Abstract
Objectives
We examined reasons for and barriers to participating in HIV voluntary counseling and testing for Asian/Pacific Islander (A/PI) men who have sex with men (MSM) in the U.S.
Methods
We collected data between June 2007 and September 2009 in a study known as Men of Asia Testing for HIV, using a cross-sectional community-based participatory design. This national study was conducted in seven U.S. metropolitan cities through a coalition of seven community-based organizations.
Results
Participants included 445 self-identified A/PI MSM aged ≥18 years. Perception of being at risk was the number one reason for testing behaviors. For first-time testers, structural barriers (e.g., language barriers with health professionals) and fear of disclosure (e.g., sexual orientation not known to parents) were deterrents for nontesting in the past. Among previously known HIV-positive men, 22% were not seeing a doctor and 19% were not taking any HIV medications.
Conclusions
HIV testing, care, and treatment policies would be less than optimal without addressing barriers to testing, including stigma related to sexual orientation, among A/PI MSM.
Men who have sex with men (MSM) continue to have the highest human immunodeficiency virus (HIV) infection rates in the U.S., with MSM of color being disproportionately affected by the disease.1 Compared with MSM of other racial/ethnic groups, Asian/Pacific Islander (A/PI) MSM have the highest proportion of acquired immunodeficiency syndrome (AIDS) cases, with a majority of them being non-U.S.-born.2,3 Research attributes this disparity to low testing rates4–6 and late diagnosis, which are often associated with advanced AIDS symptoms7 as well as late entry into care.8,9
Voluntary counseling and testing (VCT) has been a successful standard of HIV prevention since the emergence of the epidemic in the U.S. and other developed countries.10–14 This prevention strategy has also been adopted by other developing countries.15 Nonetheless, the uptake of VCT is still less then optimal.16 For example, combining multiple datasets from the 2000 to 2005 National Health Interview Surveys, Ostermann et al. found little change in lifetime and past year testing, respectively. However, individuals who perceived higher risks of contracting HIV were more likely to plan for and engage in actual HIV testing.17
Meanwhile, the literature consistently indicates that the stigma of having HIV and/or being a sexual minority is a major barrier or determinant for efficacious or effective prevention, intervention, and treatment, especially among marginalized, vulnerable, or underserved populations.18 Using a qualitative methodology (i.e., in-depth interview), Yoshioka and Schustack found three major barriers to disclosure of HIV status among a sample of HIV-positive men of Asian descent in the U.S.: (1) protection of family from shame, (2) protection of family from obligation to help, and (3) avoidance of communication regarding highly personal information (including same-sex sexual orientation).19
Collectively, these articles in the literature lend to the postulation that the effectiveness of VCT hinges on at-risk individuals having well-informed information regarding the attitudes, beliefs, and practices of HIV testing.20 Although a small number of cross-sectional studies have been conducted,6,7,9,21 little to no national data are available addressing HIV testing and HIV management among A/PI MSM. To that end, we used data from a national study of HIV prevention to describe and examine (1) reasons for and barriers to HIV testing among a national sample of A/PI MSM, and (2) care and treatment among those who have tested HIV-positive in the past, by nativity (U.S.-born vs. non-U.S.-born).
METHODS
Participant recruitment and enrollment
We used data collected between June 2007 and August 2009 from a national study that used a community-based participatory research design known as Men of Asia Testing for HIV (MATH). Eligible participants included men aged ≥18 years who self-identified as A/PI ethnicity; had sex with another man in the last 12 months; had resided in the targeted city in the last six months; were able to provide verbal and written informed consent in English, Chinese, or Vietnamese; and were willing to participate in HIV screening and confirmatory testing. Men were recruited from seven community-based organizations (CBOs) in seven metropolitan cities (Boston, Los Angeles, New York City, Oakland, Philadelphia, San Francisco, and San Jose). Two of the CBOs (in Oakland and San Jose) are primary community health-care centers serving A/PIs in their catchment areas; HIV prevention is a recent (since 2004) small addition (e.g., less than two full-time HIV prevention staff per CBO) to their services. The CBO in San Francisco is the largest AIDS service organization (ASO) targeting A/PIs in the U.S., although in the last 10 years it has expanded to include other sexual health-related services targeting this population. Similar to the one in San Francisco, the rest of the CBOs (in Boston, Los Angeles, New York City, and Philadelphia) started out as ASOs, but have expanded their services to include a variety of programs, such as language assistance and transgender programs.
