Abstract
After clinical resolution of signs and symptoms of mild traumatic brain injury (MTBI) it is still not clear if there are residual abnormalities of structural or functional brain networks. We have previously documented disrupted interhemispheric functional connectivity in “asymptomatic” concussed individuals during the sub-acute phase of injury. Testing of 15 normal volunteers (NV) and 15 subacute MTBI subjects was performed within 24 hours of clinical symptoms resolution and medical clearance for the first stage of aerobic activity. In this MRS study we report (a) both in the genu and splenium of the corpus callosum NAA/Cho and NAA/Cr ratios were significantly (p<0.05) lower in MTBI subjects shortly after the injury compared to NVs, and (b) the metabolic ratio NAA/Cho in the splenium significantly correlated with the magnitude of inter-hippocampal functional connectivity in normal volunteers, but not in MTBI. This novel finding supports our hypothesis that the functional disruption of interhemispheric brain networks in MTBI subjects results from compromised metabolic integrity of the corpus callosum and that this persists despite apparent clinical return to baseline.
Mild traumatic brain injury (MTBI) accounts for 75–90% of the 1.4 million annual incidence of traumatic brain injury (TBI) [3] in the United States. Despite being labeled “mild”, mechanical forces associated with MTBI may produce diffuse axonal injury (DAI) [17] and lead to a multitude of symptoms and neuropsychological dysfunction [29]. Conventional neuroimaging fails to identify structural changes [29]; likewise neuropsychological testing is not sensitive beyond 10 days post-injury [18]. Most cases of MTBI have spontaneous resolution of symptoms within 7–30 days, yet upwards of 38% of MTBI patients report physical, cognitive, and emotional symptoms that persist for months-to-years post-injury [4, 23, 30].
Empirical evidence gathered through postmortem, diffusion tensor imaging (DTI), and functional connectivity magnetic resonance imaging (fcMRI) studies have shown that the corpus callosum is a major predilection site in MTBI [26, 27]. The corpus callosum forms the largest and highest density commissural white matter bundle in the brain and is highly susceptible to the rotational acceleration and decelerations forces that accompany MTBI [22, 26, 27]. The corpus callosum provides a large percentage of functional connections between the right and left hemisphere. Structurally, the degree of hemispheric lateralization is established by the axons of the corpus callosum and functionally it allows for modulatory influence and the exchange of information between hemispheres [14]. Not surprising, damage to the corpus callosum has been shown to significantly alter interhemispheric functional connectivity[16, 19].
Recent work with resting state functional magnetic resonance imaging (rs-fMRI) and DTI has shown promise in expanding our understanding of human functional networks [9] and given evidence that structural and functional connectivity are closely related [11]. Although functional connectivity may reflect structural connectivity, it is not a simple one-to-one mapping and cannot distinguish direct and indirect pathways [9]. Although few studies to date have investigated both structural and functional connectivity of large-scale cognitive networks, it is important to investigate the effects of the initial injury and compensatory responses after MTBI [24]. Therefore multimodal studies combining functional and structural information are valuable in understanding how brain injury disrupts brain networks [24].
In our recent rs-fMRI research, we reported reduced interhemispheric functional connectivity in “asymptomatic” MTBI subjects [25]. Specifically the interhemispheric connectivity of the dorsolateral prefrontal cortex (DLPFC) and hippocampus were significantly (p<0.05) reduced in MTBI. We hypothesized that functional disruption of interhemispheric brain networks in MTBI subjects shortly after the injury may result from compromised structural integrity of the corpus callosum. To test this hypothesis, in this study we examined the metabolic profile of the corpus callosum via magnetic resonance spectroscopy (1H-MRS) in the sub-acute phase of MTBI. 1H-MRS spectra provide information about key neurometabolites: N-acetylaspartate (NAA), total choline (Cho), and total creatine (Cr) and are used as relative measures of neuronal integrity and metabolism, membrane structure, and glial function, respectively (Matthew et al., 2003). Decreases in NAA levels as well as increases in Cho levels are the two most common 1H-MRS findings following traumatic brain injury [21]. NAA levels are markers of neuronal integrity with low levels associated with DAI [1], while Cho levels are attributed to injury and repair of myelin in addition to inflammation [31]. In this study 1H-MRS spectra were taken from two regions of interest (ROIs), the genu and splenium of the corpus callosum. The genu of the corpus callosum connects the orbitofrontal and frontal cortices while the body and splenium connect temporal, parietal, and occipital regions [22]. From prior 1H-MRS studies of MTBI along with our earlier hypothesis we expected to find alterations in the neurometabolite ratios in the corpus callosum that correlate with the strength of interhemispheric functional connectivity. Specifically we predict there will be a decrease in NAA in levels, an increase in Cho levels while Cr levels remain relatively constant.
