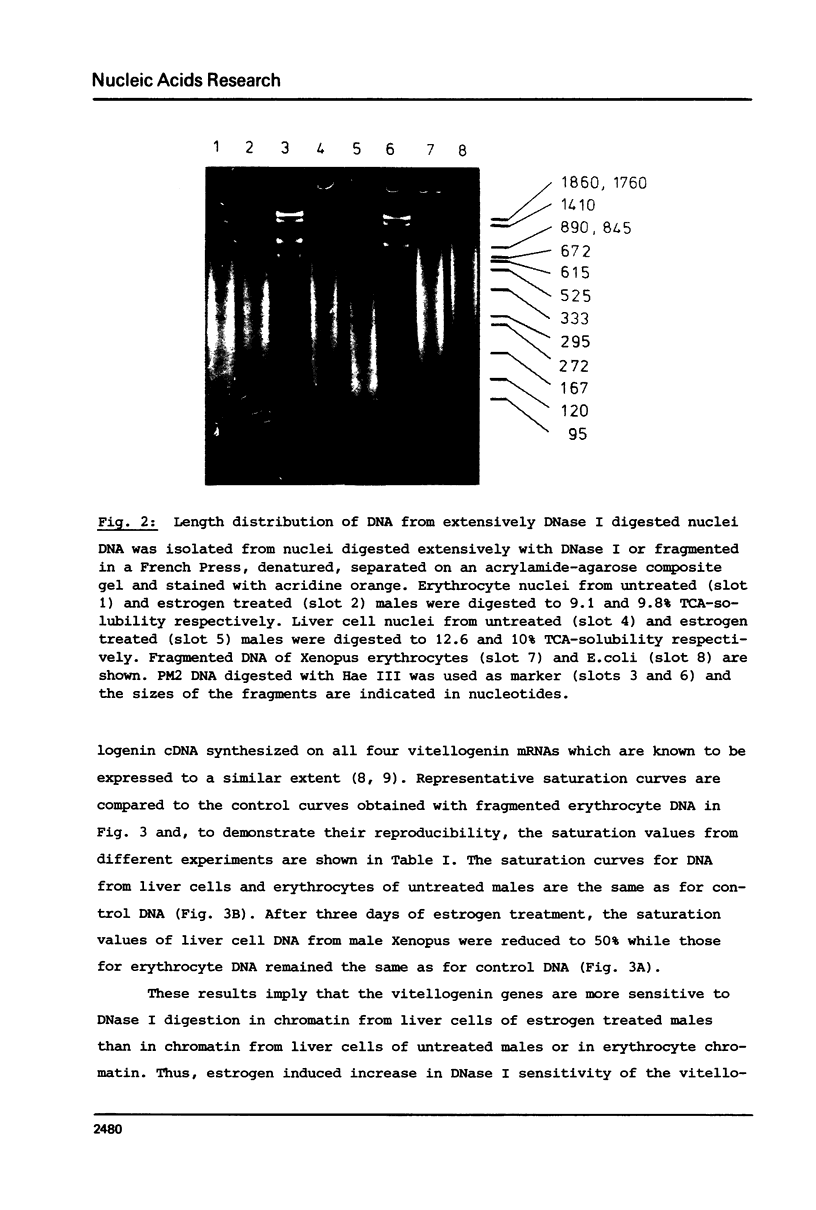
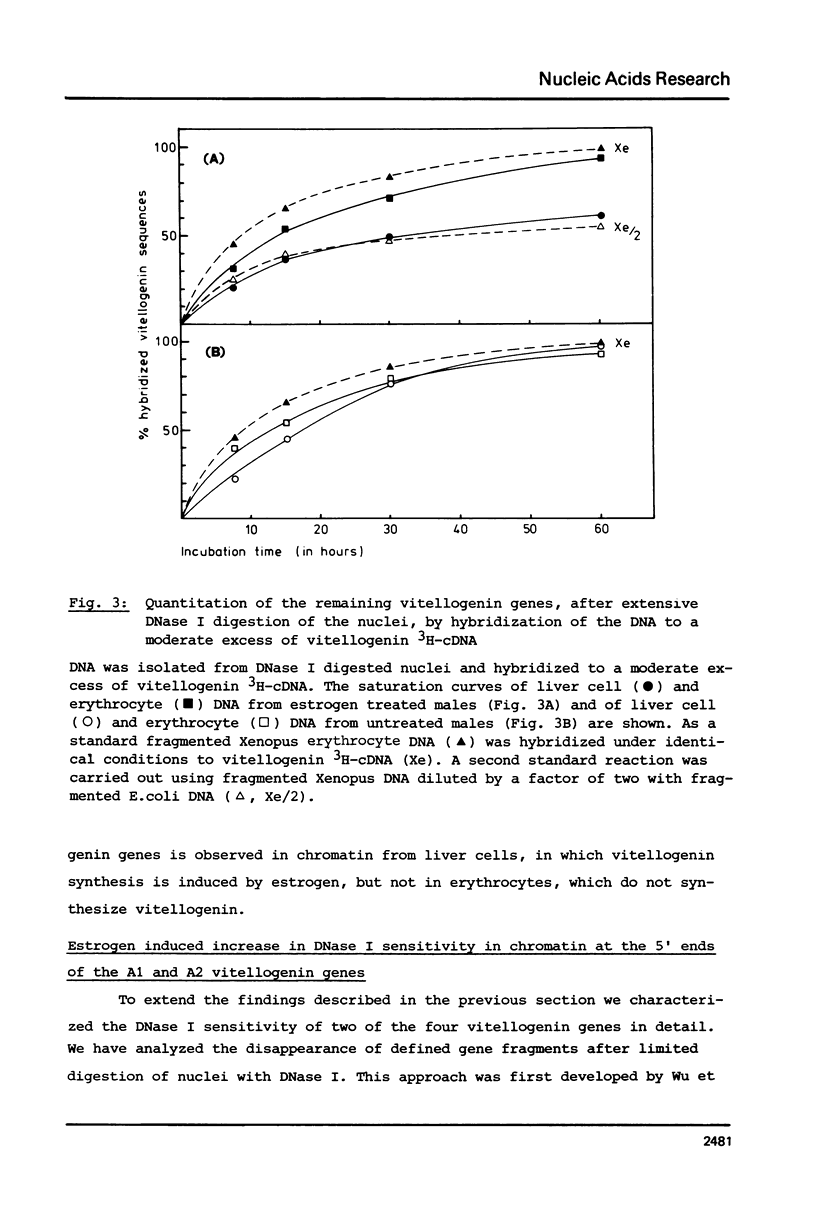
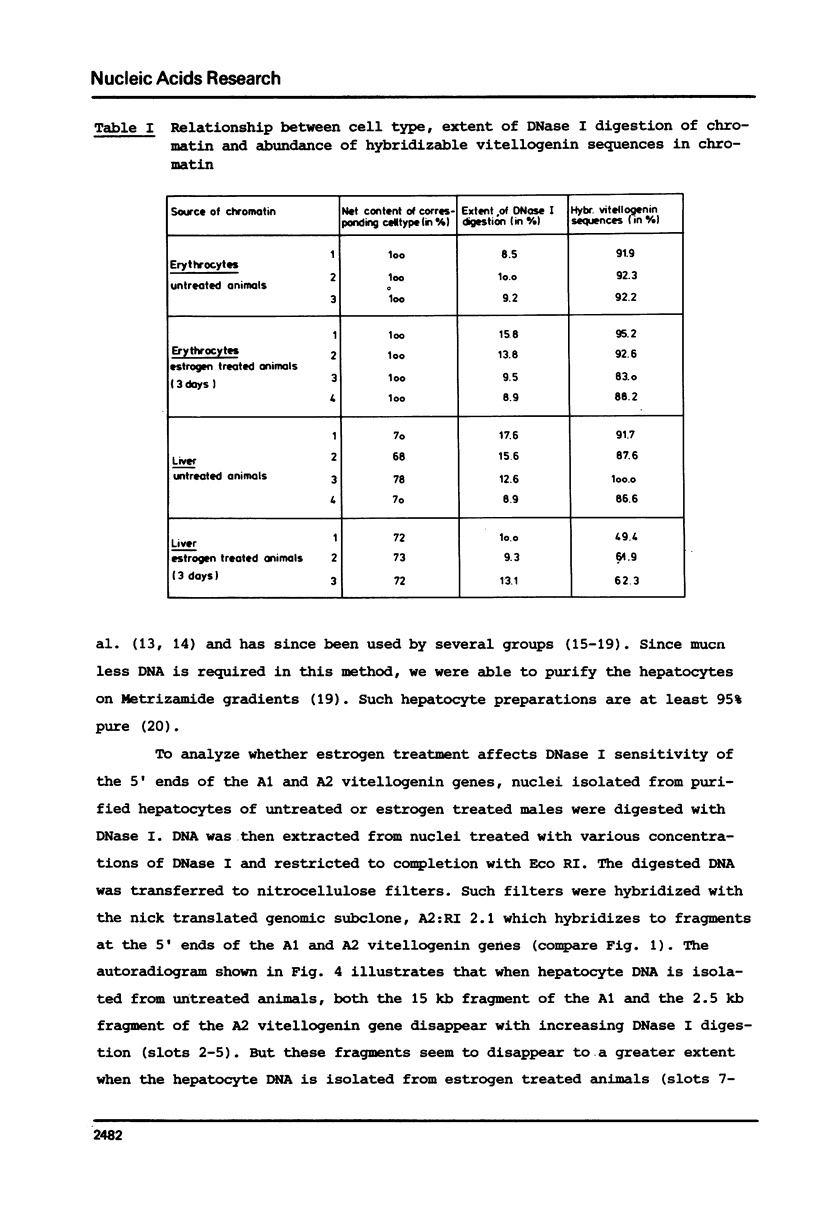
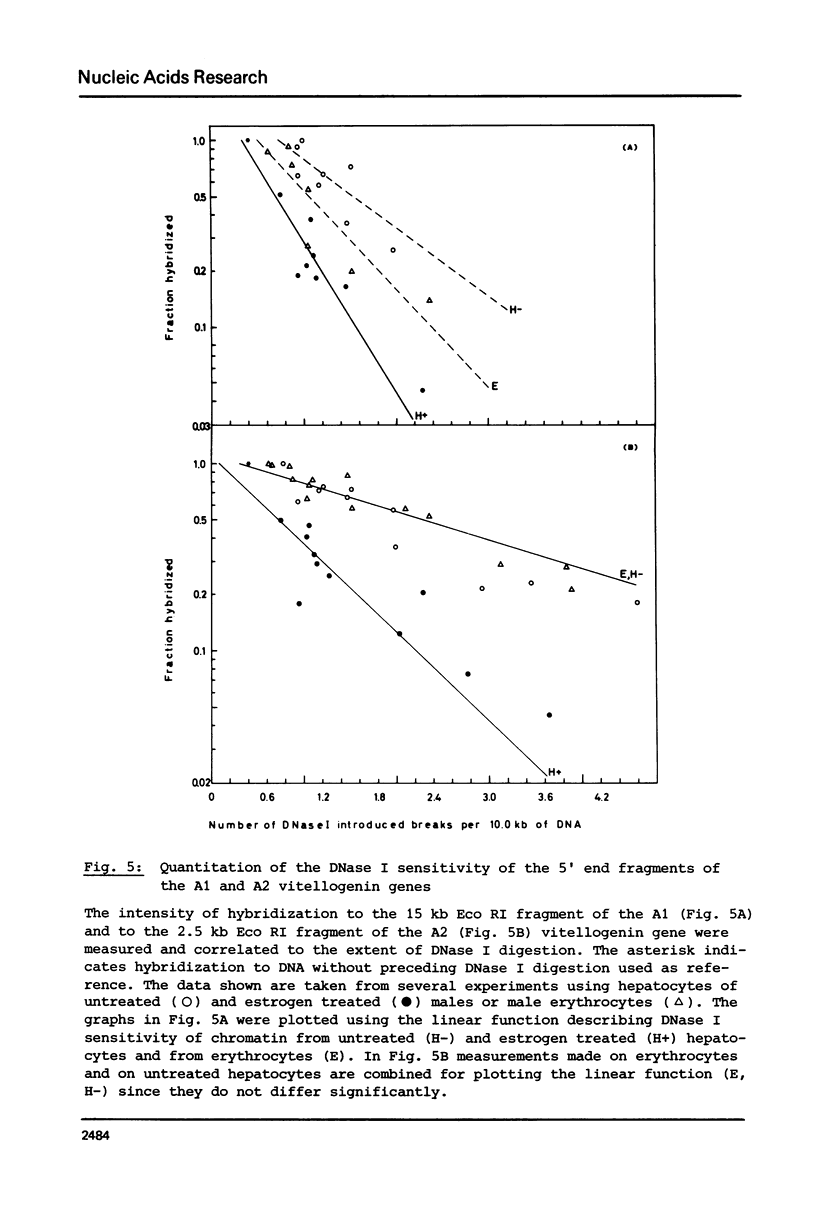
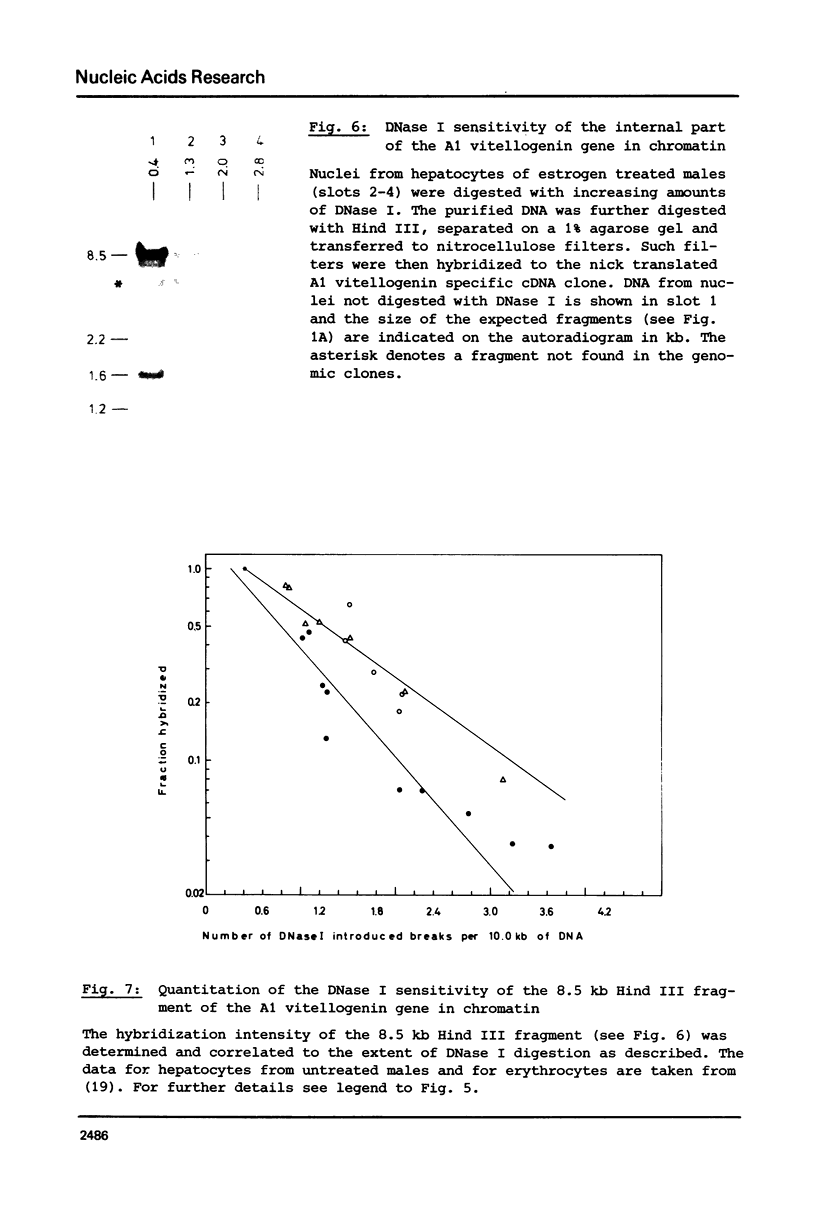
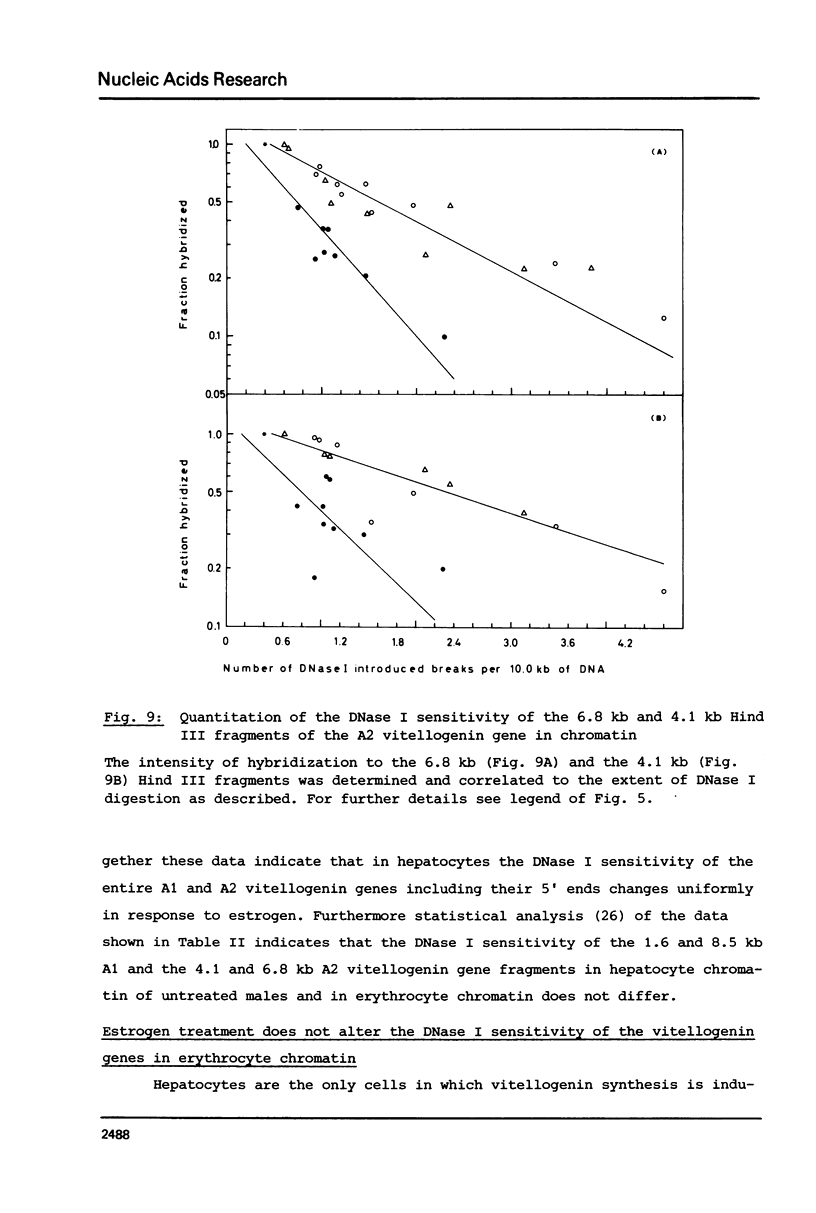
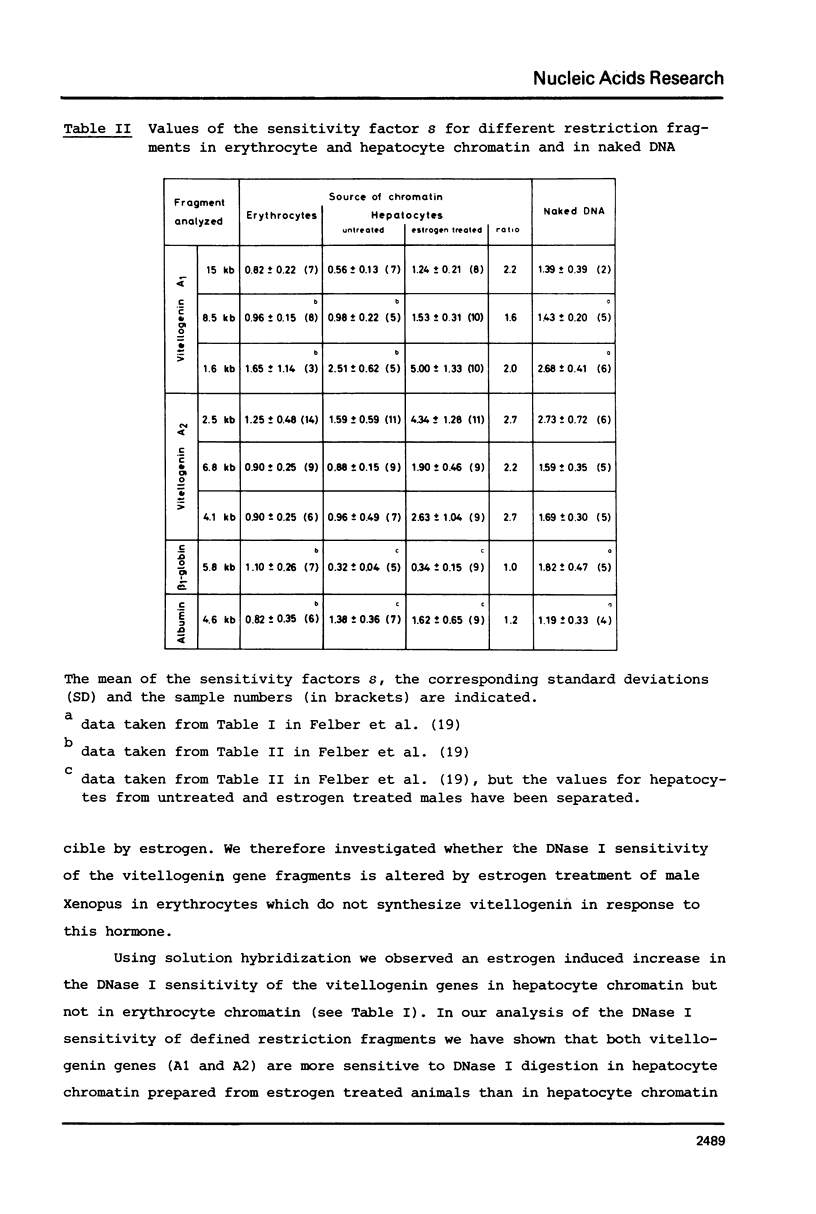
Nuclei from male Xenopus liver were digested extensively with DNase I and the residual amount of the four vitellogenin genes measured by hybridization with a moderate excess of vitellogenin cDNA. The saturation value was about twofold lower in chromatin isolated from liver cells of estrogen treated than from untreated males or from erythrocytes. Analyzing the disappearance of several defined restriction fragments specific for the A1 and A2 vitellogenin genes, after limited digestion with DNase I, suggested that the entire A1 and A2 vitellogenin genes are about twofold more sensitive to DNase I in chromatin of hepatocytes isolated from estrogen treated than from untreated