Abstract
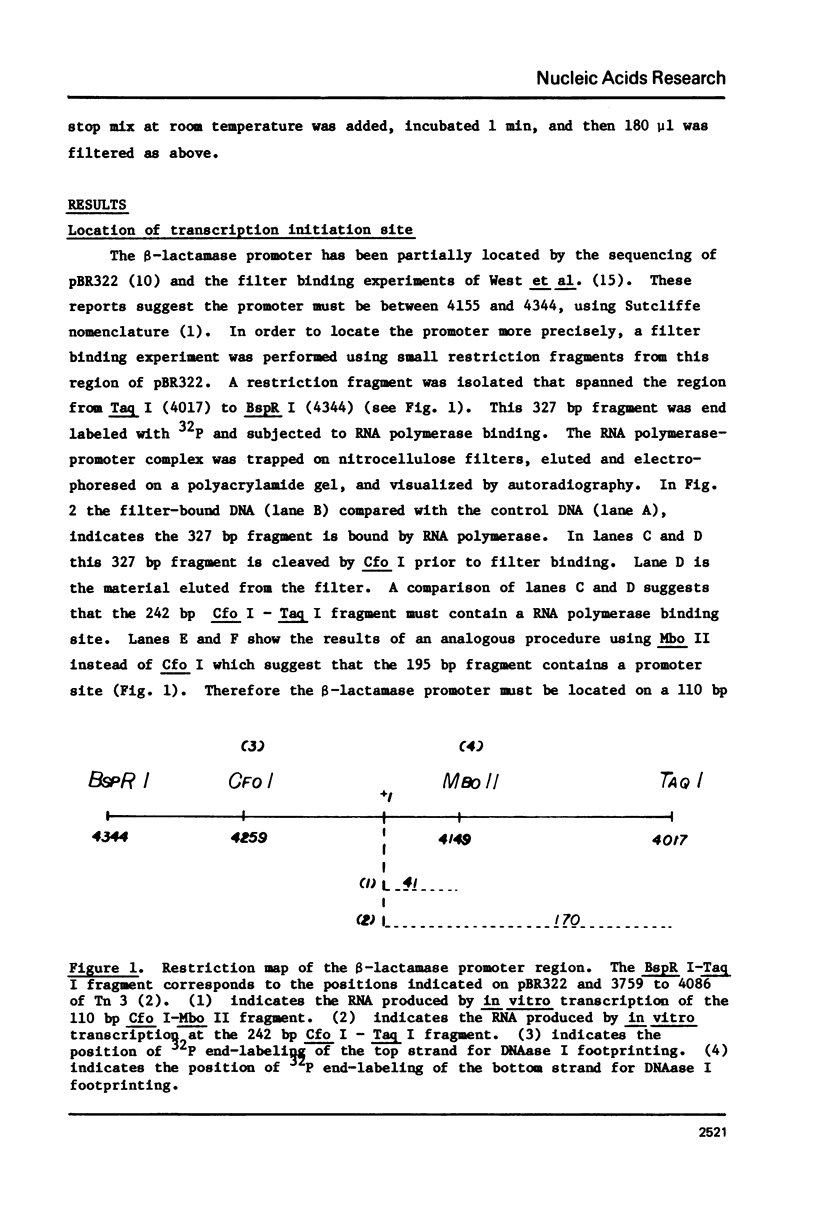
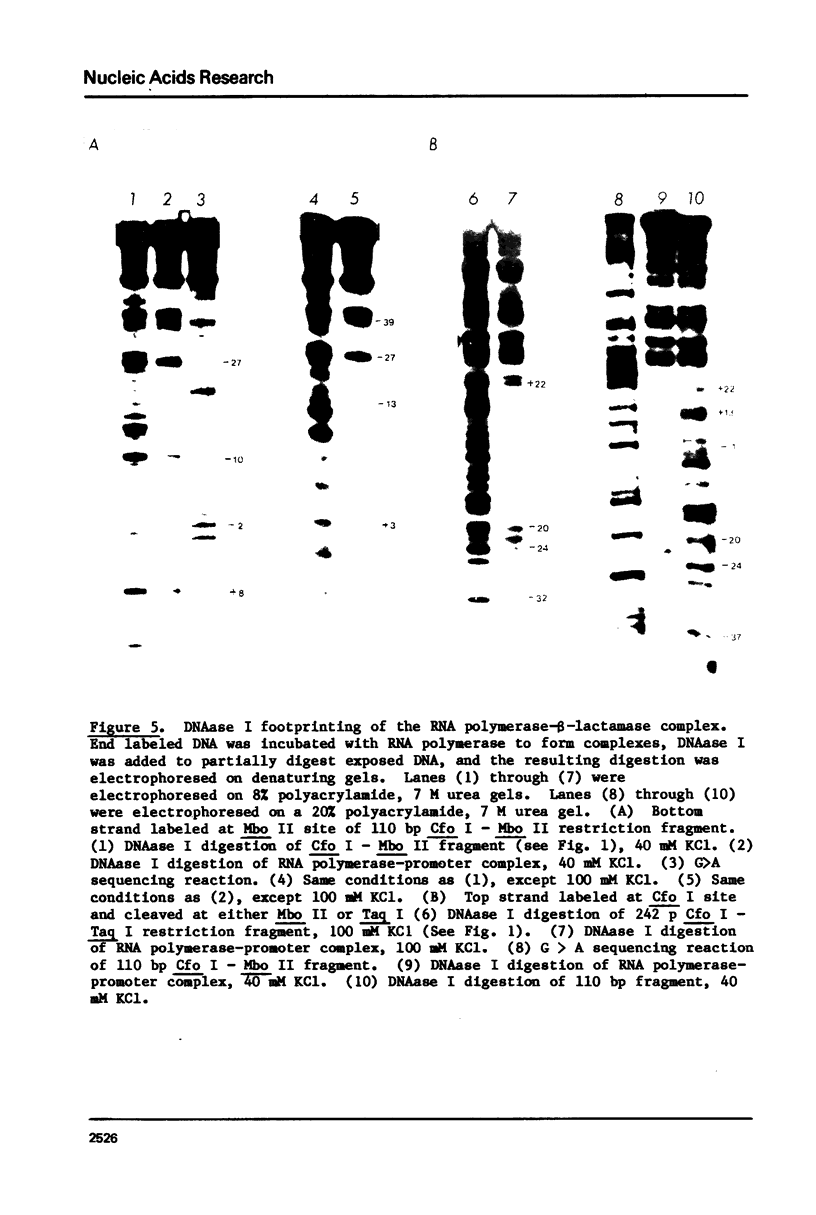
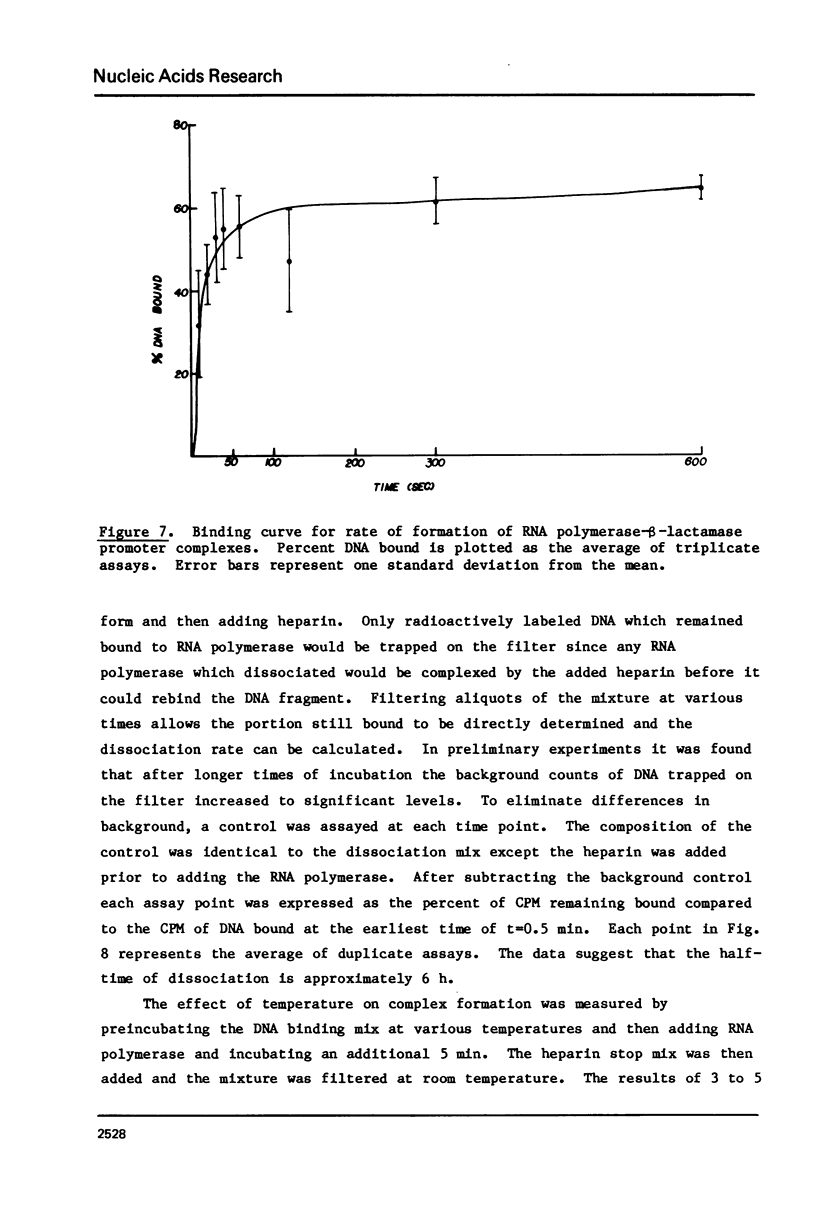
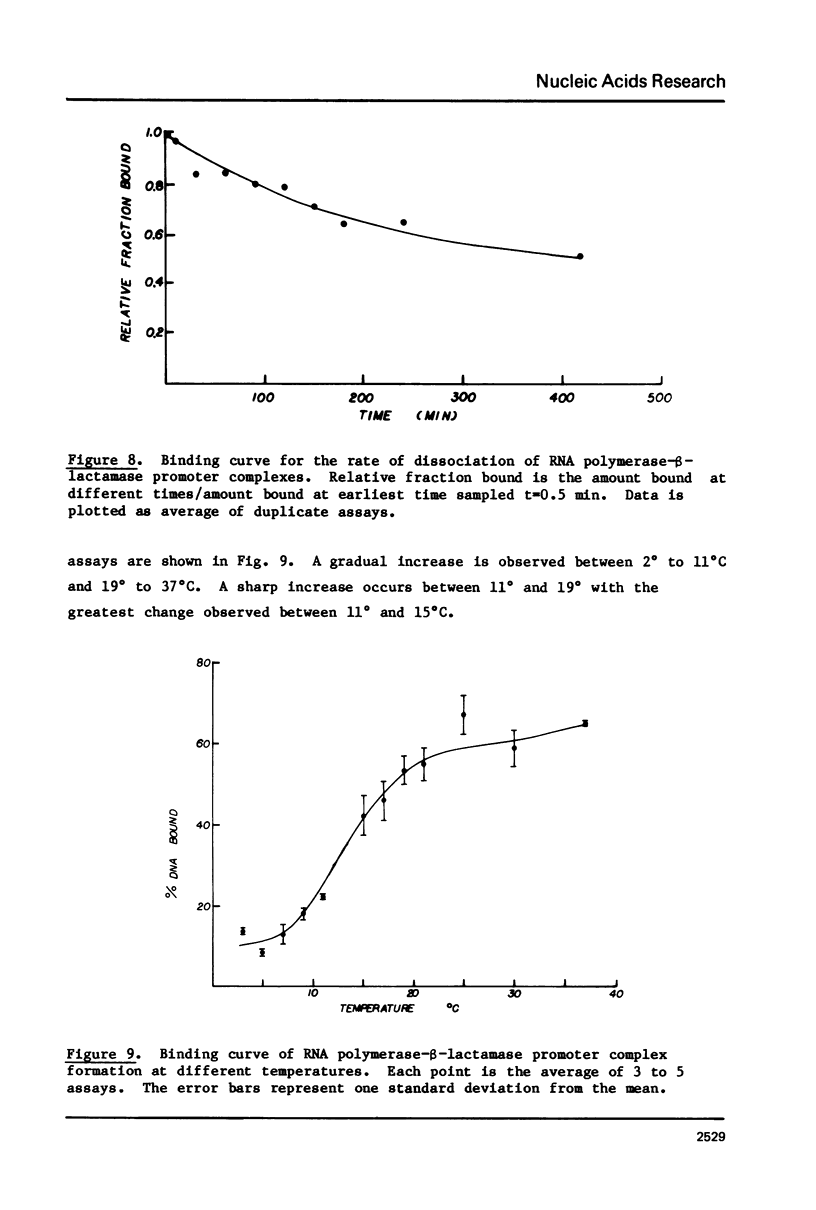
The beta-lactamase promoter of pBR322, derived from Tn3, has been characterized using several techniques. The transcription initiation site is located 35 base pairs from the translation initiation codon of beta-lactamase. The mRNA produced in vitro has a 5' pppGpA terminus. RNA polymerase bound at this start site protects a region from about -50 to +20 from DNase I cleavage using the footprinting technique. RNA polymerase binds rapidly to the beta-lactamase promoter. The half-time of association is less than one-half minute. The half-time of dissociation is approximately 6 hr. A study of the binding of RNA polymerase at different temperatures showed a large change between 11 degrees and 15 degrees C. Comparison of these parameters with those reported for other promoters is discussed.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P., Scott G. K. Partial amino acid sequence of penicillinase coded by Escherichia coli plasmid R6K. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3732–3736. doi: 10.1073/pnas.75.8.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Burman L. G., Nordström K., Boman H. G. Resistance of Escherichia coli to penicillins. V. Physiological comparison of two isogenic strains, one with chromosomally and one with episomally mediated ampicillin resistance. J Bacteriol. 1968 Aug;96(2):438–446. doi: 10.1128/jb.96.2.438-446.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Erlich H. A., Gunsalus R. P., Nunberg J. H., Kaufman R. J., Schimke R. T., Cohen S. N. Initiation of protein synthesis in bacteria at a translational start codon of mamalian cDNA: effects of the preceding nucleotide sequence. