Abstract
The presence of certain types of human papillomavirus (HPV) is a known risk factor for the development of anogenital squamous cell carcinomas (SCCs). A similar association has been hypothesized for cutaneous SCCs, although, to our knowledge, no studies to date have combined sensitive HPV DNA detection techniques with epidemiologic data controlling for known risk factors to explore the association. We designed a case–control study examining HPV prevalence using highly sensitive PCR-detection assays in tissue samples from 85 immunocompetent patients with histologically confirmed SCCs and 95 age-matched individuals without a prior history of skin cancer. A standardized interview was administered to all study subjects to collect information pertaining to potential confounding variables. The overall detection rate of HPV DNA was high in case lesions (54%) and perilesions (50%) and in both sun-exposed normal tissue (59%) and non-sun-exposed normal tissue (49%) from controls. In comparing case tissue to control tissue, there was no differential detection of HPV DNA across various HPV species. However, HPV DNA from β-papillomavirus species 2 was more likely to be identified in tumors than in adjacent healthy tissue among cases (paired analysis, odds ratio = 4.0, confidence interval = 1.3–12.0). The high prevalence of HPV DNA detected among controls suggests that HPV DNA is widely distributed among the general population. However, the differential detection of HPV β-papillomavirus species in tumors among cases suggests that certain HPV types may be involved in the progression of cutaneous SCCs.
INTRODUCTION
Cutaneous squamous cell carcinoma (SCC) is the second most common cancer among Caucasians worldwide (Preston and Stern, 1992), with an age-adjusted incidence of 100–150 per 100,000 per year (Gray et al., 1997). The known risk factors for cutaneous SCC include exposure to UV light, fair complexion, older age, male sex, smoking, chronic skin ulcers and burn scars, immunosuppression, as well as exposure to certain chemical carcinogens (Alam and Ratner, 2001; Foote et al., 2001). Human papillomavirus (HPV) has been postulated to be an additional risk factor for cutaneous SCC development (Kiviat, 1999; Biliris et al., 2000).
HPVs are common viruses that infect epithelial cells, with 108 different types identified to date (de Villiers et al., 2004). HPVs are classified into higher order “genera” (alpha, beta, and gamma contain the majority of cutaneous HPVs) and lower order “species” (Figure 1). Although it is now widely accepted that certain high-risk HPV types (mainly α-papillomaviruses) play a central role in cervical and anogenital cancers (zur Hausen, 1996; Nobbenhuis et al., 1999; Walboomers et al., 1999), the role of HPV in the development of cutaneous SCCs remains controversial. There is strong evidence implicating HPV in the pathogenesis of cutaneous SCCs in immunosuppressed individuals. Persons afflicted with epidermodysplasia verruciformis, an inherited disorder of cell-mediated immunity, are unable to adequately control infection with specific HPVs and develop large numbers of flat, atypical warts, 40–60% of which can eventually undergo malignant transformation (Majewski and Jablonska, 1997). Solid organ transplant recipients also develop large numbers of warts, primarily on sun-exposed skin, that carry a high risk of subsequent malignant transformation to cutaneous SCCs (Purdie et al., 1993; Harwood et al., 2000). HPV DNA is detected in high levels within cutaneous SCCs (up to 80%) that arise in solid organ transplant recipients (Tieben et al., 1994; Berkhout et al., 1995; Shamanin et al., 1996; de Villiers et al., 1997).
Figure 1. Phylogenetic tree containing genus α-, β-, and γ-papillomaviruses.
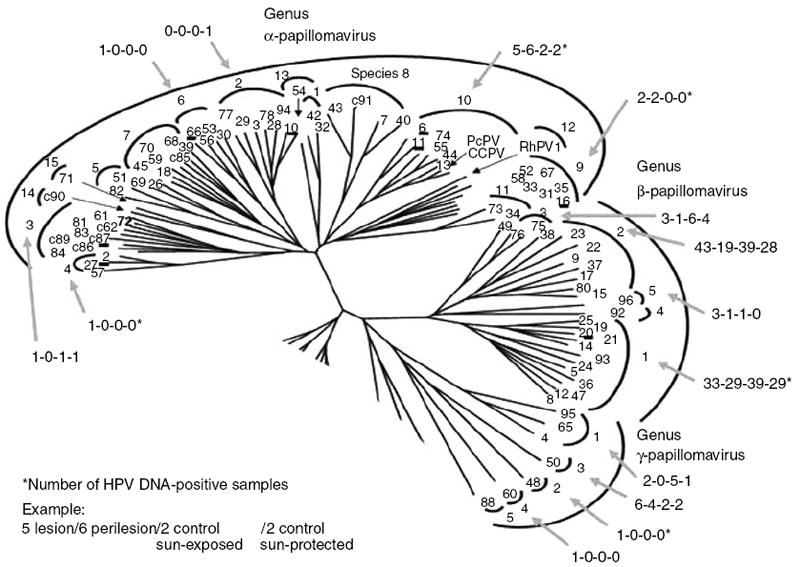
The outer circle encompasses genera, the inner circle the species, and the numbers at the ends of the branches identify type. C-numbers refer to candidate HPV types. The four numbers associated with the arrows show the total number of HPV-positive DNA samples detected in lesion, perilesion, sun-exposed and sun-protected tissues, respectively. (These numbers take into account multiple infections with the same species in a given subject and, therefore, may differ from those listed in Table 2.) (Figure is modified from a previously published version by De Villiers et al. (2004), with copyright permission granted by Elsevier.)
The mechanism by which HPV causes oncogenesis in cutaneous SCC development is unclear. The genomes of mucosal HPV types (α-papillomaviruses) containing E6 and E7 genes can replicate as extrachromosomal episomes separate from host DNA and potentially act through specific molecular changes in the E6 and E7 pathways that impair tumor suppressor genes such as p53 and Rb. HPV integration into the host genome may also cause disruption of genomic stability and facilitate further progression toward cell transformation. Alternatively, the low-risk HPV types (β- and γ-papillomaviruses) seem to require the presence of additional mutagens, such as UV radiation, to induce proliferation of infected keratinocytes. The latter may acquire mutations in tumor suppressor genes or oncogenes through other mutagens, such as UV radiation, and develop into tumors. A better understanding of the role of HPV in skin cancer arising in immunocompetent hosts can come from probing both tumor (lesion) and clinically adjacent normal skin (perilesion) for DNA, which previously studies have failed to do.
