Abstract
Estrogen exerts beneficial effects on the brain throughout life. Studies demonstrate that estrogen is neuroprotective and that reduced brain estrogen activity may influence the clinical course of Alzheimer’s disease (AD). Changes in levels of estrogen receptors have been detected in postmortem brain tissue of AD patients. Very little is known about the relationship between clinical stage and levels of estrogen receptors in postmortem brain. We hypothesized that estrogen receptor levels would be related to severity of cognitive impairment assessed proximate to death. Western blotting was used to quantify ER-α and ER-β in nuclear, cytosolic, and crude membrane fractions of superior frontal cortex from 25 AD patients. Multiple linear regression analyses adjusted for age, sex, and education showed a significant linear relationship between Mini-Mental State Examination score (MMSE) and wild-type nuclear ER-α (â = 5.463, p = 0.03), but none between MMSE and wild-type nuclear ER-β (â = 2.29, p = 0.36). We incidentally observed additional higher and lower molecular mass bands for ER-α in study subjects. Additional experiments performed on frontal cortex nuclear fractions prepared from subjects enrolled in a different study confirmed that these same bands are present in female and males with and without AD. Together our data show a relationship between wild-type ER-α and level of cognitive impairment in AD, and also suggest the possibility that variant isoforms of ER-α may be present in frontal cortex of patients with and without AD.
Keywords: Cognition, postmortem brain tissue, dementia, neuroprotection, splice variants, isoforms
INTRODUCTION
Estrogen exerts complex effects on the brain which may influence the development and progression of Alzheimer's disease (AD). In vitro and animal studies have shown that estrogen modulates proteolytic processing of β-amyloid precursor protein (β-APP) [1,2] and protects neurons from cell death produced by amyloid beta peptide (Aβ) [3,4] as well as ischemia [5,6], inflammation [7,8], oxidative stress [9,10], and excitotoxicity [11,12]. An increasing number of studies demonstrate significant but regionally variable changes in brain estrogen receptor expression and localization in patients with AD compared to persons without disease [13–20].
Two estrogen receptor subtypes, ER-α and ER-β, have been fully characterized to date. Both mediate classical nuclear genomic as well as membrane non-genomic signaling cascades. Animal [21,22] and human [23] studies reveal that ER-α and ER-β are both expressed in brain, but display different patterns of regional localization. ER-α expression is highest in hypothalamus, forebrain nuclei, and amygdala whereas ER-β expression is highest in the hippocampus and neocortex. ER-α and ER-β are co-expressed in neurons, glial cells, and vascular endothelial cells in multiple brain regions. In vitro and transgenic mouse studies show that ER-α and ER-β exert opposing effects on the cell at the level of gene transcription [24]. Accumulating data indicate that ER-α and ER-β that localize within the cell membrane mediate many of the neuroprotective effects of estrogen [25].
Currently there is very little information available about the relationship between clinical Alzheimer's disease severity and the quantity of ER-α and ER-β in postmortem brain tissue. We hypothesized that levels of estrogen receptors in frontal cortex would be related to Mini-Mental State Examination scores (MMSE) of AD patients determined proximate to death.
MATERIALS AND METHODS
Subjects
Subjects were patients evaluated at the Rush Alzheimer's Disease Center, a tertiary care referral center in Chicago. Each participant agreed to a detailed clinical evaluation and annual re-evaluation. At the time of death, a family member agreed to brain donation. The Human Investigations Subcommittee of Rush University Medical Center Institutional Review Board approved the study. All subjects met criteria for AD [26] as previously reported [27]. All subjects underwent brain autopsy and met criteria for the neuropathological diagnosis of AD following commonly accepted procedures[28] as previously described [29]. Superior frontal cortex samples were obtained from 25 subjects, 19 female and 7 male, patients with AD. MMSE scores [30] were obtained from patients at the last examination session occurring before death (mean test-death interval 14 months, s.d = 2.5). The mean age of subjects was 79.8 years, s.d. = 2.0 and the postmortem interval 5.6 hours, s.d. = 0.6). Mean frontal cortex tissue pH was 6.58, s.d.= 0.04 measured using a modification of a method published by Harrison [31].
Homogenization of Brain Tissue
Frozen superior frontal cortex tissue (0.5–1 gm) was stored at –80° C until the time of processing. Cortical gray matter was dissected away from white matter and meninges on ice, immediately immersed in ice cold homogenization buffer (20 mM HEPES, pH 7.6, 20 mM NaCl, 230 mM sucrose, 1 mM ethylene diamine tetra-acetic acid [EDTA], 1 mM dithiothreitol [DTT], COMPLETE protease inhibitor [Roche Applied Science, Indianapolis, IN], pepstatin, and soybean trypsin inhibitor and then homogenized with 0.2% butylated hydroxytoluene for each 1 gram of tissue using a Brinkman Polytron homogenizer at setting 6 for 10 seconds.
Preparation of Subcellular Fractions
The tissue homogenate was centrifuged at 1,000 × g for 15 minutes at 0 °C, and the resulting nuclear pellet washed once with homogenization buffer, removed, and saved as the nuclear fraction. The post-nuclear supernatant was divided into two separate aliquots. The first was centrifuged at 20,000 × g for 30 minutes to create a crude membrane fraction. Cytosolic fractions were prepared from the supernatant of postnuclear fractions centrifuged at 100,000 × g for 1 hour. Multiple aliquots of nuclear, cytosolic, and crude membrane fractions were prepared and frozen at −80°C until needed for analyses.