males. Using the same assay no change in the DNase I sensitivity of the two vitellogenin genes in erythrocyte chromatin was observed. Analysis of the beta 1-globin and an albumin gene demonstrated that the DNase I sensitivity of these genes in both cell types is not altered by estrogen. All these data indicate that estrogen stimulation results in an increased DNase I sensitivity specific for the vitellogenin genes in hepatocytes.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Baker H. J., Shapiro D. J. Kinetics of estrogen induction of Xenopus laevis vitellogenin messenger RNA as measured by hybridization to complementary DNA. J Biol Chem. 1977 Dec 10;252(23):8428–8434. [PubMed] [Google Scholar]
- Bellard M., Kuo M. T., Dretzen G., Chambon P. Differential nuclease sensitivity of the ovalbumin and beta-globin chromatin regions in erythrocytes and oviduct cells of laying hen. Nucleic Acids Res. 1980 Jun 25;8(12):2737–2750. doi: 10.1093/nar/8.12.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer S. R., Henshaw E. C., Berridge M. V., Tata J. R. Translation of Xenopus vitellogenin mRNA during primary and secondary induction. Nature. 1978 Jun 1;273(5661):401–403. doi: 10.1038/273401a0. [DOI] [PubMed] [Google Scholar]
- Felber B. K., Maurhofer S., Jaggi R. B., Wyler T., Wahli W., Ryffel G. U., Weber R. Isolation and translation in vitro of four related vitellogenin mRNAs of estrogen-stimulated Xenopus laevis. Eur J Biochem. 1980 Mar;105(1):17–24. doi: 10.1111/j.1432-1033.1980.tb04469.x. [DOI] [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C. D., Tata J. R. Direct induction by estradiol on vitellogenin synthesis in organ cultures of male Xenopus laevis liver. Cell. 1976 Jan;7(1):131–139. doi: 10.1016/0092-8674(76)90263-4. [DOI] [PubMed] [Google Scholar]
- Huber S., Ryffel G. U., Weber R. Thyroid hormone induces competence for oestrogen-dependent vitellogenin synthesis in developing Xenopus laevis liver. Nature. 1979 Mar 1;278(5699):65–67. doi: 10.1038/278065a0. [DOI] [PubMed] [Google Scholar]