Among immunocompetent subjects, several studies have reported variable detection rates of HPV in cutaneous SCCs ranging from 27 to 70% (Shamanin et al., 1996; Harwood et al., 2000, 2004; Iftner et al., 2003). The variability in HPV detection rates is likely technique dependent. Different PCR consensus primers have variable sensitivity for HPV types and some fail to recognize novel HPV types. An additional weakness in most published case–control studies is that they fail to capture epidemiologic data and adjust for potential confounding risk factors for skin cancer, such as sun exposure history, propensity to sunburn, exposure to chemical carcinogens, and previous history of skin cancer.
This study examines the role of HPV in cutaneous SCCs among immunocompetent subjects. Unlike previous studies that failed to use primers capable of detecting a wide range of cutaneous HPVs, we used three distinct sets of both consensus and degenerate primers to obtain maximum sensitivity for the detection of all HPV types. In addition, we collected detailed information on potential confounding variables such as skin type, history of sun exposure, and exposure to mutagens. We compared HPV detection rates within cases, looking at SCCs (lesion) as well as clinically uninvolved adjacent skin (perilesion) to examine the role of HPV in tumor progression. The strength in this study design is that it allows for the perilesional tissue to serve as the “internal control.” For comparison as an “external control,” we selected age-matched individuals with no self-reported history of skin cancer. We obtained two biopsies from each control subject, one from a sun-exposed and one from a sun-protected site, to study the effects of UV exposure on HPV prevalence. By designing the study such that there is an internal control and external control for each tumor, by ascertaining self-reported epidemiologic variables that influence skin cancer risk, and by using highly sensitive HPV primers, we aim to rigorously explore the role of HPV in cutaneous SCCs and to distinguish our research from previously published findings.
RESULTS
A total of 227 immunocompetent Caucasian individuals (132 cases and 95 controls) had skin biopsies for HPV detection assays. Of the 132 SCC cases, only 85 had confirmed SCCs upon histologic examination of the surgically excised specimen. The remaining 47 cases did not have histologically confirmed SCCs at the time of surgical treatment of their tumors and were therefore excluded from the analysis. The pathologic classification of the 85 histologically confirmed SCC cases are as follows: 52 (61%) were invasive SCCs, 3 (4%) were borderline invasive SCCs, and 30 (35%) were SCC in situ. With regard to anatomic location of the tumors, 64 (75%) arose on the head/neck, 7 (8%) on the trunk, 10 (12%) on the hands and arms, and 4 (5%) on the legs.
For the 85 histologically confirmed SCCs, we were able to collect and analyze 72 perilesional samples. Owing to surgical constraints for the repair method following Mohs micrographic surgery, we were unable to obtain perilesional tissue from 13 subjects. For the 95 controls, we obtained one sample from a sun-exposed site on the pre-auricular area and one from a sun-protected site on the upper inner arm.
Table 1 summarizes the characteristics of the study population. Cases and controls were similar in age and gender due to the frequency matching employed during subject recruitment. Cases and controls also did not significantly differ with regard to level of education, family history of cancer, or history of common warts. As a group, cases were more sun-sensitive as demonstrated by an increased propensity to sunburn (P<0.0001) and freckle (P = 0.007). Cases were also less likely to have ever used tanning beds (P = 0.005), perhaps as a result of their increased sun sensitivity, and more likely to have had a history of exposure to ionizing radiation (P = 0.04). Control subjects were more likely to have had a history of smoking (P = 0.05) and scars from a burn injury (P = 0.03).
Table 1.
Characteristics of subjects with and without cutaneous SCC
| Cases (n=85) no./total (%) | Controls (n=95) no./total (%) | P-value | |
|---|---|---|---|
| Age (mean years±SD) | 72.9±12.9 | 70.3±9.2 | 0.13 |
|
| |||
| Female gender | 22/85 (26%) | 25/95 (26%) | 0.95 |
|
| |||
| Family history of skin cancer | 11/65 (17%) | 14/91 (15%) | 0.80 |
|
| |||
| Education > high school | 39/66 (59%) | 59/93 (63%) | 0.58 |
|
| |||
| History of warts | 32/65 (49%) | 56/94 (60%) | 0.20 |
|
| |||
| Ever smoker | 38/67 (57%) | 68/95 (72%) | 0.05 |
|
| |||
| Exposure to radiation | 12/66 (18%) | 7/93 (8%) | 0.04 |
|
| |||
| Burn scars | 2/65 (3%) | 12/94 (13%) | 0.03 |
|
| |||
| History of freckling | 24/64 (38%) | 17/93 (18%) | 0.007 |
|
| |||
| Ever used tanning beds | 4/67 (6%) | 21/94 (22%) | 0.005 |
|
| |||
| Propensity to sunburn | <0.0001 | ||
| Burns, blisters | 8/66 (12%) | 7/95 (7%) | |
| Burns, no blisters | 26/66 (39%) | 12/95 (13%) | |
| Mild burn, tans | 26/66 (39%) | 41/95 (43%) | |
| No burn | 6/66 (9%) | 35/95 (37%) | |
SCC, squamous cell carcinoma.
Table 2 shows the detection rates of HPV DNA and adjusted odds ratios (ORs) for tissue from cases (lesional and perilesional) and controls (sun-exposed and sun-protected). In these analyses, the ORs are adjusted for propensity to sunburn, as the other potential confounders, such as smoking status, skin sensitivity, freckling, tanning bed use, burn scars, and exposure to radiation, were not found to substantially change the risk estimates. The data are grouped according to the number of HPV types detected per sample, as well as by HPV genera (alpha, beta, and gamma). The overall HPV detection rate was similar among cases (lesional 54%; perilesional tissue 50%), and controls (sun-exposed tissue 59%; sun-protected tissue 49%). α-Papillomavirus species 2, 3, 4, and 6, β-papillomavirus species 5, and γ-papillomavirus species 1, 2, and 4 were detected but had low prevalence (≤ 5% separately among both cases and controls). There was a trend toward a higher detection rate of the α-papillomavirus genera (mainly species 9 and 10—HPVs 6, 11, and 16) among cases than among controls (lesions compared to sun-exposed controls: OR = 3.0; confidence interval (CI) = 0.6–14.3). There were similar detection rates for the genus γ-papillomavirus among lesional (8%), perilesional (6%), and sun-exposed controls sites (7%); however, there was a marginal decreased detection rate in sun-protected control sites (3%). Among the β-papillomavirus, species 1 had similar detection rates among cases and controls. However among cases, the β-papillomavirus species 2 detection rate appeared to differ in lesional sites (34%) as compare to perilesional sites (17%). To explore this further, we performed conditional logistic regression among the 72 paired specimens (lesion versus perilesion among cases) and found that lesions harbored significantly more β-papillomavirus species 2 DNA than perilesions (OR = 4.0, CI = 1.3–12.0). The paired analyses did not show a differential detection rate within any other species or genera (Table 2).