A strict testing schedule was adhered to in order to compensate for individual differences in severity of MTBI and differential rates of recovery. Specifically, all subjects under study were scanned within 24 hours of clinical symptom resolution, return to baseline on neuropsychological testing (Balance Error Score System and Scat-2), and clearance from a medical professional for the first stage of aerobic activity. On average scanning took place 10.8 days post injury. 15 student-athletes (6 male, 9 female, mean age 20.6 +/− 1.2 years) who had recently suffered from a sports-related grade 1 MTBI (Cantu Data Driven Revised Concussion Grading Guideline, 2006) and 15 neurologically normal volunteer (NV) student-athletes with no history of MTBI (7 male, 8 female, mean age 20.4 +/− 0.8 years) were recruited for this study. The initial diagnosis of MTBI was made on the field by certified athletic trainers (AT) and as a part of the routine protocol of the Sport Concussion Program at the Pennsylvania State University. All subjects signed an informed consent form and the Institutional Review Board of the Pennsylvania State University approved this protocol. MRI scanning did not elicit any clinical symptoms and no radiological findings were observed on the anatomical T1, therefore no subjects were excluded from the study.
1H-MRS and anatomical images were acquired on a 3.0 Tesla Siemens Trio whole-body scanner (Siemens, Erlangen, Germany) using a 12 channel head coil. Three-dimensional isotropic T1 weighted magnetization prepared rapid gradient echo (MP-RAGE) anatomical images were acquired in the axial plane parallel with the anterior and posterior commisure axis covering the entire brain (0.9mm × 0.9mm × 0.9mm resolution, TE= 3.46ms, TR= 2300ms, TI= 900ms, flip angle= 9°, 160 slices, iPAT= none, NSA= 1). Three-dimensional multivoxel 1H-MRS Chemical Shift Imaging (CSI) (120mm × 120mm × 80mm Field of View, 10.0mm × 10.0mm × 12.5mm voxel size, TE=135ms, TR=1510 ms, iPAT= none, NSA= 1) was implemented to evaluate in-vivo NAA, Cho, and Cr metabolite peaks. The CSI volume of interest (VOI) was centered anteriorly/posteriorly and inferiorly/superiorly over the corpus callosum.
The 3D 1H-MRS CSI data were processed off-line using standard scanner software (spectroscopy Card, Siemens) and included zero filling, eddy current correction, water suppression, Fourier transform, baseline and phase correction post processing steps. Spectra and metabolite maps (with peaks for NAA= 2.02 ppm, Cho at 3.22 ppm, and Cr at 3.04 ppm) were automatically calculated for each voxel within the entire VOI. Within the VOI the genu and splenium of the corpus callosum were demarcated into 2 ROIs. Each ROI consisted of 6 voxels that were individually selected to be within the genu and splenium of the corpus callosum based upon anatomical T1 images overlaid with the corresponding acquired 1H-MRS voxels. NAA/Cho, NAA/Cr, and Cho/Cr ratios of these selected voxels were then averaged to come up with a mean value for each ROI [20, 28]. Minitab 16 Statistical Software (Minitab, Inc., State College, PA www.minitab.com) was used to perform statistical analysis. Metabolite ratios between the MTBI group and NV group were compared by one way ANOVA and considered significant if p < 0.05. Similar findings were found in both the genu and splenium of the corpus callosum.
