Summary
Background
Antiphospholipid syndrome is characterized by autoantibodies against cardiolipins (aCL), lupus anticoagulant, and independent β2-glycoprotein (β2GPI). Controversy exists as to whether vaccination triggers the development of anti-phospholipid antibodies (aPL) in systemic lupus erythematosus (SLE) patients.
Methods
SLE patients (101) and matched controls (101) were enrolled from 2005 to 2009 and received seasonal influenza vaccinations. Sera were tested by ELISA for aCL at baseline, 2, 6, and 12 weeks after vaccination. Vaccine responses were ranked according to an overall anti-influenza antibody response index. Individuals with positive aCL were further tested for β2GPI antibodies.
Results
SLE patients and healthy controls developed new onset aCL post-vaccination (12/101 cases and 7/101 controls, OR 1.81, p=0.34). New onset moderate aCL are slightly enriched in African American SLE patients (5/36 cases; p=0.094). The optical density (OD) measurements for aCL reactivity in patients were significantly higher than baseline at 2 weeks (p<0.05), 6 weeks (p<0.05), and 12 weeks (p<0.05) post vaccination. No new β2GPI antibodies were detected among patients with new aCL reactivity. Vaccine response was not different between patients with and without new onset aCL reactivity (p=0.43).
Conclusions
This study shows transient increases in aCL, but not anti-β2GPI responses, after influenza vaccination.
Keywords: Influenza, vaccine, antiphospholipid antibodies, systemic lupus erythematosus
Introduction
The presence of anticardiolipin antibodies (aCL) among patients with systemic lupus erythematosus (SLE), particularly the immunoglobulin G (IgG) subtype, is strongly associated with the occurrence of a thrombotic clinical event1–3. Lupus anticoagulant (LA) or aCL IgG are also detected among patients with antiphospholipid syndrome (APS) who do not meet classification criteria for SLE4. Antiphospholipid antibodies (aPL) have become part of the immunologic classification criterion for SLE by the American College of Rheumatology (ACR)5. Among aPL positive SLE patients, there is a 34% chance of developing venous thrombosis over 20 years according to a large multi-ethnic lupus cohort6. Similarly, a study by Tektonidou et al. indicated a 20.1% incidence of thrombosis over 109 months of follow up for SLE patients with persistent aCL7. SLE patients with aCL early in disease meet more SLE classification criteria and were more likely to have renal involvement, thrombocytopenia and CNS involvement8. Additionally, a correlation has been observed between developing aCL prior to SLE diagnosis and the presence of anti-Sm and anti-dsDNA autoantibodies years earlier compared to lupus patients that are aCL negative prior to diagnosis8.
Antibodies against Independent β2 glycoprotein (β2GPI), have been shown to act as a cofactor among aCL positive SLE patients in increasing thrombosis risk9–11 and are presently included in the revised Sapporo criteria for APS12. In this updated classification criteria, these aPL are required to be present on two separate determinations at least 12 weeks apart. The importance of identifying individuals at risk to develop APS derives from the relatively high morbidity and mortality seen among this group, particularly among those developing catastrophic APS13. Interestingly, catastrophic APS is oftentimes preceded by systemic infection14. Infection and the administration of vaccines have been proposed to trigger flares of disease activity or even the development of autoimmune conditions such as SLE, perhaps via molecular mimicry of microbial antigens leading to the production of pathogenic autoantibodies in SLE15–18. The presence of aCL has been documented after exposure to different infectious agents19, 20 and controversy exists as to whether vaccination triggers autoimmune reactions in SLE patients. Our study aims to determine the presence and character of these aPL (aCL and β2GPI) among SLE patients before and after administration of the seasonal influenza vaccine.