Protein Electrophoresis and Western Immunoblotting of Study Subject Samples
Levels of ER-α and ER-β in nuclear, cytosolic and crude membrane fractions were determined by western blot analysis following protein electrophoresis and electrotransfer of proteins to PVDF membranes. Samples were normalized for total protein concentration before addition to Invitrogen Laemmli sample buffer and subjected to protein electrophoresis on Invitrogen 4–12% NuPage Bis-Tris 9-well pre-cast minigels using Invitrogen NuPage MOPS SDS running buffer and Invitrogen electrophoresis apparatus.
After electrotransfer of proteins PVDF membranes were blocked in TBS-Tween20-nonfat milk blocking buffer for one hour and washed three times for five minutes prior to incubation with the primary antibodies to ER-α 1:200 (SC H-184) and ER-β 1:500 (SC H-150) for two hours at room temperature (both purchased from Santa Cruz Biotech, Santa Cruz CA). H-184 is a rabbit polyclonal antibody raised against amino acids 2–185 of ER-α of human origin and H-150 a rabbit polyclonal antibody raised against amino acids 1–150 of ER-β of human origin. The antibodies were chosen based on the results of a series of otpimization experiments that carefully compared the sensitivity and specificity of three or more candidate antibodies used for ER-α and ER-β that are commonly cited in the literature. Samples of recombinant human estrogen receptors (RP-312 for ER-α and RP-310 for ER-β, Affinity BioReagents, Golden, CO.) were run as positive controls. Non-immune serum and pre-incubation of primary antibodies with blocking peptides supplied by the manufacturer were used as negative controls.
After incubation with primary antibodies the membranes were washed three times and incubated with secondary antibody, 1:5,000 anti-rabbit IgG-HRP (Amersham Biosciences, Paskateway, NJ), for one hour. After washing three times the blots were processed using Amersham ECL-plus kits and Amersham ECL film. Bands of interest on each film were then imaged and quantified using Phoretix® ID software, version 2003.02 (Nonlinear Dynamics, Newcastle upon Tyne).
Western Blot Comparison of ER Variant Bands in Brain and Other Human Tissues
Nuclear Fraction samples prepared from medial frontal cortex of male and female Rush Religious Orders Subjects without AD and whole tissue homogenates of human breast, uterus, ovary, testes (for ER-β quantitation only) and frontal lobe obtained from ProSci (Poway, CA) adjusted to a total final protein concentration of 20 µg and were run on Invitrogen single 4–12% NuPage Bis-Tris 9-well pre-cast minigels using Invitrogen NuPage MOPS SDS running buffer run. After electrotransfer of proteins the PVDF membranes were blocked and washed as described above and then incubated at 4° C overnight with SC HC-20 1:300 for ER-α (Santa Cruz Biotech, Santa Cruz, CA) and Ab5767 1:2000 for ER-β (Abcam Inc, Cambridge, MA.) SC H-20 is an affinity purified rabbit polyclonal antibody raised against a peptide mapping at the C-terminus of the ER-α of human origin (Santa Cruz Biotech, Santa Cruz, CA) and Ab3576 a rabbit polyclonal antibody raised against a peptide mapping at amino acids 467–485 of ER-β of rat origin (Abcam Inc, Cambridge, MA). SC H-20 and Ab3567 were optimized using the methods described above. Membranes were also probed with Ab6276-100 1:7000 for β-actin (Abcam, Cambridge, MA) at room termperature for two hours to serve as a loading control. Following incubation with the primary antibodies the membranes were incubated for one hour with 1:5,000 antirabbit or anti-mouse IgG-HRP, then washed and processed using Amersham ECL-plus kits and Amersham ECL film.
Statistical Analysis
Univariate statistics were computed for ER-α and ER-β quantities within nuclear, cytosolic and membrane fractions. Because raw pixel density values of western blot bands calculated by Phoretix® ID software were exceedingly large, we converted raw values to z-scores to facilitate data interpretation. Z-scores place pixel values from different measurements on the same scale, with a mean of zero and a standard deviation of one; that is, a one unit change in the z-score represents a change of one standard deviation on the original scale. We used linear regression to examine the relationship between levels of nuclear, cytosolic, and membrane levels of ER-α and ER-β and MMSE. All linear regression models were adjusted for the effects of age, sex, and education. All regression analyses were performed using SAS Version 9 (SAS Institute Inc., 2004. SAS OnlineDoc® 9.1.3. Cary, NC: SAS Institute Inc.), and were validated graphically and analytically.