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1442–1446. doi: 10.1073/pnas.77.3.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Crombrugghe B., Pastan I., Shaw W. V., Rosner J. L. Stimulation by cyclic AMP and ppGpp of chloramphenicol acetyl transferase synthesis. Nat New Biol. 1973 Feb 21;241(112):237–239. doi: 10.1038/newbio241237a0. [DOI] [PubMed] [Google Scholar]
- Eladari M. E., Galibert F. Sequence determination of 5'-terminal and 3'-terminal T1 oligonucleotides of 18-S ribosomal RNA of a mouse cell line (L 5178 Y). Eur J Biochem. 1975 Jun 16;55(1):247–255. doi: 10.1111/j.1432-1033.1975.tb02157.x. [DOI] [PubMed] [Google Scholar]
- Hallewell R. A., Emtage S. Plasmid vectors containing the tryptophan operon promoter suitable for efficient regulated expression of foreign genes. Gene. 1980 Apr;9(1-2):27–47. doi: 10.1016/0378-1119(80)90165-1. [DOI] [PubMed] [Google Scholar]
- Heffron F., McCarthy B. J., Ohtsubo H., Ohtsubo E. DNA sequence analysis of the transposon Tn3: three genes and three sites involved in transposition of Tn3. Cell. 1979 Dec;18(4):1153–1163. doi: 10.1016/0092-8674(79)90228-9. [DOI] [PubMed] [Google Scholar]
- Hinkle D. C., Chamberlin M. J. Studies of the binding of Escherichia coli RNA polymerase to DNA. I. The role of sigma subunit in site selection. J Mol Biol. 1972 Sep 28;70(2):157–185. doi: 10.1016/0022-2836(72)90531-1. [DOI] [PubMed] [Google Scholar]
