EGF receptor stimulation in keratinocytes induces development of front–rear polarity and migration. The signaling pathways activated include activation of RhoG, recruitment of active RhoG, ELMO2, and ILK to lamellipodia, and activation of Rac1.
Abstract
Epidermal growth factor (EGF) is a potent chemotactic and mitogenic factor for epidermal keratinocytes, and these properties are central for normal epidermal regeneration after injury. The involvement of mitogen-activated protein kinases as mediators of the proliferative effects of EGF is well established. However, the molecular mechanisms that mediate motogenic responses to this growth factor are not clearly understood. An obligatory step for forward cell migration is the development of front–rear polarity and formation of lamellipodia at the leading edge. We show that stimulation of epidermal keratinocytes with EGF, but not with other growth factors, induces development of front–rear polarity and directional migration through a pathway that requires integrin-linked kinase (ILK), Engulfment and Cell Motility-2 (ELMO2), integrin β1, and Rac1. Furthermore, EGF induction of front–rear polarity and chemotaxis require the tyrosine kinase activity of the EGF receptor and are mediated by complexes containing active RhoG, ELMO2, and ILK. Our findings reveal a novel link between EGF receptor stimulation, ILK-containing complexes, and activation of small Rho GTPases necessary for acquisition of front–rear polarity and forward movement.
INTRODUCTION
The epidermal growth factor (EGF) receptor system is central for the development and postnatal homeostasis of the epidermis. Activation of EGF receptor pathways is implicated in normal epidermal renewal, regeneration after injury, modulation of immune functions, and carcinogenesis (reviewed in Pastore et al., 2008). The activity of the EGF receptor is modulated not only by direct binding of ligands such as EGF and transforming growth factor-α (TGF-α), but also via ligand-independent mechanisms. In particular, there is cooperativity between integrins and EGF receptors at sites of cell adhesion (Cabodi et al., 2004). Indeed, the interaction of integrins with extracellular matrix substrates at focal adhesions can trigger EGF-independent receptor signaling and is necessary for a full response to EGF stimulation (Bill et al., 2004), indicating the importance of cross-talk between these two types of transmembrane proteins. EGF receptor ligands can function as strong stimuli to induce forward movement in keratinocytes. In fact, TGF-α is the principal promigratory factor in human serum during epidermal regeneration after wounding (Li et al., 2006).
Cell migration requires the development of front–rear polarity, which is achieved in part through asymmetrical distribution of proteins that participate in the assembly and disassembly of focal adhesions. These proteins include integrins, paxillin, focal adhesion kinase and integrin-linked kinase (ILK; Welf and Haugh, 2011). ILK is a scaffold protein involved in cell adhesion, migration, and survival, as well as in exocytosis and mitotic spindle organization (Fielding et al., 2008; Nakrieko et al., 2008; Wickstrom et al., 2010a, 2010b). ILK also participates in the cross-talk between integrins and some receptor tyrosine kinases. For example, ILK can associate with PINCH, which in turn binds Nck-2, bridging integrins with EGF or platelet-derived growth factor receptor signaling events associated with cell survival (Hehlgans et al., 2007).
The development of front–rear polarity and forward cell movement also requires remodeling of the actin cytoskeleton. Actin cytoskeletal dynamics is modulated by the Rho family of small GTPases. During cell migration, lamellipodia form at the cell front with the contribution of active RhoG and/or Rac1, whereas RhoA participates in regulating changes at the rear of the cell (reviewed in Parsons et al., 2010). Activation of Rho GTPases occurs in a specific spatiotemporal manner following the formation of signaling complexes organized by scaffolding proteins, such as ILK. Indeed, ILK can associate with the Rac1 activators α- and β-Pix (Filipenko et al., 2005; Boulter et al., 2006), and ILK deficiency results in impaired formation of Rac1-GTP in various cell types (Liu et al., 2005; Nakrieko et al., 2008).
In epidermal keratinocytes, ILK interacts with Engulfment and Cell Motility 2 (ELMO2; Ho et al., 2009), another scaffold protein that binds Dock family members to stimulate Rac1 activation and cell motility (Gumienny et al., 2001; Katoh and Negishi, 2003; Katoh et al., 2005). ELMO2 localizes to lamellipodia and serves as a bridge between active RhoG and ILK (Ho et al., 2009). This multiprotein complex induces polarization of cultured keratinocytes in response to integrin stimulation by laminin 332. Whether other physiological signals modulate ILK/ELMO2 complexes and the roles of these signals in epithelial cell biology remain poorly understood. We now demonstrate that ILK/ELMO2 complexes are recruited to sites containing active RhoG during EGF receptor stimulation. We also identify Rac1 as a downstream effector of this novel signaling pathway and establish ILK and ELMO2 as key components in the induction of front–rear polarity in keratinocytes in response to EGF receptor activation.
RESULTS
ILK and ELMO2 cooperate to enhance EGF-induced polarization
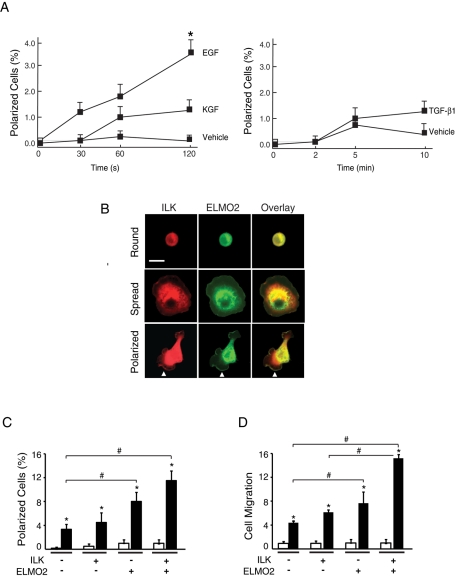
In epidermal keratinocytes and other cell types, EGF, keratinocyte growth factor (KGF), and transforming growth factor-β 1 (TGF-β1) are chemotactic stimuli that induce forward movement (Martin, 1997). Because exogenous expression of ILK and ELMO2 also induces front–rear polarity and migration in these cells (Ho et al., 2009), we investigated whether these proteins are involved in growth factor–induced cell polarization. To this end, we initially assessed the effect of EGF, KGF, and TGF-β1 on keratinocyte polarization, using an assay we previously developed (Ho et al., 2009). Subconfluent cultures were incubated in the presence or absence of growth factors, and the fraction of cells that exhibited formation of defined lamellipodia and front–rear polarity was scored at timed intervals after growth factor addition. In the absence of growth factors, <0.2% of the cells became polarized within a 2-min interval following addition of vehicle (Figure 1A). In contrast, the presence of EGF induced an 18-fold increase in the fraction of polarized cells under these conditions (Figure 1A). Although KGF and TGF-β1 can induce keratinocyte migration, they did not cause statistically significant changes in the fraction of polarized cells within the short time frame of these assays (Figure 1A), suggesting that different growth factors may induce formation of front–rear polarity with different kinetics and/or by different mechanisms.
