ABSTRACT
Porphyromonas gingivalis is a Gram-negative anaerobe that resides exclusively in the human oral cavity. Long-term colonization by P. gingivalis requires the bacteria to evade host immune responses while adapting to the changing host physiology and alterations in the composition of the oral microflora. The genetic diversity of P. gingivalis appears to reflect the variability of its habitat; however, little is known about the molecular mechanisms generating this diversity. Previously, our research group established that chromosomal DNA transfer occurs between P. gingivalis strains. In this study, we examine the role of putative DNA transfer genes in conjugation and transformation and demonstrate that natural competence mediated by comF is the dominant form of chromosomal DNA transfer, with transfer by a conjugation-like mechanism playing a minor role. Our results reveal that natural competence mechanisms are present in multiple strains of P. gingivalis, and DNA uptake is not sensitive to DNA source or modification status. Furthermore, extracellular DNA was observed for the first time in P. gingivalis biofilms and is predicted to be the major DNA source for horizontal transfer and allelic exchange between strains. We propose that exchange of DNA in plaque biofilms by a transformation-like process is of major ecological importance in the survival and persistence of P. gingivalis in the challenging oral environment.
IMPORTANCE
P. gingivalis colonizes the oral cavities of humans worldwide. The long-term persistence of these bacteria can lead to the development of chronic periodontitis and host morbidity associated with tooth loss. P. gingivalis is a genetically diverse species, and this variability is believed to contribute to its successful colonization and survival in diverse human hosts, as well as evasion of host immune defenses and immunization strategies. We establish here that natural competence is the major driving force behind P. gingivalis DNA exchange and that conjugative DNA transfer plays a minor role. Furthermore, we reveal for the first time the presence of extracellular DNA in P. gingivalis biofilms, which is most likely the source of DNA exchanged between strains within dental plaque. These studies expand our understanding of the mechanisms used by this important member of the human oral flora to transition its relationship with the host from a commensal to a pathogenic relationship.
Introduction
Horizontal DNA exchange between bacteria is a recognized adaptation mechanism to enhance bacterial survival during changing and stressful environmental conditions or during relocation from one distinct niche to another. Cooperatively with DNA replication errors, DNA exchange generates a “genomic cloud” of variants with phenotypic differences, which ensures survival of a portion of the population during a selection event, such as introduction of an antibiotic or a significant change in habitat conditions (1–4). The degree of genetic diversity found within a given bacterial species varies significantly, with some species exhibiting small amounts of diversity or a clonal population structure; in contrast, others are highly diverse, with a nonclonal population structure. Nonclonal populations are common to opportunistic human pathogens involved in chronic colonization and infections, with Helicobacter pylori and Neisseria meningitidis being perhaps the most notable examples (5, 6).
Porphyromonas gingivalis is a Gram-negative anaerobe that colonizes distinct niches in the human oral cavity, primarily the deep crypts of the tongue and the subgingival plaque biofilm (7). Stable colonization of the subgingival crevice by this oral pathogen is a major contributing factor in the development of host inflammatory responses and subsequent periodontal disease. P. gingivalis is ubiquitous within the human population and is commonly transmitted from older to younger generations within households (8–10). Successful transfer between distinct host niches likely requires this bacterium to have multiple strategies to rapidly adapt to the local environment.
There is extensive genetic variation among P. gingivalis strains, resulting in measurable phenotypic heterogeneity in behaviors ranging from biofilm formation (11) to virulence (12, 13). The population structure of P. gingivalis is nonclonal (14, 15), and it has been predicted that, as with other nonclonal opportunistic pathogens, horizontal DNA transfer is responsible for genetic variation between strains (16, 17). In a previous study using P. gingivalis type strains W83 and ATCC 33277 (18), we found that strain ATCC 33277, but not W83, is able to transfer plasmid DNA into Escherichia coli. Plasmid transfers were insensitive to treatment with DNase I, implying a mechanism most consistent with conjugation. We also documented that chromosomal DNA transfer occurs within and between P. gingivalis strains and hypothesized that the mechanism was similar to that of the plasmid transfer model (18).
Each of the genome sequences of strains W83 and ATCC 33277 (19, 20) contain genetic loci that encode homologues of the DNA transfer apparatus of the Bacteroides conjugative transposons cTnDot and cTn341 (21, 22). The Bacteroides DNA transfer locus contains 17 genes annotated alphabetically from traA to traQ (traA–Q), as well as a mobilization locus annotated as mob. The Bacteroides are the dominant anaerobes in the human intestinal flora and are close taxonomic relatives of the oral genera Porphyromonas and Prevotella (23). Extensive research has documented horizontal DNA transfer of antibiotic resistance genes throughout the human gut flora via conjugative transposons utilizing traA–Q-encoded transfer systems (24–28). Other members of phylum Bacteroidetes contain loci that encode homologues of traA–Q; however, outside the Bacteroides, the role of traA–Q is not defined. P. gingivalis type strains W83 and ATCC 33277 contain traA–Q regions that are genetically distinct from one another; in our previous study, we hypothesized that this sequence divergence was responsible for the differences in plasmid and chromosome DNA transfer frequencies between these two strains (18).
In this study, we examine the molecular mechanisms responsible for DNA exchange in the oral opportunistic pathogen P. gingivalis. Our initial working hypothesis was that P. gingivalis chromosomal DNA transfer was mediated by the traA–Q locus by a mechanism similar to chromosomal mobilization by cTns in the Bacteroides (29). However, in contrast to our expectations, we uncovered an efficient comF-dependent transformation system that is responsible for the majority of P. gingivalis DNA exchange.
RESULTS
traA–Q locus and DNA transfer.
Our initial working hypothesis was that conjugation mediated by the traA–Q locus was responsible for chromosomal DNA transfer in strain W83. To test this hypothesis, we created a W83 strain with a complete deletion of the locus traA–Q (PG1473 to PG1486) and the contiguous mobilization locus mobABC (PG1488 to PG1490) by allelic replacement of the locus with the ermF marker (see Fig. S1 in the supplemental material). Assuming the conjugation hypothesis is correct, the traA–Q-mob locus should be required for DNA transfer, as it is for Bacteroides (30). To create a chromosomal marker for the comparative analysis of DNA transfer, we inserted the tetQ gene into the intergenic region downstream of the PG1244 gene in wild-type W83 and in the traA–Q::ermF mutant, thus creating strains W83 PG1244 ΩtetQ (W83 Tetr, where Tetr indicates tetracycline resistance) and W83 PG1244 ΩtetQ traA–Q-mob::ermF (W83 Tetr Δtra). A second traA–Q deletion mutant was created in the W83 rifampin-resistant (Rifr) background, resulting in W83 Rifr Δtra (Table 1).