Broad-based recruitment strategies were used to refer participants into the study, including (1) standard outreach, such as distributing study flyers and postcards in gay bars, venues, and public sex areas; (2) announcements via mainstream (e.g., gay pride) and ethnic-specific gay events; and (3) announcements via the Internet. In short, participants in this study (n=445)constituted a purposive (i.e., deliberate and nonrandom) convenience sample of people who met the inclusion criteria. After being referred, participants scheduled a time to come to their study site. When participants arrived at their study site, they were guided through an informed consent process before all data were collected in a private office dedicated to the project.
HIV screening and confirmatory testing
A certified HIV prevention counselor initially screened all participants for HIV using OraQuick® (OraSure Technologies, Inc., Bethlehem, Pennsylvania). Those who screened HIV-negative were given additional HIV prevention messages. Those who initially screened -HIV-positive were offered a confirmatory test. For those with positive confirmatory Western blots, appropriate referrals to treatment and care were made for their HIV diagnosis.
Psychosocial and behavioral assessment: variables
While waiting for the HIV screening result, the participant completed a self-administered psychosocial and behavioral assessment (in English, Chinese, or Vietnamese), which took about 30–45 minutes to complete. The assessment contained four major sections: (1) demographic characteristics of the participants (e.g., age, ethnicity, place of birth, sexual orientation, and medical insurance); (2) psychosocial variables (e.g., degree of outness about same-sex sexual orientation to families, friends, and/or coworkers); (3) sexual health (e.g., sexual communications); and (4) sexual behaviors and HIV-related practices (e.g., unprotected anal sex, HIV testing, and HIV management).
For this study, in addition to demographic characteristics, we were interested in three major categories of variables: HIV testing behaviors, reasons for and barriers to HIV testing, and HIV management behaviors. For the HIV testing behaviors variable, participants were asked to self-report if they had ever been tested for HIV. Regardless of self-reported status, all participants underwent HIV screening and, if appropriate, confirmatory testing as described. For the reasons for and barriers to HIV testing variable, participants were asked to check off a list of reasons for HIV testing (e.g., “I started having sex with a new partner”) and barriers HIV testing (e.g., “I am afraid that the result might be positive”). For the HIV management variable, individuals who self-reported being HIV-positive with confirmed HIV-positive status in the present study were asked about how they managed their HIV condition. Specifically, care was measured by asking if the individual was being seen by a medical doctor for his HIV condition. Treatment was measured by asking if the individual was presently on any type of HIV medication (e.g., highly active antiretroviral therapy). In addition, all were asked if they had ever been diagnosed with AIDS.
Analysis
Data were stratified by nativity (i.e., non-U.S.-born vs. U.S.-born) given that more than two-thirds of A/PIs in the U.S. are non-U.S.-born.22 Descriptive statistics were used to summarize the sample, with appropriate two-tailed significance tests; p<0.05 was considered statistically significant. For the first study aim, reasons for and barriers to HIV testing were first grouped into meaningful thematic categories (e.g., disclosure) and then ranked by degree of endorsement, where 1 = most important and 5 = least important. This process was independently performed by three research staff for consensus. Significance tests were not performed on these groupings and rankings. For the second study aim, results of types of HIV management (e.g., being seen by a doctor or taking HIV medications) were presented. We used Fisher's exact test and the Student's t-test, respectively, for categorical variables and continuous variables comparing non-U.S.-born and U.S.-born individuals.