Table 2.
Rate of HPV DNA detection in subjects with and without cutaneous SCC
| Genus | Cases
|
Controls
|
ORs (95% CI)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Lesion (n=85) no. (%) | Perilesion (n=72) no. (%) | Sun (n=95) no. (%) | No sun (n=95) no. (%) | Lesion versus perilesion1 | Lesion versus sun2 | Lesion versus no sun2 | Perilesion versus sun2 | Perilesion versus no sun2 | |
| All genuses | 46 (54%) | 36 (50%) | 56 (59%) | 47 (49%) | 1.2 (0.6–2.6) | 0.9 (0.4–1.8) | 1.2 (0.6–2.5) | 0.6 (0.3–1.3) | 0.9 (0.4–1.8) |
|
| |||||||||
| α-Papillomavirus | |||||||||
| All α-papillomavirus | 7 (8%) | 7 (10%) | 3 (3%) | 4 (4%) | 0.7 (0.2–2.4) | 3.0 (0.6–14.3) | 1.9 (0.4–7.9) | 3.4 (0.6–18.3) | 2.0 (0.4–9.4) |
| 9 | 2 (2%) | 2 (3%) | 0 (0%) | 0 (0%) | 1.0 (0.1–7.1) | — | — | — | — |
| 10 | 3 (4%) | 6 (8%) | 2 (2%) | 2 (2%) | 0.2 (0.02–1.7) | 1.6 (0.2–14.2) | 0.8 (0.1–6.9) | 3.2 (0.4–22.4) | 1.8 (0.3–12.3) |
|
| |||||||||
| β-Papillomavirus | |||||||||
| All β-papillomavirus | 42 (49%) | 32 (44%) | 51 (54%) | 43 (45%) | 1.3 (0.6–2.7) | 1.0 (0.5–2.0) | 1.3 (0.6–2.5) | 0.7 (0.3–1.5) | 0.9 (0.4–1.8) |
| 1 | 24 (28%) | 25 (35%) | 29 (31%) | 24 (25%) | 0.7 (0.3–1.5) | 1.3 (0.6–2.7) | 1.3 (0.6–2.8) | 1.3 (0.6–2.8) | 1.3 (0.6–2.8) |
| 2 | 29 (34%) | 12 (17%) | 28 (29%) | 23 (24%) | 4.0 (1.3–12.0) | 1.3 (0.6–2.7) | 1.7 (0.8–3.6) | 0.5 (0.2–1.2) | 0.6 (0.3–1.5) |
| 3 | 3 (4%) | 1 (1%) | 6 (6%) | 4 (4%) | 2.0 (0.2–22.1) | 1.6 (0.3–7.4) | 2.7 (0.5–14.3) | 0.5 (0.1–5.1) | 0.9 (0.1–9.2) |
|
| |||||||||
| γ-Papillomavirus | |||||||||
| All γ-papillomavirus | 7 (8%) | 4 (6%) | 7 (7%) | 3 (3%) | 1.5 (0.4–5.3) | 1.0 (0.3–3.2) | 2.2 (0.5–9.7) | 0.6 (0.1–2.8) | 1.7 (0.3–9.3) |
| 3 | 3 (4%) | 4 (6%) | 2 (2%) | 2 (2%) | 0.8 (0.2–3.4) | 2.1 (0.3–15.3) | 1.7 (0.3–11.4) | 3.0 (0.4–21.2) | 2.6 (0.4–16.8) |
CI, confidence interval; HPV, human papillomavirus; OR, odds ratio; SCC, squamous cell carcinoma.
Paired analysis (n=72) comparing HPV detection in lesions to perilesion in cases.
Adjusted for propensity to sunburn.
Some specimens harbored multiple HPV types. We explored whether there were differential rates of detection of multiple HPV types within the same specimen for cases and controls, using multivariable logistic regression adjusted for propensity for sunburn (Table 3). Tumor from cases was more likely to have three or more HPV types detected than sun-protected controls (OR = 6.1, 95% CI = 1.4–26.1). No other patterns of differential detection were identified.
Table 3.
Multiple HPV types detected per specimen in subjects with and without cutaneous SCC
| No. of HPV types | Cases
|
Controls
|
Adjusted ORs (95% CI)
|
|||||
|---|---|---|---|---|---|---|---|---|
| Lesion (n=85) no. (%) | Perilesion (n=72) no. (%) | Sun (n=95) no. (%) | No sun (n=95) no. (%) | Lesion versus sun | Lesion versus no sun | Perilesion versus sun | Perilesion versus no sun | |
| 1 | 19 (22%) | 17 (24%) | 32 (34%) | 32 (34%) | 0.6 (0.2–1.3) | 0.7 (0.3–1.6) | 0.4 (0.2–1.2) | 0.5 (0.2–1.2) |
|
| ||||||||
| 2 | 14 (16%) | 12 (17%) | 16 (17%) | 12 (13%) | 0.9 (0.3–2.5) | 1.4 (0.5–4.1) | 1.0 (0.3–2.7) | 1.6 (0.5–4.5) |
|
| ||||||||
| 3+ | 13 (15%) | 7 (10%) | 8 (8%) | 3 (3%) | 2.5 (0.8–7.9) | 6.1 (1.4–26.1) | 1.1 (0.3–4.2) | 2.4 (0.5–11.7) |
CI, confidence interval; HPV, human papillomavirus; OR, odds ratio; SCC, squamous cell carcinoma.
Table 4 examines HPV detection rates from any tissue derived from a case (lesion or perilesion) versus any tissue derived from a control (sun-exposed or sun-protected). Overall, HPV detection was similar in cases (72%) and controls (75%) (OR = 0.8, 95% CI = 0.3–1.8). However, the distribution of HPV types in cases and controls was somewhat different. There was a marginal increased risk of infection with α-papillomavirus types (OR = 2.8, CI = 0.8–9.8) and γ-papillomavirus species 3 for cases compared to controls (OR = 2.5, CI = 0.6–10.3). β-Papillomavirus (including species 2) appeared to have similar detection rates among cases compared to controls.
Table 4.