Methods
Participant Information
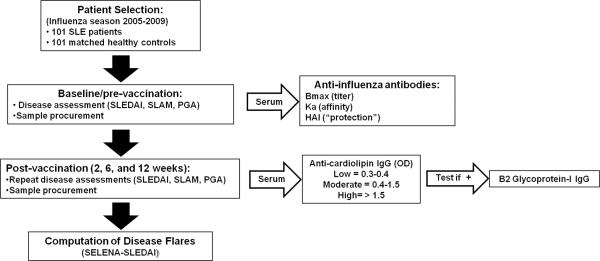
This study spanned 5 influenza seasons (2005–2009) and included a cohort of unique individuals who received the currently licensed influenza vaccine approved for use in the United States during each given year (see Figure 1 for the study design). A total of 101 patients meeting at least 4 of the 11 ACR SLE classification criteria for SLE were randomly selected after providing informed consent as designated by the Institutional Review Board of Oklahoma Medical Research Foundation (OMRF). Healthy controls were matched based on age (+/− 5 years), race, and sex. Peripheral blood was drawn and stored at −20°C pre-vaccination and 2, 6 and 12 weeks post vaccination. Patient and control demographic information, including age, sex and race, were collected. Information regarding specific SLE ACR criteria and current immunosuppressive medications of patients were also recorded.
Figure 1.
SLE and control cohort study design and evaluation scheme.
Antiphospholipid assays
IgG antibodies to the autoantigen cardiolipin were measured by enzyme linked immunosorbent assay (ELISA) for all patients for each study clinic visit. Cardiolipin in ethanol at a concentration of 0.25 μg/well was coated onto 96-well plates using a previously published protocol21. A 1:100 dilution of sera was incubated followed by incubation with anti-human IgG conjugated to alkaline phosphatase at 1:1000 (Sigma, St. Louis, MO). The aCL were measured by optical density (OD), absorbances were normalized to a standard positive control, and responses defined as low (0.30–0.40 OD), moderate (0.40–1.50 OD) and high (> 1.50 OD) reactivity. All individuals with positive aCL before and after vaccination at all time points were tested further for antibodies to β2GPI IgG using a commercial kit (Inova Diagnostics, San Diego, CA).
Clinical Characterization and Disease Assessments
Clinical evaluation and standard disease activity measures were performed by a rheumatologist for each study clinic visit. SLE disease activity index (SLEDAI), SLE activity measure (SLAM), and physician's global assessment (PGA) were performed at baseline and the 6 week and 12 week after vaccination clinic visits. The occurrences of disease flare after vaccination were then computed by using the SLE National Assessment (SELENA)-SLEDAI scores after 6 and 12 weeks.
Influenza vaccination response
Cumulative vaccine responses were ranked from the highest to lowest according to an overall anti-influenza antibody response index. The three measures of responsiveness were: 1) total amount of antibody binding native virus (Bmax), affinity (Ka, the inverse of the dissociation constant Kd), and hemagglutinin inhibition titer (HAI). The normalized average rank (or the sum of the ranks) of the three measures was used to rank order the individuals into high and low vaccine responders22.
Statistical analysis
Descriptive and correlation statistics using Graphpad prism version 5 were utilized in this study. Comparisons among different patient demographic groups were made and low, moderate, and high new onset aCL were each compared against matched controls separately by Chi-square and Fisher's exact testing. The change of aCL levels in relation to time among patients and matched controls who developed new onset aCL at 2, 6, and 12 weeks after vaccination were plotted by linear regression. Differences between levels of positive aCL for the patients and the controls were computed by one way repeated measures analysis of variance (ANOVA) and then comparisons of the mean OD levels of aCL positive patients before vaccination against each of the mean OD levels of aCL positive patients at 2, 6, and 12 weeks post vaccination were done by paired t-testing23.
Results
Influenza Vaccination can induce new onset aCL
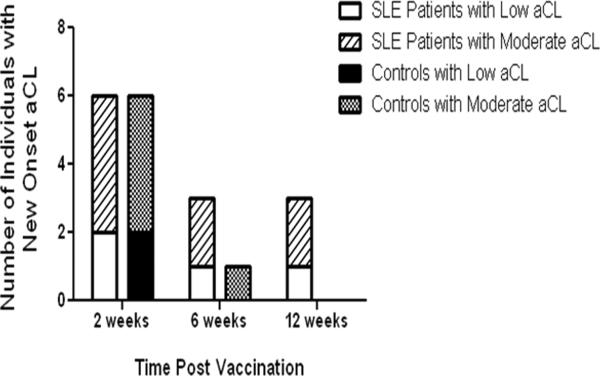
After influenza vaccination, both patients and healthy matched control individuals developed new low aCL reactivity (4/101 cases and 2/101 controls) or new moderate aCL reactivity (8/101 cases and 5/101 controls) post vaccination. No significant differences between the patients and the matched controls were observed for the development of new low or moderate aCL at 2 weeks. New low aCL reactivity was observed at 6 weeks (in one case and no controls) and at 12 weeks (in one case and no controls) post influenza vaccination. New onset moderate aCL reactivity, was also observed at 6 weeks (2/101 cases and 1/101 controls) or at 12 weeks (2/101 cases; 0/101 controls) post vaccination (Figure 2).