RESULTS
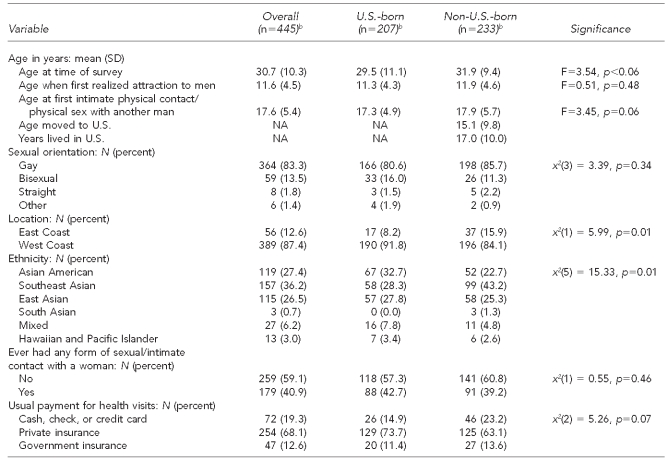
Demographic characteristics of the sample are summarized in Table 1. A roughly equal number of non-U.S.-born (52.3%) and U.S.-born (46.5%) participants enrolled in the study. Given that more than 66% of self-identified A/PIs in the U.S. are non-U.S.-born, our sample was close to representing the distribution of this population.22 Non-U.S.-born participants were significantly (p=0.01) more likely to be recruited from the East Coast (15.9%) than U.S.-born participants (8.2%). Those who self-identified as Southeast Asian were significantly more likely to be non-U.S.-born than U.S.-born (43.2% vs. 28.3%), while those who self-identified as Asian American were significantly more likely to be U.S.-born rather than non-U.S.-born (32.7% vs. 22.7%, p=0.01). These findings are consistent with the national demographic profiles of A/PIs.22
Table 1.
Demographic characteristics of Asian/Pacific Islander MSM aged ≥18 years in seven metropolitan citiesa in a study of HIV testing and management behaviors, June 2007–August 2009
aThe seven metropolitan cities included Boston, Los Angeles, New York City, Oakland, Philadelphia, San Francisco, and San Jose.
bN varies based on missing responses. Percentages many not add to 100 due to rounding.
MSM = men who have sex with men
HIV = human immunodeficiency virus
SD = standard deviation
NA = not applicable
Three demographic characteristics—age, age at first intimate physical contact with a man, and medical insurance—trended toward statistical significance. Non-U.S.-born A/PI MSM (mean = 31.9 years, p<0.06) were slightly older than U.S.-born participants (mean = 29.5 years, p<0.06). Non-U.S.-born A/PI MSM (mean = 17.9 years, p=0.06) were slightly older than U.S.-born A/PI MSM (mean = 17.3 years, p=0.06) when they had their first intimate physical contact with another man. And non-U.S.-born A/PI MSM (23.2%, p=0.07) were slightly more likely than U.S.-born A/PI MSM (14.9%, p=0.07) to pay for their health care out of pocket.
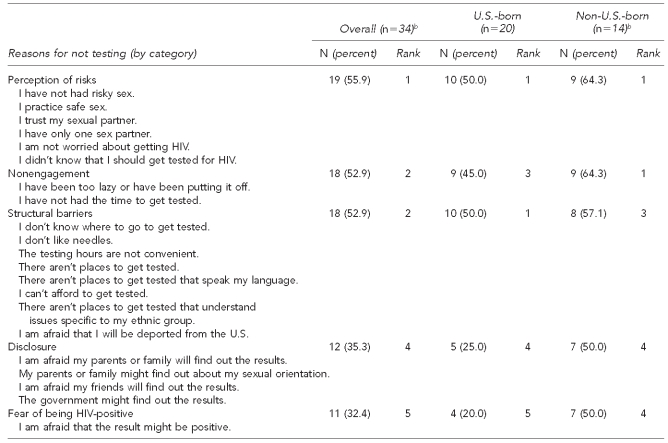
As shown in Tables 2a and 2b, a total of 35 MSM (7.9%; 14 non-U.S.-born, 20 U.S.-born, and one missing) self-reported never having been tested for HIV, and 410 MSM (92.1%; 219 non-U.S.-born, 187 U.S.-born, and four missing) reported ever having been tested for HIV in the past. None of the 35 first-time-testing participants screened HIV-positive. Of the 410 men who had been tested for HIV in the past, 335 men (81.7%; 171 non-U.S.-born, 161 U.S.-born, and three missing) self-reported being HIV-negative, 39 men (9.5%; 25 non-U.S.-born and 14 U.S.-born) self-reported being HIV-positive, and 36 men (8.8%; 23 non-U.S.-born, 12 U.S.-born, and one missing) were of unknown status at the time of the study. Of the 36 men whose status was unknown, four were screened for HIV and were subsequently confirmed HIV-positive (11.1%). Of the 39 self-reported HIV-positive men, three (7.7%; one non-U.S.-born and two U.S.-born) screened HIV-negative; unexpected, similar patterns have been observed in previous research.23 Men who self-reported being HIV-negative all screened negative. These results yielded a crude HIV prevalence of 5.6% for the total sample (data not shown).