TABLE 1 .
Bacterial strains used in this study
| Strain | Genomic marker |
Mutation | Strain abbreviation |
Source |
|---|---|---|---|---|
| P. gingivalis W83 | W83 | ATCC BAA-308 | ||
| P. gingivalis ATCC 33277 | ATCC 33277 | ATCC 33277 | ||
| P. gingivalis ATCC 53977 | ATCC 53977 | ATCC 53977 | ||
| P. gingivalis W83 | Rifr | W83 Rifr | This study | |
| P. gingivalis W83 | PG1244 ΩtetQ | W83 Tetr | This study | |
| P. gingivalis W83 | PG1244 ΩtetQ | traA–Q-mob::ermF | W83 Tetr Δtra | This study |
| P. gingivalis W83 | Rifr | traA–Q-mob::ermF | W83 Rifr Δtra | This study |
| P. gingivalis W83 | PG1244 ΩtetQ | comF::ermF | W83 Tetr ΔcomF | This study |
| P. gingivalis W83 | Rifr | comF::ermF | W83 Rifr ΔcomF | This study |
| P. gingivalis ATCC 33277 | Rifr | ATCC 33277 Rifr | This study | |
| P. gingivalis ATCC 33277 | PG1244 ΩtetQ | ATCC 33277 Tetr | This study | |
| P. gingivalis ATCC 53977 | Rifr | ATCC 53977 Rifr | This study | |
| P. gingivalis ATCC 53977 | PG1244 ΩtetQ | ATCC 53977 Tetr | This study |
To examine the involvement of the traA–Q locus in P. gingivalis DNA exchange, transfer assays with live strains were performed. For these assays, equal amounts of two strains (having Rifr or Tetr markers) were mixed, plated at a high density on agar, and incubated anaerobically for 24 h. The mixture was then collected, diluted, and plated on agar medium selective for both transfer markers (Table 2). As a result, each strain in the mixture can potentially serve as both recipient and donor of the chromosomal markers. The DNA transfer efficiencies shown are calculated as the total numbers of Tetr and Rifr offspring divided by the number of input cells and are the averages of results from three or more independent replicates (n). The DNA transfer efficiency between wild-type strains of W83 Rifr and W83 Tetr is 6.73 × 10−5 (Table 2, first row). The pairing of the W83 Tetr ∆tra mutant and the W83 Rifr Δtra mutant resulted in an average DNA transfer efficiency of 2.02 × 10−5 (Table 2, second row), which is 3-fold lower and significantly different from the wild-type efficiency (P = 0.01). However, these results do not demonstrate the complete loss of DNA transfer expected if the traA–Q-mob locus were exclusively required for DNA exchange.
TABLE 2 .
DNA transfer assays with live strains
| Strain 1 (W83) phenotype and genotype | Strain 2 (W83) phenotype and genotype | DNA transfer efficiencya | SD | nb |
|---|---|---|---|---|
| Rifr | Tetr | 6.73 × 10−5 | 4.65 × 10−5 | 10 |
| Rifr Δtra | Tetr Δtra | 2.02 × 10−5 | 6.83 × 10−6 | 8 |
| Rifr | Tetr (DNase I) | 7.81 × 10−6 | 7.45 × 10−6 | 5 |
| Rifr ΔcomF | Tetr | 9.74 × 10−6 | 9.08 × 10−6 | 3 |
| Rifr ΔcomF | Tetr ΔcomF | 4.22 × 10−7 | 3.11 × 10−8 | 3 |
| Rifr Δtra | Tetr Δtra (DNase I) | 7.81 × 10−6 | 7.45 × 10−6 | 4 |
| Rifr ΔcomF | Tetr ΔcomF (DNase I) | 4.43 × 10−7 | 1.21 × 10−7 | 4 |
DNA transfer efficiency is calculated as the total number of Tetr and Rifr offspring divided by the number of input recipient cells.
n is the number of replicates of each experiment.
The results in Table 2 are remarkably similar to those that we previously published; in that study, the DNA transfer assays utilized erythromycin and tetracycline resistance markers (18). In that case, the DNA transfer efficiency (7.6 × 10−5 [±6.6 × 10−5]) was not significantly different from the results shown here. To ensure that use of rifampin resistance as a marker does not result in high levels of background mutations, we tested P. gingivalis W83 to determine the rate of spontaneous rifampin resistance. The spontaneous mutation frequency for W83 is 2.54 × 10−9, with a standard deviation of 3.01 × 10−9, and thus does not significantly impact our DNA transfer efficiency results.
DNA uptake via transformation.
As the traA–Q-mob locus is not the only system responsible for P. gingivalis DNA transfer, we redirected our efforts to investigate the existence of other DNA exchange mechanisms in P. gingivalis. To determine if exogenous chromosomal DNA is involved in DNA exchange, we repeated the DNA transfer assay with wild-type cells and included 30 U of DNase I enzyme during the overnight anaerobic incubation of the cells on agar. If extracellular DNA (eDNA) is important for DNA exchange, the presence of DNase I will reduce the amount of free DNA available and cause a significant decrease in the number of recovered Tetr and Rifr offspring. The results from this assay show (Table 2, third row) that the DNA transfer efficiency in the presence of DNase I is reduced 7-fold relative to that in its absence (7.85 × 10−6 relative to 6.73 × 10−5, respectively). These results indicate that the presence of extracellular chromosomal DNA is important for DNA transfer.