Figure 2.
SLE patients and controls have transient new onset aCL responses post vaccination. Both SLE patients and controls develop new onset low aCL reactivity and new onset moderate aCL reactivity after influenza vaccination. SLE patients with low aCL reactivity are represented by the white bar; SLE patients with moderate aCL reactivity are respresented by the diagonally striped bar. Controls with low aCL reactivity are represented by the black bar; while controls with moderate aCL reactivity are represented by the black and white dotted bar.
Development of aCL after influenza vaccination trends toward enrichment in African American SLE patients
Of the unique 101 SLE patients, 92% are females and the average age within the cohort was 43.9 (± 14 years of age). Ethnicity of patients was determined by self report. The majority of patients were of European American (EA) descent (58%), while 36% were African American (AA) with 6% belonging to other races. Average number of SLE ACR criteria among patients was 5.7. Two AA and two EA patients tested positive for development of low aCL after vaccination, while five AA, and two EA patients developed moderate aCL after vaccination. The frequency of African Americans in the group of SLE patients with new onset aCL at moderate levels was higher than that in SLE patients who remained aCL negative after vaccination, but the difference was not statistically significant (p=0.094) (Table 1).
Table I.
Demographics of SLE patients with aCL
| aCL positive patients (Baseline cohort) | After Influenza vaccination | ||||
|---|---|---|---|---|---|
|
|
|||||
| Total (n = 101) | Low (n=6) | Moderate (n=5) | New onset low aCL (n=4) | New onset moderate aCL (n=8) | |
| Age | 43.9±14 | 47.8±19 | 31 ±8 | 46.75±16.3 | 42.1±14 |
|
| |||||
| Race: | |||||
| AA | 36 | 3 | 1 | 2 | 5 |
| EA | 59 | 3 | 4 | 2 | 2 |
| Other | 6 | 0 | 0 | 0 | 1 |
|
| |||||
| Gender: | |||||
| M | 8 | 0 | 1 | 0 | 1 |
| F | 93 | 6 | 4 | 4 | 7 |
|
| |||||
| Average Number of ACR Criteria | 5.7 | 5.3 | 7.2 | 4.8 | 5.8 |
AA, African American; EA, European American; M, Male; F, Female
Influenza vaccination can induce higher aCL reactivity
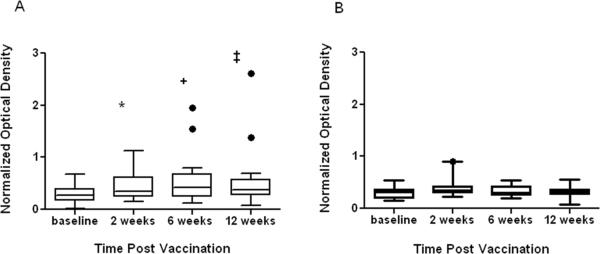
Comparing each time point from the baseline mean aCL OD level (0.284, SD 0.175) from aCL positive SLE patients, the mean aCL OD levels were significantly higher 2 weeks (0.458, SD 0.27; p=0.025), 6 weeks (0.524, SD 0.44; p=0.0102), and 12 weeks (0.519, SD 0.525; p=0.02) post vaccination. High aCL reactivity was recorded in 2 patients with low and moderate aCL before vaccination (Figure 3). No new β2GPI antibodies were detected in any subjects with new aCL reactivity post vaccination. Only one aCL positive patient before vaccination had detectable anti-β2GPI antibodies.
Figure 3.
aCL reactivity in SLE patients are higher post vaccination. The levels of positive aCL were significantly higher for SLE patients (A) two (p=0.025, shown by *), six (p=0.0102, shown by +), and 12 (p=0.02, shown by ‡) weeks post influenza vaccination. Conversely, levels of positive aCL in controls (B) were not different at any time points post vaccination. Boxplots denote the 95% confidence interval intersected by a line corresponding to the mean.High aCL reactivity was recorded only on two patients with aCL before influenza vaccination (shown in solid dots outside the boxplots A).
