Abstract
Background
HIV-infected prisoners experience poor HIV treatment outcomes post-release. Directly administered antiretroviral therapy (DAART) is a CDC-designated, evidence-based adherence intervention for drug users, yet untested among released prisoners.
Methods
Sentenced HIV-infected prisoners on antiretroviral therapy (ART) and returning to New Haven or Hartford, Connecticut were recruited and randomized 2:1 to a controlled trial (RCT) of 6 months of DAART versus self-administered therapy (SAT); all subjects received case management services. Subjects meeting DSM-IV criteria for opioid dependence were offered immediate medication-assisted treatment. Trained outreach workers provided DAART once-daily, seven days per week, including behavioral skills training during the last intervention month. Both study groups were assessed for 6 months after the intervention period. Assessments occurred within 90 days pre-release (baseline), day of release, and then monthly for 12 months. Viral load (VL) and CD4 testing was conducted baseline and quarterly; genotypic resistance testing was conducted at baseline, 6 and 12 months. The primary outcome was pre-defined as viral suppression (VL<400 copies/mL) at 6 months.
Results
Between 2004 and 2009, 279 participants were screened, of which 202 met eligibility criteria and 154 were ultimately enrolled in the study; 103 subjects were randomized to DAART and 51 to SAT. Subjects were mostly male (81.2%), people of color (87.0%), had an alcohol use disorder (39.7%), had underlying depression (54.2%), were virally suppressed (78.8%) and mean CD4=390.7 cells/mL.
Conclusions
Outcomes from this RCT will contribute greatly to HIV treatment outcomes after release from prison, a period associated with adverse HIV and other medical consequences.
Keywords: Adherence; Directly administered antiretroviral therapy; Directly observed therapy; HIV; AIDS; Substance abuse; prisoners, incarceration; DAART, antiretroviral therapy
INTRODUCTION
In 2009, 7.2 million people were involved in the criminal justice system (CJS) in the United States [1] with one sixth of all people living with HIV/AIDS (PLWHA) passing through the CJS annually [2]. As a result of the overwhelming majority of correctional inmates having substance use disorders [3, 4], including injection drug use (IDU) [5], HIV prevalence among incarcerated persons is over three times greater than among the general population.
Though not examined recently, all published studies suggest that the majority of correctional inmates learn about their HIV diagnosis [6, 7] and first initiate antiretroviral therapy (ART) while incarcerated [8], making correctional settings ideal for HIV prevention and treatment initiatives [9]. Treatment for HIV using ART results in optimal outcomes [10, 11], resulting in the reduced mortality among prisoners such that they had reached parity with non-correctional populations by 2008 [12]. Despite these successes within prison, HIV treatment outcomes deteriorate soon after release [10, 13]. Arguably, many attribute the impressive benefits of ART among prisoners to the structure of the prison environment, stabilized housing, near universal access to ART, the relative lack of drug use, the treatment of mental illness and the supervised provision of directly administered antiretroviral therapy (DAART). One of the most pressing issues facing the correctional system today is therefore sustaining the benefit of ART while HIV-infected prisoners transition to community settings [10]. After release, HIV-infected prisoners face seemingly insurmountable social and medical destabilization [13, 14]. The extent to which these factors contribute to poor HIV treatment outcomes after release are complex, yet it is imperative to both the individual and collectively to society to develop effective prison-release interventions that address the unique needs of this population that is at risk for numerous negative health consequences, including overdose and death [15]. Documented problems accessing ART [16] and relapse to drug use [17] contribute to poor post-release HIV treatment outcomes, yet post-release case management services do not ameliorate these negative health outcomes any better than pre-release planning [18].
To overcome poor post-release HIV treatment outcomes, we postulated that providing continued structure, particularly with regard to support ART adherence even in the setting of relapse to drug and alcohol use, was a necessary and important contributor to HIV treatment outcomes under circumstances that were socially and medically destabilizing. Directly administered antiretroviral therapy (DAART) has been thoroughly developed [19] and documented in randomized controlled trials (RCTs) in community settings [20, 21] and now to be designed by the Centers for Disease Control and Prevention as an evidence-based behavioral adherence intervention for drug users [22]. Among active drug users, it has been associated with improved virological and immunological outcomes that are optimized when linked to healthcare and case management [23], has high acceptability, improves adherence and retention [24] and does not promote the development of genotypic resistance mutations [25]. To determine if DAART was effective at stabilizing the treatment outcomes among HIV-infected prisoners transitioning to the community, we therefore conducted a RCT comparing DAART to self-administered therapy on virological, immunological and a number of other treatment outcomes.
METHODS
Study Design
The CONNECT trial was a two-site, RCT of HIV-infected individuals on stabilized ART who were transitioning from prison to the cities of New Haven or Hartford, Connecticut. The participants were randomized 2:1 to either: (1) directly administered antiretroviral therapy (DAART); or (2) self-administered therapy (SAT) as a control. Both groups received medication adherence counseling at baseline and at six months, and monthly interviews for 12 months. The primary outcome was achieving viral suppression at 6 months, measured at HIV-1 RNA levels < 400 copies/mL. Other outcomes of interest included: 1) changes in HIV-1 RNA levels from baseline; 2) maximum virological suppression defined as HIV-1 RNA < 50 copies/mL at 6 months; 3) change in CD4 from baseline to 6 months; 4) adherence to ART and 5) retention in care. To examine the durability of the intervention, all measures were assessed at 12 months and compared to the end of the intervention period (6 month assessment).