- Jaggi R. B., Felber B. K., Maurhofer S., Weber R., Ryffel G. U. Four different vitellogenin proteins of Xenopus identified by translation in vitro. Eur J Biochem. 1980 Aug;109(2):343–347. doi: 10.1111/j.1432-1033.1980.tb04800.x. [DOI] [PubMed] [Google Scholar]
- Kuo M. T., Mandel J. L., Chambon P. DNA methylation: correlation with DNase I sensitivity of chicken ovalbumin and conalbumin chromatin. Nucleic Acids Res. 1979 Dec 20;7(8):2105–2113. doi: 10.1093/nar/7.8.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson G. M., Tsai M. J., O'Malley B. W. Deoxyribonuclease I sensitivity of the nontranscribed sequences flanking the 5' and 3' ends of the ovomucoid gene and the ovalbumin and its related X and Y genes in hen oviduct nuclei. Biochemistry. 1980 Sep 16;19(19):4403–4441. doi: 10.1021/bi00560a004. [DOI] [PubMed] [Google Scholar]
- Mathis D., Oudet P., Chambon P. Structure of transcribing chromatin. Prog Nucleic Acid Res Mol Biol. 1980;24:1–55. doi: 10.1016/s0079-6603(08)60670-4. [DOI] [PubMed] [Google Scholar]
- May F. E., Knowland J. The role of thyroxine in the transition of vitellogenin synthesis from noninducibility to inducibility during metamorphosis in Xenopus laevis. Dev Biol. 1980 Jun 15;77(2):419–430. doi: 10.1016/0012-1606(80)90485-6. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. M., Turner P., Nienhuis A. W., Axelrod D. E., Gopalakrishnan T. V. Active conformation of the globin genes in uninduced and induced mouse erythroleukemia cells. Cell. 1978 Jul;14(3):511–521. doi: 10.1016/0092-8674(78)90237-4. [DOI] [PubMed] [Google Scholar]
- Ryffel G. U., McCarthy B. J. Complexity of cytoplasmic RNA in different mouse tissues measured by hybridization of polyadenylated RNA to complementary DNA. Biochemistry. 1975 Apr 8;14(7):1379–1385. doi: 10.1021/bi00678a006. [DOI] [PubMed] [Google Scholar]
