Centrosome separation can be completed either before or after nuclear envelope breakdown (NEB). A combination of experimental and computational approaches shows that incomplete centrosome separation at NEB decreases the accuracy of chromosome segregation and thus represents a severe threat to genome stability.
Abstract
Spindle assembly, establishment of kinetochore attachment, and sister chromatid separation must occur during mitosis in a highly coordinated fashion to ensure accurate chromosome segregation. In most vertebrate cells, the nuclear envelope must break down to allow interaction between microtubules of the mitotic spindle and the kinetochores. It was previously shown that nuclear envelope breakdown (NEB) is not coordinated with centrosome separation and that centrosome separation can be either complete at the time of NEB or can be completed after NEB. In this study, we investigated whether the timing of centrosome separation affects subsequent mitotic events such as establishment of kinetochore attachment or chromosome segregation. We used a combination of experimental and computational approaches to investigate kinetochore attachment and chromosome segregation in cells with complete versus incomplete spindle pole separation at NEB. We found that cells with incomplete spindle pole separation exhibit higher rates of kinetochore misattachments and chromosome missegregation than cells that complete centrosome separation before NEB. Moreover, our mathematical model showed that two spindle poles in close proximity do not “search” the entire cellular space, leading to formation of large numbers of syntelic attachments, which can be an intermediate stage in the formation of merotelic kinetochores.
INTRODUCTION
Accurate chromosome segregation during mitosis is critical to maintain genome stability and prevent aneuploidy. To this aim, assembly of the mitotic spindle must be coordinated with establishment of kinetochore (KT) attachment. The microtubules (MTs) of a bipolar mitotic spindle must interact with the chromosomes so that the two sister KTs on each chromosome interact with opposite spindle poles. This configuration will allow the two sister chromatids to be pulled to opposite ends of the cell upon sister chromatid separation, thus leading to the formation of two daughter cells with the correct chromosome number.
In most higher eukaryote cells interaction between spindle MTs and chromosomes is possible only after nuclear envelope breakdown (NEB). In fact, the nuclear envelope disassembles in early mitosis, and it is only after NEB that the MTs emanating from the centrosomes (spindle poles) can interact with the KTs. It has been known for many years that centrosome separation is not coordinated with NEB (Mole Bajer, 1975; Rattner and Berns, 1976). Indeed, in several different cell types centrosome separation is completed before NEB in ∼50% of mitotic cells within the same cell population, whereas in the other 50% centrosome separation is completed after NEB (Rattner and Berns, 1976; Aubin et al., 1980; Waters et al., 1993; Whitehead et al., 1996; Rosenblatt et al., 2004; Toso et al., 2009). Several studies showed that both the pre-NEB centrosome separation, referred to as the prophase pathway, and the post-NEB centrosome separation, referred to as the prometaphase pathway, rely on Eg5-dependent MT sliding (Whitehead and Rattner, 1998; Sharp et al., 1999; Rosenblatt, 2005; Tanenbaum et al., 2008; Tanenbaum and Medema, 2010; Woodcock et al., 2010). However, other mechanisms are specific to each of the two pathways (reviewed in Rosenblatt, 2005; Tanenbaum and Medema, 2010). A major mechanism of prophase centrosome separation involves the interaction between astral MTs and the nuclear envelope, possibly mediated by dynein (Gonczy et al., 1999; Robinson et al., 1999; Rosenblatt, 2005; Tanenbaum and Medema, 2010), whereas centrosome separation during prometaphase requires myosin activity at the cell cortex (Rosenblatt et al., 2004; Rosenblatt, 2005; Tanenbaum and Medema, 2010).
Whereas many studies have focused on identifying the molecules and mechanisms required for centrosome separation during these two mitotic stages, it is not known whether incomplete spindle pole separation at NEB will affect subsequent mitotic events such as establishment of KT attachment or chromosome segregation. When centrosomes achieve complete separation in prophase, at NEB the two spindle poles will be at opposite ends of the nuclear space and the MTs will grow from the centrosomes toward the chromosomes symmetrically. Conversely, when the centrosomes are not completely separated at NEB, the two spindle poles will be shifted to one side of the nuclear space and MT growth toward the chromosomes will be asymmetrical. On the basis of these differences, we speculated that incomplete spindle pole separation at NEB might lead to erroneous KT attachment and possibly chromosome missegregation. To test this hypothesis, we used a combination of experimental and computational approaches and investigated KT attachment and chromosome segregation in PtK1 cells with complete versus incomplete spindle pole separation at NEB. We found that cells with incomplete spindle pole separation at NEB exhibit KT misattachments and chromosome missegregation. In addition, our mathematical model showed that two spindle poles in close proximity do not “search” the entire cellular space, and this leads to formation of large numbers of syntelic attachments, which can be an intermediate stage in the formation of merotelic KTs.
RESULTS
Incomplete spindle pole separation at NEB is associated with elevated frequencies of anaphase lagging chromosomes
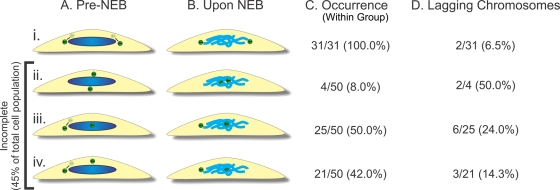
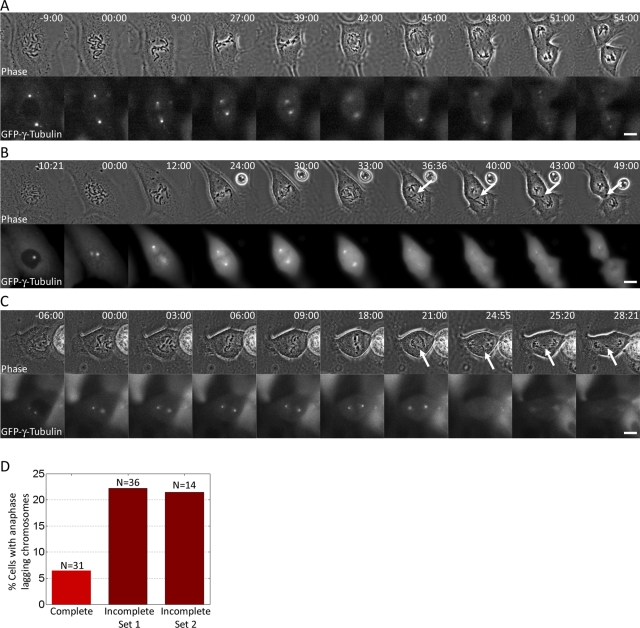
We first wanted to test the hypothesis that incomplete spindle pole separation at NEB causes KT misattachments and chromosome missegregation. Of all possible KT misattachments (monotelic, syntelic, and merotelic), merotelic attachments (a single KT bound to MTs from two spindle poles instead of just one) are known for not being detected by the spindle assembly checkpoint (Khodjakov et al., 1997a; Wise and Brinkley, 1997; Yu and Dawe, 2000; Cimini et al., 2002, 2004) and can therefore persist through mitosis into anaphase, when they can induce anaphase lagging chromosomes (Cimini et al., 2001, 2004). Thus an easy way to test our hypothesis was to determine whether increased frequencies of anaphase lagging chromosomes are found in cells with incomplete versus complete spindle pole separation at NEB. To this aim, we used PtK1 cells expressing green fluorescent protein (GFP)–γ-tubulin (a generous gift from Alexey Khodjakov, Wadsworth Center, Albany, NY; Khodjakov et al., 1997b). Although these cells were previously shown (Cimini et al., 2003b; W. T. Silkworth and D. Cimini, unpublished results) to complete centrosome separation before NEB >90% of the time, we fortuitously isolated a subclone of this cell line that exhibited incomplete spindle pole separation at NEB at higher rates and used it for this study. We performed time-lapse experiments in which the cells were imaged from prophase through late anaphase. To discriminate between complete and incomplete spindle pole separation at NEB, we measured the pole-to-pole distance upon NEB (see Materials and Methods for details) and at the end of prometaphase. Moreover, we determined the positioning of the centrosomes with respect to the chromosomes prior to and upon NEB. We found that the configuration of centrosome positioning with respect to the chromosomes prior to NEB could fall into four different categories, as diagrammed in Figure 1A, i–iv. In two of these categories (Figure 1A, i and ii), cells exhibited complete centrosome separation prior to NEB, but in one case the centrosomes were positioned along an axis parallel to the substrate (Figures 1A, i, and 2A), whereas in the other the centrosomes were positioned along an axis perpendicular to the substrate (Figure 1A, ii, Supplemental Figure S1A, and Figure 2B). Of note, in the latter category the centrosomes repositioned to the central region of the nuclear space at the time of NEB (Figure 1B, ii, Supplemental Figure S1A, and Figure 2B), thus reverting to an unseparated state. In the other two groups either the centrosomes were positioned at the edge of the nuclear space (Figure 1, A and B, iii, Supplemental Figure S1B, and Figure 2C) or one centrosome was positioned at the edge of the nuclear space and the other on the top surface of the nuclear space (Figure 1A, iv, Supplemental Figure S1C, and Figure 3B). In the latter case, the centrosome on the top