Study Setting
Project CONNECT enrolled subjects from 2004 to 2009 in two research sites in New Haven and Hartford, Connecticut. Unlike our previous studies of DAART,[19] ART was supervised once-daily, seven days per week in community settings by a trained outreach worker. Our previous studies confirmed that non-observed dosing days resulted in significantly lower adherence on those days [19]. All DAART participants were provided a mobile telephone during the 6-month intervention period to enhance daily communication between subjects and DAART outreach workers. For individuals meeting DSM-IV criteria for opioid dependence, they had the option of selecting to also receive buprenorphine as previously described [17]. Subjects prescribed buprenorphine, irrespective of randomization group, had weekly scheduled substance abuse counseling sessions for 12 weeks; the frequency of counseling sessions varied for subjects beyond the first 12 weeks, based on previously described buprenorphine counseling content[26]. For DAART subjects receiving buprenorphine, their buprenorphine was observed daily. Substance abuse counselors provided vouchers for buprenorphine prescription renewals at scheduled counseling sessions.
Study Inclusion and Exclusion Criteria
Participants were primarily recruited from the Connecticut Department of Correction (CTDOC) within 90 days prior to release. Inclusion criteria included: (1) Documentation of HIV-infection; (2) already receiving ART or eligible for it upon release; (3) ART prescribed once or twice daily; (4) incarcerated for a minimum of 90 days; (5) able to communicate in either English or Spanish; (6) returning to the cities of New Haven or Hartford; (7) at least 18 years of age; (8) capable of providing informed consent; and (9) not enrolled in any other adherence research intervention. Individuals who met eligibility requirements who were unexpectedly released from prison, but within 30 days of release, were also eligible for enrollment. Subjects who had pending charges unrelated to their current sentence or whose sentence was extended beyond the funding period were ineligible.
Protection of Human Subjects
Approvals, Confidentiality, and Data Safety and Monitoring
The Yale University Human Investigation Committee and Connecticut Department of Correction Research Advisory Committee approved the trial and it was registered at www.clinicaltrials.gov (NCT00786396). Because the trial involved released prisoners and individuals with substance use disorders with extensive criminal justice experiences, additional assurances were provided by the Office of Human Research Protection (OHRP) at the Department of Health and Human Services and a Certificate of Confidentiality from National Institutes of Health, respectively.
Specific Safety Protocols
All medications, whether dispensed as DAART or SAT, were prepared, packaged and dispensed by a licensed pharmacist. No experimental medications were used. Packaged daily pill and medication boxes were reviewed by clinical staff after preparation by the pharmacist in order to ensure that all medications were accurate to the regimen prescribed for the participant by their medical providers. Regimens were reviewed monthly with the participant and with their provider to ensure accuracy of the prescriptions. Prior to observing a participant taking their daily dose of medication, a review of the names and identification of the medication was done by the participant and research staff. This review was compared to the prescription labels on each medication box and along with a daily regimen tracking form. The tracking form also provided an opportunity to monitor the participant’s side effects, medical needs, participant’s record of non-observed medications and all attempts to be made to ensure the client received their medications for the day. Storage of all medications was kept in a locked cabinets used solely for the storage of medications.
Patient Recruitment, Enrollment, and Reimbursement
The Infectious Disease Contact Nurses (IDCN) within the Connecticut Department of Correction initiates the discharge planning process for all HIV-infected inmates beginning 90 days before expected release. This is accomplished by referring the inmates to Project TLC (Transitional Linkage to the Community) [27], a standard-of-care transitional case management program that develops a discharge plan and assists released individuals for a minimum of 30 days post-release. Project TLC assists with arranging medical appointments, ensuring that each person can continue their antiretroviral medications by completing the AIDS Drug Assistance Program application and making referral to other identified services that are needed. For the purposes of this study, a referral to Project TLC would also result in a simultaneous referral to Project CONNECT for inmates returning to New Haven and Hartford. There were two phases of recruitment. The first phase was eligibility screening and baseline data collection necessary for randomization. After the inmate signed the necessary release of information forms allowing communication between the inmate and research staff, a Project CONNECT staff member traveled to the prison as part of the pre-enrollment process, conducted the baseline assessment and described the details of the study and determined eligibility. The second phase was the formal enrollment into the randomized controlled trial, which only took place after the subject was released from the prison environment, to ensure that final enrolment was not coercive. Ideally, this occurred on the day the subject was released or as soon as possible thereafter, but within 30 days of release. If the subject remained interested in the project, he/she would sign a second consent form and a release of information form to allow further access to medical and drug treatment information for the subsequent 12 months.
Study participants received payments ranging from $10-$50 upon completing follow-up interviews; the amount varied based on the length of the survey and whether the subject had to travel for phlebotomy. No subject received payment for pre-enrollment activities or during any time of their incarceration or for participating in the DAART arm alone.