To further investigate if DNA can be introduced into P. gingivalis by a transformation-like mechanism, we modified our DNA transfer assay by incubating the donor bacteria at 80°C for 10 min to lyse the cells and release native genomic DNA. The live recipient strain was then mixed with the dead donor and treated as detailed previously. As conjugation requires both a live donor and recipient cells, conjugation would be prohibited under these conditions. The results show (Table 3, first row) that W83 is transformed by incubation with dead donor cells with an efficiency (3.17 × 10−4) that is significantly higher than the DNA transfer efficiency in the assay with live donor cells (Table 2, first row) (P = 0.02). As a control, the wild-type mixture was incubated overnight with 30 U of DNase I, which reduced the DNA transfer efficiency to 7.3 × 10−8 (Table 3, second row). Taken together, these results indicate that strain W83 has an active DNA uptake system that recognizes external DNA released from donor cells and thus appears to be naturally competent.
TABLE 3 .
DNA transfer assays with dead donor strains
| Live recipient (W83) phenotype and genotype | Dead donor (W83) phenotype and genotype | DNA transfer efficiencya | SD | nb |
|---|---|---|---|---|
| Rifr | Tetr | 3.17 × 10−4 | 2.61 × 10−4 | 10 |
| Rifr | Tetr (DNase I) | 7.30 × 10−8 | 1.41 × 10−8 | 3 |
| Rifr ΔcomF | Tetr | <10−8 | 8 | |
| Rifr | Tetr ΔcomF | 2.02 × 10−4 | 5.42 × 10−5 | 4 |
| Rifr Δtra | Tetr | 1.92 × 10−4 | 5.61 × 10−5 | 5 |
| Tetr | Rifr Δtra | 1.61 × 10−4 | 4.23 × 10−5 | 4 |
DNA transfer efficiency is calculated as the total number of Tetr and Rifr offspring divided by the number of input recipient cells.
n is the number of replicates of each experiment.
DNA exchange by transformation occurs in other P. gingivalis strains.
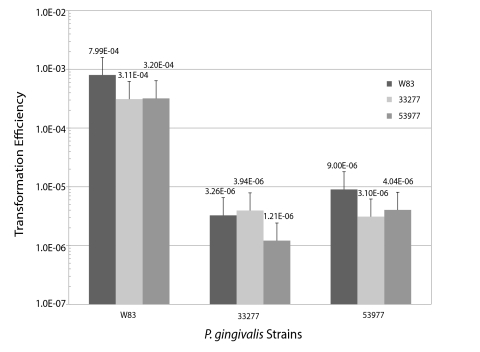
We examined two additional P. gingivalis strains for competence by utilizing strains ATCC 33277 and ATCC 53977. These strains are genetically distinct from each other and from W83. Most notably, ATCC 33277 has a traA–Q gene cluster with minimal DNA identity to the W83 traA–Q locus (15, 31); the ATCC 33277 traA–Q gene products are predicted to be more similar to those found in conjugative Bacteroides transposons. Previously, we demonstrated that strain ATCC 33277, but not W83, is able to transfer plasmid DNA to E. coli by conjugation, and this transfer was not affected by DNase I treatment (18). We created ATCC 33277 and ATCC 53977 donor strains with PG1244 ΩtetQ as the transfer marker (Table 1). Based on published genome sequences (19, 20), the PG1244 loci are 96% similar in W83 and ATCC 33277, and our PCR analysis shows it to be present in strain ATCC 53977. We then utilized the DNA transfer assay with dead donor cells to determine if strains ATCC 33277 and ATCC 53977 are competent and to compare the transformation frequencies when the DNA donor is a self or nonself strain. The results indicate that all three P. gingivalis strains tested could be transformed by both self- and nonself DNA (Fig. 1). However, in all cases, W83 was transformed at significantly higher frequencies (10−4) than either ATCC 33277 or ATCC 53977 (10−6). There was no significant difference in the transformation efficiencies between self- and nonself donors for any of the strains. Thus, our results indicated that three divergent strains of P. gingivalis all possess competence systems in which genomic DNA uptake shows no preference for the donor strain.
FIG 1 .
DNA transfer efficiency with dead donors. The DNA uptake efficiencies of experiments with three live recipient P. gingivalis strains each mixed with the same three dead donor strains are shown. Transformation efficiencies are shown on the y axis and are calculated as the number of recovered transformants (Rifr Tetr) divided by the number of input recipient cells. All recipient strains (x axis) were rifampin resistant, and all dead donor strains contained the genomic insertion PG1244 ΩtetQ.
Role of comF in DNA exchange.
The P. gingivalis W83 genome has genes that are predicted to be associated with competence, based on their identity to known competence genes in other bacterial species. One open reading frame carried by PG0158, here designated comF, is annotated as encoding an ATP-dependent DNA helicase and is predicted to possess a phosphoribosyltransferase domain, which is a distinguishing feature of ComF proteins (32–34). Another putative ComF homologue is found in the genome of strain ATCC 33277 (PGN_0270). ComF proteins are hypothesized to produce the energy required to drive translocation of DNA through the inner membrane into the cytoplasm (35). We deleted the W83 comF gene by allelic replacement with the ermF gene in the W83 Tetr and W83 Rifr strain backgrounds, resulting in strains W83 PG1244 ΩtetQ comF::ermF and W83 Rifr comF::ermF, respectively (Table 1). After confirming the mutant genotypes, we tested the phenotypes in the DNA transfer assay using a dead donor. The W83 Rifr ΔcomF mutant (Table 3, third row) was not able to incorporate the DNA transfer markers from the dead donor DNA into its chromosome at a detectable level, as no colonies from transfer assays grew on the agar plates with both antibiotics, even with an input of 5 × 108 donor and recipient cells. Using the W83 Tetr ΔcomF mutant as the dead donor strain with the W83 Rifr recipient, there was no significant effect on DNA transfer efficiency (Table 3, fourth row). These results indicate that the comF gene product is critical for W83 uptake of exogenous DNA and reinforces the concept that the major DNA exchange machinery in P. gingivalis strain W83 is competence based.
Comparison of ΔcomF and ΔtraA–Q-mob mutant phenotypes.
In order to delineate the relative roles of the traA–Q-mob locus and comF gene in P. gingivalis DNA transfer, we compared the results of DNA transfer assays with live and dead donors in both mutant backgrounds. The W83 Tetr ΔcomF mutant was paired with either W83 Rifr or the W83 Rifr ΔcomF mutant in live donor DNA transfer assays (Table 2, fourth and fifth rows). The presence of one or two ΔcomF mutants in the assay resulted in 7-fold and 159-fold reductions in the resulting numbers of Tetr Rifr colonies, respectively. This result was surprising, as the ΔcomF mutant was completely deficient in transformation in the transfer assay with dead donors, yet two live ΔcomF mutants were still able to exchange DNA. Thus, a low level of DNA transfer is present with a live donor and does not require comF.