In the genu both NAA/Cho (p=0.001) and NAA/Cr (p=0.022) ratios were significantly lower in the MTBI group compared to the NVs. However, there was no difference between groups in the Cho/Cr ratio for the genu. Similar to Genu, the splenium showed significantly lower NAA/Cho (p=0.04) and NAA/Cr (p=0.01) ratios. Again, Cho/Cr ratio was not significantly different between groups. It should be noted that despite being a reliable tool to accurately assess MTBI [28]; spatial resolution of 1H-MRS is poor and small discrepancies or delineations in metabolic information may be masked or missed [15]. In our analysis we based the results upon metabolite ratios not absolute measurements which may mask alterations if both metabolites in the ratio are equally affected [8].
In our previous study [25] we observed decreased functional interhemispheric connectivity of the dorsolateral prefrontal cortex (DLPFC) and hippocampus. Correlation analysis was performed on the significantly altered metabolic ratios, NAA/Cho and NAA/Cr, to explore if 1H-MRS values were indicative of the strength of functional interhemispheric connectivity. Indeed, the metabolic ratios at genu were correlated with DLPFC functional interhemispheric connectivity and those at splenium were correlated with the hippocampal functional interhemispheric connectivity in normal volunteers, but not in MTBI subjects. Specifically, we observed a significant correlation between the NAA/Cho ratio in the splenium and the magnitude of inter-hippocampal functional connectivity in the NV group (r = 0.639, p = 0.019). In contrast, we did not observed any trends of relationship between metabolic measures (NAA/Cho in splenium) and functional inter-hippocampal connectivity in MTBI group (r=0.125, p=0.685). Also, as can be seen from Fig.3, this lack of correlation may be a result of NAA/Cho ratios below 1.8 in the majority of MTBI subjects. This was accompanied by relatively low values (<0.75) of inter-hippocampal connectivity compared to NV.
Figure 3.
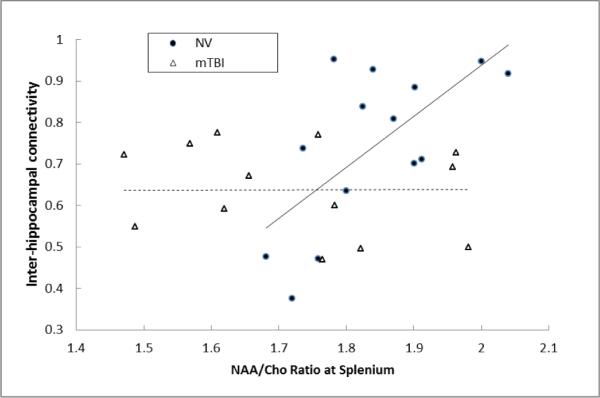
Correlation analysis for each group (NV and mTBI) of functional interhemispheric connectivity in the hippocampus with NAA/Cho ratio in the splenium.
As expected, we observed decreases in NAA levels and increases in Cho levels that resulted in reduced NAA/Cho and NAA/Cr ratios [8]. This finding is in agreement with earlier 1H-MRS studies of MTBI [2, 5, 7, 28]. The novel evidence from this study is that metabolic profiles in the corpus callosum correlate with functional inter-hippocampal connectivity only in NV but not in MTBI. Therefore, multimodal brain imaging approach may be a valuable tool not only to reveal cerebral dysfunctions in acute phase of brain injury [6], but also monitor the recovery from MTBI. It is our current research focus to track evolution of MRS measures in MTBI patients over year post-injury to address an important question of whether these patients ever return to “normal”.
While functional connectivity does not necessarily mean structural connectivity due to indirect pathways and compensatory measures, there is growing evidence that the two are related to a certain degree. In line with this notion, we have here provided additional evidence suggesting that disruptions of structural integrity may compromise the brain functional architecture [13, 19, 24]. Future research is needed to further explore this interaction. Clinically, this multimodal approach may be critical for accurate evaluation of brain injury evolution. Longitudinal and follow-up scanning combining various imaging modalities may address the important question of whether resolution of MTBI symptoms reflects the restoration of baseline brain network integrity or substitution of some compensatory function.