- Jorgensen S. E., Buch L. B., Nierlich D. P. Nucleoside triphosphate termini from RNA synthesized in vivo by Escherichia coli. Science. 1969 May 30;164(3883):1067–1070. doi: 10.1126/science.164.3883.1067. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lowe P. A., Hager D. A., Burgess R. R. Purification and properties of the sigma subunit of Escherichia coli DNA-dependent RNA polymerase. Biochemistry. 1979 Apr 3;18(7):1344–1352. doi: 10.1021/bi00574a034. [DOI] [PubMed] [Google Scholar]
- Mangel W. F., Chamberlin M. J. Studies of ribonucleic acid chain initiation by Escherichia coli ribonucleic acid polymerase bound to T7 deoxyribonucleic acid. 3. The effect of temperature on ribonucleic acid chain initiation and on the conformation of binary complexes. J Biol Chem. 1974 May 25;249(10):3007–3013. [PubMed] [Google Scholar]
- Maquat L. E., Reznikoff W. S. In vitro analysis of the Escherichia coli RNA polymerase interaction with wild-type and mutant lactose promoters. J Mol Biol. 1978 Nov 15;125(4):467–490. doi: 10.1016/0022-2836(78)90311-x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nagata S., Taira H., Hall A., Johnsrud L., Streuli M., Ecsödi J., Boll W., Cantell K., Weissmann C. Synthesis in E. coli of a polypeptide with human leukocyte interferon activity. Nature. 1980 Mar 27;284(5754):316–320. doi: 10.1038/284316a0. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Inoue A., Kita T., Numa S., Chang A. C., Cohen S. N., Nunberg J., Schimke R. T. Construction of bacterial plasmids that contain the nucleotide sequence for bovine corticotropin-beta-lipotropin precursor. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6021–6025. doi: 10.1073/pnas.75.12.6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim D. S., Bennett G. N., Yanofsky C. Escherichia coli RNA polymerase and trp repressor interaction with the promoter-operator region of the tryptophan operon of Salmonella typhimurium. J Mol Biol. 1980 Dec 5;144(2):133–142. doi: 10.1016/0022-2836(80)90029-7. [DOI] [PubMed] [Google Scholar]