FIGURE 1:
Effect of ILK and ELMO2 on EGF-induced polarization. (A) Keratinocytes were incubated in FBS- and growth factor–free medium for 4 h, followed by stimulation with growth factors for the indicated periods. The graph represents the fraction of polarized cells expressed as a percentage of the total cell population. Results are expressed as the mean + SEM (n = 3). *p < 0.05 relative to vehicle-treated cells (ANOVA). (B) Keratinocytes were transfected with vectors encoding mCherry/V5–tagged ILK and GFP-tagged ELMO2. Twenty-four hours after transfection, cells were incubated in FBS- and growth factor–free medium for 4 h, followed by stimulation with EGF (10 ng/ml) for 2 min. The cells were then processed for fluorescence microscopy to visualize and quantify round, spread, and polarized cells. Arrows indicate cell edges with colocalization of exogenous ILK and ELMO2. Bar, 50 μm. (C) Cells were transfected as in B, and the abundance of polarized keratinocytes expressing the indicated exogenous proteins and treated with EGF (black bars) or vehicle (white bars) for 2 min was determined. The dashes indicate that cells were transfected with vectors encoding mCherry and/or GFP in place of mCherry/V5-ILK and/or GFP-ELMO2, respectively. The results are expressed as the mean + SEM (n = 3). *p < 0.05 relative to cells stimulated with vehicle. #p < 0.05 relative to EGF-treated cells transfected with vectors encoding mCherry and GFP (ANOVA). (D) Cells were transfected as in C and 24 h later were trypsinized and used to measure EGF-induced chemotactic migration through Transwell inserts, as described in the Supplemental Materials and Methods. The fraction of keratinocytes expressing the indicated exogenous proteins that migrated over 6 h was determined. The results are expressed relative to the fraction of cells expressing mCherry and GFP that migrated in the absence of EGF (white bars), which is set to 1. The data are represented as the mean + SEM (n = 3). *p < 0.05 relative to cells stimulated with vehicle. #p < 0.05 relative to EGF-treated cells transfected with vectors encoding GFP and mCherry (ANOVA). The EGF-treated cell fractions are represented by black bars.
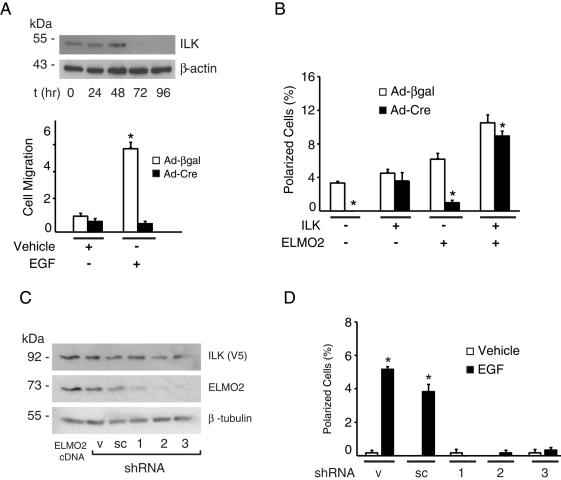
We next determined whether ILK and/or ELMO2 modulated EGF induction of cell polarization. Cultured keratinocytes exogenously expressing mCherry/V5–tagged ILK and/or green fluorescent protein (GFP)–tagged ELMO2 were incubated in the presence or absence of EGF, as described. The fractions of transfected cells that had attached but not spread (“round”), that had attached and spread (“spread”), and that had attached and developed front–rear polarity (“polarized”; Figure 1B) were determined (Ho et al., 2009). In the absence or presence of EGF, polarized cells expressing control mCherry and GFP constituted, respectively, ∼0.2 and 3.0% of the cell population, similar to values in untransfected cells (Figure 1C). EGF stimulation of cells expressing exogenous ILK did not further increase the fraction of polarized cells. In contrast, ∼8% of cells expressing exogenous ELMO2 and ∼12% of cells expressing both ILK and ELMO2 showed polarization in response to EGF (Figure 1C). Of note, the proportion of polarized cells expressing exogenous ILK and/or ELMO2 in the presence of KGF or TGF-β1 did not show significant changes relative to vehicle-treated keratinocytes (Supplemental Figure S1). Thus ILK and ELMO2 appear to cooperate to enhance EGF-induced keratinocyte polarization. Because development of front–rear polarity and EGF stimulation are associated with keratinocyte migration (Russell et al., 2003), we also investigated whether exogenous expression of ILK and/or ELMO2 had analogous enhancing effects on chemotactic migration toward EGF. Similar to its effects on cell polarity, the fraction of cells that exhibited chemotactic motility toward EGF was ∼fourfold greater in the population that exogenously expressed both ILK and ELMO2 relative to those cells expressing control vectors (Figure 1D).
Role of RhoG in recruitment of ILK/ELMO2 complexes to the plasma membrane
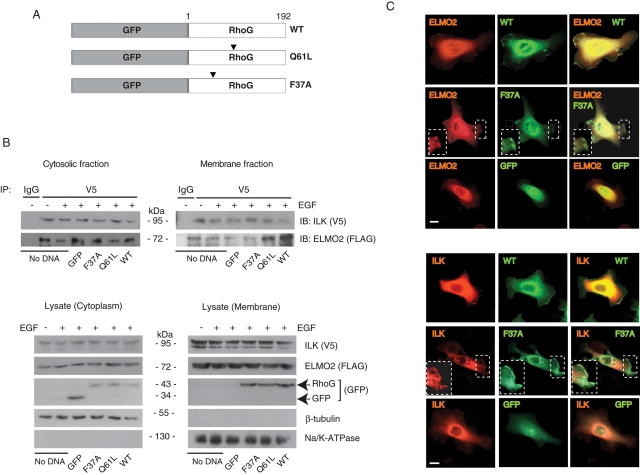
Using purified, bacterially produced proteins, we previously showed that ELMO2 serves as a bridge between ILK and active RhoG. Furthermore, activation of RhoG in keratinocytes is sufficient for localization of ELMO2 and ILK to lamellipodia formed in response to laminin 332 (Ho et al., 2009). However, whether RhoG/ELMO2/ILK complex formation and localization to the plasma membrane in live cells requires the presence of EGF is unknown. To address this issue, we analyzed complexes containing exogenously expressed mCherry/V5–tagged ILK and FLAG-tagged ELMO2 in keratinocytes cultured in the presence or absence of exogenous EGF. We found ELMO2 in ILK immunoprecipitates, from both the cytosolic and the membrane fractions, irrespective of whether this growth factor had been included in the culture medium (Figure 2B). We then investigated the ability of ILK and ELMO2 to form complexes with the RhoG proteins schematically shown in Figure 2A. Exogenously expressed ELMO2 associated with wild-type RhoG, as well as with the constitutively active mutant Q61L (Supplemental Figure S2A), both of which efficiently localize to lamellipodia (Grimsley et al., 2006; Ho et al., 2009). In contrast, ELMO2 did not interact with RhoG F37A, a mutant that localizes to the plasma membrane, but does not interact with ELMO2 (Supplemental Figure s2A; Katoh and Negishi, 2003; Santy et al., 2005; Hiramoto et al., 2006). Furthermore, wild-type and Q61L RhoG, but not the F37A mutant, were found in ILK immunoprecipitates, demonstrating that the interaction between RhoG and ELMO2 is necessary for ILK association with RhoG-containing complexes (Supplemental Figure S2B).
FIGURE 2:
Role of RhoG in formation and subcellular distribution of ILK/ELMO2 complexes. (A) Schematic of RhoG proteins used in transient transfections. Arrowheads indicate the position of mutated residues. (B) Keratinocytes were transiently transfected with vectors encoding FLAG-tagged ELMO2 and mCherry/V5–tagged ILK. Where indicated, vectors encoding GFP or GFP-tagged RhoG proteins were also included. After 4 h of incubation with the transfection reagents, the medium was replaced with serum-free medium lacking (–) or containing 10 ng/ml EGF (+). Twenty-four hours later, cell lysates were prepared, and the membrane and cytosolic fractions were subjected to immunoprecipitation using anti-V5 antibodies or an unrelated mouse immunoglobulin G (IgG), as indicated. The immune complexes were analyzed by immunoblot with anti-V5 or anti-FLAG antibodies. Lysate samples were also analyzed by immunoblot with the indicated antibodies to verify fractionation. (C) Primary mouse keratinocytes were transfected with vectors encoding wild-type or F37A GFP-tagged RhoG proteins and either FLAG-tagged ELMO2 or mCherry/V5–tagged ILK, as indicated. Twenty-four hours after transfection, cells were trypsinized and replated onto a laminin 332 matrix. The cells were processed for microscopy 3 h after replating. Exogenous ELMO2 was visualized using anti-FLAG antibodies. RhoG and ILK were visualized using, respectively, GFP and mCherry fluorescence. Protrusions in boxed areas contain RhoG F37A but not ELMO2 or ILK and are shown in the insets at higher magnification. Bar, 20 μm.