Randomization
Randomization was based upon data acquired while the subject was in prison (interview and chart review data), allowing for randomization to occur on the day of discharge from prison. Randomization was performed according to a balanced (self-adjusting) randomization scheme incorporating stratified cluster criteria [28, 29]. Randomization included the following factors: 1) returning to New Haven versus Hartford; and 2) presence or absence of an alcohol use disorder based on AUDIT criteria (>8 for men and >6 for women). Randomization was 2:1 for DAART: SAT, with oversampling the DAART group in order to allow for the potential refusal to participate in DAART should the subject not prefer it based on learning of their randomization status. Acceptability of DAART was measured as their willingness to participate in DAART after learning of their randomization status. Any subject meeting DSM-IV criteria for opioid dependence [30] for the 12-month period before incarceration was offered enrolment in buprenorphine or methadone maintenance treatment on the day of release.
Buprenorphine/naloxone was provided free of charge for all interested subjects by Reckitt Benckiser Pharmaceuticals as part of supplemental funding (R21 DA019843; Altice, PI).
Baseline Assessment and Follow-Up Interviews
Baseline information included: 1) demographic characteristics; 2) social circumstances prior to incarceration; 3) presence and severity of co-morbid medical and psychiatric conditions; 4) prior medical history and 5) HIV risk behavior in the 30 days prior to incarceration. Psychiatric and substance use disorders were assessed using the Mini International Neuropsychiatric Interview (MINI) [30] and alcohol use disorders were further measured using the Alcohol Use Disorders Inventory Test (AUDIT) [31]. Additional standardized measures at baseline and follow-up included the HIV symptom index [32], quality of life using the 36-item short-form from the Medical Outcomes Survey (SF-36) [33], the magnitude of depressive symptoms using the Self-Report Quick Inventory of Depressive Symptomatology (QIDS-SR) [34] and the Clinical Epidemiological Survey of Depression (CES-D) [35], social support scale [36], trust in physician scale [37], HIV-related stigma scale [38], neurocognitive impairment using the neuropsychological symptom inventory (NIS) [39] and the Addiction Severity Index [40] and event-level pre-incarceration HIV risk behaviors that were adapted from previous studies [41].
All subjects, after signing the final consent forms to participate, were scheduled for an interview on the day of prison-release where they were introduced to the community research team, further explained study procedures and underwent an additional survey that assessed their experiences in prison, including adherence to antiretroviral therapy using the visual analogue scale (VAS) [42], HIV risk behaviors, drug use and care-related activities. Subjects remained eligible for participation as long as this interview was conducted within 30 days post-release. All interviews were conducted using Audio Computer-Assisted Structured Interview (ACASI) methodology. On the day of release (or within 30 days), each subject underwent urine screening for opioids, cocaine, marijuana, amphetamines and methamphetamines using Reditest (Redwood, Santa Rosa, CA), phlebotomy for CD4 and HIV-1 RNA levels, and assignment to a study group.
Description of the Intervention and Control Conditions
All subjects were assigned to either DAART or SAT for a period of six months. At the end of six months, all subjects were converted to SAT and observed for an additional six months. The details of activities for the first six months for each group are described below. The protocol for both groups for the post-intervention periods (months 7-12) was identical and described subsequently.
Intervention Arm: (Directly Administered Antiretroviral Therapy, DAART)
Many of the DAART procedures have been previously described.[19] All chronically prescribed medications prescribed no more than twice-daily, including antiretroviral medications, were packaged and labeled by a pharmacist in dose packs and placed in a pillbox. For subjects selecting buprenorphine treatment, this medication was also observed as previously reported.[17] DAART subjects were observed to remove one dose per day from their weekly pre-packaged pillboxes and place their second dose (if one was prescribed) in a bottle with a Medication Event Monitoring System (MEMS, Aardex Group) recorder; in this way, the MEMS cap recorded both the morning dose by “placing” the second dose in the container and the evening dose when the subject took it later that day. Unlike our previous intervention where DAART was performed only on weekdays, DAART subjects were observed taking one dose daily seven days per week for the 6-month intervention period. DAART was conducted at selected locations throughout the cities of New Haven and Hartford. Subjects were allowed to select a location in accordance with their preferences (e.g., near their home, a drug treatment program, remote from their family, our research storefront, etc). In general, clients did not travel more than 4 blocks based on findings from our previous DAART studies.[24] DAART subjects were provided mobile telephones with voice and texting capabilities and met the outreach worker every morning between 8am and 11am. Outreach workers would observe and record all medications taken and if a client failed to show, the staff member would make multiple attempts to contact the client to guarantee that medications were delivered to them or that the client took a spare pack that was maintained in the bottle with a MEMS cap that was used to monitor medication adherence beyond observed doses, including multiple dose-per-day regimens. Up to three doses of medications, provided as “spare packs” were provided to all participants in recognition of inclement weather or unanticipated emergencies. Receipt of any more than three spare packs required meeting with the DAART outreach worker. All prescriptions were filled by a collaborating pharmacy that adhered to DAART procedures.
Previous DAART studies failed to confirm that the benefits from DAART persisted beyond the DAART intervention.[43, 44] Therefore, the outreach workers provided standardized supplemental training program that included daily recognition and naming of medications and discussion of missed doses using the Information, Motivation and Behavioral Skills model of adherence.[45] Additionally, beginning in the last month of the intervention, the outreach workers trained subjects to fill their own pillboxes, practice the names of medications, discussed methods of remembering to order refills in a timely manner and how to deal with any missed doses.