A DNA transfer assay was then performed using the W83 Tetr ΔtraA–Q-mob mutant as either the dead donor or the recipient, and no significant difference from results with the wild type was found for either condition (Table 3, fifth and sixth rows). From this we conclude that, unlike comF, the traA–Q-mob locus is not involved in DNA uptake via competence. However, as two ΔtraA–Q-mob mutants have a slight but significant reduction in DNA transfer efficiency in live-donor assays, we theorized that the ΔtraA–Q-mob mutant phenotype may represent the loss of conjugation, leaving transformation by comF as the only DNA exchange process. In the reciprocal situation, the residual DNA transfer in the ΔcomF mutant live-donor assay may result from conjugation mediated by traA–Q-mob.
To further test the theory that comF is involved in competence and that traA–Q-mob is involved in conjugation, we performed two live-donor transfer assays in the presence of DNase I. We hypothesized that two ΔtraA–Q-mob mutants in a live-donor assay would transfer DNA only via competence mechanisms and thus that the presence of DNase I in this assay would significantly reduce the number of CFU. In a parallel experiment, two ΔcomF mutants in a live-donor assay should exchange DNA only by conjugation; thus, the presence of DNase I should have no significant effect on DNA exchange efficiencies. The results from these assays are shown in Table 2 (sixth and seventh rows). Two ΔtraA–Q-mob mutants in the presence of DNase I have an average DNA transfer efficiency of 7.81 × 10−6, a 6-fold and significant reduction compared to the transfer efficiency of the non-DNase I controls (P = 0.05). However, the transfer efficiencies of two ΔcomF mutants in the presence (4.22 × 10−7) or absence (4.43 × 10−7) of DNase I are not statistically different; these results reinforce the concept that two forms of DNA transfer occur simultaneously in P. gingivalis.
Induction of natural competence in P. gingivalis.
These studies demonstrate that DNA uptake by transformation is the dominant form of DNA exchange in P. gingivalis; thus, we decided to further investigate the conditions that regulate DNA uptake. This point was of great interest to us, as in our previous study we found that purified chromosomal DNA was not taken up by P. gingivalis (18). In our DNA transfer assays with dead donor cells, native methylated DNA is released from lysed bacteria as the source of the transferred DNA. To determine if nonmethylated DNA could be transferred at similar frequencies and if this process required signaling or DNA-binding molecules from the donor lysate, a DNA transfer assay was performed with a PCR product as the source of the DNA. Specifically, 1 µg of a 3.2-kb PCR product containing the PG1244 ΩtetQ marker was incubated with a P. gingivalis recipient, in the presence or absence of bacterial cell lysate, overnight on an agar plate surface. After recovery and enumeration of the transformants, the transformation frequencies were calculated as the total number of recovered colonies per total number of recipient bacteria, with a standardized input of 1 µg of DNA. As a baseline of comparison, 1 µg of the same PCR product was electroporated into P. gingivalis, and transformation frequencies were calculated. The results of these experiments are shown in Table 4.
TABLE 4 .
DNA transfer efficiency after electroporation and transformation with a DNA fragment
| Recipient | Transfer efficiencya |
|||
|---|---|---|---|---|
| After electroporation | In a biofilm | In a biofilm with a cell lysate | In a biofilm treated with DNase I | |
| W83 | 1.04 × 10−6 (±4.9 × 10−7) | 1.81 × 10−4 (±5.2 × 10−5) | 2.66 × 10−4 (±4.8 × 10−5) | <10−8 |
| W83 ΔcomF | <10−8 | <10−8 | ND | ND |
The DNA source was a 3.2-kb PCR product containing the PG1244 ΩtetQ gene. Standard deviations are shown in parentheses. ND, not determined.
Our first observation from this experiment was that incubation of our PCR product with recipient cells on an agar plate resulted in a high transformation efficiency (1.81 × 10−4) and produced a significantly higher number of transformants than electroporation (180-fold). Additionally, the PCR product transferred equally well in the presence or absence of cell lysate. Thus, the natural competence system is tolerant to nonmethylated DNA, and there is no signal required from the donor cell lysate to activate DNA uptake. Taken together with our earlier experiments, in which high-molecular-weight chromosomal DNA was not transformative, these results suggest that the natural competence system is efficient only for uptake of low-molecular-weight DNA or requires a free 5′ DNA end.
Electroporation of the W83 Rifr ΔcomF strain compared to that of the wild type revealed a complete loss of DNA transformability in the mutant. As electroporation introduces DNA into the periplasmic space for subsequent processing and recombination, this implies that the comF product is critical for active transport of DNA across the highly selective inner membrane.
P. gingivalis biofilms contain eDNA.
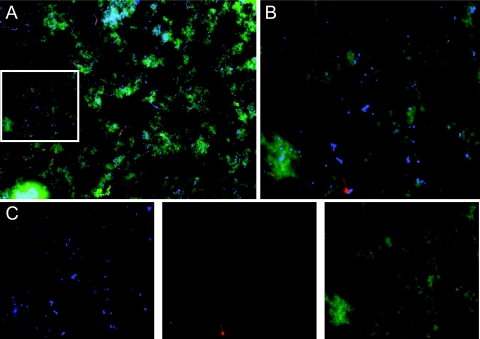
P. gingivalis is a constituent of subgingival plaque biofilms and produces an extracellular matrix. Recent studies of other species have identified extracellular DNA (eDNA) as an important constituent of some biofilm matrixes (36–39). To determine if eDNA exists in P. gingivalis biofilms, as it may represent a potential DNA transformation substrate for uptake by comF, we developed a sequential biofilm-staining protocol to allow us to differentiate between eDNA (stained with GelGreen), DNA in cells with compromised membranes (stained with propidium iodide), and DNA inside live cells (stained with DAPI [4′,6-diamidino-2-phenylindole]) using epifluorescence microscopy. As shown in Fig. 2A, copious amounts of green amorphous material can be seen surrounding distinct blue- and red-staining bacteria in 24-h biofilms. Upon closer inspection, the green eDNA is associated with both blue- and red-staining bacterial cells (Fig. 2B). There are relatively few propidium iodide-labeled bacteria, as expected for a 24-h biofilm; the majority of bacterial cells are stained with DAPI, indicating cell viability and intact membranes (Fig. 2C). To confirm that the green-staining material is eDNA, we incubated the 24-h biofilm with and without DNase I for 4 h prior to staining (Fig. S2). Incubation of the 24-h biofilms with DNase I significantly reduced the amount of green signal, without altering the relative numbers of the blue- and red-staining bacterial cells. Although DNA released from lysed bacteria in a biofilm is a likely DNA source for uptake by the ComF system, these images indicate that active secretion of DNA by live P. gingivalis into the biofilm matrix may also occur.