Paired analysis comparing HPV detection among any tissue from a case (lesion or perilesion) versus any tissue from a control (sun-exposed or sun-protected skin)
| Combined case sites (n=72) no. (%) | Combined control sites (n=95) no. (%) | OR (95% CI) | |
|---|---|---|---|
| Any HPV | 52 (72%) | 71 (75%) | 0.8 (0.3–1.8) |
|
| |||
| α-Papillomavirus | 11 (15%) | 6 (6%) | 2.8 (0.8–9.8) |
| 9 | 4 (6%) | 0 (0%) | ∞ |
| 10 | 7 (10%) | 3 (3%) | 2.3 (0.4–12.0) |
|
| |||
| β-Papillomavirus | 47 (65%) | 68 (72%) | 0.7 (0.3–1.6) |
| 1 | 36 (50%) | 39 (41%) | 1.7 (0.8–3.5) |
| 2 | 28 (39%) | 43 (45%) | 0.8 (0.4–1.6) |
| 3 | 3 (4%) | 10 (11%) | 1.2 (0.3–4.9) |
|
| |||
| γ-Papillomavirus | 10 (14%) | 9 (9%) | 1.3 (0.5–4.0) |
| 3 | 7 (10%) | 4 (4%) | 2.5 (0.6–10.3) |
CI, confidence interval; HPV, human papillomavirus; OR, odds ratio.
Lastly, we examined the risk of HPV detection and its association with anatomic site of the tumor among cases (data not shown). There were similar detection rates of HPV across anatomic sites for β-papillomaviruses and γ-papillomaviruses. However, there was a suggestion of an increased rate of detection of β-papillomavirus species 2 in head/neck lesions (39%) compared to other sites (19%) (OR = 2.7, CI = 0.8–9.0), and there was also a decreased rate of detection of β-papillomavirus species 1 in head/neck lesions (23%) compared to other sites (43%) (OR = 0.4, CI = 0.1–1.2). The α-papillomaviruses were detected in lesions on the face, scalp, and arms and did not appear to preferentially localize to a given anatomic site.
Several previously unreported putative HPV types were identified in this study, including sck1, sck5, sck6, sck7, sck8, sck17, sck25, sck27, sck29, sck30, sck46, sck4, and sck65. Table 5 lists the partial sequences of these previously unreported HPV types as well as previously described putative HPV types. Specific HPV types are allocated only after the characterization of the respective full-length genomes. We compiled data by lumping known HPV types and the related partial sequences. Of note, HPV 20 was the most frequently detected HPV type (38 overall positive samples) and HPVs 5, 9, 15, 23, and 38 all had over 15 positive samples among the cases and controls.
Table 5.
Partial sequences and related HPV types
| Genus | Species | Closest related HPV type | Putative HPV type | No. of samples positive |
|---|---|---|---|---|
| β-Papillomavirus | 1 | 5 | DL231, FA23, FA65 | 6, 6, 1 |
| 12 | FA39, X34 | 1, 1 | ||
| 19 | FA14 | 3 | ||
| 20 | DL284, DL287, sck1 | 1, 3, 1 | ||
| 24 | vs20-4, FA18, FA127, FAIMVS11 | 2, 1, 2, 1 | ||
| 25 | sck46 | 1 | ||
| 47 | FA26 | 2 | ||
| 2 | 9 | DL285, FA116 | 5, 1 | |
| 15 | sck6, DL337, FA84 | 3, 1, 4 | ||
| 17 | DL436, FA40, FA114 | 1, 5, 7 | ||
| 22 | sck5, RTRX10 | 2, 1 | ||
| 23 | FA123, sck30, vs42-1, FA16, FA118, FAIMVS13 | 1, 1, 2, 8, 1, 1 | ||
| 38 | sck27, FA51 | 1, 2 | ||
| 80 | vs92-1, X14, FA85, FA108, FAIMVS14 | 1, 2, 1, 2, 1 | ||
| 3 | 49 | sck49, sck8 | 1, 1 | |
| 75 | sck17 | 1 | ||
| 5 | 96 | FA22, FAIMVS16, FA42 | 2, 1, 1 | |
|
| ||||
| γ-Papillomavirus | 1 | 4 | FA121 | 1 |
| 65 | FA28 | 1 | ||
| 95 | FA24, FA89 | 2, 1 | ||
| 2 | 48 | FA1 | 1 | |
| 3 | 50 | sck7, sck25, sck29, FA4, FA8, FA46, FA55, FA81, FAIMVS5 | 1, 3, 1, 1, 1, 1, 1, 1, 2 | |
| 4 | 60 | sck65 | 1 | |
HPV, human papillomavirus.
DISCUSSION
Using a highly sensitive nested PCR technique, we found no difference in overall detection of HPV DNA in tissue samples collected from immunocompetent individuals with cutaneous SCC as compared to age-matched healthy controls. However, we found that among cases, tumors were more likely to have HPV DNA from the β-papillomavirus 2 species than clinically normal-appearing adjacent skin. These findings are supported by a recent paper by Forslund et al. (2007) that examined 82 SCCs and obtained paired biopsy samples from the lesion and from healthy skin from the same patient (similar to our paired analysis). They concluded that β-papillomavirus species 2 are associated with SCC. As we found similar detection rates of β-papillomavirus species 2 in our controls, we hypothesize that additional factors are necessary for SCC development in the skin.
We found that tissue samples from cases were also somewhat more likely to harbor HPV from the genus α-papillomavirus (OR = 3.0), the same genus that includes the high-risk types associated with cervical and anogenital cancer (de Villiers et al., 2004), although this difference did not reach statistical significance after controlling for the potential confounding effect of propensity to sunburn (CI = 0.6–14.3). Of note, the α-papillomavirus species 9, which includes HPV type 16 implicated in cervical cancer, was found in tissue from only two cases and was not found in any controls, which, of the genital types, harbored only low-risk HPV 6 and HPV 11. Among cases, α-papillomavirus HPV detection rates were similar in tumor samples compared to adjacent, clinically normal-appearing perilesional tissue. This finding lends support to the theory that HPV DNA in skin cancer is important for tumor initiation, but that additional factors, such as p53 mutations or alterations in proapoptotic pathways, are needed to potentiate oncogenesis.