We also examined the effects of expressing wild-type and mutant RhoG proteins on ILK/ELMO2 complexes. To this end, we analyzed the cytosolic and membrane fractions of keratinocytes cultured with exogenous EGF (10 ng/ml; Figure 2B) and transfected with vectors encoding ILK, ELMO2, and RhoG proteins. ELMO2 was present in cytoplasmic ILK immunoprecipitates, irrespective of the presence of wild-type, Q61L, or F37A RhoG. ELMO2/ILK species were also relatively abundant in membrane fractions from cells coexpressing wild-type or Q61L RhoG (Figure 2B). ELMO2/ILK species were detected in membrane fractions from cells that had not been transfected with any RhoG-encoding vectors, although at somewhat reduced levels, likely due to their interaction with endogenous RhoG (Figure 2B). It is significant that membrane-associated ELMO2/ILK levels in cells expressing RhoG F37A were similar to those observed in cells not transfected with RhoG-encoding vectors. The presence of these complexes may result from interactions with endogenous RhoG in cells that did not express the F37A mutant, as only ∼30% of cells expressing mCherry/V5-ILK and FLAG-ELMO2 also expressed any of the RhoG proteins examined (data not shown). Together, these observations are consistent with the notion that ILK/ELMO2 complexes can exist both in the membrane and in the cytoplasmic fractions, independent of the presence of exogenous EGF. Keratinocytes deposit laminin 332 matrix, which can support ELMO2/ILK localization to lamellipodia, and also express several EGF receptor agonists, including HB-EGF and TGF-α (Hashimoto, 2000). The recruitment of ELMO2/ILK complexes to the membrane in cells cultured in the absence of exogenous EGF may result from autocrine and/or paracrine stimulation by one or more of these proteins.
To complement these studies, we next examined the subcellular localization of exogenously expressed ELMO2 and ILK in the presence of RhoG proteins by fluorescence microscopy. Consistent with our biochemical analyses, ELMO2 and ILK were found at cell protrusions in cells expressing wild-type or Q61L RhoG but not in extensions where RhoG F37A was localized (Figure 2C, Supplemental Figure S3, and data not shown).
Contribution of RhoG/ELMO2/ILK complexes to EGF induction of cell polarity
RhoG activation subsequent to integrin stimulation by laminin 332 matrix promotes localization of ILK/ELMO2 complexes to the leading edge of keratinocytes (Ho et al., 2009). In addition, EGF activates RhoG in certain cell types (Katoh and Negishi, 2003; Hiramoto-Yamaki et al., 2010). These observations prompted us to examine whether formation of RhoG/ELMO2/ILK complexes is involved in EGF induction of keratinocyte polarity.
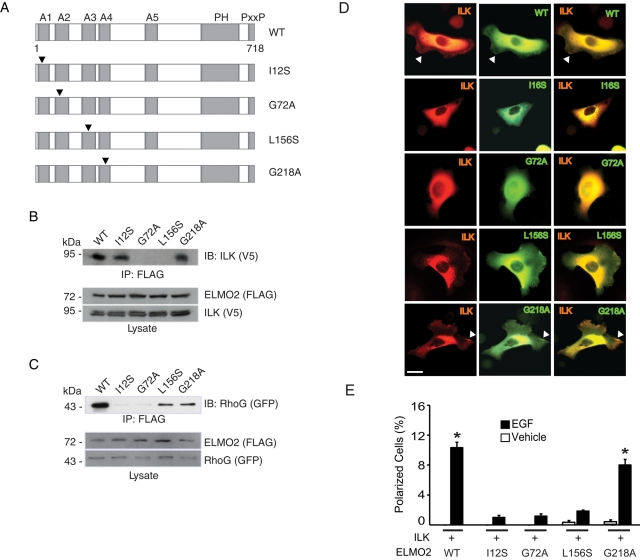
The N-terminus of ELMO2 mediates binding to both ILK and RhoG (Ho et al., 2009). Several putative ARM repeats, important for protein–protein interactions, have also been identified in silico in this region of ELMO2 (deBakker et al., 2004). We first investigated which of the ARM motifs, if any, are involved in the interactions of ELMO2 with ILK. To this end, we generated FLAG-tagged ELMO2 proteins with point mutations in residues known to be critical for ARM repeat organization (Figure 3A) and examined their ability to interact with ILK. ELMO2 G72A and L156S, with mutations that disrupt the second and third ARM repeats, respectively, could not associate with ILK (Figure 3B). In contrast, mutations that disrupted the first (ELMO2 I12S) or fourth (ELMO2 G218A) ARM repeat were without effect (Figure 3B). We also used these ELMO2 proteins to map the regions involved in binding to RhoG. We found that ELMO2 I12S and G72A, which contain mutations in the first and second ARM motifs, respectively, did not associate to any significant degree with RhoG (Figure 3C). However, ELMO2 L156S or G218A bound to RhoG. In the conditions of our experiments, we did not observe substantial, consistent differences between the apparent ability of wild-type ELMO2 and ELMO2 L156S or G218A to bind RhoG. Thus ELMO2 interaction with ILK requires its second and third ARM repeats, whereas its association with RhoG requires its first and second ARM repeats. Furthermore, and in contrast with exogenously expressed wild-type ELMO2, the mutants I12S and G72A did not localize to lamellipodial extensions in polarized keratinocytes stimulated with laminin 332 (Figure 3D and Supplemental Figure S4). In these cells, exogenously expressed mCherry/V5–tagged ILK was not found at lamellipodia either. In contrast, ELMO2 L156S or G218A were found at cell protrusions, indicating that ELMO2 association with RhoG, but not with ILK, is necessary for ELMO2 movement to cell extensions (Figure 3D and Supplemental Figure S4). ILK localized to cell edges only in the presence of ELMO2 G218A but not L156S. Together, these observations indicate that interactions with RhoG are necessary to promote movement of ILK/ELMO2 species to cell protrusions in polarized keratinocytes.
FIGURE 3:
RhoG/ELMO2/ILK complexes mediate EGF-induced keratinocyte polarization. (A) Schematic of the ELMO2 mutants used. Arrowheads indicate the position of mutated residues. A, ARM repeat; PH, pleckstrin homology domain; PxxP, proline-rich region. (B) Lysates from IMDF cells exogenously expressing mCherry/V5–tagged ILK together with FLAG-tagged ELMO2 proteins were used in immunoprecipitation assays with anti-FLAG antibodies. FLAG-ELMO2 immune complexes were resolved by SDS–PAGE and analyzed by immunoblot with anti-V5 antibodies to assess presence of ILK. The lysates were also analyzed by immunoblot with anti-FLAG or anti-V5 antibodies, as indicated. (C) Lysates from IMDF cells exogenously expressing GFP-tagged RhoG together with FLAG-tagged ELMO2 proteins were used in immunoprecipitation assays with anti-FLAG antibodies. FLAG-ELMO2 immune complexes were resolved by SDS–PAGE and analyzed by immunoblot with anti-GFP antibodies to assess presence of RhoG. The lysates were also analyzed by immunoblot with anti-FLAG or anti-GFP antibodies, as indicated. (D) Keratinocytes were transfected with vectors encoding mCherry/V5–tagged ILK and the indicated GFP-tagged ELMO2 protein. Twenty-four hours after transfection, the cells were briefly trypsinized, replated onto a laminin 332 substrate, and allowed to adhere for 3 h, prior to processing for direct fluorescence microscopy. Arrows indicate cell protrusions containing ILK and ELMO2. Bar, 20 μm. (E) Keratinocytes were transfected with vectors encoding the indicated proteins, as in D. Twenty-four hours after transfection, the cells were incubated in FBS- and EGF-free medium for 4 h, followed by stimulation with EGF or vehicle for 2 min. The cells were processed for fluorescence microscopy to assess the fraction of polarized keratinocytes. The results are expressed as the mean + SEM (n = 3). Where bars corresponding to vehicle-treated cells are absent, the fraction of polarized cells was <0.05%. *p < 0.05 relative to the corresponding vehicle-treated sample (ANOVA).