FIG 2 .
Fluorescence microscopy of P. gingivalis biofilm eDNA. P. gingivalis static biofilms were grown for 24 h and then stained and imaged with a fluorescence microscope. GelGreen-labeled extracellular DNA (diffuse green), propidium iodide-labeled dead bacterial cells (punctate red), and DAPI-labeled live cells (punctate blue). (A) Merged three-color image of a representative 24-h biofilm at 40× magnification. (B) The white boxed area in panel A is magnified here. (C) The blue, red, and green channels used in panel B are shown separately. The images shown are representative of three independent experiments.
DISCUSSION
Our results indicate that DNA transfer events in P. gingivalis are complex and that multiple mechanisms exist simultaneously to mediate DNA transfer. Our studies reveal that comF-dependent transformation by uptake of extracellular DNA is the major mechanism of horizontal DNA transfer in strain W83 and that conjugation plays a minor role in the transfer of genomic DNA. We propose that a likely extracellular DNA source is the P. gingivalis biofilm matrix, and we document the presence of extracellular DNA in 24-h P. gingivalis biofilms. The presence of redundant mechanisms to facilitate DNA exchange in this species suggests that it plays a critical role in bacterial survival in the stressful and highly competitive oral habitat.
Previously, it was assumed that P. gingivalis is not naturally competent, as recombinant DNA has traditionally been introduced by either electroporation (40, 41) or conjugation from an E. coli donor (42, 43), by using genetic tools and protocols developed for Bacteroides (44). However, molecular investigations of natural DNA transfer events in P. gingivalis have only recently been initiated. Our results here indicate that the traA–Q locus in strain W83 is involved in low-frequency conjugal transfer of chromosomal DNA, and our previous work found that plasmids were not a substrate for the W83 transfer system (18). In contrast, the traA–Q locus in P. gingivalis ATCC 33277 can mobilize plasmids by a DNase I-insensitive mechanism, and a recent study revealed the traA–Q-containing element cTnPg1 to be capable of excision and conjugation (45); both behaviors are similar to those of conjugative Bacteroides transposons. These cTn-like elements may have been shared between the oral and intestinal Bacteroidetes populations at some time in evolutionary history, and similar loci are found in the genome sequences of other members of phylum Bacteroidetes, indicating that DNA conjugation by traA–Q is widespread in this taxonomic group.
In naturally competent bacteria, specialized proteins are used for the uptake of DNA. In P. gingivalis, we show that comF is critical for DNA uptake from the exogenous environment. ComF proteins are hypothesized to produce the energy required to drive translocation of DNA through the inner membrane into the cytoplasm (35). Consistent with this, our comF mutant cannot be transformed by incubation with exogenous DNA or by electroporation. Interestingly, our studies show no bias against natural transformation of DNA from different strains or unmethylated DNA produced by PCR. This willingness to “sample” DNA from other sources is likely a major factor in the generation of genetic diversity within this species. It may also indicate that P. gingivalis is able to scavenge DNA from other species as a nutritional source, as not all DNA in a polymicrobial biofilm will be a candidate for homologous recombination into the chromosome.
From a practical perspective, P. gingivalis researchers should note that the introduction of genetic constructs via natural competence is more efficient than conjugation or electroporation. This is most likely due in part to the inherent degree of cell lysis resulting from the electric pulse during electroporation. An additional difference is that our competence assay mixtures mimic biofilms by incubation overnight on the surface of an agar plate, whereas cells recovered from electroporation are incubated in broth.
A recent transcriptome comparison analysis of planktonic versus biofilm-grown P. gingivalis revealed comF expression to be increased 2-fold in the biofilm cells (46). The same study showed that the expression of restriction-modification genes is decreased in biofilms. Thus, biofilm conditions appear to favor enhanced uptake of exogenous DNA. In parallel with this, we show here for the first time that P. gingivalis biofilms contain eDNA. As different P. gingivalis strains often cocolonize the same subgingival biofilm (47), it is possible that the biofilm lifestyle of eDNA release and natural biofilm competence underlies the reassortment of alleles into new, potentially better adapted strains of this oral anaerobe.
Our biofilm images indicate that P. gingivalis actively secretes DNA into the matrix; in other bacterial species, eDNA has been shown to represent a subset of the DNA sequences found in the genome (36–39). Secretion of select regions of DNA may represent a mechanism to control the genetic information that is shared; for example, secretion hot spots may exist near alleles that are highly variable and thus important for selection in the host environment. Future research is needed to further characterize the features of P. gingivalis eDNA and to identify the full complement of genes encoding the DNA uptake and eDNA release mechanisms in P. gingivalis biofilms.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Porphyromonas gingivalis strains (Table 1) were grown anaerobically at 37°C in a Coy anaerobic chamber under an atmosphere of 86% nitrogen-10% carbon dioxide-4% hydrogen. The culture medium was Trypticase soy broth (TSB) supplemented with 5% yeast extract, 2% sodium bicarbonate, 7.5 µM hemin, and 3 µM menadione. TSB blood agar plates (BAP) were made with the addition of 5% sheep’s blood and 1.5% agarose. P. gingivalis strains resistant to rifampin were selected by serial passage on BAP containing increasing amounts of the antibiotic. Selection for antibiotic-resistant P. gingivalis was with 30 µg/ml rifampin, 15 µg/ml erythromycin, or 1 µg/ml tetracycline. Dual resistance was selected with 30 µg/ml rifampin and 5 µg/ml erythromycin or 30 µg/ml rifampin and 1 µg/ml tetracycline.