The high prevalence of HPV DNA detected in sun-exposed skin among our controls (59%) confirms findings from earlier studies suggesting that HPV DNA is widely distributed on the skin in the general population (Boxman et al., 1997; Astori et al., 1998; Forslund et al., 1999; Antonsson et al., 2000; Hazard et al., 2007). Studies among immunocompetent individuals have found HPV DNA in 67–74% of plucked hairs (Boxman et al., 2000; de Koning et al., 2007) and in 69–80% of skin swabs (Antonsson et al., 2000; Hazard et al., 2007). Similarly, antibodies to HPV have been detected in 53% of the sera in healthy controls (Karagas et al., 2006). These studies, combined with our data, suggest that HPV DNA is widespread and that occult infections can arise on clinically normal-appearing skin.
Our HPV detection rate among SCC tumors differs somewhat from the findings from a case–control study using degenerate PCR primers to detect HPV in skin biopsy samples (Iftner et al., 2003). That study detected a similar overall HPV DNA rate in cases (60%, n = 72), but with a higher prevalence (21%) of high-risk genital α-papillomavirus genera compared to the rate in our study (8%). Further, in contrast to our study, they rarely detected HPV in healthy controls (4.7%, n = 106). The authors in that study suggest that the low HPV rate in control tissue may be because the normal skin was disinfected first, resulting in a reduction of skin surface contamination.
Recent studies that have attempted to link seroevidence of specific HPV types with SCC status in immunocompetent individuals have had mixed findings. In a case–control study looking at serum, HPV 8 seropositivity (β-papillomavirus species 1) was positively associated with SCC status, whereas HPV 15 seropositivity (β-papillomavirus species 2) was negatively correlated with SCC status (Masini et al., 2003). In a recent case–control study, HPV antibodies were more frequently detected in SCC patients than in control subjects (OR = 1.6, 95% CI = 1.2–2.3), especially seropositivity to β-papillomaviruses but not α-papillomaviruses (Karagas et al., 2006). This study was limited in that it detected seropositivity as measured by ELISA to only a small fraction of cutaneous HPVs (only 16 types tested) without a correlation of presence of HPV DNA in the tissue. Our methodology involved a more sensitive detection assay of active infection (presence of DNA, not antibodies), and we did not detect differential rates of the genus β-papillomavirus in our cases as compared to controls.
Tumors from cases were more likely to harbor three or more HPV types (“multiple HPVs”) as compared to sun-protected control skin (OR = 6.1, 95% CI = 1.4–26.1) but not sun-exposed control skin (OR = 2.5, 95% CI = 0.8–7.9). The profile of multiple HPV detection rates for perilesional tissue (10%) was similar to that of sun-exposed skin in controls (8%) (OR = 1.1). The similar rates of multiple HPVs detected in clinically normal perilesional skin (which is often sun-exposed, as SCCs arise mostly on sun-exposed skin) and sun-exposed skin in controls very likely suggests that this effect is mediated by UV light. It has previously been shown that UV exposure in the skin induces local T-cell-mediated immunosuppression (Hersey et al., 1983; Yoshiawa et al., 1990; Kelly et al., 2000). Therefore, areas of the body exposed to higher doses of UV light may be less able to fight HPV and more likely to have multiple types detected. Alternatively, UV may have a direct effect on viral replication (de Villiers, 1999). We provide further support for the interaction of UV light with HPV detection in our study by noting a somewhat greater prevalence of HPV in sun-exposed (59%) as compared to sun-protected skin (49%) among our control population. Our findings are also supported by Forslund et al. (2007) in a recently published study, which also notes that HPV DNA was associated with sun-exposure, both for the lesions (OR = 4.45, 95% CI = 2.44–8.11) and for the healthy skin samples (OR = 3.65, 95% CI = 1.79–7.44). The effects of UV light may have important consequences for host resistance to HPV infection and HPV persistence. Further studies on UV-specific DNA markers in relation to HPV status may shed more light on this interaction.
A limitation of our study is that by setting out to detect a broad spectrum of HPV DNA, we did not evaluate transcriptional activity or assess viral load. Demonstrating active infection can be performed using in situ hybridization to identify replicating DNA or RNA. Purdie et al. (2005) has previously demonstrated the feasibility of looking at transcripts in SCCs by in situ hybridization but noted a low transcriptional activity in SCCs as well as in warts, suggesting that in situ hybridization may underestimate active HPV infection. In our study, given the multitude of HPV species detected, including some previously unreported types for which there were no available clones, doing in situ hybridization was not feasible. Similarly, we did not determine the viral load in our samples, given the multitude of types that were detected. Future studies need to be performed that measure the transcriptional activity and expression of specific viral genes as well as the viral load to quantify HPV activity and assess its role in cutaneous carcinogenesis.
In summary, we found that in immunocompetent hosts, cutaneous infection with HPV is prevalent but is not a necessary factor for SCC development. Similar rates of HPV were detected in tissue samples from cases as compared to controls. However, among cases, tumors were more likely to have HPV DNA isolated from β-papillomavirus species 2 than adjacent healthy tissue (paired analysis, OR = 4.0, CI = 1.3–12.0). The high prevalence of HPV DNA detected among controls suggests that HPV DNA is widely distributed among the general population. However, the differential detection of HPV β-papillomavirus species in tumors among cases suggests that certain HPV types may be involved in the progression of cutaneous SCCs. There may be an interaction with HPV and UV light, either through the induction of oncogenes such as p53 or through the inactivation of tumor suppressor genes such as Rb that can potentiate carcinogenesis. Given the widespread occurrence of HPV, future studies need to be performed that measure the expression of certain viral genes so that the activity of HPV can be quantified. Likewise, information on type-specific humoral immune response activities over time could help elucidate the interaction of these viral proteins with host cell functions.
MATERIALS AND METHODS
Subject eligibility and enrollment
Inclusion and exclusion criteria
Caucasian men and women over the age of 55 years who were not immunosuppressed (owing to organ transplantation or immunosuppressive therapy for cancer) were eligible. Subjects were excluded if they had a history of genetic syndromes that would increase susceptibility to damage from UV exposure, including xeroderma pigmentosum, nevoid basal cell syndrome, albinism, epidermodysplasia verruciformis, epidermolysis bullosa dystrophica, dyskeratosis congenital, and psoralen with UV light treatment. Digit and mucosal lip lesions were also excluded. All subjects provided informed consent according to procedures approved by the Institutional Review Boards of the University of Washington and other participating institutions. The study was conducted according to the Declaration of Helsinki Principles.
Subject recruitment
Cases (N = 132) were recruited from patients with a histologically confirmed cutaneous SCC (lip, finger, and genital tumors were excluded) who were undergoing surgical resection of their SCC at dermatology clinics at the University of Washington Medical Center, the VA Puget Sound Health Care System, Group Health Cooperative, and a private medical practice. Controls (N = 95) were individuals without a prior history of skin cancer who were recruited by a letter sent from their primary care providers and were matched to the cases by age ± 5 years.