surface repositioned toward the center of the nuclear space upon NEB (Figure 1B, iv, and Supplemental Figure S1C). Because of the reduced pole-to-pole distance upon NEB (i.e., the time when kinetochore–microtubule interaction becomes possible), cells displaying centrosome configurations like those depicted in Figure 1B, ii–iv (and shown in Supplemental Figure S1), were classified as cells with incomplete centrosome separation. Cells with complete centrosome separation displayed centrosome positioning like that depicted in Figure 1B, i (and shown in Figures 2A and 3A). After an initial characterization of centrosome positioning in the overall population, an additional set of cells with incomplete centrosome separation was selectively imaged (see Materials and Methods for details) to confirm the results obtained in the initial screening. When all the data were taken together, we found that for cells with incomplete centrosome separation the mean interpolar distance upon NEB was 7.33 ± 2.02 (mean ± SD; N = 50), which was <75% the distance measured at the end of prometaphase (13.63 ± 1.98 μm; Supplemental Figure S2). In cells with complete centrosome separation the mean pole-to-pole distance was 15.81 ± 4.99 μm (N = 31) upon NEB and 12.63 ± 1.97 μm at the end of prometaphase. Our measurements are consistent with previously reported pole-to-pole distances of 12–15 μm in metaphase PtK1 cells (Brinkley and Cartwright, 1971; Cameron et al., 2006). We also measured the distance across the long axis of the “nuclear space” (i.e., region occupied by the chromosomes) at NEB and found an average size of 16.27 ± 3.39 μm (N = 67). This indicates that, whereas in cells with complete spindle pole separation the centrosomes were positioned at opposite ends of the nuclear space, in cells with incomplete spindle pole separation, the pole-to-pole distance at NEB was less than half that of the nuclear space. This suggests that the positioning of the centrosomes in such cells may cause a single KT to face both spindle poles and thus establish a merotelic attachment, which, if not corrected, will result in a lagging chromosome in anaphase. Indeed, cells with incomplete spindle pole separation upon NEB exhibited high frequencies of anaphase lagging chromosomes, which overall were ∼3.5-fold the frequency of anaphase lagging chromosomes in cells with complete spindle pole separation (Figures 1D and 2D). In agreement with previous observations (Toso et al., 2009), there was no significant difference in the duration of mitosis for cells with complete versus incomplete spindle pole separation (data not shown). Consistently, numerical simulations showed that at small initial distances between spindle poles, there are more persistent merotelic attachments, but those do not induce a mitotic delay (Cimini et al., 2002). Monotelic attachments, which can cause a mitotic delay, disappear in the simulations just 2–3 min more slowly in cells with incomplete spindle pole separation compared with cells with initial pole-to-pole distances of 12–15 μm, thus delaying mitosis insignificantly. Taken together, these data reveal that cells that start prometaphase with incompletely separated centrosomes/spindle poles are more likely than those with complete spindle pole separation to exhibit chromosome segregation defects in the form of anaphase lagging chromosomes (Figures 1D and 2).
FIGURE 1:
Analysis of centrosome positioning before and immediately after NEB. (A, B) Diagrams showing the different types of centrosome arrangements before (A) and upon NEB (B) in PtK1 cells with complete (i) or incomplete (ii–iv) centrosome separation. The arrow between a shadowed and a dark image of the centrosome in i, iii, and iv indicates that the centrosome could be positioned at any point between the two. Note that in those cases in which the centrosomes achieve positions at the top and bottom of the nucleus before NEB (A, ii), they do not persist in such positions upon NEB, and instead they move toward the center of the nuclear space. See Supplemental Figure S1 for examples of cells with different centrosome configurations. (C) Rates of occurrence of the various configurations of centrosome positioning within the complete (i) and incomplete (ii–iv) spindle pole separation groups. (D) Rates of anaphase lagging chromosomes in cells from each subgroup.
FIGURE 2:
Incomplete spindle pole separation at NEB is associated with elevated frequencies of anaphase lagging chromosomes. (A–C) Still images from time-lapse movies of PtK1 cells expressing GFP–γ-tubulin. Phase-contrast images are shown in the top row and single focal plane GFP images are shown in the bottom row. A cell in which the spindle poles were fully separated at NEB is shown in A. In the cell shown in B, the centrosomes were separated along the z-axis of the cell prior to NEB (−10:21-min time point; only one centrosome is in focus in the image) but moved toward the center of the nuclear space and in very close proximity upon NEB (00:00-min time point). The same cell exhibits an anaphase lagging chromosome (arrow). In the cell shown in C, the centrosomes were shifted to one side of the nuclear space prior to and upon NEB. One lagging chromosome (arrow) was present in anaphase. (D) Frequencies of anaphase lagging chromosomes in cells with complete and incomplete spindle pole separation at NEB. The Incomplete Set 2 data refer to an additional set of cells with incomplete centrosome separation imaged by four-dimensional (three-dimensional plus time) time-lapse microscopy (see Materials and Methods for details). Scale bars, 10 μm.
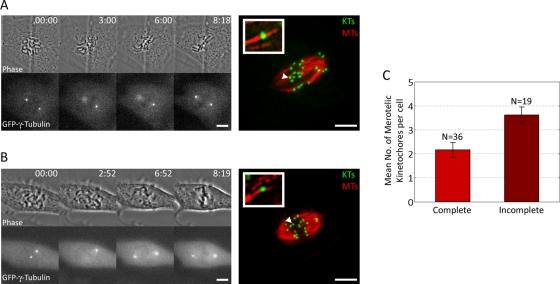
FIGURE 3:
Incomplete spindle pole separation at NEB promotes formation of merotelic kinetochore attachments. (A, B) Examples of GFP–γ-tubulin PtK1 cells imaged by time-lapse microscopy from NEB to late prometaphase, then immunostained for KTs and MTs, relocalized, and imaged by confocal microscopy (far right). A cell with complete spindle pole separation at NEB is shown in A, whereas a cell with incomplete spindle pole separation is shown in B. Insets represent 225% enlargements of the KTs indicated by the arrowheads. (C) The histogram shows that prometaphase cells with incomplete spindle pole separation at NEB exhibit higher (t test, p < 0.01) numbers of merotelic KTs than do cells with complete spindle pole separation at NEB. Error bars represent standard errors of the mean. Scale bars in the time-lapse images, 10 μm. Scale bars in the fixed cell images, 5 μm.
Incomplete spindle pole separation at NEB promotes formation of merotelic kinetochore attachments
Differences in frequencies of anaphase lagging chromosomes may be due to either differences in the rate of formation (Cimini et al., 2001, Cimini et al., 2003b; Kline-Smith et al., 2004; Salmon et al., 2005; Ganem et al., 2009; Silkworth et al., 2009) or differences in the rate of correction of merotelic KT attachments (Cimini et al., 2006; DeLuca et al., 2006; Bakhoum et al., 2009; Thompson et al., 2010). To test whether formation of merotelic KTs was more frequent in cells with incomplete versus complete spindle pole separation, we performed a set of experiments in which GFP–γ-tubulin PtK1 cells were followed by time-lapse microscopy from (or before) NEB until mid prometaphase, at which time the cells were fixed and immunostained for KTs and MTs (Figure 3, A and B). The same cells were then relocalized, imaged by high-resolution confocal microscopy, and analyzed to determine the number of merotelic KTs. The mean pole-to-pole distance at NEB in cells with incomplete and complete spindle pole separation was found to be 7.09 ± 2.79 (N = 19) and 15.83 ± 3.86 μm (N = 36), respectively, values comparable to those found in the experiments described earlier. Cells with incomplete spindle pole separation at NEB were found to possess significantly (t test, p < 0.01) higher numbers of merotelic KTs in prometaphase than cells with complete spindle pole separation (Figure 3C). These results show that cells in which spindle pole separation is not complete upon NEB, and which thus undergo spindle bipolarization during prometaphase, are more likely to establish KT misattachments than are cells in which spindle pole separation is complete at NEB. Our data also showed that overall the spindle poles were incompletely separated upon NEB in ∼45% (N = 122) of our PtK1 cell clone, similar to what was found in HeLa, MCDKII, and PtK2 cells (Rattner and Berns, 1976; Aubin et al., 1980; Whitehead et al., 1996; Rosenblatt et al., 2004; Toso et al., 2009; Woodcock et al., 2010), making these cells a good model in which to study this phenomenon.