Table 2a.
Reasons for not testing for HIV among a study of Asian/Pacific Islander MSM aged ≥18 years in seven metropolitan cities,a June 2007–August 2009
aThe seven metropolitan cities included Boston, Los Angeles, New York City, Oakland, Philadelphia, San Francisco, and San Jose.
bThe number varies based on missing responses.
HIV = human immunodeficiency virus
MSM = men who have sex with men
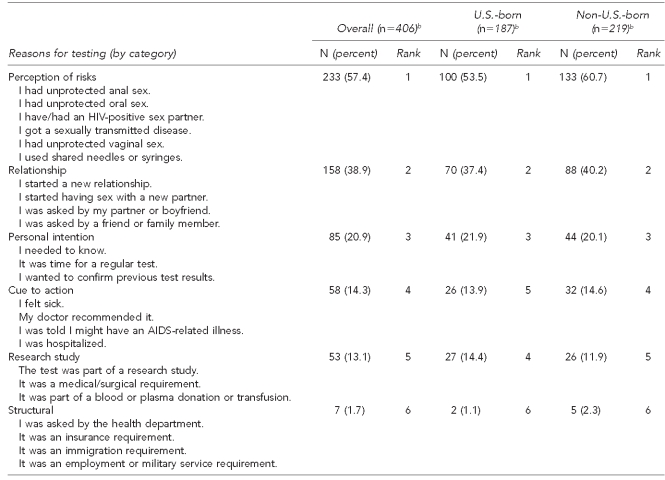
Table 2b.
Reasons for testing for HIV among a study of Asian/Pacific Islander MSM aged ≥18 years in seven metropolitan cities,a June 2007–August 2009
aThe seven metropolitan cities included Boston, Los Angeles, New York City, Oakland, Philadelphia, San Francisco, and San Jose.
bThe number varies based on missing responses.
HIV = human immunodeficiency virus
MSM = men who have sex with men
AIDS = acquired immunodeficiency syndrome
For the first study aim, participants were asked to check off a list of reasons (all that apply) regarding their motivations for and barriers to HIV testing (Tables 2a and 2b). Both types of participants—naïve testers (those testing for the first time in this study) and non-naïve testers (those who had tested before)—ranked perception of risks as the number one reason for their testing behaviors. That is, naïve testers cited low-to-no risks (e.g., “I am not worried about getting HIV”) for their prior nontesting behavior, whereas non-naïve testers cited having high-risk behaviors (“I had unprotected anal sex”) for their testing behavior.
Other reasons cited by naïve testers included laziness (e.g., “I have been too lazy or have been putting it off”), structural barriers (e.g., “I don't know where to go to get tested” or “I can't afford to get tested”), fear of disclosure (e.g., “I am afraid my parents or family will find out the results”), and fear of being HIV-positive. Among MSM who had previously been tested, other reasons for testing behavior included relationship involvement, personal intention, cues to action (e.g., doctor recommendation), research study involvement, or employment requirements. Though cues to action, such as a doctor recommendation, ranked as the fourth reason overall for HIV testing, this reason was consistent with past research; A/PIs in the U.S. tend to first find out their HIV/AIDS status when they are sick, and testing—often for the first time ever—is conducted per their doctor's request.8
With respect to the second study aim, Table 3 presents characteristics of MSM who self-reported being HIV-positive with confirmed HIV-positive status in the present study. The average CD-4 counts of the total sample was 488 (range: 89–984), with CD-4 counts averaging 480 for non-U.S.-born and 506 for U.S.-born MSM. Twenty-two percent of HIV-positive men (six non-U.S.-born and two U.S.-born) were not seeing a medical doctor for their HIV condition. The average CD-4 count of this group was 336 (data not shown), all falling under the latest International AIDS Society (IAS) guidelines for treatment (i.e., they should have medical consultation). Even more alarming, 19% of the HIV-positive men (six non-U.S.-born and one U.S.-born MSM) were not taking any medications for their HIV condition. The average CD-4 count of this subgroup was 468 (range: 200–850; data not shown); all but one met the latest IAS guideline for treatment (i.e., asymptomatic with CD4 counts <500 or symptomatic).24 Likely due to the small sample size, none of the comparisons between non-U.S.-born and U.S.-born individuals were statistically significant.