SLE patients with aCL have similar disease flare frequencies and vaccine responses as aCL negative patients
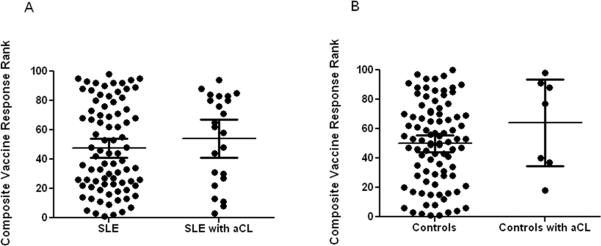
Forty three out of 101 (42.6%) patients developed disease flares after vaccination. No significant differences were observed between the aCL positive and negative patients when examining the frequency of post vaccination disease flare (8/23 aCL positive vs. 35/78 aCL negative; p=0.47). Among individuals who flared, only three patients developed new onset aCL while five patients were aCL positive prior to vaccination. When examining aCL and vaccine response no statistical difference was observed between SLE patients without aCL and patients with aCL (p=0.43) (Figure 4).
Figure 4.
SLE patients with or without new onset aCL have similar vaccine responses. There is no difference in the vaccine response (equal contribution of Bmax, Ka, and HAI) of SLE patients with and without aCL (A) or in controls with and without aCL (B). The error bars on the graphs represent 95% confidence interval with a line corresponding to the mean vaccine response.
Discussion
Autoantibody production against phospholipid binding proteins has been documented as a consequence of exposure to infectious agents24, 25 but conflicting reports still exist about the appearance of these aPL after vaccine administration. In healthy subjects given the Hepatitis B recombinant vaccine who exhibited changes in aPL values, a significant increase was observed in β2GPI values but not in aCL values26. After influenza vaccination, transient appearance of autoantibodies and progressively increased levels of aCL or anti-β2GPI were demonstrated in only approximately 8% of 92 apparently healthy adults after vaccination27. This low rate of aCL positivity among healthy individuals was also seen in our study. Seven out of 101 healthy controls developed new onset low (n=2) or moderate (n=5) aCL after receiving the vaccine. Susceptible individuals may have an increased risk of developing a sustained autoimmune response and subsequent production of these autoantibodies after vaccination. Several case reports have recounted the occurrence of an autoimmune condition and production of aPL after influenza vaccination28, 29.
Among SLE patients, production of aCL has been documented in one patient after pneumococcal vaccination30 and among nine patients after influenza vaccination31. Looking further into the effect of influenza vaccination among SLE patients, a report by Tarjan et al. showed a remarkable elevation in the levels of β2GPI antibodies in aPL positive and negative patients, but did not show any clinical consequences in patients with previous aPL positivity32. SLE patients with aCL tend to have these antibodies preceding initial clotting events by several years. Furthermore, the presence of early, pre-diagnosis aCL in this susceptible group correlates with a more varied and severe SLE clinical course and younger age at diagnosis21. In this study, the titer of aCL antibody reactivity was significantly higher among SLE patients after vaccination at all time points.
Among SLE patients with APS, aPL have been demonstrated to be directed against epitopes expressed on β2GPI but not cardiolipin in contrast to aPL associated with infectious diseases33–35. β2GPI binds to anionic phospholipids including cardiolipin adhering to activated platelets and inhibiting the contact activation of blood coagulation36, 37. Anti-β2GPI have been shown to recognize different β2GPI epitopes including a major antigenic region recognized by monoclonal aPL isolated from APS patients38. In addition, the group of de Laat et al. have shown that pathogenic aPL bind to a cryptic epitope (G40–R43) on the first domain of β2GPI39. In our study, none of the individuals who developed aCL responses after vaccination had detectable β2GPI antibodies. No matched control and only one SLE patient with aCL reactivity prior to vaccination had detectable levels of anti-β2GPI. This could reflect the apparently benign nature of aCL observed in healthy individuals in the general population as well as the aCL induced by infectious agents, and potentially by influenza vaccine administration seen in our study. The significantly higher aCL reactivity seen among SLE patients in this study after vaccination, although almost uniformly non-reactive to β2GPI antibodies, can be further evaluated beyond 12 weeks to determine persistence of aPL in multiple years.