Data collection
Interview procedures
A standardized interview was administered to all study subjects to collect information pertaining to the following potential confounding variables: (i) demographic or behavioral information, including age, sex, ethnicity, income, and exposure to tobacco smoke, arsenic, hydrocarbons, or radiation; (ii) physical characteristics such as propensity to sunburn and freckle; (iii) history of pertinent dermatological conditions, including actinic keratosis, burn scars, ulcers, and warts; and (iv) lifetime history of sun exposure, including a history of tanning bed use, and number of blistering sunburns.
Physical examination
All control subjects had a full skin examination of their face, neck, scalp, ears, back, torso, buttocks, arms, and legs performed by a dermatologist (MMA) to insure absence of lesions suspicious for skin cancer. If suspicious lesions were identified, the subject was deemed ineligible and an appropriate medical referral was initiated.
Collection of skin specimens
Cases
A small portion of the residual SCC as well as adjacent clinically uninvolved perilesional skin (Figure 2) was collected from each subject and divided into thirds. One third was placed in 10% neutral buffered formalin for histopathologic confirmation of SCC at the time of the surgery (hematoxylin and eosin stain), another third placed into standard viral transport media, and the final portion placed into ethanol.
Figure 2. SCC of the ear demonstrating lesional and perilesional tissue.
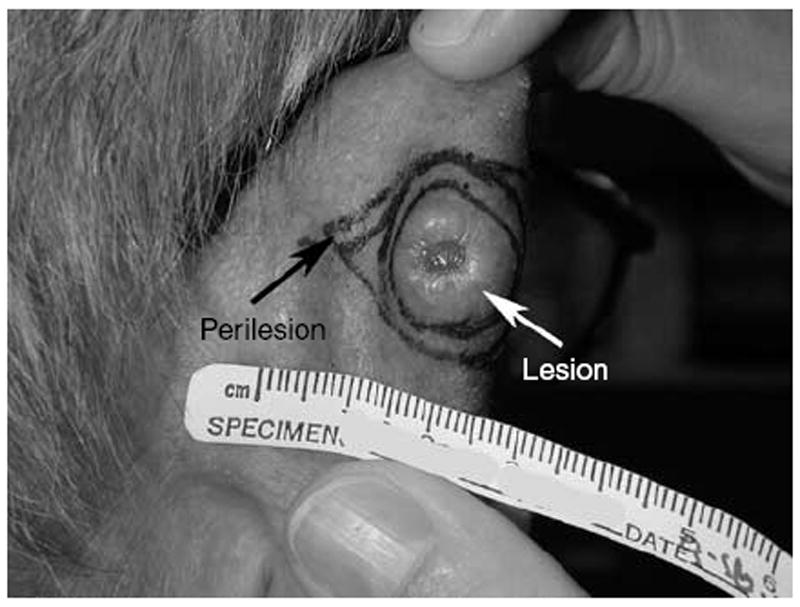
Controls
For each subject, punch biopsies of clinically normal skin were obtained from a sun-exposed (pre-auricular) site as well as from a sun-protected site from the upper inner arm. After intradermal anesthesia with 1 ml of 1% lidocaine with epinephrine at 1:100,000, a 4-mm punch biopsy was inserted to the level of the subcutaneous fat. Each specimen was divided into thirds. One third was placed in 10% neutral buffered formalin for histopathologic evaluation (hematoxylin and eosin stain), another third placed into standard viral transport media for HPV PCR-detection assays, and the final portion placed into ethanol for future analyses. The defect was closed with two simple interrupted 5-0 fast-absorbing gut sutures.
Laboratory methods
Histopathology methods
Routine paraffin-embedded tissue was prepared from the biopsy specimens and then sectioned and stained with hematoxylin and eosin to confirm the diagnosis.
HPV detection
DNA from the biopsies was extracted with buffer-saturated phenol–chloroform–isoamyl alcohol and subsequently precipitated with ethanol. Three different PCR protocols were used to amplify HPV DNA (Snijders et al., 1991; Berkhout et al., 1995; Forslund et al., 1999). The first protocol used primers originally designed for detection of epidermodysplasia verruciformis and epidermodysplasia verruciformis-related cutaneous HPV types, but by modifying the amplification conditions, almost all known HPV types were amplified (de Villiers, 1997). The second protocol allowed for detection of mucosal HPVs (Snijders et al., 1991) and the third is designed to detect all known cutaneous HPV types (Forslund et al., 1999).
Protocol 1
DNA (100 ng per 50 μl reaction volume per sample) was denatured at 94 °C for 9 minutes and then immediately followed by 40-cycle amplification in the Thermal Cycler (Perkin Elmer Cetus, Norwalk, CT). Each cycle consisted of denaturation (94 °C for 1 minute), annealing (50 °C for 2 minutes), and extension (72 °C for 1 minute), with a final extension at 72 °C for 5 minutes in the last cycle followed by cooling down to 4 °C. The reaction mixture contained 1 mm dNTP, 50 pmol of each primer, 1.25 U Ampli Taq Gold (Perkin Elmer), and supplied buffer with 2.5 mm of MgCl2. An aliquot of 2–3 μl PCR product was used for the subsequent nested PCR amplification, using the same conditions as described above. Aliquots of the first, as well as the nested amplification products, were separated on a 1.5% agarose gel. If visible bands were of expected sizes (first 460 bp, nested 380 bp) in both reaction products, both products were eluted from the gel for further analysis.
Protocol 2
DNA (100 ng per 50 μl reaction volume per sample) was amplified with a pair of primers GP5 + and GP6 +, which generated HPV fragments of 140–150 bp. Amplification was completed through 40 cycles (94 °C for 1 minute, annealing at 40 °C for 2 minutes, and extension at 72 °C for 1.5 minutes), with an initial heating for 9 minutes at 94 °C and an end extension for 4 minutes at 72 °C. The reaction mixture contained 1 mm dNTP, 50 pmol of each primer, 1.25 U Ampli Taq Gold (Perkin Elmer), and supplied buffer with 2.5 mm of MgCl2.