Merotelic kinetochore attachments can form through a syntelic intermediate
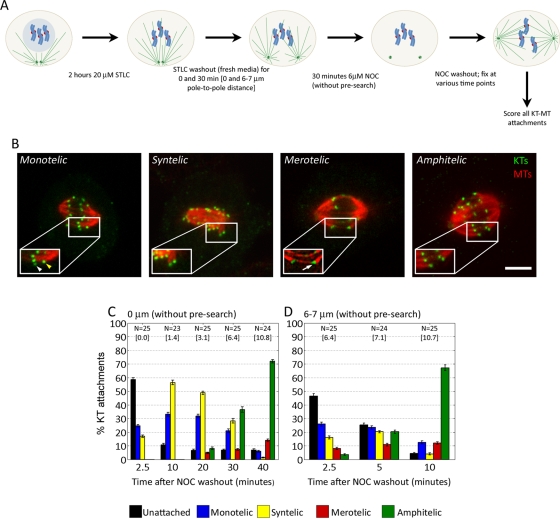
We next wanted to understand how incomplete spindle pole separation influences the number and types of KT attachments formed and how merotelic attachments arise. To this aim, we experimentally inhibited spindle pole separation by inhibiting the kinesin Eg5 by a 2 h S-trityl-l-cysteine (STLC) treatment (Skoufias et al., 2006). We then washed out the STLC and added nocodazole (NOC) to completely disassemble the MTs. Next we washed out the NOC and fixed the cells at regular time intervals (Figure 4A). Pilot experiments were performed to identify the minimum dose of NOC and length of treatment that induced MT disassembly but did not result in centrosome displacement or assembly of ectopic sites of MT nucleation after NOC washout. This experimental design allowed spindle pole separation to occur simultaneously with establishment of KT–MT interactions (i.e., search and capture occurs while the spindle poles are moving apart), as would normally happen in untreated cells. Finally, we determined the number and types of KT attachments at different time points until the end of prometaphase (Figure 4, B and C). We found that at early time points there were large numbers of unattached chromosomes and that the attached chromosomes exhibited either syntelic or monotelic attachments (Figure 4C). At later time points, both merotelic and amphitelic attachments increased, whereas syntelic and monotelic decreased (Figure 4C), suggesting that some of the syntelic attachments were converted into merotelic ones. These results suggest that merotelic attachments can form through a syntelic intermediate and that this mechanism may explain the higher frequency of merotelic attachments in cells with incomplete spindle pole separation compared with cells achieving complete centrosome separation before NEB. The types (monotelic and syntelic) of KT attachments (disregarding the unattached chromosomes) at the early time points of our experiment were very similar to those found in cells arrested with Eg5 inhibitors (Kapoor et al., 2000; Khodjakov et al., 2003). Moreover, we performed an experiment in which cells were washed out of STLC after a 2-h treatment, fixed at regular time intervals, and analyzed for numbers and types of KT attachments (initial pole-to-pole distance 0 μm, with presearch). We found that the changes in numbers and types of KT attachments over time (Supplemental Figure S3A) were very similar to those observed in cells in which spindle pole separation and KT attachment occurred simultaneously (initial pole-to-pole distance 0 μm, without presearch). Although this experiment was not representative of the events occurring under physiological conditions, it provided useful information for the development of our mathematical model (see later discussion and the Supplemental Material).
FIGURE 4:
Pole-to-pole distance at NEB determines the types and numbers of KT attachments that form in early prometaphase. (A) Flowchart of experimental protocol used for the data summarized in C and D. (B) Examples of different types of KT attachments in PtK1 cells. The images are single focal planes or maximum-intensity projections of two to four optical sections around the chromosome of interest. Insets are 150% enlarged views of the KT or KT pair in the boxed region. White and yellow arrowheads denote the unattached and attached sister KT, respectively. White arrow denotes merotelic KT. Scale bar, 5 μm. (C) Frequencies of different types of KT attachments in cells that were first treated with STLC for 2 h, then washed out and incubated in NOC for 30 min, and finally washed out of the NOC and fixed at subsequent time points. In these cells, the spindle poles started at a distance of 0 μm and gradually separated during MT assembly (spindle poles moving apart from 0 μm without presearch). (D) Frequencies of different types of KT attachments in cells that were first treated with STLC for 2 h, washed out and reincubated in STLC-free media for 30 min (to allow the spindle poles to move apart to a distance of 6–7 μm), then incubated in NOC for 30 min, and finally washed out of the NOC and fixed at subsequent time points. In these cells, the spindle poles started at a distance of 6–7 μm and gradually separated during MT assembly (to mimic incomplete spindle pole separation in untreated PtK1 cells). N, number of cells analyzed for each experimental point. For each cell, the type of KT attachment was determined for all the chromosomes (8.98 ± 1.36, mean ± SD) that could be clearly visualized. The numbers in square brackets represent the average pole-to-pole distance (in μm) at each time point.
Because the pole-to-pole distance upon NEB in untreated cells with incomplete spindle pole separation is never 0 μm, we further modified the experiment. We reincubated the cells in drug-free media between the STLC washout and the NOC treatment to allow enough time for the spindle poles to move apart to a distance of 6–7 μm (Figure 4A), which represents the pole-to-pole distance in untreated cells with incomplete spindle pole separation at NEB (Supplemental Figure S1). We found that an initial pole-to-pole distance of 6–7 μm still resulted in a large number of syntelic chromosomes but overall generated a more random distribution of different types of KT attachments at early time points after NOC washout (see 2.5- and 5-min time points in Figure 4D). It is also noteworthy that in this case, as opposed to when the search starts with spindle poles very close to each other (0-μm distance, Figure 4C), both amphitelic and merotelic attachments formed at early time points, suggesting that merotelic KT misattachments do not necessarily form through a syntelic intermediate. Finally, we also performed the experiment with an initial pole-to-pole distance of 2–3 μm and found that the numbers and types of KT attachments observed at early time points (Supplemental Figure S4) were intermediate between those observed with initial distances of 0 and 6–7 μm. In this case, both amphitelic and merotelic attachments appeared early but at lower frequencies than when the initial distance was 6–7 μm. In all experiments, amphitelic attachments increased over time at the expense of syntelic and monotelic ones, as expected due to attachment of unattached KTs and correction of misattachments. An increase in merotelic attachments was also observed, which may be due to the conversion of syntelic attachments into merotelic ones due to partial correction of syntelic attachments. Finally, it is also worth noting that in all cases (but particularly for the 0- and 2- to 3-μm pole-to-pole distance) there was an initial increase in the number of syntelic attachments (see Figure 4C, 2.5 and 10 min; Figure 4D, 2.5 and 5 min; and Supplemental Figure S4, 2.5 and 5 min). This suggests not only that when the pole-to-pole distance is small chromosomes tend to establish syntelic attachments, but also that correction of syntelic attachments is not efficient when the spindle poles are not fully separated.