RESULTS
ER-α and ER-β are Detectable in Nuclear, Cytosolic, and Membrane Fractions
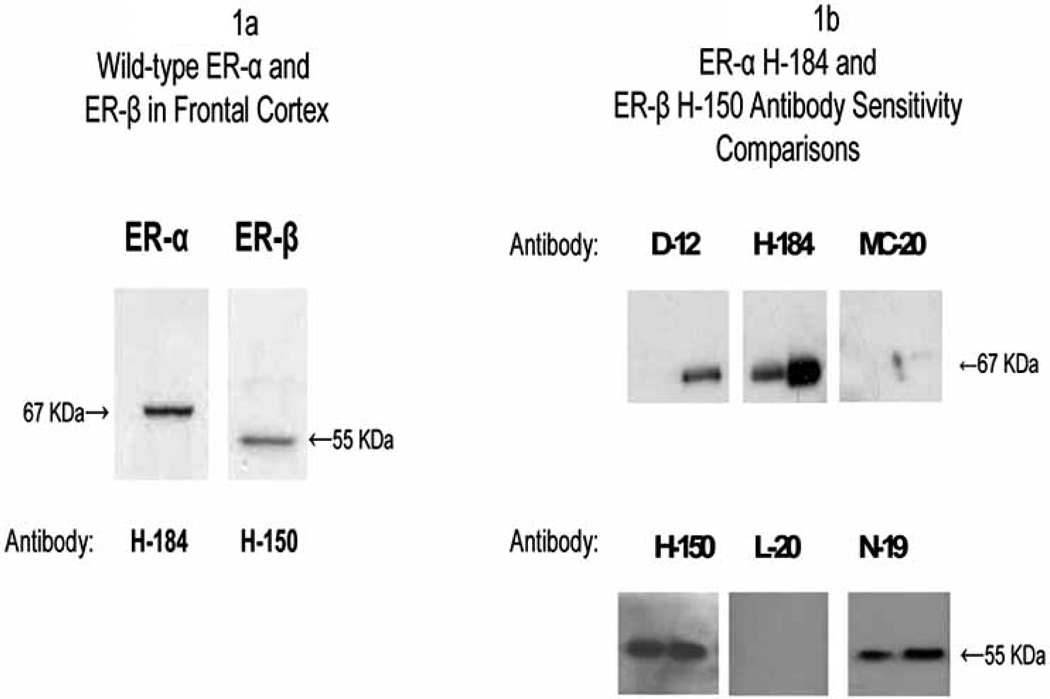
Specific ER-α and ER-β bands were present in each of the three subcellular fractions. Fig. 1a displays western blots of frontal cortex nuclear fractions that show the 65–67 kDa band for ER-α and a 52–55 kDa band for ER-β detected using the antibodies SC H-184 for ER-α and SC H-150 for ER-β (see the Materials and Methods section for details on antibody optimization). The location of the bands correspond to bands observed with recombinant human ER-α and ER-β and approximate the estimated molecular mass of wild-type (unmodified) ER-α and ER-β detected by other investigators [32]. Fig. 1b displays western blots comparing the sensitivity of SC H-184 and SC H-150 antibodies and several other antibodies using serial dilutions of recombinant ER-α and ER-β. Western blots of serial dilutions of nuclear fractions of frontal cortex run under the same conditions showed a similar favorable pattern of sensitivity of H-184 and H-150 for detection of wild-type ER-α and ER-β.
Fig. (1a-1b).
1a) Western blots of frontal cortex nuclear fractions probed for wild-type ER-α with SC H-184 and wild-type ER-β with SC H-150 show a 65–67 kDa band for ER-α and a 52–55 kDa band for ER-β respectively; 1b) Top figure shows western blots of recombinant ER-α run at 10 and 50 ng concentrations to compare the sensitivity of SC H-184 1:200 to that of SC D-12, 1:200, and SC MC-20, 1:200; bottom figure shows western blots of recombinant ER-β run at 0.5 and 1 µg concentrations to compare the sensitivity of SC H-150 1:500 to SC L-20, 1:500 and SC N-19, 1:500. All primary antibody incubations were conducted at room temperature for two hours.
Levels of Nuclear ER-α, But Not Nuclear ER-β, Are Related to MMSE Score
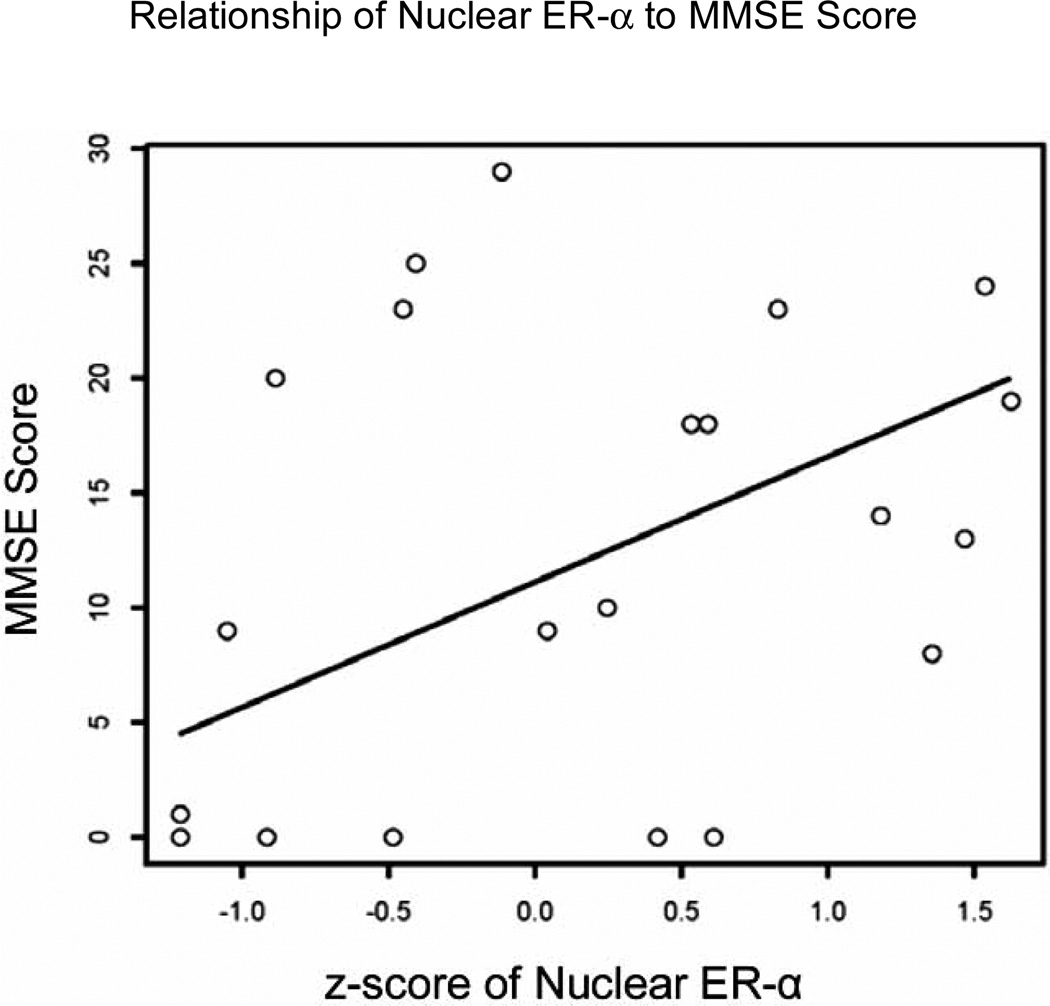
We performed linear regression analyses to determine the relationship between levels of wild-type ER-α and ER-β in each of the subcellular fractions with MMSE measured at the last testing session. The regression models controlled for the effects of age, sex, and education. Table 1 summarizes the results of these analyses. As can be seen, levels of nuclear ER-α were significantly associated with MMSE (â = 13.231, p =0.032). Fig. 2 displays a scatterplot of MMSE scores plotted against z-scores of nuclear ER-α and a predicted regression line from the model, using average values for the other covariates (age, sex, and education). For these analyses we eliminated one subject from the dataset who had a value of ER-α that had a disproportionately large influence on the analyses of this relatively small number of subjects. When the outlier was included in analyses, the relationship between nuclear ER-α and MMSE was marginally significant (â = 4.478, p = 0.083). In contrast, levels of nuclear ER-β were not significant predictors of MMSE (see Table 1).