Following the identification of critical residues in ELMO2 that allowed us to distinguish its interactions with RhoG from those with ILK, we investigated whether RhoG/ELMO2/ILK species are implicated in induction of cell polarization by EGF. Exogenous ILK and ELMO2 proteins were expressed in these cells, and the fraction of keratinocytes that acquired front–rear polarity upon stimulation by EGF was scored. Expression of ILK together with wild-type or G218A ELMO2 resulted in 8- to 10-fold increases in the fraction of polarized cells in response to EGF (Figure 3E). In contrast, no significant increase in polarized cell numbers was observed in EGF-treated cells coexpressing ILK with ELMO2 I12S, G72A, or L156S (Figure 3E). Thus EGF induction of cell polarity appears to be mediated via RhoG/ELMO2/ILK complexes.
EGF receptor kinase activity and induction of cell polarization
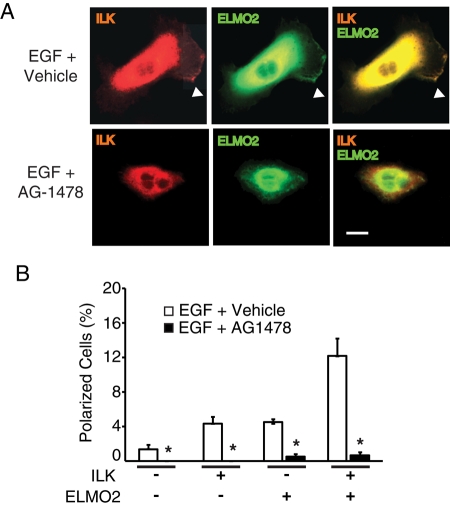
We next asked whether signaling through the EGF receptor tyrosine kinase is necessary for ILK/ELMO2 induction of cell polarization. To address this issue, we used keratinocytes expressing exogenous ILK and/or ELMO2. The cells were incubated in fetal bovine serum (FBS) and EGF-free medium for 4 h in the presence of the EGF receptor inhibitor AG1478 (2 ng/ml, final), followed by addition of EGF. In agreement with previous results, exogenous ILK/ELMO2 increased the fraction of polarized cells observed in the presence of EGF, but this effect was abrogated by AG1478 (Figure 4 and Supplemental Figure S5). We also observed decreased spreading in AG1478-treated cells (Figure 4), suggesting that EGF receptor activity may play other roles in keratinocytes in addition to polarization via ILK/ELMO2.
FIGURE 4:
EGF receptor tyrosine kinase activity is necessary for EGF-induced polarization. (A) Keratinocytes were transfected with vectors encoding mCherry/V5–tagged ILK and GFP-tagged ELMO2. Twenty-four hours after transfection, the cells were incubated in FBS- and EGF-free medium for 4 h but in the presence of AG1478 or DMSO (0.1%; vehicle control). The cells were then stimulated with EGF for 2 min and processed for fluorescence microscopy. Arrowheads indicate cell protrusions in which ILK and ELMO2 colocalize. Bar, 20 μm. (B) Quantification of polarized cells treated as described in A. The results are expressed as the mean + SEM (n = 3). Where bars corresponding to vehicle-treated cells are absent, the fraction of polarized cells was <0.05%. *P < 0.05 relative to DMSO-treated cells (ANOVA).
ILK and ELMO2 are required for EGF induction of keratinocyte polarization
To assess the importance of endogenous ILK in induction of cell polarization by EGF, we analyzed the effect of EGF stimulation on ILK-deficient keratinocytes. To this end, we isolated cells from Ilkf/f mice, which contain Ilk alleles that can be inactivated by expression of Cre recombinase (Nakrieko et al., 2008). ILK protein in cells infected by Cre-encoding adenoviruses is undetectable by 72 h postinfection (Figure 5A). We first tested the ability of keratinocytes to migrate chemotactically toward EGF in modified Boyden chamber assays and observed that this growth factor induced a sixfold increase in the fraction of ILK-expressing cells that migrated. In contrast, ILK-deficient keratinocytes showed negligible migration, irrespective of whether EGF was present as a chemotactic stimulus (Figure 5A). Furthermore, ILK-deficient cells exhibited defective spreading and did not show polarization or formation of cell extensions upon stimulation by EGF (Figure 5B and Supplemental Figure S6). This behavior contrasted with the increased proportion of polarized cells observed in control ILK-expressing cultures infected with a β-galactosidase–encoding adenovirus (Ad-βgal) upon treatment with this growth factor (Figure 5B and Supplemental Figure S6). The presence of exogenously expressed human ILK in ILK-deficient mouse keratinocytes restored the ability of the cells to polarize in response to EGF, and the fraction of polarized ILK-deficient cells exogenously expressing both ILK and ELMO2 was ∼80% of that found in Ad-βgal–infected cells expressing those two proteins (Figure 5B and Supplemental Figure S6). Of note, transient transfection of ILK-deficient keratinocytes with a vector encoding ELMO2 was not sufficient to restore EGF-induced polarization (Figure 5B), indicating not only that ILK is a necessary component in this process, but also that increased polarization induced by exogenous ELMO2 occurs mainly, if not exclusively, through ILK-dependent mechanisms.
FIGURE 5:
Requirement for ILK and ELMO2 in EGF-induced polarization. (A) Ilkf/f keratinocytes were infected with adenovirus encoding Cre recombinase (Ad-Cre). Cell lysates were prepared at the indicated times after infection, resolved by SDS–PAGE, and analyzed by immunoblot with antibodies against endogenous ILK and β-actin (as loading control). Replicate cultures were infected with Ad-Cre or adenoviruses encoding β-galactosidase (Ad-βgal), and 72 h postinfection were trypsinized and used to measure EGF-induced chemotactic migration through Transwell inserts, as described in the Supplemental Materials and Methods. The fraction of keratinocytes that migrated through the inserts over 6 h was determined. The results are expressed relative to the fraction of cells infected with Ad-βgal that migrated in the absence of EGF, which is set to 1. The data represent the mean + SEM (n = 3). *p < 0.05 relative to Ad-βgal–infected cells stimulated with vehicle (ANOVA). (B) Ilkf/f keratinocytes were infected with Ad-Cre or Ad-βgal. Twenty-four hours after infection, cells were cotransfected with vectors encoding mCherry/V5–tagged ILK and/or GFP-tagged ELMO2. Twenty-four hours after transfection, cells were incubated in FBS- and EGF-free medium for 4 h, followed by stimulation with EGF for 2 min. The cells were processed for fluorescence microscopy, and the fraction of polarized cells was scored. Negative signs indicate cells that were transfected with vectors encoding only GFP- and/or mCherry. Results are expressed as the mean + SEM (n = 5). *p < 0.05 relative to cells infected with Ad-βgal (ANOVA). (C) Keratinocytes were transiently transfected with vectors encoding mCherry/V5–tagged ILK and either FLAG-tagged ELMO2 or with plasmids encoding control or ELMO2 shRNA sequences. Cell extracts were isolated 48 h after transfection, resolved by SDS–PAGE, and analyzed by immunoblot with antibodies against exogenous ILK, endogenous ELMO2 (except for the sample transfected with ELMO2-encoding vectors, which shows the total levels of exogenous pus endogenous proteins) or β-tubulin, used as protein loading control. sc, scrambled shRNA; v, vector only. The numbers indicate shRNA1, 2, or 3. (D) Cells transfected with shRNA-encoding vectors, as in C, were incubated in FBS- and EGF-free medium for 4 h, followed by stimulation with EGF for 2 min. The cells were processed for fluorescence microscopy, and the fraction of polarized cells was scored. Results are expressed as the mean + SEM (n = 3). Where bars are absent, the fraction of polarized cells was <0.05%. *p < 0.05 relative to cells treated with vehicle (ANOVA).