Control Arm: (Self-Administered Therapy, SAT)
SAT subjects received their monthly refills from the research pharmacy, which were then checked by the research staff and a MEMS cap was placed on the anchoring antiretroviral medication component. Anchoring medications included either the non-nucleoside reverse transcriptase inhibitor or protease inhibitor medication included in all regimens. Medication refills were scheduled and provided during the monthly structured interview when MEMS cap recordings could be downloaded. Subjects selecting to receive buprenorphine received their medications as SAT weekly for the first 12 weeks and then monthly thereafter. No cell phones were provided to SAT subjects.
Post Intervention Period
After the 6-month intervention period, all subjects were followed for an additional six months to determine the durability of the DAART intervention (post-intervention effect). During this time, refills for both study groups were provided by the research pharmacy and monitored using MEMS; these were the same procedures used for the SAT group during the intervention period. Table 1 summarizes the schedule and contents of the study visits.
Table 1.
Schedule and contents of study visits
| Activity | Study Arm |
Pre- Release |
Day of Release |
Month | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||||
| Structured Interview using ACASI |
Both | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Standardized adherence counseling |
Both | X | X | ||||||||||||
| Standardized dherence training |
DAART | X | X | X | X | X* | |||||||||
| MEMS Cap Download |
Both | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Urine Toxicology |
Both | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| CD4 lymphocyte |
Both | X | X | X | X | X | |||||||||
| HIV-1 RNA level |
Both | X | X | X | X | X | |||||||||
| HIV-1 Genotypic resistance testing |
Both | X | X | ||||||||||||
Both: refers to DAART and SAT groups
ACASI: Audio Computer Assisted Self interview
X*: intensified adherence training during the last month of the DAART intervention
Adherence Counseling
Baseline adherence counseling was provided for all participants regardless of study arm assignment. Counseling consisted of a standardized, 15-minute video that addressed the importance of medication adherence [46], including the association of adherence on viral load, CD4 counts, development of genotypic resistance mutations and dealing with missed doses. At six months, all subjects reviewed the video again and were provided with a comic-style pamphlet produced by Visionary Health Concepts, titled “Blocking the Mutant Invasion” [47].
Laboratory Monitoring
Blood samples
Phlebotomy was performed at pre-scheduled intervals to assess HIV-1 RNA levels (Amplicor 1.5; Roche), CD4 (FACS; Quest) lymphocyte count, and HIV-1 genotypic resistance mutations. All subjects underwent baseline phlebotomy. HIV-1 RNA and CD4 were assessed quarterly and genotypic resistance testing was monitored at 6- and 12-month interview. Quarterly, a standard chemical (including electrolytes, renal function, lipid profile and liver enzymes) and hematological (complete blood count) was also performed as part of routine clinical practice. In cases where the subject’s HIV provider performed phlebotomy, we used laboratory data that were provided by the clinic. Laboratory values were assigned to a designated time point (e.g. 6 months) if they were drawn within 6 weeks of the designated time period. A maximum of 50 mL was drawn at any visit; discard samples were stored and frozen at −80° C. All results were first reviewed by the medical director (SAS) and faxed to the primary HIV care provider. Any laboratory values that involved a Grade III or IV toxicity were discussed by phone with the primary HIV provider to come up with a treatment plan.
Urine drug testing
Urine toxicology screens were conducted monthly for monitoring relapse to drug use using the 6-panel Reditest (Redwood, Santa Rosa, CA); testing for buprenorphine was conducting using the Buprenorphine Reditest (Redwood, Santa Rosa, CA). Positive urine tests are indicative of active drug use of opioids, cocaine, amphetamine, buprenorphine and benzodiazepines in the previous 72-hour period and marijuana during the previous 42 days.
Sample size and power calculations
We calculated the sample size needed to detect this effect size with 80% power and a two-sided significance level of 0.05. Assuming alpha = 0.05, beta = 0.20, a compound symmetry true correlation structure of 0.5 (the most conservative, based on our results from the earlier study [48], constant intervention effects during intervention and then during post-intervention, the maximum attrition rate of 35%, and 2:1 randomization, the sample size required was estimated to be 149.
Planned statistical analyses
Statistical testing and modeling will be conducted for each outcome discussed. All p-values will result from two-tailed tests, and a p-value < 0.05 will be considered significant. Data from this randomized controlled trial of DAART versus SAT among released prisoners will be analyzed using both an intent-to-treat analysis (including all subjects randomized into either one of the two arms) and an on-treatment analysis (including only those subjects who participated in the arm to which they were assigned). Comparison of baseline characteristics will be evaluated using the Mantel-Haenszel chi-square test for categorical variables and F test of analysis of variance for continuous scale data. Kruskal-Wallis testing and a Dunn multiple comparison test will be performed to determine any statistically significant changes observed in HIV-1 RNA levels (VL) and CD4 lymphocyte counts. Final linear regression models will incorporate covariates with a univariate outcome p<.10. The primary outcome is having a VL<400 copies/mL at 6 months, assessed using a Cox proportional hazards model with secondary variables as covariates. The secondary outcome is VL<50 copies/mL. Change in log10 HIV-1 RNA from baseline to specified time-related outcomes (e.g. 12, 24, 48 weeks) will be analyzed using Buckley-James distribution-free model, which accounts for censored data. Analysis of change in mean CD4 lymphocyte count from baseline to time endpoints will be performed using the Wilcoxon rank test, stratified by daily vs. twice daily antiretroviral regimen. Spearman’s rank correlation (ro) will be used to test for associations between data with a binomial (bimodal) distribution (i.e. adherence, VL, CD4 count with binary outcome). The same test will be used to assess the correlation between self-reported adherence to treatment and MEMS cap data. Pill counts will be used to verify the MEMS cap data and improve the model fit in comparison with self-report. Sensitivity analysis of different adherence measures (e.g. MEMS, VAS) will be incorporated.