Computational modeling reveals that two spindle poles in close proximity do not search the entire cellular space
To better understand how initial spindle pole distance affects establishment of KT–MT attachments, we took advantage of a computational model we recently developed to simulate spindle assembly in realistic geometry (Paul et al., 2009). We adapted this model to our present experimental system (PtK1 cells) by taking into account a number of parameters, including size of the nuclear space (volume occupied by the chromosomes at NEB), chromosome number, and chromosome size (see the Supplemental Material for information about parameters used in the simulations). We reverse engineered the process of spindle assembly by using the quantitative experimental data described in the preceding section and shown in Figure 4, C and D, and Supplemental Figure S3A. Specifically, we simulated (using the same method as in Paul et al., 2009) the stochastic search for KTs by MTs growing from two poles (Figure 5A), the distance between which was changing as in the three experimental setups (Figure 4, C and D, and Supplemental Figure S3A). In the course of spindle assembly, the monotelically attached chromosomes rotated so that the uncaptured KT became shielded from the capturing pole (Rieder and Alexander, 1990; Nicklas, 1997), thereby decreasing the probability of syntelic and merotelic attachments. Furthermore, the monotelically attached chromosomes moved away from the capturing pole, simulating CENP-E–dependent congression of monotelic chromosomes (Kapoor et al., 2006; Cai et al., 2009), further decreasing the probability of syntelic and merotelic attachment and increasing the chance of amphitelic attachment by the opposing spindle pole. This bias of monotelic chromosomes to move away from the capturing pole increased with increasing pole-to-pole distances, reflecting the increase in number of k-fibers along which the monotelic chromosomes can move (see the Supplemental Material for details). Time-course simulations performed with such a computational model (details in the Supplemental Material) closely reproduced the experimental results for all initial pole-to-pole distances (Figure 5, B and C) and revealed that there are “blind spots” for each of the poles that are maximal when the poles are in close proximity, in which case each pole essentially searches in its own half-space (Figure 5, A and D). Note that these blind spots are not an artificial assumption but are simply the consequence of the steric hindrance that MTs of one centrosome present for MTs growing from the other centrosome when they are in close proximity. As the distance between the poles increases, each pole “sees” increasing fractions of chromosomes, until, when spindle pole separation is complete (∼10 μm), almost all chromosomes are seen from both poles (Figure 5D). This “shielding” effect would explain our finding that there is a lag phase in the disappearance (correction) of syntelic attachments in cells with incomplete spindle pole separation (Figures 4, C and D, and Supplemental Figures S3A and S4). Indeed, when the spindle poles are very close to each other a syntelic chromosome would likely only be seen by one pole and therefore would keep forming syntelic attachments even if initially corrected. Finally, the mathematical model also explains why merotelic attachments (for which a single KT must be seen by both poles) do not form right away when the spindle poles are in close proximity (Figure 4C and Supplemental Figure S3A) but start forming at early time points for increasing pole-to-pole distances (Supplemental Figure S4 and Figure 4D). The simulations also demonstrated that MTs attached to syntelic chromosomes have to be unstable, detaching after ∼2 min, because otherwise the number of erroneous attachments would be too great. Finally, the model indicates that chromosome rotations and movements toward the spindle equator after the first capture are important for both rapid and accurate spindle assembly.
FIGURE 5:
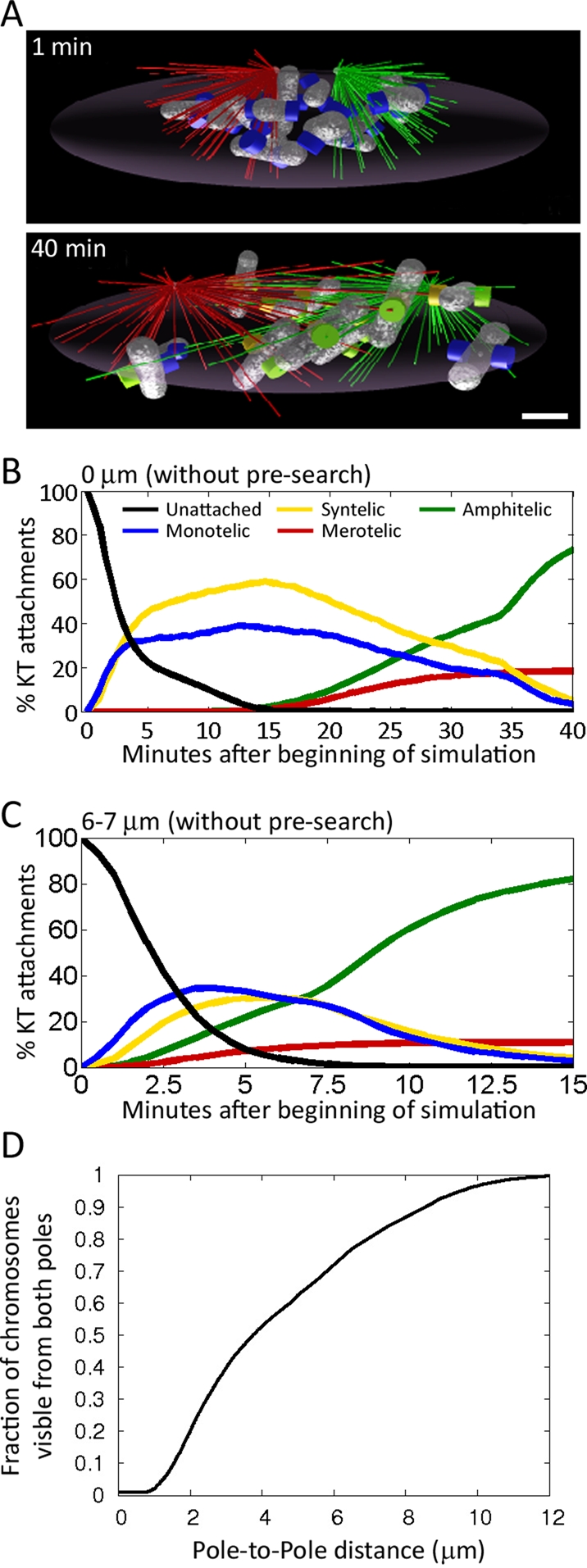
Computer simulations of spindle assembly from different initial spindle pole distances can closely reproduce experimental results. (A) Snapshots of computer simulations of spindle assembly in a cell with incomplete spindle pole separation at NEB (top). MTs from different poles are shown in green and red to differentiate between their spindle pole of origin. Unattached KTs are blue; captured KTs are green. The complete separation of MTs coming from opposite poles in the top image is an exemplification of the “shielding” effect occurring between the two poles when the pole-to-pole distance is very small (see the text for details). (B, C) Time-course simulations of spindle assembly in the presence of a shielding effect between the two centrosomes. (B) Simulation starting with a spindle pole distance of 0 μm (as in Figure 4C). (C) Simulation starting with a spindle pole distance of 6 μm (as in Figure 4D). The simulation results closely reproduce the experimental results both at the initial pole-to-pole distance of 0 μm and at an initial distance of 6 μm. (D) The graph shows the correlation between pole-to-pole distance and the fraction of chromosomes seen from both poles.
It is worth noting that the model invoking the shielding effect (Figure 5D) between the two spindle poles could reproduce the experimental results significantly better than alternative models, especially for very small initial pole-to-pole distances. Indeed, a model in which the two poles were allowed to search in the whole space (i.e., no shielding effect; Supplemental Figure S5, A and B) could not reproduce the experimental results in the case of an initial pole-to-pole distance of 0 μm (Supplemental Figure S5A). Specifically, this model resulted in the formation of larger numbers of merotelic KTs at early time points and low numbers of amphitelic KTs at later time points (compare Figure 4C and Supplemental Figure S5A). Results that better fit the experimental data were obtained when the latter model was modified by allowing merotelic and amphitelic KTs to frequently disassemble at small interpolar distances and to become more stable with increasing pole-to-pole distances. Although this modification produced better results (Supplemental Figure S5, C and D), it could not reproduce the changes in frequency of monotelic and syntelic attachments over time for cells starting with an initial pole-to-pole distance of 0 μm (compare Figure 4C and Supplemental Figure S5C).
Finally, we computationally tested additional conditions to account for observations reported in previous studies. Specifically, we varied the inter-KT angle to simulate spindle assembly in the presence of “plastic” KTs (Loncarek et al., 2007), we increased the KT target size to simulate the long k-fibers that form in cells arrested in mitosis by Eg5 inhibition (Khodjakov et al., 2003), and we introduced small unevenness in chromosome distribution. None of these modifications led to better fits of the model results to the experimental data (see the Supplemental Material for details). This does not necessarily mean that the phenomena tested do not play any role in the spindle assembly process, but that they may be more subtle than the other mechanisms/processes considered in this study and/or that the information about dynamic correlations between chromosome and centrosome positions and KT orientations in our hands is insufficient to detect an effect.