We also investigated whether ELMO2 is required for EGF induction of cell polarization. For these studies, we first screened three different short hairpin RNA (shRNA) sequences directed to the mouse ELMO2 mRNA for their ability to reduce ELMO2 protein levels in keratinocytes. Transient transfection of vectors encoding these shRNAs, but not vectors containing control sequences, substantially reduced the levels of total ELMO2 protein 48 h following transfection (Figure 5C). In the absence of EGF, ELMO2-deficient cells remained attached and spread and did not show any appreciable differences from ELMO2-expressing cells (Supplemental Figure S7). However, and similar to ILK-deficient keratinocytes, knockdown of ELMO2 prevented induction of cell polarization by EGF (Figure 5D and Supplemental Figure S7). Together, these results indicate that ILK and ELMO2 are obligatory downstream effectors of EGF receptor stimulation of keratinocyte polarization.
Rac1 mediates EGF induction of polarization via ILK/ELMO2 complexes
Rac1 activation plays key roles in formation of lamellipodia and development of front–rear polarity (Parri and Chiarugi, 2010). In addition, EGF stimulation, as well as ILK- and ELMO2-containing complexes, can participate in Rac1 activation in various cell types (Ho et al., 2009; Hiramoto-Yamaki et al., 2010; Samson et al., 2010). Consequently, we examined whether ILK/ELMO2 species can modulate EGF-induced Rac1 activation in epidermal keratinocytes.
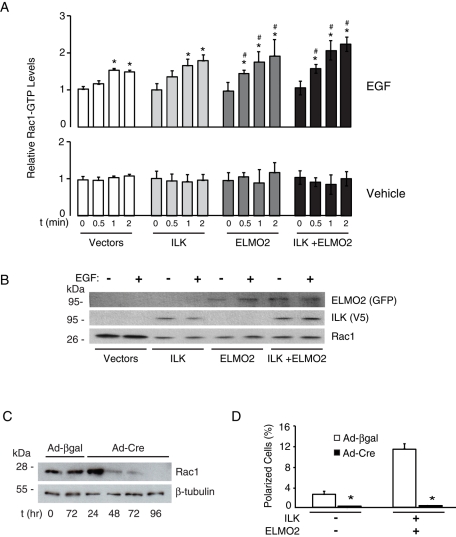
Treatment of keratinocytes with EGF resulted in Rac1 activation within 30 s and peaked at 1 min, as measured by increases in Rac1-GTP levels in cell lysates (Figure 6A). Exogenous expression of ILK and/or ELMO2 significantly enhanced EGF-induced Rac1 activation over the same period (Figure 6, A and B). Increases in Rac1-GTP levels depended on EGF stimulation, as they did not appreciably change in vehicle-treated cells, irrespective of the presence or absence of exogenous ILK and/or ELMO2 (Figure 6, A and B). Thus ILK/ELMO2 promote Rac1 activation in response to EGF.
FIGURE 6:
Role of Rac1 in EGF- and ILK/ELMO2–induced polarization. (A) Keratinocytes were transfected with vectors encoding the indicated proteins and, 24 h later, were incubated in FBS- and EGF-free medium for 4 h. The cells were then stimulated with EGF or vehicle for the indicated times. Cell lysates were prepared and levels of active Rac1 (Rac1-GTP) were determined. The data are expressed relative to Rac1-GTP levels in samples transfected with GFP- and mCherry-encoding plasmids (Vectors) in the absence of EGF and collected at t = 0, which is set to 1. Bars represent average + SEM (n = 3). *p < 0.05 relative to vehicle-treated cells transfected with Vectors (ANOVA). (B) Samples of the cell lysates used for A and collected after 2 min of EGF treatment were resolved by SDS–PAGE and immunoblotted using the indicated antibodies. (C) Rac1f/f keratinocytes were infected with Ad-βgal or Ad-Cre. Cell extracts were prepared at the indicated times after infection, resolved by SDS–PAGE, and analyzed by immunoblot with antibodies against Rac1 or β-tubulin. (D) Rac1f/f keratinocytes were infected with Ad-Cre or Ad-βgal. Twenty-four hours after infection, cells were cotransfected with vectors encoding mCherry and GFP (–) or mCherry/V5–tagged ILK, as well as with GFP-tagged ELMO2. Twenty-four hours after transfection, cells were incubated in FBS- and EGF-free medium for 4 h, followed by stimulation with EGF (10 ng/ml) for 2 min. The cells were processed for fluorescence microscopy, and the fraction of polarized cells was scored. Results are expressed as the mean + SEM (n = 3). *p < 0.05 relative to cells infected with Ad-βgal (ANOVA).
The EGF-induced activation of Rac1 in cells exogenously expressing ILK and ELMO2 suggests Rac1 may mediate ILK/ELMO2 modulation of cell polarization. To address this issue, we used Rac1f/f keratinocytes. Ad-Cre treatment of these cells resulted in Rac1 gene inactivation, followed by decreases of >90% in Rac1 protein levels by 48 h following infection, which were associated with defective cell spreading (Figure 6C and data not shown). Treatment of Rac1-deficient keratinocytes with EGF did not result in formation of polarized cells with broad lamellipodia, irrespective of whether exogenous ILK and ELMO2 were expressed in these cultures (Figure 6D and Supplemental Figure S8). Instead, EGF induced formation of short, small cell protrusions to which exogenous ILK and ELMO2 localized. We conclude that induction of lamellipodial extensions associated with cell polarization by ILK/ELMO2 upon EGF receptor stimulation depends on Rac1 activation and that Rac1 is not necessary to recruit ILK/ELMO2 to cell protrusions.
Requirement for β1 integrins in EGF-induced polarization modulated by ILK/ELMO2
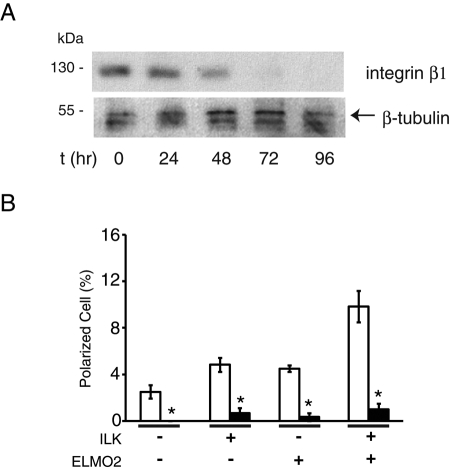
Growth factor receptors can activate multiple signaling pathways, some of which involve cross-talk with integrins. These last can modulate responses to growth factors by regulating receptor trafficking and/or signaling. Indeed, in several adherent cell types, β1 integrins and EGF receptors act cooperatively to promote adhesion, spreading, development of front–rear polarity, and migration in response to EGF (Caswell et al., 2008). The existence of this cross-talk, together with the known role of ILK in mediating some of the responses to β1 integrin stimulation, prompted us to investigate whether β1 integrins are necessary for lamellipodia formation and cell polarization in response to EGF. We isolated and cultured primary keratinocytes from Intb1f/f mice and infected them with Ad-Cre. We observed that, by 48 and 72 h following infection, integrin β1 protein levels had decreased, respectively, by ∼50 and 90% (Figure 7A). Within the time frame of this experiment, integrin β1–deficient keratinocytes remained attached to the laminin 332 matrix these cells normally produce, likely through α6β4 integrins. However, treatment of these cells with EGF failed to trigger substantial formation of broad lamellipodia and front–rear polarity. This defect was not corrected by exogenous expression of ILK and/or ELMO2 (Figure 7B), although these two proteins colocalized at those cell protrusions that were rarely observed in integrin β1–deficient keratinocytes (Supplemental Figure S9). Together, these observations are consistent with the concept that β1 integrins and EGF receptors coordinately function to engage ILK/ELMO2 species and to activate Rac1 to promote cell polarization.