RESULTS
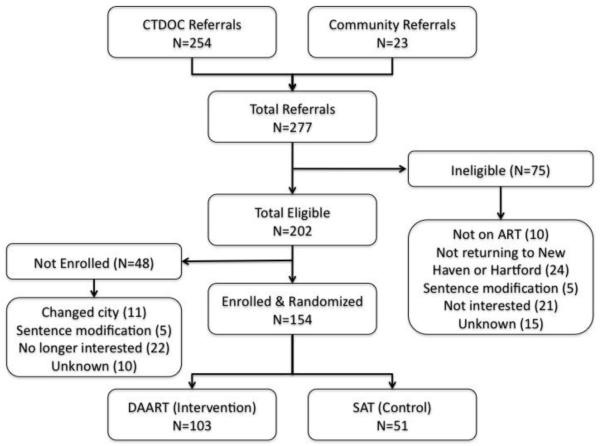
Screening and baseline interviews occurred between January 2005 and December 2009. A total of 277 participants were referred, most of which (92%) came directly from the Connecticut Department of Correction (CTDOC). Of these, 202 were eligible and 154 enrolled in the study (Figure 1). The main reasons for exclusion or not enrolling was not planning to return to either New Haven or Hartford. This occurred primarily because inmates were either offered a relocation program before release or it was stipulated as part of their community re-entry program. Lack of interest in enrolling in the study was related to either not being interested in research in general or not being interested in an adherence intervention (or both). Though not captured systematically, these individuals either felt that their adherence was not a problem or that they did not have an interest in being involved in a research study (data not shown). Table 2 illustrates the baseline characteristics of the participants.
Figure 1.
Flow chart of study recruitment, enrollment and completion.
DAART: Directly Administered Antiretroviral Therapy; SAT: Self Administered Therapy; ART: antiretroviral therapy; Change in status: sentence modification or prolonged by new charges
Table 2.
Baseline characteristics of CONNECT study samples, N (%) for categorical variables and mean ± SD for continuous variables
| DAART Arm (N= 103) |
SAT Arm (N= 51) |
Total Sample Population (N=154) |
P value | |
|---|---|---|---|---|
| Gender | 0.541 | |||
| Male | 85 (82.5%) | 40 (78.4%) | 125 (81.2%) | |
| Female | 18 (17.5%) | 11 (21.6%) | 29 (18.8%) | |
| Mean Age, years (SD) | 46.41 ±6.38 | 43.84 ±7.47 | 45.56 ±6.85 | 0.110 |
| Ethnicity | 0.606 | |||
| White | 15 (14.6%) | 5 (9.80 %) | 20 (13.0%) | |
| Black | 56 (54.4%) | 27 (52.9%) | 83 (53.9%) | |
| Hispanic | 32 (31.1%) | 19 (37.3%) | 51 (33.1%) | |
| Study Site | 0.332 | |||
| New Haven | 55 (53.4%) | 23 (45.1%) | 78 (50.6%) | |
| Hartford | 48 (46.6%) | 28 (54.9%) | 76 (49.4%) | |
| Homelessness | 0.312 | |||
| Near homeless | 49 (50.0%) | 31 (63.3%) | 80 (54.4%) | |
| Homeless | 28 (28.57%) | 10 (20.4%) | 38 (25.9%) | |
| Median months of Incarceration |
7 | 7 | 7 | 0.674 |
| Buprenorphine use | 36 (36.0%) | 14 (27.5%) | 50 (33.1%) | 0.291 |
| DSM-IV psychiatric axis I diagnoses (MINI) |
42 (42.0%) | 24 (47.1%) | 66 (43.7%) | 0.553 |
| Mood disorders | 36 (36.0%) | 18 (35.3%) | 54 (35.8%) | 0.932 |
| Anxiety disorders | 23 (23.0%) | 18 (35.3%) | 41 (27.2%) | 0.108 |
| Psychotic disorders | 11 (11.0%) | 7 (13.7%) | 18 (11.9%) | 0.625 |
| Hazardous Drinking | 0.097 | |||
| None | 65 (65.0%) | 26 (51.0%) | 91 (60.3%) | |
| Hazardous | 35 (35.0%) | 16 (49.0%) | 60 (39.7%) | |
| ASI-Alcohol | 0.241 ±0.143 | 0.217 ±0.129 | 0.233 ±0.138 | 0.094 |
| ASI-Drug | 0.154 ±0.191 | 0.199 ±0.207 | 0.169 ±0.197 | 0.234 |
| Mean CES-D score | 17.42 ±10.61 | 19.80 ±11.06 | 18.24 ±10.79 | 0.027 |
| CES-D (≥16) | 49 (52.7%) | 28 (57.1%) | 77 (54.2%) | 0.612 |
| QIDS-SR | 8.16 ±4.97 | 8.65 ±5.25 | 8.33 ±5.05 | 0.254 |
| Social Support Scale | 65.06 ±23.81 | 62.06 ±21.83 | 64.05 ±23.14 | 0.056 |
| Trust in Physician | 67.64 ±6.68 | 68.33 ±5.45 | 67.87 ±6.28 | 0.407 |
| Dosing schedule | ||||
| Once daily | 82 (81.2%) | 44 (89.8%) | 126 (84.0%) | 0.177 |
| Twice daily | 19 (18.8%) | 5 (10.2%) | 24 (16.0%) | |
| Baseline antiretroviral therapy regimens |
||||
| NNRTI+NRTIs | 36 (35.6%) | 16 (32.7%) | 52 (34.7%) | 0.917 |
| Boosted PI+NRTIs | 51 (50.5%) | 25 (51.0%) | 76 (50.7%) | |
| Non-boosted PI+NRTIs | 9 (8.9%) | 6 (12.2%) | 15 (10.0%) | |
| Others | 5 (5.0%) | 2 (4.1%) | 7 (4.7%) | |
| Viral Load | N= 101 | N= 50 | N= 151 | 0.309 |
| HIV-1 RNA < 400 copies/mL |
82 (81.2%) | 37 (74.0%) | 119 (78.8%) | |
| Viral Load | N= 101 | N= 50 | N= 151 | 0.378 |
| HIV-1 RNA < 50 copies/mL |