Molecular biology.
DNA cloning, sequencing, PCR amplification, E. coli plasmid purification, and other common molecular biology techniques were carried out by standard procedures (48). Total DNA was purified from P. gingivalis using the Promega Wizard genomic DNA purification kit, with further purification by phenol-chloroform extraction if necessary. DNA amplification by PCR was done using the high-fidelity ExTaq polymerase (TaKaRa Bio, Inc.). Primers were synthesized by Integrated DNA Technologies (IDT). Primers used in this study are listed in Table 1 in the supplemental material. DNA sequencing was performed by SeqWright DNA Technology Services (Houston, TX). Sequence assembly and DNA and protein analysis were done with CLC Combined Workbench, v3 (CLC bio).
Construction of P. gingivalis allelic-exchange mutants.
Allelic-exchange mutants of P. gingivalis W83 were generated by PCR amplification of ~1-kb fragments upstream and downstream of the region to be deleted and then by creating fusion PCR products with the ermF marker according to a protocol derived from the work of Kuwayama et al. (49). The PCR product was cloned into the pCR2-TOPO vector for DNA sequencing (Invitrogen). Confirmed constructs were then digested with SacI, and the linear DNA was electroporated into P. gingivalis (40). Alternatively, fusion PCR products were sequenced and electroporated directly into W83 without cloning. Transformants were selected on erythromycin and confirmed by PCR or Southern hybridization. Mutant strains used in this study are listed in Table 1, and the genetic maps for relevant wild-type and mutant loci are shown in Fig. S1.
Construction of P. gingivalis genomic markers for DNA transfer assays.
For this study, we created two genomic markers to test for chromosomal DNA transfer. A tetQ marker is located in a large intergenic region between PG1244 and PG1245 and is designated PG1244 ΩtetQ. We use the symbol Ω to mean a gene insertion near the indicated gene rather than in it. This gene insertion was created using a PCR fusion method as described for the mutant strains above. As our second marker, we created Rifr strains by serial passage on agar plates containing the antibiotic. Rifampin resistance results from spontaneous mutation of the rpoB gene, which is gene PG0394 in strain W83.
P. gingivalis DNA transfer assays with live strains.
P. gingivalis-to-P. gingivalis DNA transfer assays with a live donor were performed by combining mid-log-phase cultures (optical density at 600 nm [OD600], 0.8 to 1.0) at equal ratios of both strains (5 × 108 cells each), pelleting the mixed cultures, resuspending the cells in 50 µl of TSB, dropping the mixture onto prereduced BAP, and incubating the resulting bacterial pellet for 24 h anaerobically at 37°C. Chimeric P. gingivalis offspring were selected by resuspending the bacterial pellet into TSB and incubating the recovered bacteria for 10 to 14 days anaerobically on BAP containing both rifampin and tetracycline. DNA transfer efficiencies were calculated by dividing the total number of offspring by the number of input recipient bacterial cells. Controls were individual P. gingivalis strains resuspended in 50 µl TSB and treated identically to the mixed-strain samples.
P. gingivalis DNA transfer assays with dead donor cells.
P. gingivalis-to-P. gingivalis DNA transfer assays with dead donors were performed similarly to the assays with live cells, except that the mid-log-phase donor cells were heat killed by incubation at 80°C for 10 min and were then placed on ice for 10 min prior to being mixed with live recipient cells. The mixtures were subsequently treated as in the assays with live cells. Controls were individual P. gingivalis strains resuspended in 50 µl TSB and treated identically to the mixed-strain samples. To confirm cell death in the dead donors, aliquots of donor controls were also plated on nonselective media. As with the assays of live cells, transformants were enumerated after dilution on selective media. DNA transfer efficiencies were calculated by dividing the total number of offspring by the number of input recipient bacterial cells.
Determination of spontaneous mutation frequency.
The P. gingivalis strains were spread on BAP and grown for 24 h. Three tubes containing 2 ml of TSB were inoculated with one independent colony each obtained from the BAP and incubated anaerobically overnight. One hundred microliters of a 10–6 dilution of the overnight cultures was seeded onto BAP, and 500 µl of the overnight cultures was plated onto BAP containing rifampin (30 µg/ml). Colony counts were performed after 7 days of anaerobic incubation. Mutation frequency values are reported as the number of rifampin-resistant colonies in proportion to the total viable count, and the final value was obtained from three independent experiments.
P. gingivalis biofilm microscopy.
Bacterial strains were grown to early log phase and diluted to an OD600 of 0.2 in biofilm medium (50% TSB-50% PBS). Five hundred microliters of diluted culture was added to CultureWell chambered cover glass 8-well slides (Grace Bio Labs) and incubated anaerobically and statically for 24 h at 37°C. Biofilms on the cover glass slides were stained for the detection of extracellular DNA. The supernatant was gently removed with a drawn-out Pasteur pipette, 250 µl of a 0.1× solution of GelGreen (Biotium; molecular mass [MM], >1,000 Da) in N18 solution (10 mM glucose, 10 mM HEPES, 140 mM NaCl, 0.03 mM CaCl2, 2 mM MgCl2, 5 mM KCl, pH 7.5) was added to each well, and plates were incubated for 30 min with gentle rocking. An aliquot of propidium iodide (Sigma; MM = 668 Da) was then added to each well, resulting in a final 0.1× (3 µM) concentration, and plates were incubated for an additional 30 minutes. Finally, an aliquot of DAPI (Sigma; MM = 350 Da; final concentration 300 nM) was added to each well, and plates were incubated for an additional 15 minutes. The supernatant was gently aspirated, the slide chambers were removed, and the wells were mounted in PermaFluor hard-set mounting medium (Thermo Scientific). Biofilm images were recorded at a 40× magnification using a Nikon Eclipse 80i epifluorescence microscope fitted with a Nikon DS-Qi1 digital camera, using the DAPI, fluorescein isothiocyanate (FITC), and Texas Red excitation/emission filter sets and Nikon NIS-Elements imaging software. Images were processed for contrast using ImageJ (NIH). In some experiments, biofilms were incubated with 100 U of DNase I for 4 h prior to extracellular DNA staining.
Statistics.