Table 1.
Multiple Linear Regression Analyses that Examined Display the Relationship Between MMSE Scores and z-Scores of (a) Wild-Type ER-α, and (b) Wild-Type ER-β Levels in Nuclear, Cytosolic, and Membrane Fractions of Frontal Cortex. Regression Models are Adjusted for the Effects of Age, Sex, and Level of Education.
| ER α Fraction | N | β | p-value |
|---|---|---|---|
| Nuclear | 24 | 13.231 | 0.032* |
| Cytosolic | 25 | 2.752 | 0.190 |
| Membrane | 25 | 3.067 | 0.154 |
| ER β Fraction | N | B | p-value |
| Nuclear | 25 | 1.210 | 0.615 |
| Cytosolic | 25 | −2.716 | 0.218 |
| Membrane | 25 | −0.082 | 0.997 |
p ≤ 0.05.
Fig. (2).
A scatterplot and predicted linear regression line adjusted for age, sex, and years of education displays the relationship between z-scores of wild-type nuclear ER-α levels and total MMSE score.
Because of the variability in MMSE test-death intervals, we also examined whether it altered the significance of the relationship between MMSE and nuclear ER-α. Linear regression analyses with MMSE test-death interval added as a term in the model did not change the significance of the relationship between ER-α and MMSE (â = 4.809, p = 0.0278). As an additional check, we ran an additional model including a term for the interaction between ER-α and MMSE test-death interval to explicitly test whether the relationship between nuclear ER-α and MMSE differed by interval. The interaction was not significant (â = −1.914, p = 0.3486).
Potential Variant ER-α Isoforms in Frontal Cortex
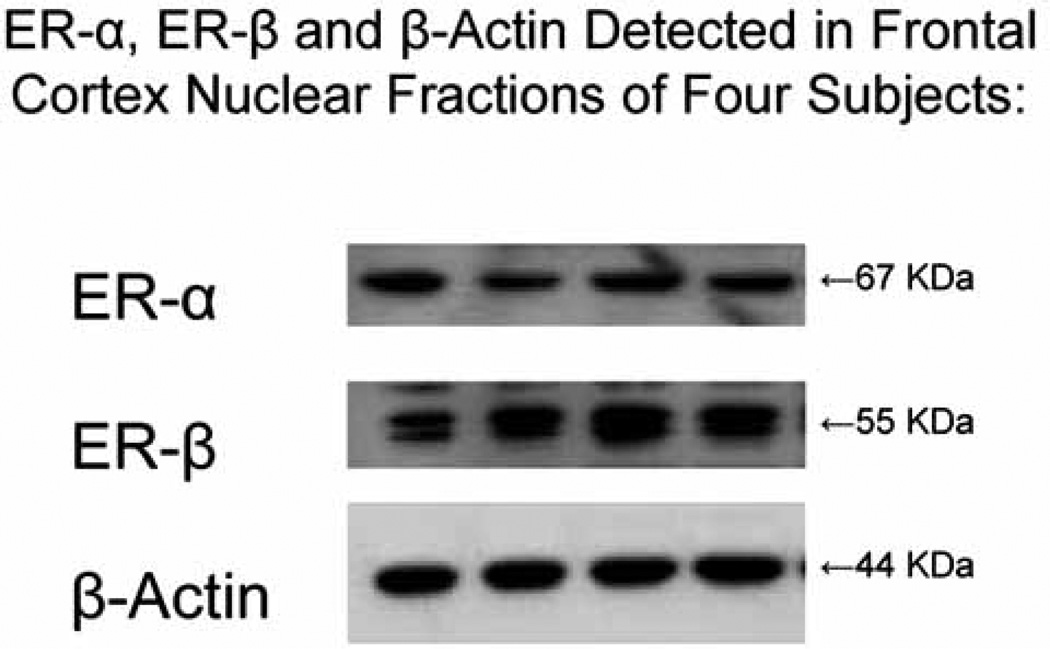
In addition to the expected band observed for wild-type ER-α we consistently detected additional antibody-specific bands in AD subjects that migrated at a lower or higher molecular mass. Data from human studies have demonstrated the existence of splice variants of ER-α as well as ER-β in normal and neoplastic tissues of multiple organs including breast, uterus, ovary and bone [32]. To determine whether the additional bands that we observed on western blots of AD subjects are present in subjects without AD, we prepared frontal cortex nuclear fractions from a subset of male and female subjects of the Rush Religious Orders Study, some of whom had AD proximate to death and some of whom had no evidence of cognitive impairment (NCI) at their last clinical evaluation proximate to death [see Bennett et al. 2005 [33] for details of the Study]. We used SC H-20 to detect ER-α (Santa Cruz Biotech, Santa Cruz CA) and Ab5767 (Abcam Inc, Cambridge, MA) to detect ER-β. Fig. 3 displays western blots of frontal cortex nuclear fraction samples of four representative Religious Orders Study subjects with NCI probed sequentially for wild-type ER-α, ER-β and β-actin as a loading control.