55 (54.5%) | 31 (62.0%) | 876(57.0 %) | |
| Log HIV-1 RNA (among VL>50 copies/mL) |
2.28 ±0.98 | 2.32 ± 1.11 | 2.30 ± 1.02 | 0.576 |
| CD4+ lymphocytes (cells/mL) |
408.7 ±252.7 | 354.5 ± 198.8 | 390.7 ± 236.9 | 0.420 |
DAART: directly administered antiretroviral therapy; SAT: self-administered antiretroviral therapy; NNRTI: non-nucleoside reverse transcriptase inhibitor; PI: protease inhibitor; SD=standard deviation; QIDS-SR= Self-Report Quick Inventory of Depressive Symptomatology
DAART, compared to SAT subjects were similar with regard to demographic characteristics, social instability upon release, addiction severity and a number of other characteristics. DAART subjects were statistically more likely, however, to have a higher mean CES-D score, but did not differ from SAT subjects with regard to meeting criteria for depression using standard cut-off with the CES-D and mean QIDS-SR score. Similarly, the two groups did not differ in terms of type of ART regimen or baseline viral load or CD4 lymphocyte status. Thus, subjects were generally well-balanced.
DISCUSSION
HIV-infected prisoners have many unmet and challenging needs as they transition from the correctional to the community setting. Examples include homelessness, lack of social support, unemployment, and poor access to antiretroviral medications, medical care, psychiatric services or substance abuse treatment.[10, 14] It is clear that case management alone is sufficient to optimize HIV treatment outcomes after release from prison.[10, 18] Innovative community-based programs that make regular contacts with drug users (e.g., drug treatment, prisons, and needle exchange programs) have had an important place in the detection and successful treatment of TB and other infectious diseases among these high-risk individuals [49, 50]. Although transitional case management programs have improved linkage to care in the community, for some jail and prison release programs [51, 52] they have unfortunately not demonstrated benefit in regards to HIV treatment outcomes upon release from prison [53].
Released inmates are vulnerable to resuming risky sexual and injection-related behaviors [15, 54, 55]. Both idealized modeling [56] and empiric data [57] suggest that high levels of adherence and suppression of HIV replication can markedly reduce HIV transmission. Thus, it is imperative that the high levels of viral suppression that are achieved during incarceration persist after release to the community. Empiric data, however, suggest that the period immediately after release from prison is associated with increased morbidity and mortality, particularly from drug overdose and relapse and from HIV-related complications.[15] This is particularly true for sustaining HIV suppression [10, 13]. A number of factors may contribute to poor HIV-related incomes, including those amenable to intervention [58], including poor prescription refills, potentially associated with decreased access to antiretroviral therapy [16], relapse to drug use [17] and non-adherence and/or non-persistence to antiretroviral treatment [59].
Directly observed therapy (DOT) has been proven effective in the public health management of tuberculosis [60]. In contrast to tuberculosis treatment, however, HIV treatment is chronic and characterized by lifelong medication use and sometimes the need to dose medications more than once-daily, which poses obstacles to the implementation of DAART [61]. Evidence suggests, however, that DAART provides virological and immunological benefits to drug-using populations during the period of the intervention [20, 21, 62-68]. Furthermore, these benefits are conferred without producing higher rates of antiretroviral drug resistance [25, 69]. DAART is thus emerging as a critical tool for improving outcomes in vulnerable populations such as recently released HIV-infected inmates.