- Ryffel G. U. Synthesis of vitellogenin, an attractive model for investigating hormone-induced gene activation. Mol Cell Endocrinol. 1978 Dec;12(3):237–246. doi: 10.1016/0303-7207(78)90082-5. [DOI] [PubMed] [Google Scholar]
- Ryffel G. U., Wahli W., Weber R. Quantitation of vitellogenin messenger RNA in the liver of male Xenopus toads during primary and secondary stimulation by estrogen. Cell. 1977 May;11(1):213–221. doi: 10.1016/0092-8674(77)90332-4. [DOI] [PubMed] [Google Scholar]
- Stalder J., Groudine M., Dodgson J. B., Engel J. D., Weintraub H. Hb switching in chickens. Cell. 1980 Apr;19(4):973–980. doi: 10.1016/0092-8674(80)90088-4. [DOI] [PubMed] [Google Scholar]
- Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell. 1980 Jun;20(2):451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- Tata J. R., Baker B. S., Deeley J. V. Vitellogenin as a multigene family. Not all Xenopus vitellogenin genes may be in an "expressible" configuration. J Biol Chem. 1980 Jul 25;255(14):6721–6726. [PubMed] [Google Scholar]
- Tata J. R., Smith D. F. Vitellogenesis: a versatile model for hormonal regulation of gene expression. Recent Prog Horm Res. 1979;35:47–95. doi: 10.1016/b978-0-12-571135-7.50006-0. [DOI] [PubMed] [Google Scholar]
- Wahli W., Dawid I. B., Wyler T., Jaggi R. B., Weber R., Ryffel G. U. Vitellogenin in Xenopus laevis is encoded in a small family of genes. Cell. 1979 Mar;16(3):535–549. doi: 10.1016/0092-8674(79)90028-x. [DOI] [PubMed] [Google Scholar]
- Wahli W., Dawid I. B., Wyler T., Weber R., Ryffel G. U. Comparative analysis of the structural organization of two closely related vitellogenin genes in X. laevis. Cell. 1980 May;20(1):107–117. doi: 10.1016/0092-8674(80)90239-1. [DOI] [PubMed] [Google Scholar]
- Wangh L. J., Osborne J. A., Hentschel C. C., Tilly R. Parenchymal cells purified from Xenopus liver and maintained in primary culture synthesize vitellogenin in response to estradiol-17 beta and serum albumin in response to dexamethasone. Dev Biol. 1979 Jun;70(2):479–499. doi: 10.1016/0012-1606(79)90040-x. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Wu C., Bingham P. M., Livak K. J., Holmgren R., Elgin S. C. The chromatin structure of specific genes: I. Evidence for higher order domains of defined DNA sequence. Cell. 1979 Apr;16(4):797–806. doi: 10.1016/0092-8674(79)90095-3. [DOI] [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]
- Wu C., Wong Y. C., Elgin S. C. The chromatin structure of specific genes: II. Disruption of chromatin structure during gene activity. Cell. 1979 Apr;16(4):807–814. doi: 10.1016/0092-8674(79)90096-5. [DOI] [PubMed] [Google Scholar]
- Zasloff M., Camerini-Otero R. D. Limited DNase I nicking as a probe of gene conformation. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1907–1911. doi: 10.1073/pnas.77.4.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]