DISCUSSION
Incomplete centrosome separation at NEB and kinetochore misattachment
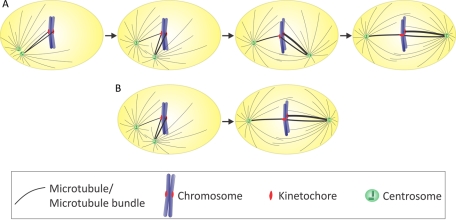
Our data reveal that the degree of centrosome separation upon NEB plays a critical role in determining the number and types of KT attachments that form in early prometaphase. Specifically, we showed that when the centrosomes are not located at opposite ends of the xy-axis of the nuclear space at NEB there is an increase in formation of KT misattachments (Figures 3 and 4, C and D) and consequently an increase in chromosome missegregation (Figures 1 and 2). Our data also indicate that incomplete spindle pole separation at NEB can lead to the formation of merotelic attachments through two different mechanisms (Figure 6): 1) when the spindle poles are very close to each other (<2–3 μm), chromosomes tend to become syntelically attached (Figure 6A). Merotelic attachments will then be formed by partial correction of syntelic attachments during spindle bipolarization (Figure 6A, third and fourth steps). Indeed, our in silico data indicate that syntelic attachments are much more unstable than merotelic and amphitelic ones. This suggests that, in vivo, syntelic attachments may be corrected more efficiently than merotelic ones, as previously suggested by others (Khodjakov and Rieder, 2009; Nezi and Musacchio, 2009; Yang et al., 2009). Such an efficient correction may in turn explain why merotelic attachments are observed much more frequently than syntelic ones in tissue culture cells (Hauf et al., 2003; Cimini, 2008). Efficient correction of syntelic attachments may also be required for spindle bipolarization. Indeed, if Aurora B kinase is inhibited in PtK1 cells recovering from an STLC arrest, both correction of syntelic attachments and bipolarization are inhibited/delayed in most cells (W. T. Silkworth, I. K. Nardi, and D. Cimini, unpublished results). 2) If the spindle poles are incompletely separated upon NEB but far enough from each other (≥2–3 μm), then merotelic attachments can also form through a second mechanism (Figure 6B), in which a single KT would be seen by and establish attachment with both spindle poles directly, without going through a syntelic intermediate. Of interest, even though a certain degree of centrosome separation (6–7 μm) is always achieved before NEB, in many cells (∼45%) this separation does not reach the maximum possible distance. Such partial spindle pole separation is enough to increase the rate of KT misattachment formation and chromosome missegregation compared with cells exhibiting complete spindle pole separation (>10 μm) at NEB (Figures 1 and 2).
FIGURE 6:
Merotelic KT attachments can form through two different mechanisms in cells with incomplete spindle pole separation at NEB. (A) When the spindle poles are very close to each other, chromosomes tend to become syntelically attached. Merotelic attachments will then be formed after partial correction of syntelic attachments during spindle bipolarization (third and fourth steps). (B) If the spindle poles are incompletely separated but far enough from each other, a single KT can be seen by and establish attachment with both spindle poles right away, without going through a syntelic intermediate.
Prophase versus prometaphase centrosome separation
As described here and in other studies (Rattner and Berns, 1976; Aubin et al., 1980; Whitehead et al., 1996; Kapoor et al., 2000; Rosenblatt et al., 2004; Kaseda et al., 2009b; Toso et al., 2009), spindle length and/or structure at the end of prometaphase are not affected by the position of the centrosomes at NEB because centrosome separation can be completed after NEB. It has been proposed that there is substantial redundancy during bipolar spindle assembly aimed at ensuring accurate chromosome segregation (Rosenblatt, 2005; Tanenbaum and Medema, 2010), and the existence of mechanisms that allow completion of centrosome separation either in prophase or in prometaphase was believed to exemplify such redundancy (Whitehead et al., 1996; Kaseda et al., 2009a; Toso et al., 2009). However, we show here that, although cells in which centrosome separation is not completed before NEB do not exhibit permanent defects of spindle organization or structure, they do exhibit increased rates of chromosome missegregation compared with cells in which centrosome separation is completed before NEB. Thus the mechanisms responsible for prometaphase centrosome separation may represent a backup system that ensures progression of mitosis in spite of the potential risk. Of interest, during the early stages of embryonic development, the spindle poles are completely separated at NEB (Robinson et al., 1999; Rosenblatt, 2005), indicating that prophase centrosome separation is highly prevalent in this context. It is likely that the completion of centrosome separation before NEB at this stage of development ensures the accuracy of chromosome segregation required for development of a healthy adult organism. Similarly, RPE1 cells, known for maintaining a stable diploid chromosome number, were found to achieve complete centrosome separation prior to NEB 100% of the time (Magidson et al., 2011).
Although traditionally the existence of two different pathways has been invoked to explain how centrosome separation can be completed either before or after NEB, our data on the pole-to-pole distance upon NEB (Supplemental Figure S2) do not seem to support a two-pathway model. Indeed, if centrosome separation occurred through one of two alternative pathways, one would expect to find a bimodal distribution of pole-to-pole distances at NEB. Instead, we found a continuous distribution of distances upon NEB, suggesting that there are not two distinct pathways, but rather a number of mechanisms, some of which can be used in prophase (MT/nuclear envelope interaction), some in prometaphase (myosin activity at the cell cortex), and some in both (Eg5-dependent MT sliding). Nevertheless, it does appear that at least in some cell types centrosome separation and NEB are sufficiently uncoordinated that the number of cells in which centrosome separation is incomplete at NEB is relatively high (Rattner and Berns, 1976; Aubin et al., 1980; Waters et al., 1993; Whitehead et al., 1996; Rosenblatt et al., 2004; Toso et al., 2009). At this time we have only limited information on how prevalent incomplete centrosome separation at NEB is in different cell types. However, we have preliminary data showing that in several different cancer cell lines a large proportion of cells exhibit incomplete centrosome separation in early mitosis (W. T. Silkworth, I. K. Nardi, and D. Cimini, unpublished results). Thus it is possible that under normal conditions (e.g., early development and untransformed or primary cells) there would be a selective pressure against delayed centrosome separation due to its association with chromosome missegregation and aneuploidy, which may, in turn, reduce cell viability. However, situations in which there are high proliferation rates and misregulation of many physiological pathways, as in cancer cells, centrosomes may fail to achieve complete separation before NEB much more frequently. Of interest, incomplete spindle pole separation in cancer cells may represent a mechanism associated with chromosomal instability in addition to previously identified ones, such as transient spindle multipolarity (Ganem et al., 2009; Silkworth et al., 2009) and inefficient correction of KT misattachments (Bakhoum et al., 2009).
Why does incomplete spindle pole separation at NEB induce kinetochore misattachments and chromosome missegregation?