In a recent systematic review of DAART, however, Ford et al [70] concluded that directly observed antiretroviral therapy offers no benefit over self-administered treatment, and called into question the use of such an approach to support adherence in general patient populations. On the other hand, the same review did mention marginal benefit of directly observed therapy in groups that were judged to be at high risk of non-adherence [70] and two subsequent meta-analysis confirmed the benefits of DAART, especially among HIV-infected persons with substance use disorders [69, 71]. There is an ongoing debate on DAART benefits, with DAART proponents pointing out that it should not be implemented on patients without known problematic adherence and that trials of DAART should focus only on patients who are likely to derive benefit [72].
Resisting drug relapse is another tremendous challenge for released prisoners. 85% of released prisoners relapse to opioid and alcohol use upon release to the community, regardless of the time of incarceration [73, 74]. Active opioid use has been found to be highly associated with non-adherence to antiretroviral therapy and increases morbidity and mortality as well as increased risk-taking behaviors [75-77]. Medication-assisted therapy (MAT), including methadone, buprenorphine and extended released naltrexone (XR-NTX) therapy for opioid dependence and XR-NTX for alcohol use disorders [78, 79], is another measure which can help decrease drug and alcohol use, time to relapse, criminal activity and HIV risk behaviors and increase retention in treatment [80-83]. Future prisoner-release interventions should include MAT part of a comprehensive intervention to improve ART adherence and HIV treatment outcomes [58].
Findings from this study will answer many important questions. First, does DAART confer more benefit than standard of care in maintaining the benefits of ART conferred during incarceration. Second, can the benefits of DAART be maintained beyond the intervention period? Third, can the use of buprenorphine for the subset of subjects who meet criteria for opioid dependence improve outcomes with or without the added contribution of DAART? Fourth, are there other covariates that contribute to improved (e.g. MAT) or worsened outcomes (e.g. alcohol use or untreated psychiatric conditions that are highly prevalent in this population) that must be simultaneously addressed. Fifth, with newer ART regimens, does DAART promote or retard development of resistance to ART? Last, can DAART confer protection for HIV transmission despite prevalent HIV risk behaviors after release to the community? Until such questions are answered, it is imperative to utilize the best evidence available to create effective prison-release interventions for people living with HIV/AIDS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- [1].Glaze LE. Bureau of Justice Statistics. U.S. Department of Justice; Washington, D.C.: 2010. Correctional Populations in the United States, 2009. [Google Scholar]
- [2].Spaulding AC, Seals RM, Page MJ, Brzozowski AK, Rhodes W, Hammett TM. HIV/AIDS among inmates of and releasees from US correctional facilities, 2006: declining share of epidemic but persistent public health opportunity. PLoS One. 2009;4:e7558. doi: 10.1371/journal.pone.0007558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Maruschak LM. Bureau of Justice Statistics. U.S. Department of Justice; Washington: 2009. HIV in Prisons, 2007-08. [Google Scholar]
- [4].Satcher Johnson A, Hu X, Hughes D, Campsmith M, Hall I, Prejean J, et al. The HIV/AIDS Surveillance Report. The HIV/AIDS Surveillance Report. Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Coordinating Center for Infectious Diseases, Centers for Disease Control and Prevention (CDC), U.S. Department of Health and Human Services; Atlanta: 2007. [Google Scholar]
- [5].Mumola C. Substance abuse and treatment of state and federal prisoners, 1997. Bureau of Justice Statistics, US Department of Justice, Office of Justice Programs; Washington, DC: 1999. [Google Scholar]
- [6].Altice FL, Mostashari F, Selwyn PA, Checko PJ, Singh R, Tanguay S, et al. Predictors of HIV infection among newly sentenced male prisoners. J Acquir Immune Defic Syndr. 1998;18:444–53. doi: 10.1097/00042560-199808150-00005. [DOI] [PubMed] [Google Scholar]
- [7].Altice FL, Marinovich A, Khoshnood K, Blankenship KM, Springer SA, Selwyn PA. Correlates of HIV infection among incarcerated women: implications for improving detection of HIV infection. J Urban Health. 2005;82:312–26. doi: 10.1093/jurban/jti055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Altice FL, Mostashari F, Friedland GH. Trust and the acceptance of and adherence to antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;28:47–58. doi: 10.1097/00042560-200109010-00008. [DOI] [PubMed] [Google Scholar]
- [9].Flanigan TP, Zaller N, Beckwith CG, Bazerman LB, Rana A, Gardner A, et al. Testing for HIV, sexually transmitted infections, and viral hepatitis in jails: still a missed opportunity for public health and HIV prevention. J Acquir Immune Defic Syndr. 2010;55:S78–83. doi: 10.1097/QAI.0b013e3181fbc94f. [DOI] [PubMed] [Google Scholar]
- [10].Springer SA, Pesanti E, Hodges J, Macura T, Doros G, Altice FL. Effectiveness of antiretroviral therapy among HIV-infected prisoners: reincarceration and the lack of sustained benefit after release to the community. Clin Infect Dis. 2004;38:1754–60. doi: 10.1086/421392. [DOI] [PubMed] [Google Scholar]