Table 3.
Care and treatment characteristics of HIV-positive Asian/Pacific Islander MSM in seven metropolitan cities,a June 2007–August 2009
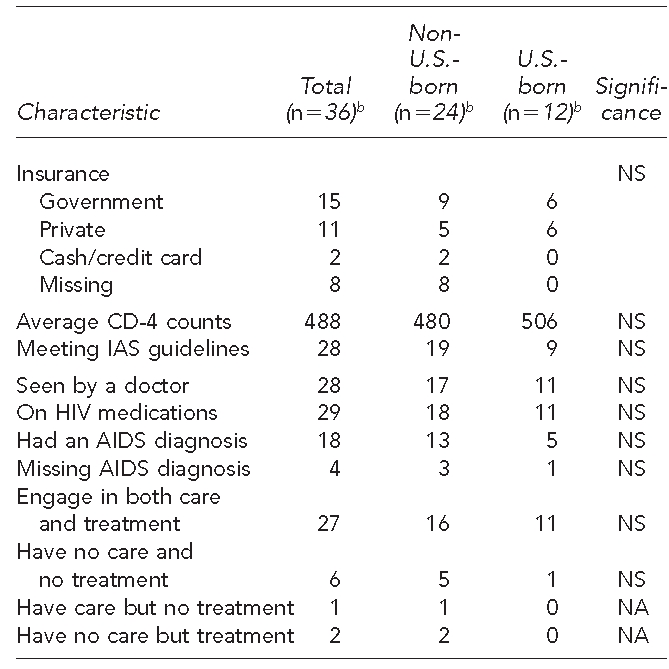
aThe seven metropolitan cities included Boston, Los Angeles, New York City, Oakland, Philadelphia, San Francisco, and San Jose.
bThe number varies based on missing responses.
HIV = human immunodeficiency virus
MSM = men who have sex with men
NS = not significant
IAS = International AIDS Society
AIDS = acquired immunodeficiency syndrome
NA = not applicable
DISCUSSION
In this study, we examined (1) reasons for and barriers to HIV testing among a national sample of A/PI MSM and (2) care and treatment among those who tested HIV-positive. Although we used a purposive convenience sample, the diversity of the study sites, including cities with the largest A/PI populations, gives credence to these findings. Several demographic characteristics differentiated non-U.S.-born from U.S.-born A/PI MSM, including residence and geographic region of origin, as well as, to some extent, their age at first intimate sexual contact with another man and health-care access. However, our sample size did not allow us to further explore differential effects on HIV-related issues due to nativity.
Combining those who self-reported ever having been tested for HIV but who did not know their HIV status (n=36) and those who had never been tested for HIV (n=35) resulted in a finding of almost 16% of participants not knowing their status, which was slightly better than the overall national estimate of 21%, but much better than the A/PI estimate of 30%.25 It should be noted that our sample was recruited from CBOs that were familiar with or had extensive experience with HIV prevention targeting A/PIs; the estimate of unknown HIV status (16%) may be an underestimate of the general A/PI MSM population as a whole.