The varying levels of aCL reactivity seen among each of the subjects in this study on all time points before and after Influenza vaccination reinforces the transient nature of aPL reported among several studies which requires interaction between innate and acquired immunity in promoting thrombotic risk40, 41. No specific clinical or laboratory variables have been identified to be associated with SLE flare following influenza vaccination42, 43. Vaccines may trigger short term generation of autoantibodies however, this does not have long term affects on SLE disease course as seen in our study.
Acknowledgements
We would like to thank Scott Stewart, Jourdan Anderson and Beverly Hurt for the technical support as well as Drs. Amy Dedeke, Morris Reichlin, John Harley, Linda Zacharias, Craig Carson, Ana Ahluwahlia Kumar, other referring providers and all of the individuals that agreed to participate in this study. This study was made possible by the Kirkland Scholar Award Program at The Hospital for Special Surgery in New York City and is funded exclusively by Rheuminations, Inc., a non-profit foundation dedicated to supporting research leading to the treatment and cure of lupus. Additionally, this work was supported in part by the Lou Kerr Chair in Biomedical Research, as well as grants from NIAMS, NIAID and NCRR (P30 RR031152, P30 AR053483, HHSN266200500026C, U19A1082714).
Footnotes
Disclosures The authors have no financial disclosures related to this manuscript.
References
- 1.Hughes G. Thrombosis, abortion, cerebral disease, and the lupus anticoagulant. Br Med J (Clinc Res Ed) 1983;287:1088–1089. doi: 10.1136/bmj.287.6399.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris E, Chan J, Asherson R, et al. Thrombosis, recurrent fetal loss, and thrombocytopenia. Predicitive value of the anticardiolipin antibody test. Arch Intern Med. 1986;146:2153–2156. [PubMed] [Google Scholar]
- 3.Harris E, Gharavi A, Boey M, et al. Anticardiolipin antibodies: Detection by radioimmunoassay and association with thrombosis in systemic lupus erythematosus. Lancet. 1983;322:1211–1214. doi: 10.1016/s0140-6736(83)91267-9. [DOI] [PubMed] [Google Scholar]
- 4.Hughes G. Hughes syndrome: antiphospholipid syndrome. J R Coll Physicians Lond. 1998;32:260–264. [PMC free article] [PubMed] [Google Scholar]
- 5.Hochberg M. Updating the American College of Rheumatology Revised Criteria for the Classification of Systemic Lupus Erythematosus. Arthritis and Rheumatism. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 6.Somers E, Magder L, Petri M. Antiphospholipid antibodies and incidence of venous thrombosis in a cohort of patients with systemic lupus erythematosus. J Rheumatol. 2002;29:2531–2536. [PubMed] [Google Scholar]
- 7.Tektonidou M, Laskari K, Panagiotakos D, Moutsopoulos H. risk factors for thrombosis and primary thrombosis prevention in patients with systemic lupus erythematosus with or without antiphospholipid antibodies. Arthritis Rheum. 2009;61:29–36. doi: 10.1002/art.24232. [DOI] [PubMed] [Google Scholar]
- 8.McClain MT, Arbuckle MR, Heinlen LD, et al. The prevalence, onset, and clinical significance of antiphospholipid antibodies prior to diagnosis of systemic lupus erythematosus. Arthritis Rheum. 2004;50:1226–1232. doi: 10.1002/art.20120. [DOI] [PubMed] [Google Scholar]
- 9.Tsutsumi A, Matsuura E, Ichikawa K, et al. Antibodies to beta 2-glycoprotein 1 and clinical manifestiations in patients with systemic lupus erythematosus. Arthritis Rheum. 1996;39:1466–1474. doi: 10.1002/art.1780390905. [DOI] [PubMed] [Google Scholar]