Fig. (3).
Displays western blots of frontal cortex nuclear fractions of four representative Religious Orders Study subjects without AD that are probed sequentially for ER-α, ER-β and β-actin as a loading control. ER-α is detected by SC H-20, ER-β by Ab5767 and β-actin by Ab6276-100. Each of the samples was adjusted to contain 20 µg total protein.
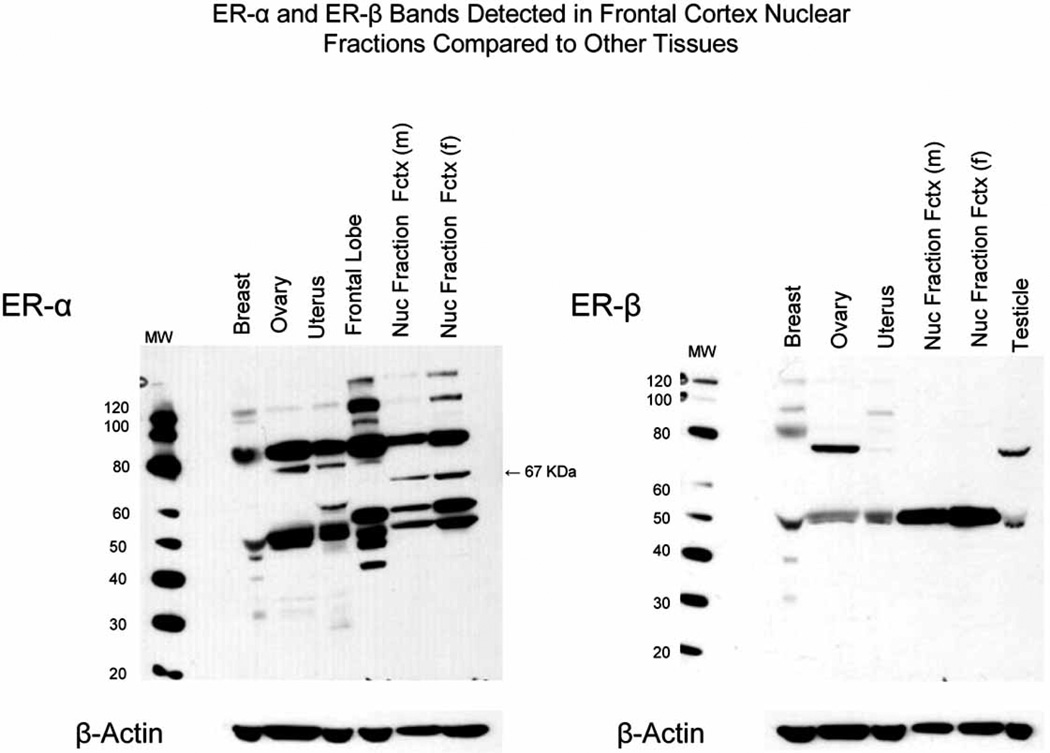
In order to compare western blot band patterns found in brain tissue to those found in other human tissues we probed samples of frontal cortex nuclear fractions run along with samples of human breast, uterus, ovary and frontal cortex homogenate for ER-α and samples of nuclear fractions run with breast, uterus, ovary and testicle homogenate for ER-β. All samples were adjusted to contain the same amount of total protein. The western blot in Fig. 4a shows ER-α bands detected in medial frontal cortex nuclear fractions of male and female subjects with NCI compared to samples of frontal lobe, breast, ovary and uterus homogenates. Bands for wild-type ER-α (67 kDa) are present in nuclear fractions of frontal cortex, but absent in other samples at this exposure time, a finding that is most likely explained by a relative enrichment of wild-type ER-α in the nuclear fractions compared to whole tissue homogenates. However, bands several-fold greater in density than wild-type ER-α are present at estimated molecular masses of 80, 50 and 46 kDa in frontal cortex nuclear fractions as well as whole frontal lobe homogenate. Bands of approximately 80 and 46 kDa can also be seen in samples of breast, ovary and uterus.
Fig. (4a–b).
4a) Displays a western blot of samples of frontal cortex nuclear fractions of a male and female subject without AD (nuclear fraction fctx) and samples of frontal lobe, breast, ovary and uterus homogenate probed for ER-α with SC HC-20; and 4b) samples of frontal cortex nuclear fractions, breast, ovary, uterus and testicle probed for ER-β with Ab5767. Each sample was adjusted to contain 20 µg total protein. Also displayed below each blot are western blots of the same gels that were probed with Ab6276-100 for β-actin as a loading control.
In contrast to the complex band pattern observed for ER-α, the western blot ER-β shown in Fig. 4b shows a single prominent band for wild type ER-β (52–55 kDa) in frontal cortex nuclear fractions of male and female subjects with NCI and homogenates of frontal lobe, breast, ovary, uterus and testicle.
DISCUSSION
Estrogen exerts potent neuroprotective effects against beta amyloid peptide toxicity, oxidative stress, excitoxicity, and inflammation. We hypothesized that there would be a relationship between levels of estrogen receptors and severity of cognitive impairment in persons with AD. Multiple linear regression analyses that were adjusted for age, sex and education showed that levels of nuclear fraction ER-α, but not nuclear fraction ER-β in frontal cortex, were related to MMSE scores measured proximate to death.