FIGURE 7:
Integrin β1 is required for EGF- and ILK/ELMO2–induced polarization. (A) Intb1f/f keratinocytes were infected with Ad-Cre. Cell lysates prepared at the indicated times after infection were resolved by SDS–PAGE and analyzed by immunoblot analysis with antibodies against integrin β1 or β-tubulin. (B) Intb1f/f keratinocytes were infected with Ad-Cre or Ad-βgal and, 48 h later, were transfected with vectors encoding mCherry/V5–tagged ILK and/or GFP-tagged ELMO2. Twenty-four hours after transfection, the cells were incubated for 4 h in FBS- and EGF-free medium, followed by EGF stimulation for 2 min. Keratinocytes were then processed for fluorescence microscopy to score the fraction of polarized cells, shown in the graph. Results are expressed as the mean + SEM (n = 3). Minus signs indicate cells transfected with vectors encoding mCherry and GFP. *p < 0.05 relative to cells infected with Ad-βgal (ANOVA).
DISCUSSION
In this study, we identified EGF as a physiological signal that induces recruitment of RhoG, ELMO2, and ILK to the plasma membrane to induce formation of lamellipodia and keratinocyte polarization, which also results in forward migration. In these cells, there appears to be a degree of selectivity in the mechanisms that couple EGF signaling with ILK/ELMO2 species. This is evidenced by the inability of exogenous ILK/ELMO2 to enhance cell polarization in response to stimulation by other growth factors, such as TGF-β1 and KGF. These observations are in keeping with the reported activation of multiple guanine-nucleotide exchange factors that stimulate RhoG in response to EGF but not to other growth factors (Samson et al., 2010). Of note, EGF receptor stimulation has also been shown to induce activation of the EphA2 receptor tyrosine kinase, which in turn recruits RhoG/ELMO2 to promote migration of mammary carcinoma cells (Hiramoto-Yamaki et al., 2010). Whether ILK is a component of the EphA2/RhoG/ELMO2 complexes in breast carcinoma cells remains to be investigated but could potentially place ILK as a key therapeutic target. Similarly, the possible involvement of EphA2 in EGF induction of keratinocyte polarization remains to be investigated.
Although ILK has been implicated in various growth factor signaling pathways (Hehlgans et al., 2007), the direct involvement of ILK in EGF induction of cell polarization and forward movement is a novel function for this scaffold protein. ELMO2 has recently been reported to be a key player in EGF-induced membrane ruffling and polarization (Hiramoto-Yamaki et al., 2010), and our findings provide evidence for a link between ILK and ELMO2 in EGF-induced cell polarization. It is significant that the formation of ILK/ELMO2 complexes can occur in the absence of EGF receptor stimulation (Ho et al., 2009). Whether EGF receptor tyrosine kinase activity is sufficient for recruitment of this complex to the plasma membrane and/or subsequent downstream events remains to be established.
EGF receptor stimulation can rapidly activate RhoG, allowing it to interact with ELMO2 at the plasma membrane (Katoh and Negishi, 2003; Hiramoto-Yamaki et al., 2010). It is significant that, as shown in the present study, expression of RhoG F37A, which does not interact with ELMO2 and inhibits cell polarization and migration, does not interfere with ILK/ELMO2 association in cytoplasmic fractions. This suggests that RhoG activation downstream from EGF may serve to recruit ILK/ELMO2 to the plasma membrane rather than to catalyze the association between those two proteins.
ILK and ELMO2 have been independently implicated in regulation of Rac1 activity: the former, in complexes that contain α- or β-Pix, and the latter as a direct guanine-exchange factor when bound to Dock proteins (Katoh and Negishi, 2003; Ho et al., 2009; Hiramoto-Yamaki et al., 2010). In our studies, Rac1 gene inactivation inhibits EGF-induced polarization. We observed similar effects upon expression of an ELMO2 deletion mutant incapable of interacting with Dock proteins (data not shown), suggesting that Rac1 may be activated directly downstream of ILK/ELMO2, potentially by ILK/ELMO2/Dock complexes. ELMO2/ILK may also serve as a hub to recruit other Rac1-activating proteins to induce formation of cell extensions and forward movement. How exactly Rac1 is activated downstream from ILK/ELMO2 remains to be determined.
Targeted inactivation of the Ilk gene in epidermal keratinocytes severely affects their ability to spread, acquire front–rear polarity, and migrate (Lorenz et al., 2007; Nakrieko et al., 2008), emphasizing the importance of ILK for cell motion. Similarly, keratinocytes treated with ELMO2 shRNAs showed impaired ability to polarize in response to EGF, although they did not exhibit detectable defects in cell adhesion or spreading. These observations are consistent with the proposal that ELMO2 plays a minor role, if any, in keratinocyte attachment and spreading onto laminin 332 but is a key component of cell polarization in response to EGF. Alternatively, in the absence of ELMO2, ELMO1 may mediate some of these functions. Phenotypic redundancy between these two ELMO proteins has been suggested to occur in ELMO1-null mice (Elliott et al., 2010).
The existence of cross-talk mechanisms between integrins and growth factor receptors is well established. Loss of β1 integrin impairs keratinocyte adhesion, spreading, and forward movement (Raghavan et al., 2003). We now show that β1 integrin works in conjunction with EGF receptors to induce keratinocyte polarization. β1 integrins are essential for EGF signaling in various cell types, as they modulate endosomal trafficking of EGF receptors, which is necessary for optimal activation (Morello et al., 2011). β1 integrins are also involved in formation and activation of complexes containing N-WASP and Cdc42, which are required for chemotaxis induced by platelet-derived growth factor (King et al., 2011). The precise role of β1 integrins in the EGF/RhoG/ELMO2/ILK pathway may occur at various levels and is an important area for future research.
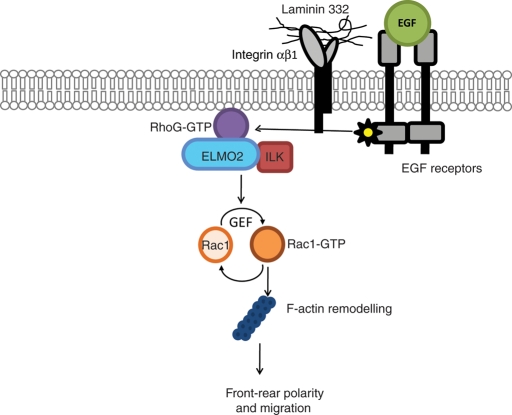
In conclusion, our data are consistent with a model in which EGF receptor stimulation promotes activation and recruitment of RhoG at the plasma membrane (Figure 8). Active RhoG then associates with ELMO2/ILK complexes, resulting in activation of Rac1, formation of lamellipodia, and cell polarization, followed by forward migration. Aspects of this model that remain important areas for future research are how β1 integrins modulate this process and the events that link RhoG/ELMO2/ILK to Rac1 activation. ELMO2 forms complexes with several Dock proteins, including Dock4, which directly activate Rac1 (Ueda et al., 2008). Whether this mechanism also functions in the context of the ILK/ELMO2 species remains to be defined.
FIGURE 8:
Proposed model of EGF induction of keratinocyte polarization. EGF receptor stimulation mediates activation of RhoG and its localization to the plasma membrane. Active RhoG recruits ILK/ELMO2 complexes already present in the cytosol. The activation of RhoG and subsequent recruitment of ILK/ELMO2 complexes may be mediated through EGF receptor kinase activity, as well as by integrin β1 ligation by extracellular matrix substrates. RhoG/ILK/ELMO2 complexes localized to the cell edge may activate Rac1 through ELMO2-associated guanine nucleotide exchange factors (GEF) or through recruitment of other ILK-interacting proteins. In turn, activation of Rac1 at the cell edge leads to F-actin reorganization, lamellipodia formation, acquisition of front–rear polarity, and directional migration.
MATERIALS AND METHODS
Mouse strains, cell culture, and transfections
The mouse strains used were as follows: CD-1, B6;129-Itgb1tm1Efu/J (stock number 004605; Jackson Laboratory, Bar Harbor ME; termed Itgb1f/f), in which loxP sites flanking exon 3 of the Itgb1 gene that encodes integrin β1 have been introduced (Raghavan et al., 2000); Ilktm1Star, with engineered loxP sites downstream from exons 4 and 12 of the Ilk gene (termed Ilkf/f; Terpstra et al., 2003), and Rac1tm1.1Djk, with loxP sites flanking exon 1 of the Rac1 gene (termed Rac1f/f; Glogauer et al., 2003).