Research by Ross et al. provides a possible explanation for the somewhat unexpected finding of the few who self-reported being HIV-positive but screened HIV-negative. They interviewed one of their subjects who self-reported being positive but tested negative and, according to the authors, “This individual indicated he had understood a ‘positive’ result to mean a good result; that is, no evidence of HIV infection, in the sense of colloquial English rather than serology, suggests that this misunderstanding may be the source of the significant inaccuracy in self-report.”23 Unlike Ross et al., we did not conduct qualitative interviews with our participants; however, given that more than half of the sample was non-U.S.-born, the possibility that language may have played a role in the participants' misunderstanding of the survey is not too farfetched.
Perhaps the most important findings pertained to (1) reasons/barriers and (2) care/treatment. Perception of risks (e.g., risk for contracting HIV) was the major reason for engagement or nonengagement of HIV testing, a finding that is consistent with the literature.17 This finding underscores the importance of reinforcing the message of safer practices, including regular HIV testing.
Structural barriers (e.g., “I don't know where to get tested”) were also found to be major obstacles for naïve testers. Consistent with research by Do et al.,21 knowledge of testing sites was associated with HIV testing behavior. Given that we are in the third decade of the HIV epidemic, the fact that a portion of naïve testers still cited lack of knowledge of how to access HIV testing is a cause for alarm. Compounding this finding, some naïve testers cited not being able to afford to get tested as a structural barrier, indicating that there is perhaps a misconception about costs among some participants, as many HIV testing sites offer free or low-cost testing.26
Individuals who had never been tested also cited fear of disclosure as a deterrent to HIV testing. It seems that letting families and friends know about their HIV status and/or sexual orientation may be reasons for participants' hesitancy to test. Meanwhile, for those non-U.S.-born MSM who might be undocumented (we did not inquire about citizenship in the study), potential government or job surveillance might be a deterrent for taking an HIV test via a standard channel (e.g., a public health clinic). Perhaps our research study based in the A/PI community may have been perceived as a safe environment for these non-U.S.-born individuals to find out their HIV status. Obermeyer and Osborn argued that “… conditions under which testing is administered document the effect of provider-client interactions on the utilization of testing. Clients' responses are influenced by providers' background characteristics (such as gender or ethnic group), attitudes, and perseverance and the extent to which they are trusted by their clients.”20
Findings on engagement in care and treatment were more complex. Due to the small sample size, none of the statistical comparisons by nativity were significant. Nonetheless, the fact that a sizable proportion of HIV-positive individuals was not being seen by a doctor (22%), not on HIV medication (19%), or both (17%) is a major public health concern. Collectively, these statistics suggest that knowing one's HIV status (via VCT) is only a beginning, and that other psychosocial determinants need to be examined to promote early care and treatment, as well as compliancy.
CONCLUSIONS
These findings are timely, as prominent international public health entities such as the IAS, Centers for -Disease Control and Prevention, and National Institutes of Health all argue for the efficacy or effectiveness of the “Seek, Test, and Treat” (also known as Seek and Treat) paradigm, based on “mathematical modeling approaches”27 as a form of HIV prevention. This study further contributes to a much needed, but sorely -lacking, debate with empirical evidence to reassess this broad-based HIV prevention initiative with a critical lens.18
Four major public health implications pertain to HIV VCT from these collective findings. First, the fact that three individuals self-reported being HIV-positive but tested negative underscores the importance of knowing one's status via an objective measure (i.e., testing). Second, risk perception (i.e., lack of it among those who had never been tested or having risks among those who had ever been tested) continues to speak to the importance of the necessity of basic HIV testing education. Third, removing structural barriers (e.g., having more convenient hours) should be a priority for reaching out to vulnerable or underserved populations, including MSM of Asian descent. Fourth, for those who have never been tested for HIV, fear of disclosure (and, by extension, stigma) continues to be an obstacle for testing, a finding that still resonates from research conducted more than a decade ago.16
We argue that the public health benefit of a mechanical or biological implementation of the paradigm of VCT would be less than optimal without the inclusion of appropriate sociocultural components (e.g., reducing stigma and improving health-seeking among non-U.S.-born individuals), especially among racial/ethnic minority groups and/or marginalized populations.
Footnotes
Preparation of this article was supported in part by grants from the National Institutes of Health (R01HD046354—Frank Wong) and the Emory Center for AIDS Research (P30 AI050409—Eric Nehl and Frank Wong). Georgetown University's Institutional Review Board (#2004-130) approved this study.