To the best of our knowledge ours is the first study to use western blotting to investigate the relationship between cognitive function in subjects with AD and levels of ER-α and ER-β present in postmortem frontal cortex. Data from immunocytochemical studies of postmortem brain tissue of AD and control subjects show that there are significant differences between subjects with AD and controls that differ by brain region. Levels of both ER-α and ER-β were found to be increased in nucleus basalis of Meynert [13] and the vertical limb of the diagonal band of Broca [16]. In medial mamillary nucleus, levels of ER-α were increased, but there was no change in the level of ER-β expression. In hippocampus, neurons staining for ER-α were found to be decreased in two different studies [17,34]. Lu and colleagues [34] also examined ER-β expression in hippocampus and found it to be decreased in AD. In contrast, Savaskan et. al. [19] found an increase in hippocampal ER-β, but was unable to detect any ER-α.
The primary aim of the study was to quantify levels of wild-type ER-α and ER-β in frontal cortex using western blotting. In addition to a wild-type band, we unexpectedly detected antibody-specific higher and lower molecular mass ER-α bands in frontal cortex of subjects with and without AD. We hypothesized that these might represent variant isoforms of ER-α. We excluded the possibility of sample preparation- related proteolytic degradation and show that bands of approximately the same molecular mass are present in samples of human frontal lobe homogenate and homogenates of breast, ovary and uterus. Our findings should not be surprising because mRNA splice variants of ER-α as well as ER-β have been identified in neoplastic as well as normal human and animal tissues [32,35–39]. Osterlund et al., [40,41], using in situ hybridization histochemistry examined the distribution of variant mRNA isoforms of ER-α and ER-β in human brain and detected multiple variants transcribed from alternative promoters. Perlman and colleagues [42], using nested RT-PCR, detected 12 different splice variants of ER-α in normal human frontal cortex. 83% of adult humans expressed at least one variant; single exon 2, 5 and 7 deletion variants were most frequently expressed in human frontal cortices. In a small scale study Ishunina and colleagues using quantitative RT-PCR on RNA isolated from the tubero-mammalary region of 5 controls and 6 AD patients, detected single exon 2, 4 and 7 deletion variants in all of the subjects, but did not find any AD-associated differences in expression [43]. Recently the same group of investigators has examined a larger group of subjects and show that ER-α mRNA splice variants are present in hippocampus of women and men with and without AD and that both wild-type and alternatively spliced ER-α mRNA are downregulated in subjects with AD [44].
The results of our study must be viewed with several caveats in mind. First, the sample size is relatively small and predominantly female, characteristics that limit our ability to detect more subtle associations between MMSE and estrogen receptors in other fractions and any gender-specific effects. Second, in light of the regional differences found in immunocytochemical studies, it is possible that a similar study conducted on another brain region might yield different findings.
In conclusion, our study shows that there is a relationship between MMSE and levels of wild-type nuclear fraction ER-α in superior frontal cortex of patients with AD. This finding raises the question of whether decreased levels of ER-α and/or changes in the balance between cellular ER-α and ER-β-mediated signaling might compromise the neuroprotective effects of estrogen on the brain and thereby contribute to the clinical progression of Alzheimer's disease. We also present data that suggest that variant forms of ER-α are present in frontal cortex of subjects with and without Alzheimer’s disease. Future larger scale studies of multiple brain regions from postmortem brain tissue of subjects with and without AD will be needed to confirm the presence of potential variant ER-α isoforms and define the relationship between levels of wild-type and variant estrogen receptors in different subcellular compartments and measures of cognitive function and hallmark neuropathological changes found in AD.
ACKNOWLEDGMENTS
We thank the staff and patients of the Rush Alzheimer's Disease Center and the Religious Orders Study. This work was supported by grants from the Northwestern Memorial Hospital Foundation (JFK) and the National Institutes of Health P30AG10161 and R01AG15819 (DAB), and R03AG19493-01 (JFK). We also thank Woojeong Bang, M.S., and Todd Beck, M.S., for their assistance in statistical programming.