All experiments were repeated independently at least three times. Data were analyzed for statistical significance (P ≤ 0.05) using an unpaired t test with Excel software (Microsoft).
SUPPLEMENTAL MATERIAL
Mutant constructs. (Top) Wild-type traA–Q-mob region (PG1473 to PG1490) of P. gingivalis strain W83 (yellow) and allelic replacement of traA–Q-mob region with ermF (green); (bottom) wild-type comF (PG0158) region of P. gingivalis strain W83 (red) and allelic replacement of comF with ermF (green). Download Figure S1, PDF file, 0.2 MB.
DNase I treatment of 24-h biofilms. (A) Twenty-four-hour P. gingivalis biofilm stained sequentially with GelGreen, propidium iodide, and DAPI; (B) 24-h P. gingivalis biofilm treated with 100 U of DNase I for 4 h prior to the staining protocol. Download Figure S2, PDF file, 1.3 MB.
PCR primers.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant DE-019634 from the National Institute for Dental and Craniofacial Research to G.D.T.
We thank the reviewers for constructive criticism of the manuscript.
Footnotes
Citation Tribble GD, et al. 2012. Natural competence is a major mechanism for horizontal DNA transfer in the oral pathogen Porphyromonas gingivalis. mBio 3(1):e00231-11. doi:10.1128/mBio.00231-11.
REFERENCES
- 1. Ehrlich GD, et al. 2010. The distributed genome hypothesis as a rubric for understanding evolution in situ during chronic bacterial biofilm infectious processes. FEMS Immunol. Med. Microbiol. 59:269–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gogarten JP, Townsend JP. 2005. Horizontal gene transfer, genome innovation and evolution. Nat. Rev. Microbiol. 3:679–687 [DOI] [PubMed] [Google Scholar]
- 3. Gupta S, Maiden MC. 2001. Exploring the evolution of diversity in pathogen populations. Trends Microbiol. 9:181–185 [DOI] [PubMed] [Google Scholar]
- 4. Frost LS, Leplae R, Summers AO, Toussaint A. 2005. Mobile genetic elements: the agents of open source evolution. Nat. Rev. Microbiol. 3:722–732 [DOI] [PubMed] [Google Scholar]
- 5. Kang J, Blaser MJ. 2006. Bacterial populations as perfect gases: genomic integrity and diversification tensions in Helicobacter pylori. Nat. Rev. Microbiol. 4:826–836 [DOI] [PubMed] [Google Scholar]
- 6. Feil EJ, Maiden MC, Achtman M, Spratt BG. 1999. The relative contributions of recombination and mutation to the divergence of clones of Neisseria meningitidis. Mol. Biol. Evol. 16:1496–1502 [DOI] [PubMed] [Google Scholar]
- 7. Lamont RJ, Jenkinson HF. 1998. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 62:1244–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ozmeriç N, Preus HR, Olsen I. 1999. Intrafamilial transmission of black-pigmented, putative periodontal pathogens. Anaerobe 5:571–577 [DOI] [PubMed] [Google Scholar]
- 9. Tuite-McDonnell M, et al. 1997. Concordance of Porphyromonas gingivalis colonization in families. J. Clin. Microbiol. 35:455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McClellan DL, Griffen AL, Leys EJ. 1996. Age and prevalence of Porphyromonas gingivalis in children. J. Clin. Microbiol. 34:2017–2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stinson MW, Safulko K, Levine MJ. 1991. Adherence of Porphyromonas (Bacteroides) gingivalis to Streptococcus sanguis in vitro. Infect. Immun. 59:102–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Neiders ME, et al. 1989. Heterogeneity of virulence among strains of Bacteroides gingivalis. J. Periodontal Res. 24:192–198 [DOI] [PubMed] [Google Scholar]
- 13. Van Steenbergen TJ, Delemarre FG, Namavar F, De Graaff J. 1987. Differences in virulence within the species Bacteroides gingivalis. Antonie Van Leeuwenhoek 53:233–244 [DOI] [PubMed] [Google Scholar]
- 14. Enersen M, Olsen I, van Winkelhoff AJ, Caugant DA. 2006. Multilocus sequence typing of Porphyromonas gingivalis strains from different geographic origins. J. Clin. Microbiol. 44:35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Igboin C, Griffen A, Leys E. 2009. Analysis of Porphyromonas gingivalis strain diversity. J. Clin. Microbiol. 47:3073–3081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frandsen EV, Poulsen K, Curtis MA, Kilian M. 2001. Evidence of recombination in Porphyromonas gingivalis and random distribution of putative virulence markers. Infect. Immun. 69:4479–4485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koehler A, et al. 2003. Multilocus sequence analysis of Porphyromonas gingivalis indicates frequent recombination. Microbiology 149:2407–2415 [DOI] [PubMed] [Google Scholar]
- 18. Tribble GD, Lamont GJ, Progulske-Fox A, Lamont RJ. 2007. Conjugal transfer of chromosomal DNA contributes to genetic variation in the oral pathogen Porphyromonas gingivalis. J. Bacteriol. 189:6382–6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Naito M, et al. 2008. Determination of the genome sequence of Porphyromonas gingivalis strain ATCC 33277 and genomic comparison with strain W83 revealed extensive genome rearrangements in P. gingivalis. DNA Res. 15:215–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nelson KE, et al. 2003. Complete genome sequence of the oral pathogenic bacterium Porphyromonas gingivalis strain W83. J. Bacteriol. 185:5591–5601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bacic M, et al. 2005. Genetic and structural analysis of the Bacteroides conjugative transposon CTn341. J. Bacteriol. 187:2858–2869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bonheyo G, Graham D, Shoemaker NB, Salyers AA. 2001. Transfer region of a Bacteroides conjugative transposon, CTnDOT. Plasmid 45:41–51 [DOI] [PubMed] [Google Scholar]
- 23. Paster BJ, Dewhirst FE, Olsen I, Fraser GJ. 1994. Phylogeny of Bacteroides, Prevotella, and Porphyromonas spp. and related bacteria. J. Bacteriol. 176:725–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arzese AR, Tomasetig L, Botta GA. 2000. Detection of tetQ and ermF antibiotic resistance genes in Prevotella and Porphyromonas isolates from clinical specimens and resident microbiota of humans. J. Antimicrob. Chemother. 45:577–582 [DOI] [PubMed] [Google Scholar]