- 10.Amengual O, Atsumi T, Khamashta M, Koike T, Hughes G. Specificity of ELISA for antibody to beta 2-glycoprotein I in patients with systemic lupus erythematosus. Br J Rheumatol. 1996;35:1239–1243. doi: 10.1093/rheumatology/35.12.1239. [DOI] [PubMed] [Google Scholar]
- 11.Koike T, Matsuura e. Anti-beta 2-glycoprotein 1 antibody: specificity and clinical significance. Lupus. 1996;35:1239–1243. doi: 10.1177/096120339600500508. [DOI] [PubMed] [Google Scholar]
- 12.Miyakis S, Lockshin M, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 13.Espinosa G, Cervera R. Antiphospholipid syndrome: frequency, main causes, and risk factors of mortality. Nat Rev Rheumatol. 2010;6:296–300. doi: 10.1038/nrrheum.2010.47. [DOI] [PubMed] [Google Scholar]
- 14.Asherson R, Cervera R, Piette J, et al. Catastrophoic antiphospholipid syndrome: Clues to the pathogenesis from a series of 80 patients. Medicine (Baltimore) 2001;80:355–377. doi: 10.1097/00005792-200111000-00002. [DOI] [PubMed] [Google Scholar]
- 15.James J, Scofield R, Harley J. Lupus humoral autoimmunity after short peptide immunization. Ann N Y Acad Sci. 1997;815:124–127. doi: 10.1111/j.1749-6632.1997.tb52054.x. [DOI] [PubMed] [Google Scholar]
- 16.Molina V, Shoenfeld Y. Infection, vaccines, and other environmental triggers of autoimmunity. Autoimmunity. 2005;38:235–245. doi: 10.1080/08916930500050277. [DOI] [PubMed] [Google Scholar]
- 17.Orbach H, Agmon-Levin N, Zandman-Goddard G. Vaccines and autoimmune diseases of the adult. Discov Med. 2010;9:90–97. [PubMed] [Google Scholar]
- 18.James J, Harley J, Scofield R. Epstine-Barr virus and systemic lupus erythematosus. Curr Opin Rheumatol. 2006;18:462–467. doi: 10.1097/01.bor.0000240355.37927.94. [DOI] [PubMed] [Google Scholar]
- 19.Santiago M, Cossermelli W, Tuma MP, MN, Oliveira R. Anticardiolipin antibodies in patients with infectious disease. Clin Theumatol. 1986;8:23–28. doi: 10.1007/BF02031064. [DOI] [PubMed] [Google Scholar]
- 20.Vaarala P, Palosuo T, Kleemola M, Aho K. Anticardiolipin response in acute infections. Clin Immunol Immunopathol. 1986;41:8–15. doi: 10.1016/0090-1229(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.McClain M, Arbuckle M, Heinlen L, et al. The Prevalence, Onset, and Clinical Significiance of Antiphospholipid Antibodies Prior to Diagnosis of Systemic Lupus Erythematosus. Arthritis Rheum. 2004;50:1226–1232. doi: 10.1002/art.20120. [DOI] [PubMed] [Google Scholar]
- 22.Feng J, Gulati U, Zhang X, et al. Antibody quantity versus quality after influenza vaccination. Vaccine. 2009;27:6358–6362. doi: 10.1016/j.vaccine.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirkman T. Statistics to use. 2010 Oct 21; www.physics.csbsju.edu/stats/
- 24.Gharavi A, Peirangeli S, Espinola R, et al. Antiphospholipid antibodies induced in mice by immunization with cytomegalovirus-derived peptide cause thrombosis and activation of endothelial cells in vivo. Arthritis Rheum. 2002;46:545–552. doi: 10.1002/art.10130. [DOI] [PubMed] [Google Scholar]
- 25.Blank M, Krause I, Fridkin M, et al. Bacterial induction of autoantibodies to B2-glycoprotein-I accounts for the infectious etiology of antiphospholipid syndrome. J Clin Invest. 2002;109:797–804. doi: 10.1172/JCI12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinuc P, Avcin T, B B, et al. Anti-phospholipid antibodies following vaccination with recombinant hepatitis B vaccine. Clin Exp Immunol. 2005;142:377–380. doi: 10.1111/j.1365-2249.2005.02923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toplak N, Kveder T, Trampus-Bakija A, et al. Autoimmune response following annual influenza vaccination in 92 apparently healthy adults. Autoimmun Rev. 2008;8:134–138. doi: 10.1016/j.autrev.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Mormile R, D'Alterio V, Treccagnoli G, Sorrentino P. Henoch-Schonlein purpura with antiphospholipid antibodies after inlfuenza vaccination : houw fearful is it in children? Vaccine. 2004;23:567–568. doi: 10.1016/j.vaccine.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe T, Onda H. Henoch-Schonlein purpura with antiphospholipid antibodies following an influenza vaccination. Pediatr Nephrol. 2001;16:458–459. doi: 10.1007/s004670100569. [DOI] [PubMed] [Google Scholar]