In conclusion, MTBI produces an imbalance in brain metabolites that is not yet restored to pre-injury levels when there has been the initial clinical symptom resolution and a return to baseline on neuropsychological testing. Similar findings have been recently reported in the literature [10, 28]. There is growing evidence through advanced neuroimaging techniques that despite a return to premorbid status (based upon current clinical measures), there are still residual deficits within brain structural and functional networks [12, 18, 25, 32] in the sub-acute phase of MTBI. This should be a major clinical concern when clearing athletes for sport participation, since subtle alteration of brain networks due to prior history of MTBI may put an individual at a higher risk from subsequent brain injuries.
Highlights
-
>
1H-MRS was used to investigate the integrity of the corpus callosum in subactue MTBI
-
>
Reduced ratios for NAA/Cho and NAA/Cr were observed in the MTBI population
-
>
NV had a significant correlation of NAA/Cho ratio with interhippocampal functional connectivity
Figure 1.
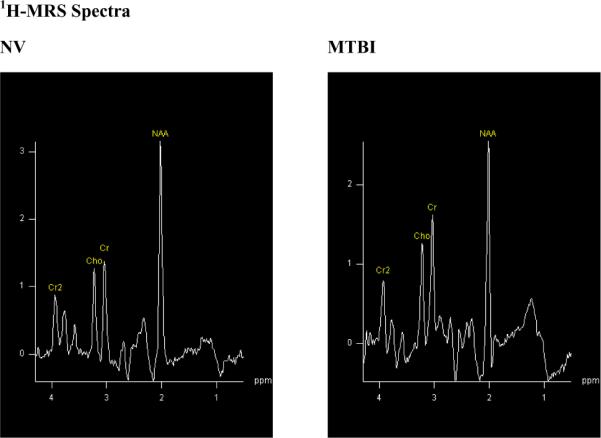
Examples of 1H-MRS spectra acquired from one voxel for MTBI and NV group. Notice the increased Cho and decreased NAA peaks in the MTBI subjects compared to NV.
Figure 2.
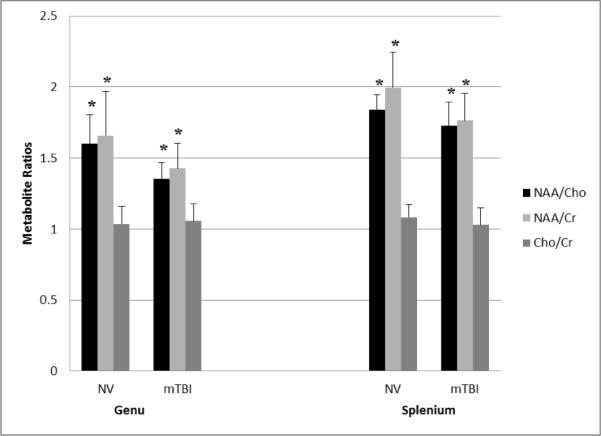
Bar graph of average brain metabolite ratios for each group (NV and MTBI) in each ROI (genu and splenium) * indicates that ratio is significant (p<0.05).