- Oppenheim D. S., Yanofsky C. Functional analysis of wild=type and altered tryptophan operon promoters of Salmonella typhimurium in Escherichia coli. J Mol Biol. 1980 Dec 5;144(2):143–161. doi: 10.1016/0022-2836(80)90030-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Scherer G. E., Walkinshaw M. D., Arnott S. A computer aided oligonucleotide analysis provides a model sequence for RNA polymerase-promoter recognition in E.coli. Nucleic Acids Res. 1978 Oct;5(10):3759–3773. doi: 10.1093/nar/5.10.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A., Galas D. J. The interaction of RNA polymerase and lac repressor with the lac control region. Nucleic Acids Res. 1979 Jan;6(1):111–137. doi: 10.1093/nar/6.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg P. H., Nüsslein C., Schaller H. Interaction of RNA polymerase with promoters from bacteriophage fd. Eur J Biochem. 1977 Mar 15;74(1):107–113. doi: 10.1111/j.1432-1033.1977.tb11372.x. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Stahl S. J., Chamberlin M. J. An expanded transcriptional map of T7 bacteriophage. Reading of minor T7 promoter sites in vitro by Escherichia coli RNA polymerase. J Mol Biol. 1977 Jun 5;112(4):577–601. doi: 10.1016/s0022-2836(77)80165-4. [DOI] [PubMed] [Google Scholar]
- Stefano J. E., Ackerson J. W., Gralla J. D. Alterations in two conserved regions of promoter sequence lead to altered rates of polymerase binding and levels of gene expression. Nucleic Acids Res. 1980 Jun 25;8(12):2709–2723. doi: 10.1093/nar/8.12.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano J. E., Gralla J. Lac UV5 transcription in vitro. Rate limitation subsequent to formation of an RNA polymerase-DNA complex. Biochemistry. 1979 Mar 20;18(6):1063–1067. doi: 10.1021/bi00573a020. [DOI] [PubMed] [Google Scholar]