Protocol 3
DNA (100 ng per 50μl reaction volume per sample) was amplified using the FAP59 and FAP64 primers. Amplicons of 480 pb were generated. Amplification was performed after an initial heating for 8 minutes at 94 °C followed by 45 cycles each of 1.5 minutes at 94 °C for denaturation, 1.5 minutes at 50 °C for annealing, and 1.5 minutes at 72 °C for extension. The reaction was completed with a final extension cycle of 5 minutes at 72 °C. The reaction mixture contained 200 μm of dNTPs and 50 pmol of each primer, and MgCl2 was added to a final concentration of 3.5 mm.
Analysis of amplified products generated by the three protocols
An aliquot of amplified products for each sample was subjected to 1.5 or 2% agarose gel electrophoresis and visualized after EtBr staining. If only one band of the expected size was visible, the rest of the PCR products from that sample were purified directly using High Pure PC Product Purification kit (Roche, Roche Applied Science, Indianapolis, IN). If more than one band was visible, the rest of the PCR products from that sample were separated on another agarose gel. The excised gel segments containing the expected bands were purified using the Genomed-Gel extraction Spinkit (Genomed, St Louis, MO). The resulting products were cloned into a plasmid vector using either TA Cloning Kit (Invitrogen, Carlsbad, CA) or the TOPO-TA-Cloning Kit (Invitrogen). All ligations were performed overnight at 14 °C. Clones containing target inserts were identified by restriction enzyme digestion and analyzed by gel electrophoresis. Up to 10 clones per sample were subjected to sequencing.
Sequencing was performed by an ABI Model Sequences using Big Dye terminator chemistry (Perkin Elmer Applied Biosystems Division, Waltham, MA). The sequences were compared to the DNA sequences of all known HPV types in the European Molecular Biology Laboratory database (European Bioinformatics Institute, Cambridge, UK) and GenBank (National Center for Biotechnology Information, NIH, Bethesda, MD). An HPV type was defined by less than 90% homology within the L1 open reading frame to any other known type. The putative, previously unreported HPV types were sequenced in full on both DNA strands for complete identification.
Statistical methods
Differences between cases and controls were assessed using χ2 analyses to compare categorical variables and Student’s t-test to compare continuous measures. The risk of SCC associated with HPV was estimated using multivariable logistic regression, adjusting for propensity to sunburn. Conditional logistic regression was also used to assess the risk of SCC among paired specimens (lesional versus perilesional within cases). A two-sided 0.05 level test determined statistical significance for all analyses. All analyses were conducted using SAS 9.1 (SAS Institute Inc., Cary, NC).
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health NRSA Individual Postdoctoral Fellowship (1 F32 AR47552, Dr Asgari), the National Cancer Institute (R01 CA084087, Dr Kiviat), as well as the Ministry of Health, Berlin (Dr E-M de Villiers).
Abbreviations
- CI
confidence interval
- HPV
human papillomavirus
- OR
odds ratio
- SCC
squamous cell carcinoma
Footnotes
CONFLICT OF INTEREST The authors state no conflict of interest.
References
- Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:975–83. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- Antonsson A, Forslund O, Ekberg H, Sterner G, Hansson BG. The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of these viruses. J Virol. 2000;74:11636–41. doi: 10.1128/jvi.74.24.11636-11641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astori G, Lavergne D, Benton C, Hockmayr B, Egawa K, Garbe C, et al. Human papillomaviruses are commonly found in normal skin of immunocompetent hosts. J Invest Dermatol. 1998;110:752–5. doi: 10.1046/j.1523-1747.1998.00191.x. [DOI] [PubMed] [Google Scholar]
- Berkhout RJ, Tieben LM, Smits HL, Bavinck JN, Vermeer BJ, ter Schegget J. Nested PCR approach for detection and typing of epidermodysplasia verruciformis-associated human papillomavirus types in cutaneous cancers from renal transplant recipients. J Clin Microbiol. 1995;33:690–5. doi: 10.1128/jcm.33.3.690-695.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biliris KA, Koumantakis E, Dokianakis DN, Sourvinos G, Spandidos DA. Human papillomavirus infection of non-melanoma skin cancers in immunocompetent hosts. Cancer Lett. 2000;161:83–8. doi: 10.1016/s0304-3835(00)00596-6. [DOI] [PubMed] [Google Scholar]
- Boxman IL, Berkhout RJ, Mulder LH, Wolkers MC, Bouwes Bavinck JN, Vermeer BJ, et al. Detection of human papillomavirus DNA in plucked hairs from renal transplant recipients and healthy volunteers. J Invest Dermatol. 1997;108:712–5. doi: 10.1111/1523-1747.ep12292090. [DOI] [PubMed] [Google Scholar]
- Boxman IL, Russell A, Mulder LH, Bavinck JN, Schegget JT, Green A. Case–control study in a subtropical Australian population to assess the relation between non-melanoma skin cancer and epidermodysplasia verruciformis human papillomavirus DNA in plucked eyebrow hairs. The Nambour Skin Cancer Prevention Study Group. Int J Cancer. 2000;86:118–21. doi: 10.1002/(sici)1097-0215(20000401)86:1<118::aid-ijc18>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- de Koning MN, Struijk L, Bavinck JN, Kleter B, ter Schegget J, Quint WG, et al. Betapapillomaviruses frequently persist in the skin of healthy individuals. J Gen Virol. 2007;88:1489–95. doi: 10.1099/vir.0.82732-0. [DOI] [PubMed] [Google Scholar]
- de Villiers EM. Papillomavirus and HPV typing. Clin Dermatol. 1997;15:199–206. doi: 10.1016/s0738-081x(96)00164-2. [DOI] [PubMed] [Google Scholar]
- de Villiers EM. Human papillomavirus. Introduction. Semin Cancer Biol. 1999;9:377. doi: 10.1006/scbi.1999.0140. [DOI] [PubMed] [Google Scholar]