Acknowledgement
This work was supported by National Institutes of Health Grant RO1 NS056227-01A2 “Identification of Athletes at Risk for Traumatic Brain Injury”.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Babikian T, Freier MC, Ashwal S, Riggs ML, Burley T, Holshouser BA. MR spectroscopy: Predicting long-term neuropsychological outcome following pediatric TBI. Journal of Magnetic Resonance Imaging. 2006;24:801–811. doi: 10.1002/jmri.20696. [DOI] [PubMed] [Google Scholar]
- [2].Belanger HG. Recent Neuroimaging Techniques in Mild Traumatic Brain Injury. The journal of neuropsychiatry and clinical neurosciences. 2007;19:5–20. doi: 10.1176/jnp.2007.19.1.5. [DOI] [PubMed] [Google Scholar]
- [3].Bergman K, Bay E. Mild Traumatic Brain Injury/Concussion: A Review for ED Nurses. Journal of Emergency Nursing. 2010;36:221–230. doi: 10.1016/j.jen.2009.07.001. [DOI] [PubMed] [Google Scholar]
- [4].Broglio SP. Neurocognitive performance of concussed athletes when symptom free. Journal of athletic training. 2007;42:504. [PMC free article] [PubMed] [Google Scholar]
- [5].Cecil KM. Proton magnetic resonance spectroscopy for detection of axonal injury in the splenium of the corpus callosum of brain-injured patients. Journal of neurosurgery. 1998;88:795–801. doi: 10.3171/jns.1998.88.5.0795. [DOI] [PubMed] [Google Scholar]
- [6].Gasparovic C, Yeo R, Mannell M, Ling J, Elgie R, Phillips J, Doezema D, Mayer A. Neurometabolite Concentrations in Gray and White Matter in Mild Traumatic Brain Injury: A 1H–Magnetic Resonance Spectroscopy Study. Journal of Neurotrauma. 2009 doi: 10.1089/neu.2009.0896. 110306202455053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Govind V. Whole-Brain Proton MR Spectroscopic Imaging of Mild-to-Moderate Traumatic Brain Injury and Correlation with Neuropsychological Deficits. Journal of Neurotrauma. 2010;27:483–496. doi: 10.1089/neu.2009.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Govindaraju V. Volumetric proton spectroscopic imaging of mild traumatic brain injury. American journal of neuroradiology : AJNR. 2004;25:730. [PMC free article] [PubMed] [Google Scholar]
- [9].Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-State Functional Connectivity Reflects Structural Connectivity in the Default Mode Network. Cerebral Cortex. 2008;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Henry LC, Tremblay J, Tremblay S, Lepore N, Theoret H, Ellemberg D, Lassonde M. Acute and Chronic Changes in Diffusivity Measures after Sports Concussion. Journal of Neurotrauma. 2011 doi: 10.1089/neu.2011.1836. 110824121127008. [DOI] [PubMed] [Google Scholar]
- [11].Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proceedings of the National Academy of Sciences. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Johnson B, Zhang K, Gay M, Horovitz S, Hallett M, Sebastianelli W, Slobounov S. Alteration of brain default network in subacute phase of injury in concussed individuals: Resting-state fMRI study. NeuroImage. 2011 doi: 10.1016/j.neuroimage.2011.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Johnston JM. Loss of Resting Interhemispheric Functional Connectivity after Complete Section of the Corpus Callosum. The Journal of neuroscience. 2008;28:6453–6458. doi: 10.1523/JNEUROSCI.0573-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kompus K, Kalpouzos G, Westerhausen R. The size of the anterior corpus callosum correlates with the strength of hemispheric encoding-retrieval asymmetry in the ventrolateral prefrontal cortex. Brain Research. 2011 doi: 10.1016/j.brainres.2011.08.052. [DOI] [PubMed] [Google Scholar]
- [15].Marino S, Ciurleo R, Bramanti P, Federico A, Stefano N. 1H-MR Spectroscopy in Traumatic Brain Injury. Neurocritical Care. 2010;14:127–133. doi: 10.1007/s12028-010-9406-6. [DOI] [PubMed] [Google Scholar]
- [16].de la Plata C.D. Marquez. Deficits in Functional Connectivity of Hippocampal and Frontal Lobe Circuits After Traumatic Axonal Injury. Archives of neurology (Chicago) 2011;68:74–84. doi: 10.1001/archneurol.2010.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Maruta J, Suh M, Niogi SN, Mukherjee P, Ghajar J. Visual Tracking Synchronization as a Metric for Concussion Screening. The Journal of Head Trauma Rehabilitation. 2010;25:293–305. doi: 10.1097/HTR.0b013e3181e67936. 210.1097/HTR.1090b1013e3181e67936. [DOI] [PubMed] [Google Scholar]