Cultures of primary mouse keratinocytes isolated from 2- to 4-d-old mice were established and were transfected with polyethyleneimine (PEI; 1 mg/ml 25-kDa linear PEI, pH 7.0, catalog number 23966; Polysciences, Warrington, PA) as described (Ho et al., 2009; Dagnino et al., 2010). Transfected cells were cultured for 24 h prior to processing for microscopy or lysate preparation. The spontaneously immortalized dermal fibroblast line IMDF (Apostolova et al., 2002) was cultured in HyQ DMEM-RS (HyClone, Logan, UT) supplemented with 8% FBS.
Adenovirus infections
Primary keratinocyte cultures were infected with adenovirus encoding Cre recombinase (Ad-Cre) or β-galactosidase (Ad-βgal) at a multiplicity of infection (MOI) of 50 in Ca2+-free EMEM supplemented with bovine serum albumin (BSA; 2.5%) as described (Nakrieko et al., 2008). Under these conditions, ≥95% of the keratinocytes were infected without detectable reduction in cell viability. In experiments involving adenoviral infection followed by plasmid transfections, these last were conducted either 48 h (for Intb1f/f keratinocytes) or 24 h (for Ilkf/f or Rac1f/f cells) postinfection.
Stimulation by laminin 332 matrix and growth factors
For extracellular matrix stimulation, keratinocytes were briefly trypsinized 24 h following transfection and were resuspended in FBS- and Ca+-free EMEM supplemented with 2.5% BSA. The cells were plated onto laminin 332 matrix–coated coverslips (Nakrieko et al., 2008) and were cultured for 3 h to allow attachment and spreading, prior to processing for fluorescence microscopy (Ivanova et al., 2007). For growth factor stimulation experiments, subconfluent cells cultured on laminin 332 matrix were transfected, and, 24 h later, the growth medium was replaced with FBS-, EGF-, and Ca2+-free EMEM supplemented with 2.5% BSA. Four hours later, cells were stimulated by addition of EGF (10 ng/ml, final), KGF (20 ng/ml, final), or TGF-β1 (10 ng/ml) for intervals indicated in individual experiments. To inhibit EGF-receptor signaling, keratinocytes were incubated in FBS-, EGF-, and Ca2+-free EMEM containing AG1478 (30 ng/ml, final, T4182; Sigma-Aldrich, St. Louis, MO) for 4 h prior to stimulation with EGF.
Fluorescence and light microscopy
Twenty-four hours following transfection, keratinocytes were used for experiments indicated in individual figures and were processed for epifluorescence microscopy as described (Ivanova et al., 2007; Ho et al., 2009). For experiments assessing polarization, cells were fixed in 4% paraformaldehyde and stained with Alexa Fluor 350–conjugated phalloidin. At least 100 cells per sample were scored. Fluorescence photomicrographs were obtained with a Leica (Wetzlar, Germany) DMIRBE fluorescence microscope equipped with an Orca-ER digital camera (Hamamatsu Photonics, Hamamatsu, Japan), using Volocity 4.3.2 software (Improvision, Coventry, United Kingdom). Light microscopy images were obtained with a Zeiss Axio Imager Z1 microscope equipped with a Zeiss AxioCam ICc 1 camera, using AxioVision 4.6.3 software (Zeiss, Thornwood, NY). All results shown are representative of at least three experiments conducted with duplicate or triplicate samples.
Rac1 activation assays
Levels of Rac1-GTP were measured using a luminescence-based GLISA Rac1 Activation Assay Biochem Kit (Cytoskeleton, Denver, CO). Cells were lysed following the manufacturer's protocol, and 0.5 μg of lysate per sample was used. Luminescence (440 nm) was measured with a Safire2 Microplate Detection System (Tecan, Männedorf, Switzerland). Luminescence units in each sample were expressed after subtraction of the background units measured in protein-free lysis buffer. Treatments were conducted on duplicate samples, and experiments were repeated at least three times. Statistical significance was set to p < 0.05 (analysis of variance [ANOVA]).
Supplementary Material
Acknowledgments
We thank G. Di Guglielmo for helpful comments on the manuscript. This work was supported by funds from the Canadian Institutes of Health Research (to L.D.). E.H. was the recipient of a studentship from the London Strategic Training Initiative in Cancer Research and Technology Transfer Program, funded by the Canadian Institutes of Health Research.
Abbreviations used:
- BSA
bovine serum albumin
- EGF
epidermal growth factor
- ELMO
Engulfment and Cell Motility
- FBS
fetal bovine serum
- GFP
green fluorescent protein
- ILK
integrin-linked kinase
- KGF
keratinocyte growth factor
- MOI
multiplicity of infection
- PEI
polyethyleneimine
- TGF-β1
transforming growth factor-β 1
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E11-07-0596) on December 7, 2011.
REFERENCES
- Apostolova MD, Ivanova IA, Dagnino C, D'souza SJA, Dagnino L. Active import and export mechanisms regulate E2F-5 subcellular localization. J Biol Chem. 2002;277:34471–34479. doi: 10.1074/jbc.M205827200. [DOI] [PubMed] [Google Scholar]
- Bill HM, Knudsen B, Moores SL, Muthuswamy SK, Rao VR, Brugge JS, Miranti CK. Epidermal growth factor receptor-dependent regulation of integrin-mediated signaling and cell cycle entry in epithelial cells. Mol Cell Biol. 2004;24:8586–8599. doi: 10.1128/MCB.24.19.8586-8599.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter E, Grall D, Cagnol S, Van Obberghen-Schilling E. Regulation of cell-matrix adhesion dynamics and Rac1 by integrin-linked kinase. FASEB J. 2006;20:1489–1491. doi: 10.1096/fj.05-4579fje. [DOI] [PubMed] [Google Scholar]
- Cabodi S, Moro L, Bergatto E, Boeri Erba E, Di Stefano P, Turco E, Tarone G, Defilippi P. Integrin regulation of epidermal growth factor (EGF) receptor and of EGF-dependent responses. Biochem Soc Trans. 2004;32:438–442. doi: 10.1042/BST0320438. [DOI] [PubMed] [Google Scholar]
- Caswell PT, Chan M, Lindsay AJ, McCaffrey MW, Boettiger D, Norman JC. Rab-coupling protein coordinates recycling of alpha5beta1 integrin and EGFR1 to promote cell migration in 3D microenvironments. J Cell Biol. 2008;183:143–155. doi: 10.1083/jcb.200804140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagnino L, Ho E, Chang WY. Expression and analysis of exogenous proteins in epidermal cells. Methods Mol Biol. 2010;585:93–105. doi: 10.1007/978-1-60761-380-0_8. [DOI] [PubMed] [Google Scholar]
- deBakker CD, et al. Phagocytosis of apoptotic cells is regulated by a UNC-73/TRIO-MIG-2/RhoG signaling module and armadillo repeats of CED-12/ELMO. Curr Biol. 2004;14:2208–2216. doi: 10.1016/j.cub.2004.12.029. [DOI] [PubMed] [Google Scholar]
- Elliott MR, Zheng S, Park D, Woodson RI, Reardon MA, Juncadella IJ, Kinchen JM, Zhang J, Lysiak JJ, Ravichandran KS. Unexpected requirement for ELMO1 in clearance of apoptotic germ cells in vivo. Nature. 2010;467:333–337. doi: 10.1038/nature09356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding AB, Dobreva I, McDonald PC, Foster LJ, Dedhar S. Integrin-linked kinase localizes to the centrosome and regulates mitotic spindle organization. J Cell Biol. 2008;180:681–689. doi: 10.1083/jcb.200710074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipenko NR, Attwell S, Roskelley C, Dedhar S. Integrin-linked kinase activity regulates Rac- and Cdc42-mediated actin cytoskeleton reorganization via alpha-PIX. Oncogene. 2005;24:5837–5849. doi: 10.1038/sj.onc.1208737. [DOI] [PubMed] [Google Scholar]