- de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- de Villiers EM, Lavergne D, McLaren K, Benton EC. Prevailing papillomavirus types in non-melanoma carcinomas of the skin in renal allograft recipients. Int J Cancer. 1997;73:356–61. doi: 10.1002/(sici)1097-0215(19971104)73:3<356::aid-ijc9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Foote JA, Harris RB, Giuliano AR, Roe DJ, Moon TE, Cartmel B, et al. Predictors for cutaneous basal- and squamous-cell carcinoma among actinically damaged adults. Int J Cancer. 2001;95:7–11. doi: 10.1002/1097-0215(20010120)95:1<7::aid-ijc1001>3.0.co;2-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forslund O, Antonsson A, Nordin P, Stenquist B, Hansson BG. A broad range of human papillomavirus types detected with a general PCR method suitable for analysis of cutaneous tumours and normal skin. J Gen Virol. 1999;80:2437–43. doi: 10.1099/0022-1317-80-9-2437. [DOI] [PubMed] [Google Scholar]
- Forslund O, Iftner T, Andersson K, Lindelof B, Hradil E, Nordin P, et al. Viraskin Study Group. Cutaneous human papillomaviruses found in sun-exposed skin: beta-papillomavirus species 2 predominates in squamous cell carcinoma. J Infect Dis. 2007;196:876–83. doi: 10.1086/521031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray DT, Suman VJ, Su WP, Clay RP, Harmsen WS, Roenigk RK. Trends in the population-based incidence of squamous cell carcinoma of the skin first diagnosed between 1984 and 1992. Arch Dermatol. 1997;133:735–40. [PubMed] [Google Scholar]
- Harwood CA, Surentheran T, McGregor JM, Spink PJ, Leigh IM, Breuer J, et al. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289–97. doi: 10.1002/1096-9071(200007)61:3<289::aid-jmv2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Harwood CA, Surentheran T, Sasieni P, Proby CM, Bordea C, Leigh IM, et al. Increased risk of skin cancer associated with the presence of epidermodysplasia verruciformis human papillomavirus types in normal skin. Br J Dermatol. 2004;150:949–57. doi: 10.1111/j.1365-2133.2004.05847.x. [DOI] [PubMed] [Google Scholar]
- Hazard K, Karlsson A, Andersson K, Ekberg H, Dillner J, Forslund O. Cutaneous human papillomaviruses persist on healthy skin. J Invest Dermatol. 2007;127:116–9. doi: 10.1038/sj.jid.5700570. [DOI] [PubMed] [Google Scholar]
- Hersey P, Haran G, Hasic E, Edwards A. Alteration of T cell subsets and induction of suppressor T cell activity in normal subjects after exposure to sunlight. J Immunol. 1983;131:171–4. [PubMed] [Google Scholar]
- Iftner A, Klug SJ, Garbe C, Blum A, Stancu A, Wilczynski SP, et al. The prevalence of human papillomavirus genotypes in nonmelanoma skin cancers of nonimmunosuppressed individuals identifies high-risk genital types as possible risk factors. Cancer Res. 2003;63:7515–9. [PubMed] [Google Scholar]
- Karagas MR, Nelson HH, Sehr P, Waterboer T, Stukel TA, Andrew A, et al. Human papillomavirus infection and incidence of squamous cell and basal cell carcinomas of the skin. J Natl Cancer Inst. 2006;98:389–95. doi: 10.1093/jnci/djj092. [DOI] [PubMed] [Google Scholar]
- Kelly DA, Young AR, McGregor JM, Seed PT, Potten CS, Walker SL. Sensitivity to sunburn is associated with susceptibility to ultraviolet radiation-induced suppression of cutaneous cell-mediated immunity. J Exp Med. 2000;191:561–6. doi: 10.1084/jem.191.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviat NB. Papillomaviruses in non-melanoma skin cancer: epidemiological aspects. Semin Cancer Biol. 1999;9:397–403. doi: 10.1006/scbi.1999.0143. [DOI] [PubMed] [Google Scholar]
- Majewski S, Jablonska S. Skin autografts in epidermodysplasia verruciformis: human papillomavirus-associated cutaneous changes need over 20 years for malignant conversion. Cancer Res. 1997;57:4214–6. [PubMed] [Google Scholar]
- Masini C, Fuchs PG, Gabrielli F, Stark S, Sera F, Ploner M, et al. Evidence for the association of human papillomavirus infection and cutaneous squamous cell carcinoma in immunocompetent individuals. Arch Dermatol. 2003;139:890–4. doi: 10.1001/archderm.139.7.890. [DOI] [PubMed] [Google Scholar]
- Nobbenhuis MA, Walboomers JM, Helmerhorst TJ, Rozendaal L, Remmink AJ, Risse EK, et al. Relationship of human papillomavirus status to cervical lesions and consequences for cervical cancer screening: a prospective study. Lancet. 1999;354:20–5. doi: 10.1016/S0140-6736(98)12490-X. [DOI] [PubMed] [Google Scholar]
- Preston DS, Stern RS. Nonmelanoma cancers of the skin. N Engl J Med. 1992;327:1649–62. doi: 10.1056/NEJM199212033272307. [DOI] [PubMed] [Google Scholar]
- Purdie KJ, Sexton CJ, Proby CM, Glover MT, Williams AT, Stables JN, et al. Malignant transformation of cutaneous lesions in renal allograft patients: a role for human papillomavirus. Cancer Res. 1993;53:5328–33. [PubMed] [Google Scholar]
- Purdie KJ, Surentheran T, Sterling JC, Bell L, McGregor JM, Proby CM, et al. Human papillomavirus gene expression in cutaneous squamous cell carcinomas from immunosuppressed and immunocompetent individuals. J Invest Dermatol. 2005;125:98–107. doi: 10.1111/j.0022-202X.2005.23635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamanin V, zur Hausen H, Lavergne D, Proby CM, Leigh IM, Neumann C, et al. Human papillomavirus infections in nonmelanoma skin cancers from renal transplant recipients and nonimmunosuppressed patients. J Natl Cancer Inst. 1996;88:802–11. doi: 10.1093/jnci/88.12.802. [DOI] [PubMed] [Google Scholar]
- Snijders PJ, Meijer CJ, Walboomers JM. Degenerate primers based on highly conserved regions of amino acid sequence in papillomaviruses can be used in a generalized polymerase chain reaction to detect productive human papillomavirus infection. J Gen Virol. 1991;72:2781–6. doi: 10.1099/0022-1317-72-11-2781. [DOI] [PubMed] [Google Scholar]
- Tieben LM, Berkhout RJ, Smits HL, Bouwes Bavinck JN, Vermeer BJ, Bruijn JA, et al. Detection of epidermodysplasia verruciformis-like human papillomavirus types in malignant and premalignant skin lesions of renal transplant recipients. Br J Dermatol. 1994;131:226–30. doi: 10.1111/j.1365-2133.1994.tb08496.x. [DOI] [PubMed] [Google Scholar]
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Yoshiawa T, Rae V, Bruins-Slot W, Van den Berg JW, Taylor JR, Streilein JW. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J Invest Dermatol. 1990;95:530–6. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Papillomavirus infections—a major cause of human cancers. Biochim Biophys Acta. 1996;1288:F55–78. doi: 10.1016/0304-419x(96)00020-0. [DOI] [PubMed] [Google Scholar]