- [18].Mayer AR, Mannell MV, Ling J, Gasparovic C, Yeo RA. Functional connectivity in mild traumatic brain injury. Human Brain Mapping. 2011:n/a–n/a. doi: 10.1002/hbm.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Meyerand ME, Quigley M, Cordes D, Haughton V, Turski P, Seth R, Moritz C. Role of the corpus callosum in functional connectivity. American journal of neuroradiology : AJNR. 2003;24:208–212. [PMC free article] [PubMed] [Google Scholar]
- [20].Nakabayashi M. Neural injury and recovery near cortical contusions: a clinical magnetic resonance spectroscopy study. Journal of neurosurgery. 2007;106:370–377. doi: 10.3171/jns.2007.106.3.370. [DOI] [PubMed] [Google Scholar]
- [21].Ross BD. 1H MRS in acute traumatic brain injury. Journal of Magnetic Resonance Imaging. 1998;8:829–840. doi: 10.1002/jmri.1880080412. [DOI] [PubMed] [Google Scholar]
- [22].Rutgers DR, Fillard P, Paradot G, Tadie M, Lasjaunias P, Ducreux D. Diffusion Tensor Imaging Characteristics of the Corpus Callosum in Mild, Moderate, and Severe Traumatic Brain Injury. American Journal of Neuroradiology. 2008;29:1730–1735. doi: 10.3174/ajnr.A1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sedney CL, Orphanos J, Bailes JE. When to Consider Retiring an Athlete After Sports-Related Concussion. Clinics in Sports Medicine. 2011;30:189–200. doi: 10.1016/j.csm.2010.08.005. [DOI] [PubMed] [Google Scholar]
- [24].Sharp DJ, Beckmann CF, Greenwood R, Kinnunen KM, Bonnelle V, De Boissezon X, Powell JH, Counsell SJ, Patel MC, Leech R. Default mode network functional and structural connectivity after traumatic brain injury. Brain. 2011;134:2233–2247. doi: 10.1093/brain/awr175. [DOI] [PubMed] [Google Scholar]
- [25].Slobounov SM, Gay M, Zhang K, Johnson B, Pennell D, Sebastianelli W, Horovitz S, Hallett M. Alteration of brain functional network at rest and in response to YMCA physical stress test in concussed athletes: RsFMRI study. NeuroImage. 2011;55:1716–1727. doi: 10.1016/j.neuroimage.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smits M, Houston GC, Dippel DWJ, Wielopolski PA, Vernooij MW, Koudstaal PJ, Hunink MGM, Lugt A. Microstructural brain injury in post-concussion syndrome after minor head injury. Neuroradiology. 2010;53:553–563. doi: 10.1007/s00234-010-0774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sponheim SR, McGuire KA, Kang SS, Davenport ND, Aviyente S, Bernat EM, Lim KO. Evidence of disrupted functional connectivity in the brain after combat-related blast injury. NeuroImage. 2011;54:S21–S29. doi: 10.1016/j.neuroimage.2010.09.007. [DOI] [PubMed] [Google Scholar]
- [28].Vagnozzi R, Signoretti S, Cristofori L, Alessandrini F, Floris R, Isgro E, Ria A, Marziale S, Zoccatelli G, Tavazzi B, Del Bolgia F, Sorge R, Broglio SP, McIntosh TK, Lazzarino G. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 2010;133:3232–3242. doi: 10.1093/brain/awq200. [DOI] [PubMed] [Google Scholar]
- [29].Vanderploeg RD, Belanger HG, Curtiss G. Mild Traumatic Brain Injury and Posttraumatic Stress Disorder and Their Associations With Health Symptoms. Archives of Physical Medicine and Rehabilitation. 2009;90:1084–1093. doi: 10.1016/j.apmr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- [30].Witt ST, Lovejoy DW, Pearlson GD, Stevens MC. Decreased prefrontal cortex activity in mild traumatic brain injury during performance of an auditory oddball task. Brain Imaging and Behavior. 2010;4:232–247. doi: 10.1007/s11682-010-9102-3. [DOI] [PubMed] [Google Scholar]
- [31].Yeo RA, Gasparovic C, Merideth F, Ruhl D, Doezema D, Mayer AR. A Longitudinal Proton Magnetic Resonance Spectroscopy Study of Mild Traumatic Brain Injury. Journal of Neurotrauma. 2011;28:1–11. doi: 10.1089/neu.2010.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang K, Johnson B, Pennell D, Ray W, Sebastianelli W, Slobounov S. Are functional deficits in concussed individuals consistent with white matter structural alterations: combined FMRI & DTI study. Experimental Brain Research. 2010;204:57–70. doi: 10.1007/s00221-010-2294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