- Strauss H. S., Burgess R. R., Record M. T., Jr Binding of Escherichia coli ribonucleic acid polymerase holoenzyme to a bacteriophage T7 promoter-containing fragment: selectivity exists over a wide range of solution conditions. Biochemistry. 1980 Jul 22;19(15):3496–3504. doi: 10.1021/bi00556a014. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3737–3741. doi: 10.1073/pnas.75.8.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlin B. E., Nordström K. R plasmid gene dosage effects in Escherichia coli K-12: copy mutants of the R plasmic R1drd-19. Plasmid. 1977 Nov;1(1):1–7. doi: 10.1016/0147-619x(77)90003-8. [DOI] [PubMed] [Google Scholar]
- Villa-Komaroff L., Efstratiadis A., Broome S., Lomedico P., Tizard R., Naber S. P., Chick W. L., Gilbert W. A bacterial clone synthesizing proinsulin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3727–3731. doi: 10.1073/pnas.75.8.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R. W., Jr, Rodriguez R. L. Construction and characterization of E. coli promoter-probe plasmid vectors. II. RNA polymerase binding studies on antibiotic-resistance promoters. Gene. 1980 May;9(3-4):175–193. doi: 10.1016/0378-1119(90)90321-h. [DOI] [PubMed] [Google Scholar]
- Yang H. L., Heller K., Gellert M., Zubay G. Differential sensitivity of gene expression in vitro to inhibitors of DNA gyrase. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3304–3308. doi: 10.1073/pnas.76.7.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]