- Glogauer M, Marchal CC, Zhu F, Worku A, Clausen BE, Foerster I, Marks P, Downey GP, Dinauer M, Kwiatkowski DJ. Rac1 deletion in mouse neutrophils has selective effects on neutrophil functions. J Immunol. 2003;170:5652–5657. doi: 10.4049/jimmunol.170.11.5652. [DOI] [PubMed] [Google Scholar]
- Grimsley CM, Lu M, Haney LB, Kinchen JM, Ravichandran KS. Characterization of a novel interaction between ELMO1 and ERM proteins. J Biol Chem. 2006;281:5928–5937. doi: 10.1074/jbc.M510647200. [DOI] [PubMed] [Google Scholar]
- Gumienny TL, et al. CED-12/ELMO, a novel member of the CrkII/Dock180/Rac pathway, is required for phagocytosis and cell migration. Cell. 2001;107:27–41. doi: 10.1016/s0092-8674(01)00520-7. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. Regulation of keratinocyte function by growth factors. J Dermatol Sci. 2000;24((Suppl 1)):S46–S50. doi: 10.1016/s0923-1811(00)00141-9. [DOI] [PubMed] [Google Scholar]
- Hehlgans S, Haase M, Cordes N. Signalling via integrins: implications for cell survival and anticancer strategies. Biochim Biophys Acta. 2007;1775:163–180. doi: 10.1016/j.bbcan.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Hiramoto K, Negishi M, Katoh H. Dock4 is regulated by RhoG and promotes Rac-dependent cell migration. Exp Cell Res. 2006;312:4205–4216. doi: 10.1016/j.yexcr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Hiramoto-Yamaki N, Takeuchi S, Ueda S, Harada K, Fujimoto S, Negishi M, Katoh H. Ephexin4 and EphA2 mediate cell migration through a RhoG-dependent mechanism. J Cell Biol. 2010;190:461–477. doi: 10.1083/jcb.201005141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho E, Irvine T, Vilk GJ, Lajoie G, Ravichandran KS, D'souza SJ, Dagnino L. Integrin-linked kinase interactions with ELMO2 modulate cell polarity. Mol Biol Cell. 2009;20:3033–3043. doi: 10.1091/mbc.E09-01-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova IA, Vespa A, Dagnino L. A novel mechanism of E2F1 regulation via nucleocytoplasmic shuttling: determinants of nuclear import and export. Cell Cycle. 2007;6:2186–2195. doi: 10.4161/cc.6.17.4650. [DOI] [PubMed] [Google Scholar]
- Katoh H, Hiramoto K, Negishi M. Activation of Rac1 by RhoG regulates cell migration. J Cell Sci. 2005;119:56–65. doi: 10.1242/jcs.02720. [DOI] [PubMed] [Google Scholar]
- Katoh H, Negishi M. RhoG activates Rac1 by direct interaction with the Dock180-binding protein ELMO. Nature. 2003;424:461–464. doi: 10.1038/nature01817. [DOI] [PubMed] [Google Scholar]
- King SJ, Worth DC, Scales TME, Monypenny J, Jones JE, Parsons M. Beta-1 integrins regulate fibroblast chemotaxis through control of N_WASP stability. EMBO J. 2011;30:1705–1718. doi: 10.1038/emboj.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fan J, Chen M, Li W, Woodley DT. Transforming growth factor-alpha: a major human serum factor that promotes human keratinocyte migration. J Invest Dermatol. 2006;126:2096–2105. doi: 10.1038/sj.jid.5700350. [DOI] [PubMed] [Google Scholar]
- Liu E, et al. Targeted deletion of integrin-linked kinase reveals a role in T-cell chemotaxis and survival. Mol Cell Biol. 2005;25:11145–11155. doi: 10.1128/MCB.25.24.11145-11155.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz K, Grashoff C, Torka R, Sakai T, Langbein L, Bloch W, Aumalley M, Fassler R. Integrin-linked kinase is required for epidermal and hair follicle morphogenesis. J Cell Biol. 2007;177:501–513. doi: 10.1083/jcb.200608125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Morello V, Cabodi S, Sigismund S, Camacho-Leal MP, Repetto D, Volante M, Papotti M, Turco E, Defilippi P. beta1 integrin controls EGFR signaling and tumorigenic properties of lung cancer cells. Oncogene. 2011;30:4087–4096. doi: 10.1038/onc.2011.107. [DOI] [PubMed] [Google Scholar]
- Nakrieko KA, Welch I, Dupuis H, Bryce DM, Pajak A, St-Arnaud RS, Dedhar S, D'souza SJA, Dagnino L. Impaired hair follicle morphogenesis and polarized keratinocyte movement upon conditional inactivation opf integrin-linked kinase in the epidermis. Mol Biol Cell. 2008;19:1462–1473. doi: 10.1091/mbc.E07-06-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parri M, Chiarugi P. Rac and Rho GTPases in cancer cell motility control. Cell Commun Signal. 2010;8:23. doi: 10.1186/1478-811X-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Horwitz AR, Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol. 2010;11:633–643. doi: 10.1038/nrm2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore S, Mascia F, Mariani V, Girolomoni G. The epidermal growth factor receptor system in skin repair and inflammation. J Invest Dermatol. 2008;128:1365–1374. doi: 10.1038/sj.jid.5701184. [DOI] [PubMed] [Google Scholar]
- Raghavan S, Bauer C, Mundschan G, Li Q, Fuchs E. Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Bio. 2000;150:1149–1160. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan S, Vaezi A, Fuchs E. A role for αβ1 integrins in focal adhesion function and polarized cytoskeletal dynamics. Dev Cell. 2003;5:415–427. doi: 10.1016/s1534-5807(03)00261-2. [DOI] [PubMed] [Google Scholar]
- Russell AJ, Fincher EF, Millman L, Smith R, Vela V, Waterman EA, Dey CN, Guide S, Weaver VM, Marinkivich MP. a6b4 integrin regulates keratinocyte chemotaxis through differential GTPase activation and antagonism of a3b1 integrin. J Cell Sci. 2003;116:3543–3556. doi: 10.1242/jcs.00663. [DOI] [PubMed] [Google Scholar]
- Samson T, Welch C, Monaghan-Benson E, Hahn KM, Burridge K. Endogenous RhoG is rapidly activated after epidermal growth factor stimulation through multiple guanine-nucleotide exchange factors. Mol Biol Cell. 2010;21:1629–1642. doi: 10.1091/mbc.E09-09-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santy LC, Ravichandran KS, Casanova JE. The DOCK180/ELMO complex couples ARNO-mediated Arf6 activation to the downstream activation of Rac1. Curr Biol. 2005;15:1749–1754. doi: 10.1016/j.cub.2005.08.052. [DOI] [PubMed] [Google Scholar]
- Terpstra L, Prud'homme J, Arabian A, Takeda S, Karsenty G, Dedhar S, St-Arnaud R. Reduced chondrocyte proliferation and chondrodysplasia in mice lacking the integrin-linked kinase in chondrocytes. J Cell Biol. 2003;162:139–148. doi: 10.1083/jcb.200302066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda S, Fujimoto S, Hiramoto K, Negishi M, Katoh H. Dock4 regulates dendritic development in hippocampal neurons. J Neurosci Res. 2008;86:3052–3061. doi: 10.1002/jnr.21763. [DOI] [PubMed] [Google Scholar]
- Welf ES, Haugh JM. Signaling pathways that control cell migration: models and analysis. Wiley Interdiscip Rev Syst Biol Med. 2011;3:231–240. doi: 10.1002/wsbm.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom SA, et al. Integrin-linked kinase controls microtubule dynamics required for plasma membrane targeting of caveolae. Dev Cell. 2010a;19:574–588. doi: 10.1016/j.devcel.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom SA, Lange A, Montanez E, Fassler R. The ILK/PINCH/parvin complex: the kinase is dead, long live the pseudokinase! EMBO J. 2010b;29:281–291. doi: 10.1038/emboj.2009.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.