REFERENCES
- 1.Chang D, Kwan J, Timiras PS. Estrogens influence growth maturation and amyloid beta-peptide production in neuroblastoma cells and in a beta-APP transfected kidney 293 cell line. Adv Exp Med Biol. 1997;429:261–271. doi: 10.1007/978-1-4757-9551-6_19. [DOI] [PubMed] [Google Scholar]
- 2.Manthey D, Heck S, Engert S, Behl C. Estrogen induces a rapid secretion of amyloid beta precursor protein via the mitogen-activated protein kinase pathway. Eur J Biochem. 2001;268:4285–4291. doi: 10.1046/j.1432-1327.2001.02346.x. [DOI] [PubMed] [Google Scholar]
- 3.Guerra B, Diaz M, Alonso R, Marin R. Plasma membrane oestrogen receptor mediates neuroprotection against beta-amyloid toxicity through activation of Raf-1/MEK/ERK cascade in septal-derived cholinergic SN56 cells. J Neurochem. 2004;91:99–109. doi: 10.1111/j.1471-4159.2004.02695.x. [DOI] [PubMed] [Google Scholar]
- 4.Fitzpatrick JL, Mize AL, Wade CB, Harris JA, Shapiro RA, Dorsa DM. Estrogen-mediated neuroprotection against beta-amyloid toxicity requires expression of estrogen receptor alpha or beta and activation of the MAPK pathway. J Neurochem. 2002;82:674–682. doi: 10.1046/j.1471-4159.2002.01000.x. [DOI] [PubMed] [Google Scholar]
- 5.Pare G, Krust A, Karas RH, Dupont S, Aronovitz M, Chambon P, Mendelsohn ME. Estrogen Receptor-alpha Mediates the Protective Effects of Estrogen Against Vascular Injury. Circ Res. 2002;90:1087–1092. doi: 10.1161/01.res.0000021114.92282.fa. [DOI] [PubMed] [Google Scholar]
- 6.Carswell HV, Macrae IM, Gallagher L, Harrop E, Horsburgh KJ. Neuroprotection by a selective estrogen receptor beta agonist in a mouse model of global ischemia. Am J Physiol Heart Circ Physiol. 2004;287:H1501–H1504. doi: 10.1152/ajpheart.00227.2004. [DOI] [PubMed] [Google Scholar]
- 7.Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, Krust A, Dupont S, Ciana P, Chambon P, Maggi A. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci USA. 2003;100:9614–9619. doi: 10.1073/pnas.1531957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker AE, Brautigam VM, Watters JJ. Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor beta. Endocrinology. 2004;145:5021–5032. doi: 10.1210/en.2004-0619. [DOI] [PubMed] [Google Scholar]
- 9.Mize AL, Shapiro RA, Dorsa DM. Estrogen receptor-mediated neuroprotection from oxidative stress requires activation of the mitogen-activated protein kinase pathway. Endocrinology. 2003;144:306–312. doi: 10.1210/en.2002-220698. [DOI] [PubMed] [Google Scholar]
- 10.Behl C, Skutella T, Lezoualc'h F, Post A, Widmann M, Newton CJ, Holsboer F. Neuroprotection against oxidative stress by estrogens: structure-activity relationship. Mol Pharmacol. 1997;51:535–541. [PubMed] [Google Scholar]
- 11.Sato K, Matsuki N, Ohno Y, Nakazawa K. Estrogens inhibit l-glutamate uptake activity of astrocytes via membrane estrogen receptor alpha. J Neurochem. 2003;86:1498–1505. doi: 10.1046/j.1471-4159.2003.01953.x. [DOI] [PubMed] [Google Scholar]
- 12.Weaver CE, Jr, Park-Chung M, Gibbs TT, Farb DH. 17beta-Estradiol protects against NMDA-induced excitotoxicity by direct inhibition of NMDA receptors. Brain Res. 1997;761:338–341. doi: 10.1016/s0006-8993(97)00449-6. [DOI] [PubMed] [Google Scholar]
- 13.Ishunina TA, Swaab DF. Increased expression of estrogen receptor alpha and beta in the nucleus basalis of Meynert in Alzheimer's disease. Neurobiol Aging. 2001;22:417–426. doi: 10.1016/s0197-4580(00)00255-4. [DOI] [PubMed] [Google Scholar]
- 14.Ishunina TA, Wouda J, Fisser B, Swaab DF. Sex differences in estrogen receptor alpha and beta expression in vasopressin neurons of the supraoptic nucleus in elderly and Alzheimer's disease patients: no relationship with cytoskeletal alterations. Brain Res. 2002;951:322–329. doi: 10.1016/s0006-8993(02)03269-9. [DOI] [PubMed] [Google Scholar]
- 15.Ishunina TA, van Heerikhuize JJ, Ravid R, Swaab DF. Estrogen receptors and metabolic activity in the human tuberomamillary nucleus: changes in relation to sex aging and Alzheimer's disease. Brain Res. 2003;988:84–96. doi: 10.1016/s0006-8993(03)03347-x. [DOI] [PubMed] [Google Scholar]
- 16.Ishunina TA, Swaab DF. Increased neuronal metabolic activity and estrogen receptors in the vertical limb of the diagonal band of Broca in Alzheimer's disease: relation to sex and aging. Exp Neurol. 2003;183:159–172. doi: 10.1016/s0014-4886(03)00138-9. [DOI] [PubMed] [Google Scholar]
- 17.Hu XY, Qin S, Lu YP, Ravid R, Swaab DF, Zhou JN. Decreased estrogen receptor-alpha expression in hippocampal neurons in relation to hyperphosphorylated tau in Alzheimer patients. Acta Neuropathol (Berl) 2003;106:213–220. doi: 10.1007/s00401-003-0720-3. [DOI] [PubMed] [Google Scholar]
- 18.Lu YP, Zeng M, Swaab DF, Ravid R, Zhou JN. Colocalization and alteration of estrogen receptor-alpha and -beta in the hippocampus in Alzheimer's disease. Hum Pathol. 2004;35:275–280. doi: 10.1016/j.humpath.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Savaskan E, Olivieri G, Meier F, Ravid R, Muller-Spahn F. Hippocampal estrogen beta-receptor immunoreactivity is increased in Alzheimer's disease. Brain Res. 2001;908:113–119. doi: 10.1016/s0006-8993(01)02610-5. [DOI] [PubMed] [Google Scholar]