The work presented here indicates that if the centrosomes are not in diametrically opposed positions along the xy-axis of the nuclear space at the time of NEB, KTs are likely to establish erroneous attachments (Figures 3–5). A consequence of the high rates of misattachment formation is chromosome missegregation at later mitotic stages (Figures 1 and 2). Specifically, high rates of merotelic KT formation are expected to result in high rates of chromosome missegregation (Cimini et al., 2003b; Ganem et al., 2009; Silkworth et al., 2009) because merotelic KT attachments are not detected by the spindle assembly checkpoint (Khodjakov et al., 1997a; Wise and Brinkley, 1997; Yu and Dawe, 2000; Cimini et al., 2002, 2004). Over the years, several different factors have been shown to promote KT misattachments (in particular merotelic). These include altered centromere/chromosome architecture (Stear and Roth, 2002; Cimini et al., 2003a; Gregan et al., 2007; Samoshkin et al., 2009; Manning et al., 2010) and defects in mitotic spindle morphology/assembly (Heneen, 1975; Sluder et al., 1997; Ganem et al., 2009; Paul et al., 2009; Silkworth et al., 2009). In this study, we show that a delay in spindle bipolarization also leads to increased rates of merotelic KT formation (Figures 3–5). Specifically, both our experimental and computational data show that cells with incomplete spindle pole separation upon NEB establish a large number (20–60%) of syntelic attachments (Figures 3 and 4 and Supplemental Figure S4). However, our data suggest that it is not the prometaphase bipolarization per se that induces KT misattachment but rather the relative positions of the two centrosomes/spindle poles at the time of NEB with respect to the nuclear space. In fact, if the two spindle poles are not completely separated upon NEB, the early prometaphase spindle will be skewed and/or will have spindle poles positioned very close to each other. In two types of centrosome positioning configurations (Figure 1B, iii and iv) we observed both a reduced pole-to-pole distance and a skewed placement of the centrosomes toward one-half of the nuclear space. In a third type, initially separated centrosomes (Figure 1A, ii) moved to the center of the nuclear space upon NEB (Figure 1B, ii), which resulted in a symmetrical configuration but in very short pole-to-pole distance. This behavior was observed in only a small number of cells (<5% of the overall mitotic cell population), and it appears to exemplify a difference between PtK1 cells and several human cell types. Indeed, it was previously shown that in human cells the centrosomes can reach diametrically opposing positions along the z-axis before NEB. As opposed to what we observe in PtK1 cells, in human cells the centrosomes persist in those positions after NEB, and the spindle assembles perpendicular to the substrate (Chaly and Brown, 1988; Mosgoller et al., 1991; Nagele et al., 1995; Magidson et al., 2011). This results in the chromosomes at the metaphase plate frequently appearing in what is known as a wheel-shaped rosette (Nagele et al., 1995) or the toroidal configuration (Magidson et al., 2011). In our cells, when the centrosomes are positioned along the z-axis prior to NEB, they move toward the center of the nuclear space upon NEB (Figure 1, A and B, ii, and Supplemental Figure S1A), thus preventing spindle assembly perpendicular to the substrate. As a result, we never observed metaphase PtK1 cells with chromosomes in a rosette configuration. Thus, in human cells prophase centrosome separation along the z-axis is not expected to affect KT attachment or chromosome segregation (Magidson et al., 2011). Conversely, in PtK1 cells centrosome separation along the z-axis is not maintained upon NEB, and therefore it results in short pole-to-pole distances upon NEB, which are associated with KT misattachment and chromosome missegregation (this study). In summary, we find that incomplete centrosome separation upon NEB can result in asymmetrical positioning of the spindle poles with respect to the nuclear space at the onset of prometaphase and in all cases is characterized by a short pole-to-pole distance. As indicated by our mathematical model, the two spindle poles can only see a fraction of the chromosomes at short pole-to-pole distances (Figure 5D), and this gives rise to large numbers of KT misattachments.
Recent studies showed that transient spindle multipolarity promotes formation of merotelic attachments in cancer cells (Ganem et al., 2009; Paul et al., 2009; Silkworth et al., 2009). Like transient multipolarity, incomplete spindle pole separation upon NEB leads to a temporary geometric defect of the mitotic spindle. However, although both situations will result in elevated rates of chromosome missegregation, they are mechanistically very different. In the case of a cell with two unseparated centrosomes, each chromosome can likely be seen by only one centrosome, and this results in high frequencies of syntelic attachments. Conversely, within a multipolar spindle each chromosome is likely seen by more than one centrosome, favoring merotelic attachment over syntelic.
MATERIALS AND METHODS
Cell culture and drug treatments
Both PtK1 cells (a generous gift from Ted Salmon, University of North Carolina, Chapel Hill, NC) and PtK–GFP–γ-tubulin (a generous gift from Alexey Khodjakov, Wadsworth Center, Albany, NY) were maintained in Ham's F-12 medium (Life Technologies, Carlsbad, CA). The culture medium was supplemented with 10% fetal bovine serum, penicillin, streptomycin, and amphotericin B (antimycotic). Cells were grown in a 37°C, 5% CO2, humidified incubator. For experiments, cells were grown on sterile coverslips inside 35-mm Petri dishes. Cells at ∼80% confluency were incubated in media containing 20 μM STLC for 2 h and either fixed (0 μm with presearch) or washed out of the drug. STLC washout was performed by washing the cells in fresh media four times. After washout, cells were either immediately incubated in media containing 6 μM NOC for 30 min (0 μm without presearch) or reincubated in drug-free media for 15 min (2–3 μm without presearch) or 30 min (6–7 μm without presearch) before the NOC treatment. NOC was then washed out, and cells were either fixed immediately (2.5 min) or reincubated in drug-free media and fixed at regular time intervals.
Fixation and immunostaining
Cells were rapidly rinsed in 1× PHEM buffer (60 mM PIPES, 25 mM Hepes, 10 mM EGTA, 2 mM MgCl2) and incubated in ice-cold 95% methanol with 5 mM ethylene glycol tetraacetic acid for 5 min first and then again for 20 min at −20°C. Subsequently, cells were washed in phosphate-buffered saline (PBS) and then blocked in 10% boiled goat serum for 1 h at room temperature. The coverslips were then incubated overnight at 4°C in primary antibodies diluted in 5% boiled goat serum. Cells were finally washed in PBST (PBS with 0.05% Tween 20), incubated in secondary antibodies diluted in 5% boiled goat serum for 1 h at room temperature, washed again, stained with 4′,6-diamidino-2-phenylindole, and mounted in an antifade solution containing 90% glycerol and 0.5% N-propyl gallate. Primary antibodies were diluted as follows: human anticentromere antigen (Antibodies Incorporated, Davis, CA), diluted 1:100; mouse anti–α-tubulin (DM1A; Sigma-Aldrich, St. Louis, MO), diluted 1:500. Secondary antibodies were diluted as follows: X-rhodamine goat anti-human (Jackson ImmunoResearch Laboratories, West Grove, PA), diluted 1:100; and Alexa 488 goat anti–mouse (Molecular Probes, Invitrogen, Carlsbad, CA), diluted 1:400.
Live-cell imaging
Ptk1 cells expressing GFP–γ-tubulin were grown on coverslips to ∼70% confluency and mounted into a Rose chamber (Rieder and Hard, 1990) with a top coverslip. The chamber was injected with L-15 medium supplemented with 4.5 g/l glucose. Experiments were performed on a Nikon (Melville, NY) Eclipse Ti inverted microscope equipped with phase-contrast transillumination, transmitted light shutter, Lumen 200PRO fluorescence illumination system, ProScan automated stage (Prior Scientific, Rockland, MA), and HQ2 CCD camera (Photometrics, Tucson, AZ). Cells were maintained at ∼36°C by means of an Air Stream stage incubator (Nevtek, Williamsville, VA). Image acquisition, shutters, z-axis position, and Lumen 200PRO fluorescence illumination system were all controlled by NIS Elements AR software (Nikon) on a PC. To determine the time of NEB, the cells were monitored by phase contrast every 1–2 min, whereas simultaneous phase contrast and fluorescence images were acquired at a single focal plane every 3 min for a 1- to 2-h period with a Plan Apochromatic 60×, 1.4–numerical aperture (NA) phase-contrast objective. Extra images were acquired immediately following NEB, at which time the positions of the centrosomes were also recorded. To record the centrosome coordinates prior to and upon NEB, the experiment was manually switched from time-lapse to live imaging, the centrosome positions were identified by focusing up and down along the z-axis, and the coordinates were recorded. Time-lapse imaging was then resumed. To further confirm centrosome positioning and pole-to-pole distances, an additional set of cells with incomplete spindle pole separation was imaged by four-dimensional (three-dimensional plus time) microscopy. For these cells, z-series optical sections were obtained in seven 1.2-μm steps using a Plan Apochromatic 60×, 1.4-NA phase-contrast objective. After completion of spindle pole separation, images were acquired at a single focal plane to follow the cells into anaphase. The collected images were subsequently analyzed to 1) determine centrosome positions with respect to the nuclear space, 2) measure pole-to-pole distances before and upon NEB, and 3) determine the chromosome segregation phenotype. For relocalization experiments, cells were grown on coverslips photoetched with an alphanumeric grid pattern (Electron Microscopy Sciences, Hatfield, PA) and imaged until mid prometaphase (∼10 min after NEB), at which time the specific alphanumeric grid was recorded, and the cells were removed from the microscope and immediately fixed and immunostained as described. After immunostaining, cells were relocalized (using previously recorded alphanumeric grid), imaged, and analyzed as described in the following section.