- 25. Leng Z, Riley DE, Berger RE, Krieger JN, Roberts MC. 1997. Distribution and mobility of the tetracycline resistance determinant tetQ. J. Antimicrob. Chemother. 40:551–559 [DOI] [PubMed] [Google Scholar]
- 26. Lacroix JM, Walker CB. 1996. Detection and prevalence of the tetracycline resistance determinant tet Q in the microbiota associated with adult periodontitis. Oral Microbiol. Immunol. 11:282–288 [DOI] [PubMed] [Google Scholar]
- 27. Nikolich MP, Hong G, Shoemaker NB, Salyers AA. 1994. Evidence for natural horizontal transfer of tetQ between bacteria that normally colonize humans and bacteria that normally colonize livestock. Appl. Environ. Microbiol. 60:3255–3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lépine G, Lacroix JM, Walker CB, Progulske-Fox A. 1993. Sequencing of a tet(Q) gene isolated from Bacteroides fragilis 1126. Antimicrob. Agents Chemother. 37:2037–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Whittle G, Hamburger N, Shoemaker NB, Salyers AA. 2006. A Bacteroides conjugative transposon, CTnERL, can transfer a portion of itself by conjugation without excising from the chromosome. J. Bacteriol. 188:1169–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li LY, Shoemaker NB, Salyers AA. 1995. Location and characteristics of the transfer region of a Bacteroides conjugative transposon and regulation of transfer genes. J. Bacteriol. 177:4992–4999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen T, et al. 2004. Comparative whole-genome analysis of virulent and avirulent strains of Porphyromonas gingivalis. J. Bacteriol. 186:5473–5479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Busch S, Rosenplänter C, Averhoff B. 1999. Identification and characterization of ComE and ComF, two novel pilin-like competence factors involved in natural transformation of Acinetobacter sp. strain BD413. Appl. Environ. Microbiol. 65:4568–4574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Londoño-Vallejo JA, Dubnau D. 1993. ComF, a Bacillus subtilis late competence locus, encodes a protein similar to ATP-dependent RNA/DNA helicases. Mol. Microbiol. 9:119–131 [DOI] [PubMed] [Google Scholar]
- 34. Nakasugi K, Svenson CJ, Neilan BA. 2006. The competence gene, comF, from Synechocystis sp. strain PCC 6803 is involved in natural transformation, phototactic motility and piliation. Microbiology 152:3623–3631 [DOI] [PubMed] [Google Scholar]
- 35. Johnsborg O, Eldholm V, Håvarstein LS. 2007. Natural genetic transformation: prevalence, mechanisms and function. Res. Microbiol. 158:767–778 [DOI] [PubMed] [Google Scholar]
- 36. Harmsen M, Lappann M, Knøchel S, Molin S. 2010. Role of extracellular DNA during biofilm formation by Listeria monocytogenes. Appl. Environ. Microbiol. 76:2271–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lappann M, et al. 2010. A dual role of extracellular DNA during biofilm formation of Neisseria meningitidis. Mol. Microbiol. 75:1355–1371 [DOI] [PubMed] [Google Scholar]
- 38. Thomas VC, et al. 2009. A fratricidal mechanism is responsible for eDNA release and contributes to biofilm development of Enterococcus faecalis. Mol. Microbiol. 72:1022–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vilain S, Pretorius JM, Theron J, Brözel VS. 2009. DNA as an adhesin: Bacillus cereus requires extracellular DNA to form biofilms. Appl. Environ. Microbiol. 75:2861–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smith CJ. 1995. Genetic transformation of Bacteroides spp. using electroporation. Methods Mol. Biol. 47:161–169 [DOI] [PubMed] [Google Scholar]
- 41. Yoshimoto H, Takahashi Y, Hamada N, Umemoto T. 1993. Genetic transformation of Porphyromonas gingivalis by electroporation. Oral Microbiol. Immunol. 8:208–212 [DOI] [PubMed] [Google Scholar]
- 42. Chen T, et al. 2000. Identification and cloning of genes from Porphyromonas gingivalis after mutagenesis with a modified Tn4400 transposon from Bacteroides fragilis. Infect. Immun. 68:420–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Progulske-Fox A, Oberste A, Drummond C, McArthur WP. 1989. Transfer of plasmid pE5-2 from Escherichia coli to Bacteroides gingivalis and B. intermedius. Oral Microbiol. Immunol. 4:132–134 [DOI] [PubMed] [Google Scholar]
- 44. Salyers AA, Bonheyo G, Shoemaker NB. 2000. Starting a new genetic system: lessons from Bacteroides. Methods 20:35–46 [DOI] [PubMed] [Google Scholar]
- 45. Naito M, et al. 2011. Characterization of the Porphyromonas gingivalis conjugative transposon CTnPg1: determination of the integration site and the genes essential for conjugal transfer. Microbiology 157:2022–2032 [DOI] [PubMed] [Google Scholar]
- 46. Lo AW, et al. 2009. Comparative transcriptomic analysis of Porphyromonas gingivalis biofilm and planktonic cells. BMC Microbiol. 9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Leys EJ, Smith JH, Lyons SR, Griffen AL. 1999. Identification of Porphyromonas gingivalis strains by heteroduplex analysis and detection of multiple strains. J. Clin. Microbiol. 37:3906–3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 49. Kuwayama H, et al. 2002. PCR-mediated generation of a gene disruption construct without the use of DNA ligase and plasmid vectors. Nucleic Acids Res. 30:E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mutant constructs. (Top) Wild-type traA–Q-mob region (PG1473 to PG1490) of P. gingivalis strain W83 (yellow) and allelic replacement of traA–Q-mob region with ermF (green); (bottom) wild-type comF (PG0158) region of P. gingivalis strain W83 (red) and allelic replacement of comF with ermF (green). Download Figure S1, PDF file, 0.2 MB.
DNase I treatment of 24-h biofilms. (A) Twenty-four-hour P. gingivalis biofilm stained sequentially with GelGreen, propidium iodide, and DAPI; (B) 24-h P. gingivalis biofilm treated with 100 U of DNase I for 4 h prior to the staining protocol. Download Figure S2, PDF file, 1.3 MB.
PCR primers.