- 20.Ishunina TA, Kamphorst W, Swaab DF. Changes in metabolic activity and estrogen receptors in the human medial mamillary nucleus: relation to sex aging and Alzheimer's disease. Neurobiol Aging. 2003;24:817–828. doi: 10.1016/s0197-4580(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 21.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Kritzer MF. Regional laminar and cellular distribution of immunoreactivity for ER alpha and ER beta in the cerebral cortex of hormonally intact adult male and female rats. Cereb Cortex. 2002;12:116–128. doi: 10.1093/cercor/12.2.116. [DOI] [PubMed] [Google Scholar]
- 23.Kruijver FP, Balesar R, Espila AM, Unmehopa UA, Swaab DF. Estrogen-receptor-beta distribution in the human hypothalamus: similarities and differences with ER alpha distribution. J Comp Neurol. 2003;466:251–277. doi: 10.1002/cne.10899. [DOI] [PubMed] [Google Scholar]
- 24.Lindberg MK, Moverare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, Ohlsson C. Estrogen Receptor ER-beta Reduces ER-alpha-Regulated Gene Transcription Supporting a "Ying Yang" Relationship between ER-alpha and ER-beta in Mice. Mol Endocrinol. 2003;17:203–208. doi: 10.1210/me.2002-0206. [DOI] [PubMed] [Google Scholar]
- 25.Beyer C, Pawlak J, Karolczak M. Membrane receptors for oestrogen in the brain. J Neurochem. 2003;87:545–550. doi: 10.1046/j.1471-4159.2003.02042.x. [DOI] [PubMed] [Google Scholar]
- 26.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 27.Wilson RS, Gilley DW, Bennett DA, Beckett LA, Evans DA. Person-specific paths of cognitive decline in Alzheimer's disease and their relation to age. Psychol Aging. 2000;15:18–28. doi: 10.1037//0882-7974.15.1.18. [DOI] [PubMed] [Google Scholar]
- 28.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 29.Bennett DA, Cochran EJ, Saper CB, Leverenz JB, Gilley DW, Wilson RS. Pathological changes in frontal cortex from biopsy to autopsy in Alzheimer's disease. Neurobiol Aging. 1993;14:589–596. doi: 10.1016/0197-4580(93)90043-b. [DOI] [PubMed] [Google Scholar]
- 30.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Harrison PJ, Heath PR, Eastwood SL, Burnet PW, McDonald B, Pearson RC. The relative importance of premortem acidosis and postmortem interval for human brain gene expression studies: selective mRNA vulnerability and comparison with their encoded proteins. Neurosci Lett. 1995;200:151–154. doi: 10.1016/0304-3940(95)12102-a. [DOI] [PubMed] [Google Scholar]
- 32.Herynk MH, Fuqua SAW. Estrogen Receptor Mutations in Human Disease. Endocr Rev. 2004;25:869–898. doi: 10.1210/er.2003-0010. [DOI] [PubMed] [Google Scholar]
- 33.Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005;64:834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- 34.Lu YP, Zeng M, Swaab DF, Ravid R, Zhou JN. Colocalization and alteration of estrogen receptor-alpha and -beta in the hippocampus in Alzheimer's disease. Hum Pathol. 2004;35:275–280. doi: 10.1016/j.humpath.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Toran-Allerand CD. Minireview: A plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- 36.Flouriot G, Griffin C, Kenealy M, Sonntag-Buck V, Gannon F. Differentially Expressed Messenger RNA Isoforms of the Human Estrogen Receptor-{alpha} Gene Are Generated by Alternative Splicing and Promoter Usage. Mol Endocrinol. 1998;12:1939–1954. doi: 10.1210/mend.12.12.0209. [DOI] [PubMed] [Google Scholar]
- 37.Hirata S, Shoda T, Kato J, Hoshi K. Isoform/variant mRNAs for sex steroid hormone receptors in humans. Trends Endocrinol Metab. 2003;14:124–129. doi: 10.1016/s1043-2760(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 38.Petersen DN, Tkalcevic GT, Koza-Taylor PH, Turi TG, Brown TA. Identification of Estrogen Receptor beta 2 A Functional Variant of Estrogen Receptor {beta} Expressed in Normal Rat Tissues. Endocrinology. 1998;139:1082–1092. doi: 10.1210/endo.139.3.5840. [DOI] [PubMed] [Google Scholar]
- 39.Saunders PT, Millar MR, Macpherson S, Irvine DS, Groome NP, Evans LR, Sharpe RM, Scobie GA. ERbeta1 and the ERbeta2 splice variant (ERbetacx/beta2) are expressed in distinct cell populations in the adult human testis. J Clin Endocrinol Metab. 2002;87:2706–2715. doi: 10.1210/jcem.87.6.8619. [DOI] [PubMed] [Google Scholar]
- 40.Osterlund MK, Gustafsson JA, Keller E, Hurd YL. Estrogen receptor beta (ERbeta) messenger ribonucleic acid (mRNA) expression within the human forebrain: distinct distribution pattern to ERalpha mRNA. J Clin Endocrinol Metab. 2000;85:3840–3846. doi: 10.1210/jcem.85.10.6913. [DOI] [PubMed] [Google Scholar]
- 41.Osterlund MK, Keller E, Hurd YL. The human forebrain has discrete estrogen receptor [alpha] messenger RNA expression: high levels in the amygdaloid complex. Neuroscience. 1999;95:333–342. doi: 10.1016/s0306-4522(99)00443-1. [DOI] [PubMed] [Google Scholar]
- 42.Perlman WR, Matsumoto M, Beltaifa S, Hyde TM, Saunders RC, Webster MJ, Rubinow DR, Kleinman JE, Weickert CS. Expression of estrogen receptor alpha exon-deleted mRNA variants in the human and non-human primate frontal cortex. Neuroscience. 2005;134:81–95. doi: 10.1016/j.neuroscience.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 43.Ishunina TA, Swaab DF, Fischer DF. Estrogen receptor-alpha splice variants in the medial mamillary nucleus of Alzheimer's disease patients: identification of a novel MB1 isoform. J Clin Endocrinol Metab. 2005;90:3757–3765. doi: 10.1210/jc.2004-1858. [DOI] [PubMed] [Google Scholar]
- 44.Ishunina TA, Fischer DF, Swaab DF. Estrogen receptor α its splice variants in the hippocampus in aging and Alzheimer's diseasae. doi: 10.1016/j.neurobiolaging.2006.07.024. [DOI] [PubMed] [Google Scholar]