Confocal microscopy and image analysis
Immunofluorescently stained cells were imaged with a Swept Field Confocal system (Prairie Technologies, Middleton, WI) on a Nikon Eclipse TE-2000U inverted microscope. The microscope was equipped with a 100×, 1.4-NA Plan Apochromatic phase-contrast objective lens, phase-contrast transillumination, transmitted light shutter, and automated ProScan stage (Prior Scientific). The confocal head was equipped with filters for illumination at 488, 568, and 647 nm from a 400-mW argon laser and a 150-mW krypton laser. Digital images were acquired with an HQ2 CCD camera (Photometrics). Image acquisition, shutter, z-axis position, laser lines, and confocal system were all controlled by NIS Elements AR software (Nikon) on a PC. The z-series optical sections through each cell analyzed were obtained at 0.6-μm steps. For determination of the numbers of merotelic KTs in relocalized cells and unattached, monotelic, amphitelic, syntelic, and merotelic attachments in the fixed-cell time-course experiments, acquired images were analyzed as previously described (Silkworth et al., 2009).
Supplementary Material
Acknowledgments
We thank Alexey Khodjakov for providing the PtK–GFP–γ-tubulin cells. We thank the members of the Cimini lab for helpful discussion. I.K.N. was a recipient of a Fralin Summer Undergraduate Research Fellowship and an Atlantic Coast Conference Undergraduate Research Scholars Award. This work was supported by National Science Foundation Grant MCB-0842551 to D.C. and National Institutes of Health Grant GM068952 to A.M.
Abbreviations used:
- KT
kinetochore
- MT
microtubule
- NEB
nuclear envelope breakdown
- NOC
nocodazole
- STLC
S-trityl-l-cysteine
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-02-0095) on November 30, 2011.
REFERENCES
- Aubin JE, Osborn M, Weber K. Variations in the distribution and migration of centriole duplexes in mitotic PtK2 cells studied by immunofluorescence microscopy. J Cell Sci. 1980;43:177–194. doi: 10.1242/jcs.43.1.177. [DOI] [PubMed] [Google Scholar]
- Bakhoum SF, Genovese G, Compton DA. Deviant kinetochore microtubule dynamics underlie chromosomal instability. Curr Biol. 2009;19:1937–1942. doi: 10.1016/j.cub.2009.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley BR, Cartwright J., Jr Ultrastructural analysis of mitotic spindle elongation in mammalian cells in vitro. Direct microtubule counts. J Cell Biol. 1971;50:416–431. doi: 10.1083/jcb.50.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, O'Connell CB, Khodjakov A, Walczak CE. Chromosome congression in the absence of kinetochore fibres. Nat Cell Biol. 2009;11:832–838. doi: 10.1038/ncb1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron LA, Yang G, Cimini D, Canman JC, Kisurina-Evgenieva O, Khodjakov A, Danuser G, Salmon ED. Kinesin 5-independent poleward flux of kinetochore microtubules in PtK1 cells. J Cell Biol. 2006;173:173–179. doi: 10.1083/jcb.200601075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaly N, Brown DL. The prometaphase configuration and chromosome order in early mitosis. J Cell Sci. 1988;91(3):325–335. doi: 10.1242/jcs.91.3.325. [DOI] [PubMed] [Google Scholar]
- Cimini D. Merotelic kinetochore orientation, aneuploidy, and cancer. Biochim Biophys Acta. 2008;1786:32–40. doi: 10.1016/j.bbcan.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Cimini D, Cameron LA, Salmon ED. Anaphase spindle mechanics prevent mis-segregation of merotelically oriented chromosomes. Curr Biol. 2004;14:2149–2155. doi: 10.1016/j.cub.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Cimini D, Fioravanti D, Salmon ED, Degrassi F. Merotelic kinetochore orientation versus chromosome mono-orientation in the origin of lagging chromosomes in human primary cells. J Cell Sci. 2002;115:507–515. doi: 10.1242/jcs.115.3.507. [DOI] [PubMed] [Google Scholar]
- Cimini D, Howell B, Maddox P, Khodjakov A, Degrassi F, Salmon ED. Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J Cell Biol. 2001;153:517–527. doi: 10.1083/jcb.153.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimini D, Mattiuzzo M, Torosantucci L, Degrassi F. Histone hyperacetylation in mitosis prevents sister chromatid separation and produces chromosome segregation defects. Mol Biol Cell. 2003a;14:3821–3833. doi: 10.1091/mbc.E03-01-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimini D, Moree B, Canman JC, Salmon ED. Merotelic kinetochore orientation occurs frequently during early mitosis in mammalian tissue cells and error correction is achieved by two different mechanisms. J Cell Sci. 2003b;116:4213–4225. doi: 10.1242/jcs.00716. [DOI] [PubMed] [Google Scholar]
- Cimini D, Wan X, Hirel CB, Salmon ED. Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr Biol. 2006;16:1711–1718. doi: 10.1016/j.cub.2006.07.022. [DOI] [PubMed] [Google Scholar]
- DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell. 2006;127:969–982. doi: 10.1016/j.cell.2006.09.047. [DOI] [PubMed] [Google Scholar]
- Ganem NJ, Godinho SA, Pellman D. A mechanism linking extra centrosomes to chromosomal instability. Nature. 2009;460:278–282. doi: 10.1038/nature08136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonczy P, Pichler S, Kirkham M, Hyman AA. Cytoplasmic dynein is required for distinct aspects of MTOC positioning, including centrosome separation, in the one cell stage Caenorhabditis elegans embryo. J Cell Biol. 1999;147:135–150. doi: 10.1083/jcb.147.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregan J, Riedel CG, Pidoux AL, Katou Y, Rumpf C, Schleiffer A, Kearsey SE, Shirahige K, Allshire RC, Nasmyth K. The kinetochore proteins Pcs1 and Mde4 and heterochromatin are required to prevent merotelic orientation. Curr Biol. 2007;17:1190–1200. doi: 10.1016/j.cub.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, Heckel A, van Meel J, Rieder CL, Peters JM. The small molecule hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003;161:281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneen WK. Kinetochores and microtubules in multipolar mitosis and chromosome orientation. Exp Cell Res. 1975;91:57–62. doi: 10.1016/0014-4827(75)90140-8. [DOI] [PubMed] [Google Scholar]
- Kapoor TM, Lampson MA, Hergert P, Cameron L, Cimini D, Salmon ED, McEwen BF, Khodjakov A. Chromosomes can congress to the metaphase plate before biorientation. Science. 2006;311:388–391. doi: 10.1126/science.1122142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor TM, Mayer TU, Coughlin ML, Mitchison TJ. Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. J Cell Biol. 2000;150:975–988. doi: 10.1083/jcb.150.5.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaseda K, McAinsh A, Cross RA. A countdown clock in mitotic prophase: design logic for dual pathway mitosis. Presented at American Society for Cell Biology 2009 meeting; 5–9 December 2009; 2009a. Available at: http://f1000.com/posters/browse/summary/100 (accessed 4 January 2012) [Google Scholar]
- Kaseda K, McAinsh AD, Cross RA. Walking, hopping, diffusing and braking modes of kinesin-5. Biochem Soc Trans. 2009b;37:1045–1049. doi: 10.1042/BST0371045. [DOI] [PubMed] [Google Scholar]