- 30.Elkayam O, Paran D, Burke M, et al. Pneumococcal vaccination of patients with systemic lupus erythematosus: effects on generation of autoantibodies. Autoimmunity. 2005;38:493–496. doi: 10.1080/08916930500285725. [DOI] [PubMed] [Google Scholar]
- 31.Abu-Shakra M, Press J, Buskila D, Sukenik S. Influenza virus vaccination of patients with SLE: Effects on generation of autoantibodies. Clin Rheumatol. 2002;21:369–372. doi: 10.1007/s100670200099. [DOI] [PubMed] [Google Scholar]
- 32.Tarjan P, Sipka S, Lakos G, et al. Influenza vaccination and the production of anti-phospholipid antibodies in patients with systemic lupus erythematosus. Scand J Immunol. 2006;35:241–243. doi: 10.1080/03009740500474552. [DOI] [PubMed] [Google Scholar]
- 33.Hunt J, McNeil H, Morgan G, Crameri R, Krilis S. A phopholipid-beta 2-glycoprotein I complex in an antigen for anticardiolipin antibodies occurring in autoimmune disease but not with infection. Lupus. 1992;1:75–81. doi: 10.1177/096120339200100204. [DOI] [PubMed] [Google Scholar]
- 34.Cabral A, Cabiedes J, Alarcon-Segovia D. Heterogeneity of antibodies to beta 2-glycoprotein 1 from patients with systemic lupus erythematosus. Lupus. 2004;13:182–187. doi: 10.1191/0961203303lu531oa. [DOI] [PubMed] [Google Scholar]
- 35.Matsuura E, Igarashi Y, Fujimoto M, et al. Heterogeneity of anticardiolipin antibodies defined by the anticardiolipin cofactor. J Immunol. 1992;148:3885–3891. [PubMed] [Google Scholar]
- 36.Matsuura E, Shen L, Matsunami Y, et al. Pathophysiology of beta2-glycoprotein I in antiphospholipid syndrome. Lupus. 2010;19:379–384. doi: 10.1177/0961203310361352. [DOI] [PubMed] [Google Scholar]
- 37.Nakaya Y, Schaefer E, Brewer HJ. Activation of human post heparine lipoprotein lipase by apolipoprotein H (beta 2-glycoprotein I) Biochem Biophys Res Commun. 1980;95:1168–1172. doi: 10.1016/0006-291x(80)91595-8. [DOI] [PubMed] [Google Scholar]
- 38.George J, Gilburd B, Hojnik M, et al. Target recognition of B2-glycoprotein I (B2GPI)-dependent anticardiolipin antibodies: evidence for involvement of the fourth domain of B2GPI in antibody binding. J Immunol. 1998;160:3917–3923. [PubMed] [Google Scholar]
- 39.de Laat B, de Groot P. Autoantbodies directed against domain I of beta 2-glycoprotein I. Curr Rheumatol Rep. 2011;13:70–76. doi: 10.1007/s11926-010-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cervera R, Asherson R. Antiphospholipid syndrome associated with infections: clinical and microbiological characteristics. Immunobiology. 2005;210:735–741. doi: 10.1016/j.imbio.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Rauch J, Dieude M, Saubang R, Levine J. The dual role of innate immunity in the antiphospholipid syndrome. Lupus. 2010;19:347–353. doi: 10.1177/0961203310361492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abu-Shakra M. Safety of vaccination of patients with systemic lupus erythematosus. Lupus. 2009;18:1205–1208. doi: 10.1177/0961203309346507. [DOI] [PubMed] [Google Scholar]
- 43.Abu-Shakra M, Zalmanson S, Neumann L, et al. Influenza virus vaccination of patients with systemic lupus erythematosus: effects on disease activity. J Rheumatol. 2000;27:1681–1685. [PubMed] [Google Scholar]