The Men of Asia Testing for HIV (MATH) Study Consortium consists of the following organizations: AIDS Services in Asian Communities, Philadelphia, Pennsylvania; Asian Americans for Community Involvement, San Jose, California; Asian Health Services, Oakland, California; the Asian & Pacific Islander Coalition on HIV/AIDS, New York, New York; the Asian Pacific AIDS Intervention Team, Los Angeles, California; the Asian and Pacific Islander American Health Forum, San Francisco, California; the Asian & Pacific Islander Wellness Center, San Francisco; Massachusetts Asians and Pacific Islanders for Health, Boston, Massachusetts; Georgetown University, Washington, D.C.; George Washington University, Washington, D.C.; and Emory University, Atlanta, Georgia.
REFERENCES
- 1.Centers for Disease Control and Prevention (US) HIV among gay, bisexual and other men who have sex with men (MSM) 2010. Sep, [cited 2011 May 11]. Available from: URL: http://www.cdc.gov/hiv/topics/msm/index.htm.
- 2.Centers for Disease Control and Prevention (US) HIV/AIDS among Asians and Pacific Islanders. 2008. Aug, [cited 2010 Apr 9]. Available from: URL: http://www.cdc.gov/hiv/resources/factsheets/PDF/API.pdf.
- 3.Raymond HF, Chen S, Truong HM, Knapper KB, Klausner JD, Choi KH, et al. Trends in sexually transmitted diseases, sexual risk behavior, and HIV infection among Asian/Pacific Islander men who have sex with men, San Francisco, 1999-2005. Sex Transm Dis. 2007;34:262–4. doi: 10.1097/01.olq.0000237854.25406.ad. [DOI] [PubMed] [Google Scholar]
- 4.MacKellar DA, Valleroy LA, Secura GM, Bartholow BN, McFarland W, Shehan D, et al. Repeat HIV testing, risk behaviors, and HIV seroconversion among young men who have sex with men: a call to monitor and improve the practice of prevention. J Acquir Immune Defic Syndr. 2002;29:76–85. doi: 10.1097/00042560-200201010-00011. [DOI] [PubMed] [Google Scholar]
- 5.Do TD, Chen S, McFarland W, Secura GM, Behel SK, McHellar DA, et al. HIV testing patterns and unrecognized HIV infection among young Asian and Pacific Islander men who have sex with men in San Francisco. AIDS Educ Prev. 2005;17:540–54. doi: 10.1521/aeap.2005.17.6.540. [DOI] [PubMed] [Google Scholar]
- 6.Prevalence and awareness of HIV infection among men who have sex with men—21 cities, United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;59(37):1201–7. [PubMed] [Google Scholar]
- 7.Adih WK, Campsmith M, Williams CL, Hardnett FP, Hughes D. Epidemiology of HIV among Asians and Pacific Islanders in the United States, 2001-2008. J Int Assoc Physicians AIDS Care (Chic) 2011;10:150–9. doi: 10.1177/1545109711399805. [DOI] [PubMed] [Google Scholar]
- 8.Eckholdt HM, Chin J. Pneumocystis carinii pneumonia in Asians and Pacific Islanders. Clin Infect Dis. 1997;24:1265–7. doi: 10.1093/clinids/24.6.1265. [DOI] [PubMed] [Google Scholar]
- 9.Eckholdt HM, Chin JJ, Manzon-Santos JA, Kim DD. The needs of Asians and Pacific Islanders living with HIV in New York City. AIDS Educ Prev. 1997;9:493–504. [PubMed] [Google Scholar]
- 10.Denison JA, O'Reilly KR, Schmid GP, Kennedy CE, Sweat MD. HIV voluntary counseling and testing and behavioral risk reduction in developing countries: a meta-analysis, 1990-2005. AIDS Behav. 2008;12:363–73. doi: 10.1007/s10461-007-9349-x. [DOI] [PubMed] [Google Scholar]