- Khodjakov A, Cole RW, McEwen BF, Buttle KF, Rieder CL. Chromosome fragments possessing only one kinetochore can congress to the spindle equator. J Cell Biol. 1997a;136:229–240. doi: 10.1083/jcb.136.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A, Cole RW, Rieder CL. A synergy of technologies: combining laser microsurgery with green fluorescent protein tagging. Cell Motil Cytoskeleton. 1997b;38:311–317. doi: 10.1002/(SICI)1097-0169(1997)38:4<311::AID-CM1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Khodjakov A, Copenagle L, Gordon MB, Compton DA, Kapoor TM. Minus-end capture of preformed kinetochore fibers contributes to spindle morphogenesis. J Cell Biol. 2003;160:671–683. doi: 10.1083/jcb.200208143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodjakov A, Rieder CL. The nature of cell-cycle checkpoints: facts and fallacies. J Biol. 2009;8:88. doi: 10.1186/jbiol195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline-Smith SL, Khodjakov A, Hergert P, Walczak CE. Depletion of centromeric MCAK leads to chromosome congression and segregation defects due to improper kinetochore attachments. Mol Biol Cell. 2004;15:1146–1159. doi: 10.1091/mbc.E03-08-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loncarek J, Kisurina-Evgenieva O, Vinogradova T, Hergert P, La Terra S, Kapoor TM, Khodjakov A. The centromere geometry essential for keeping mitosis error free is controlled by spindle forces. Nature. 2007;450:745–749. doi: 10.1038/nature06344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magidson V, O'Connell CB, Loncarek J, Paul R, Mogilner A, Khodjakov A. The spatial arrangement of chromosomes during prometaphase facilitates spindle assembly. Cell. 2011;146:555–567. doi: 10.1016/j.cell.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning AL, Longworth MS, Dyson NJ. Loss of pRB causes centromere dysfunction and chromosomal instability. Genes Dev. 2010;24:1364–1376. doi: 10.1101/gad.1917310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mole Bajer J. The role of centrioles in the development of the astral spindle (newt) Cytobios. 1975;13:117–140. [Google Scholar]
- Mosgoller W, Leitch AR, Brown JK, Heslop-Harrison JS. Chromosome arrangements in human fibroblasts at mitosis. Hum Genet. 1991;88:27–33. doi: 10.1007/BF00204924. [DOI] [PubMed] [Google Scholar]
- Nagele R, Freeman T, McMorrow L, Lee HY. Precise spatial positioning of chromosomes during prometaphase: evidence for chromosomal order. Science. 1995;270:1831–1835. doi: 10.1126/science.270.5243.1831. [DOI] [PubMed] [Google Scholar]
- Nezi L, Musacchio A. Sister chromatid tension and the spindle assembly checkpoint. Curr Opin Cell Biol. 2009;21:785–795. doi: 10.1016/j.ceb.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Nicklas RB. How cells get the right chromosomes. Science. 1997;275:632–637. doi: 10.1126/science.275.5300.632. [DOI] [PubMed] [Google Scholar]
- Paul R, Wollman R, Silkworth WT, Nardi IK, Cimini D, Mogilner A. Computer simulations predict that chromosome movements and rotations accelerate mitotic spindle assembly without compromising accuracy. Proc Natl Acad Sci U S A. 2009;106:15708–15713. doi: 10.1073/pnas.0908261106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner JB, Berns MW. Centriole behavior in early mitosis of rat kangaroo cells (PTK2) Chromosoma. 1976;54:387–395. doi: 10.1007/BF00292817. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Alexander SP. Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J Cell Biol. 1990;110:81–95. doi: 10.1083/jcb.110.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Hard R. Newt lung epithelial cells: cultivation, use, and advantages for biomedical research. Int Rev Cytol. 1990;122:153–220. doi: 10.1016/s0074-7696(08)61208-5. [DOI] [PubMed] [Google Scholar]
- Robinson JT, Wojcik EJ, Sanders MA, McGrail M, Hays TS. Cytoplasmic dynein is required for the nuclear attachment and migration of centrosomes during mitosis in Drosophila. J Cell Biol. 1999;146:597–608. doi: 10.1083/jcb.146.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J. Spindle assembly: asters part their separate ways. Nat Cell Biol. 2005;7:219–222. doi: 10.1038/ncb0305-219. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J, Cramer LP, Baum B, McGee KM. Myosin II-dependent cortical movement is required for centrosome separation and positioning during mitotic spindle assembly. Cell. 2004;117:361–372. doi: 10.1016/s0092-8674(04)00341-1. [DOI] [PubMed] [Google Scholar]
- Salmon ED, Cimini D, Cameron LA, DeLuca JG. Merotelic kinetochores in mammalian tissue cells. Philos Trans R Soc Lond B Biol Sci. 2005;360:553–568. doi: 10.1098/rstb.2004.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoshkin A, Arnaoutov A, Jansen LE, Ouspenski I, Dye L, Karpova T, McNally J, Dasso M, Cleveland DW, Strunnikov A. Human condensin function is essential for centromeric chromatin assembly and proper sister kinetochore orientation. PLoS ONE. 2009;4:e6831. doi: 10.1371/journal.pone.0006831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Yu KR, Sisson JC, Sullivan W, Scholey JM. Antagonistic microtubule-sliding motors position mitotic centrosomes in Drosophila early embryos. Nat Cell Biol. 1999;1:51–54. doi: 10.1038/9025. [DOI] [PubMed] [Google Scholar]
- Silkworth WT, Nardi IK, Scholl LM, Cimini D. Multipolar spindle pole coalescence is a major source of kinetochore mis-attachment and chromosome mis-segregation in cancer cells. PLoS ONE. 2009;4:e6564. doi: 10.1371/journal.pone.0006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoufias DA, DeBonis S, Saoudi Y, Lebeau L, Crevel I, Cross R, Wade RH, Hackney D, Kozielski F. S-trityl-L-cysteine is a reversible, tight binding inhibitor of the human kinesin Eg5 that specifically blocks mitotic progression. J Biol Chem. 2006;281:17559–17569. doi: 10.1074/jbc.M511735200. [DOI] [PubMed] [Google Scholar]
- Sluder G, Thompson EA, Miller FJ, Hayes J, Rieder CL. The checkpoint control for anaphase onset does not monitor excess numbers of spindle poles or bipolar spindle symmetry. J Cell Sci. 1997;110:421–429. doi: 10.1242/jcs.110.4.421. [DOI] [PubMed] [Google Scholar]
- Stear JH, Roth MB. Characterization of HCP-6, a C. elegans protein required to prevent chromosome twisting and merotelic attachment. Genes Dev. 2002;16:1498–1508. doi: 10.1101/gad.989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanenbaum ME, Macurek L, Galjart N, Medema RH. Dynein, Lis1 and CLIP-170 counteract Eg5-dependent centrosome separation during bipolar spindle assembly. EMBO J. 2008;27:3235–3245. doi: 10.1038/emboj.2008.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanenbaum ME, Medema RH. Mechanisms of centrosome separation and bipolar spindle assembly. Dev Cell. 2010;19:797–806. doi: 10.1016/j.devcel.2010.11.011. [DOI] [PubMed] [Google Scholar]
- Thompson SL, Bakhoum SF, Compton DA. Mechanisms of chromosomal instability. Curr Biol. 2010;20:R285–R295. doi: 10.1016/j.cub.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toso A, Winter JR, Garrod AJ, Amaro AC, Meraldi P, McAinsh AD. Kinetochore-generated pushing forces separate centrosomes during bipolar spindle assembly. J Cell Biol. 2009;184:365–372. doi: 10.1083/jcb.200809055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters JC, Cole RW, Rieder CL. The force-producing mechanism for centrosome separation during spindle formation in vertebrates is intrinsic to each aster. J Cell Biol. 1993;122:361–372. doi: 10.1083/jcb.122.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead CM, Rattner JB. Expanding the role of HsEg5 within the mitotic and post-mitotic phases of the cell cycle. J Cell Sci. 1998;111((Pt 17)):2551–2561. doi: 10.1242/jcs.111.17.2551. [DOI] [PubMed] [Google Scholar]
- Whitehead CM, Winkfein RJ, Rattner JB. The relationship of HsEg5 and the actin cytoskeleton to centrosome separation. Cell Motil Cytoskeleton. 1996;35:298–308. doi: 10.1002/(SICI)1097-0169(1996)35:4<298::AID-CM3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Wise DA, Brinkley BR. Mitosis in cells with unreplicated genomes (MUGs): spindle assembly and behavior of centromere fragments. Cell Motil Cytoskeleton. 1997;36:291–302. doi: 10.1002/(SICI)1097-0169(1997)36:3<291::AID-CM9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Woodcock SA, Rushton HJ, Castaneda-Saucedo E, Myant K, White GR, Blyth K, Sansom OJ, Malliri A. Tiam1-Rac signaling counteracts Eg5 during bipolar spindle assembly to facilitate chromosome congression. Curr Biol. 2010;20:669–675. doi: 10.1016/j.cub.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Kenny AE, Brito DA, Rieder CL. Cells satisfy the mitotic checkpoint in Taxol, and do so faster in concentrations that stabilize syntelic attachments. J Cell Biol. 2009;186:675–684. doi: 10.1083/jcb.200906150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HG, Dawe RK. Functional redundancy in the maize meiotic kinetochore. J Cell Biol. 2000;151:131–142. doi: 10.1083/jcb.151.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.