- 11.Rotheram-Borus MJ, Swendeman D, Chovnick G. The past, present, and future of HIV prevention: integrating behavioral, biomedical, and structural intervention strategies for the next generation of HIV prevention. Annu Rev Clin Psychol. 2009;5:143–67. doi: 10.1146/annurev.clinpsy.032408.153530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Z, Sullivan SG, Wang Y, Rotheram-Borus MJ, Detels R. Evolution of China's response to HIV/AIDS. Lancet. 2007;369:679–90. doi: 10.1016/S0140-6736(07)60315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS. 2006;20:1447–50. doi: 10.1097/01.aids.0000233579.79714.8d. [DOI] [PubMed] [Google Scholar]
- 14.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39:446–53. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 15.Menzies N, Abang B, Wanyenze R, Nuwaha F, Mugisha B, Coutinho A, et al. The cost and effectiveness of four HIV counseling and testing strategies in Uganda. AIDS. 2009;23:395–401. doi: 10.1097/QAD.0b013e328321e40b. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector: progress report September 2009. Geneva: WHO; 2009. [Google Scholar]
- 17.Ostermann J, Kumar V, Pence BW, Whetten K. Trends in HIV testing and differences between planned and actual testing in the United States, 2000-2005. Arch Intern Med. 2007;167:2128–35. doi: 10.1001/archinte.167.19.2128. [DOI] [PubMed] [Google Scholar]
- 18.Mahajan AP, Sayles JN, Patel VA, Remien RH, Sawires SR, Ortiz DJ, et al. Stigma in the HIV/AIDS epidemic: a review of the literature and recommendations for the way forward. AIDS. 2008;22(Suppl 2):S67–79. doi: 10.1097/01.aids.0000327438.13291.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshioka MR, Schustack A. Disclosure of HIV status: cultural issues of Asian patients. AIDS Patient Care STDs. 2001;15:77–82. doi: 10.1089/108729101300003672. [DOI] [PubMed] [Google Scholar]
- 20.Obermeyer CM, Osborn M. The utilization of testing counseling for HIV: a review of the social and behavioral evidence. Am J Public Health. 2007;97:1762–74. doi: 10.2105/AJPH.2006.096263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Do TD, Hudes ES, Proctor K, Han CS, Choi KH. HIV testing trends and correlates among young Asian and Pacific Islander men who have sex with men in two U.S. cities. AIDS Educ Prev. 2006;18:44–55. doi: 10.1521/aeap.2006.18.1.44. [DOI] [PubMed] [Google Scholar]
- 22.Pew Hispanic Center. The American community—Asians: 2004. Washington: Department of Commerce, Census Bureau (US); 2007. Feb, [cited 2010 Apr 9]. Also available from: URL: http://www.census.gov/prod/2007pubs/acs-05.pdf. [Google Scholar]
- 23.Ross MW, Loxley W, Wodak A, Buzolic A, Monheit B, Stowe A. Drug users' self-reported false-positive HIV status. Am J Public Health. 1993;83:1349–50. doi: 10.2105/ajph.83.9.1349-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson MA, Aberg JA, Cahn P, Montaner JS, Rizzardini G, Telenti A, et al. Antiretroviral treatment of adults with HIV infection: 2010 recommendations of the International AIDS Society—USA Panel. JAMA. 2010;304:321–33. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 25.Campsmith ML, Rhodes PH, Hall HI, Green TA. Undiagnosed HIV prevalence among adults and adolescents in the United States at the end of 2006. J Acquir Immune Defic Syndr. 2010;53:619–24. doi: 10.1097/QAI.0b013e3181bf1c45. [DOI] [PubMed] [Google Scholar]
- 26.Schwarcz S, Richards TA, Frank H, Wenzel C, Chin HL, Chin CS, et al. Identifying barriers to HIV testing: personal and contextual factors associated with late HIV testing. AIDS Care. 2011;23:892–900. doi: 10.1080/09540121.2010.534436. [DOI] [PubMed] [Google Scholar]
- 27.Montaner JS, Lima VD, Barrios R, Yip B, Wood E, Kerr T, et al. Association of highly antiretroviral therapy coverage, population viral load, and yearly new diagnosis in British Columbia, Canada: a population-based study. Lancet. 2010;376:532–9. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]