- [11].Springer SA, Friedland GH, Doros G, Pesanti E, Altice FL. Antiretroviral treatment regimen outcomes among HIV-infected prisoners. HIV Clinical Trials. 2007;8:205–12. doi: 10.1310/hct0804-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Maruschak LM, Beaver R. HIV in Prisons, 2008. U.S. Department of Justice; Washington, D.C.: 2009. [Google Scholar]
- [13].Stephenson BL, Wohl DA, Golin CE, Tien HC, Stewart P, Kaplan AH. Effect of release from prison and re-incarceration on the viral loads of HIV-infected individuals. Public Health Rep. 2005;120:84–8. doi: 10.1177/003335490512000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Springer SA, Spaulding AC, Meyer JP, Altice FL. Public health implications of adequate transitional care for HIV-infected Prisoners: Five essential components. Clin Infect Dis. 2011;53:469–79. doi: 10.1093/cid/cir446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Binswanger IA, Stern MF, Deyo RA, Heagerty PJ, Cheadle A, Elmore JG, et al. Release from prison--a high risk of death for former inmates. N Engl J Med. 2007;356:157–65. doi: 10.1056/NEJMsa064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Baillargeon J, Giordano TP, Rich JD, Wu ZH, Wells K, Pollock BH, et al. Accessing antiretroviral therapy following release from prison. JAMA. 2009;301:848–57. doi: 10.1001/jama.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Springer SA, Chen S, Altice FL. Improved HIV and Substance Abuse Treatment Outcomes for Released HIV-Infected Prisoners: The Impact of Buprenorphine Treatment. J Urban Health. 2010;87:592–602. doi: 10.1007/s11524-010-9438-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wohl DA, Scheyett A, Golin CE, White B, Matuszewski J, Bowling M, et al. Intensive Case Management Before and After Prison Release is No More Effective Than Comprehensive Pre-Release Discharge Planning in Linking HIV-Infected Prisoners to Care: A Randomized Trial. AIDS Behav. 2011;15:356–64. doi: 10.1007/s10461-010-9843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Altice FL, Mezger JA, Hodges J, Bruce RD, Marinovich A, Walton M, et al. Developing a directly administered antiretroviral therapy intervention for HIV-infected drug users: implications for program replication. Clin Infect Dis. 2004;38(Suppl 5):S376–87. doi: 10.1086/421400. [DOI] [PubMed] [Google Scholar]
- [20].Altice FL, Maru DS, Bruce RD, Springer SA, Friedland GH. Superiority of directly administered antiretroviral therapy over self-administered therapy among HIV-infected drug users: a prospective, randomized, controlled trial. Clin Infect Dis. 2007;45:770–8. doi: 10.1086/521166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Macalino GE, Hogan JW, Mitty JA, Bazerman LB, Delong AK, Loewenthal H, et al. A randomized clinical trial of community-based directly observed therapy as an adherence intervention for HAART among substance users. AIDS. 2007;21:1473–7. doi: 10.1097/QAD.0b013e32811ebf68. [DOI] [PubMed] [Google Scholar]
- [22].Centers for Disease Control and Prevention . Compendium of Evidence-Based HIV Behavioral Interventions: Medication Adherence Chapter. Atlanta: 2011. [Google Scholar]
- [23].Smith-Rohrberg D, Mezger J, Walton M, Bruce RD, Altice FL. Impact of enhanced services on virologic outcomes in a directly administered antiretroviral therapy trial for HIV-infected drug users. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S48–53. doi: 10.1097/01.qai.0000248338.74943.85. [DOI] [PubMed] [Google Scholar]
- [24].Maru DS, Bruce RD, Walton M, Mezger JA, Springer SA, Shield D, et al. Initiation, adherence, and retention in a randomized controlled trial of directly administered antiretroviral therapy. AIDS Behav. 2008;12:284–93. doi: 10.1007/s10461-007-9336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Maru DS, Kozal MJ, Bruce RD, Springer SA, Altice FL. Directly administered antiretroviral therapy for HIV-infected drug users does not have an impact on antiretroviral resistance: results from a randomized controlled trial. J Acquir Immune Defic Syndr. 2007;46:555–63. doi: 10.1097/qai.0b013e318158c0bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Copenhaver MM, Bruce RD, Altice FL. Behavioral counseling content for optimizing the use of buprenorphine for treatment of opioid dependence in community-based settings: a review of the empirical evidence. Am J Drug Alcohol Abuse. 2007;33:643–54. doi: 10.1080/00952990701522674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Altice F, Khoshnood K. Transitional case management as a strategy for linking HIV-infected prisoners to community health and social services (Project TLC) Connecticut Department of Public Health; Hartford, CT: 1997. [Google Scholar]
- [28].Piaggio G, Carroli G, Villar J, Pinol A, Bakketeig L, Lumbiganon P, et al. Methodological considerations on the design and analysis of an equivalence stratified cluster randomization trial. Stat Med. 2001;20:401–16. doi: 10.1002/1097-0258(20010215)20:3<401::aid-sim801>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- [29].Zelen M. A new design for randomized clinical trials. N Engl J Med. 1979;300:1242–5. doi: 10.1056/NEJM197905313002203. [DOI] [PubMed] [Google Scholar]
- [30].Sheehan D, Lecrubier Y, Sheehan KH, Janavs J, Weiller E, Keskiner A, et al. The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. European Psychiatry. 1997;12:232–41. [Google Scholar]
- [31].Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- [32].Kilbourne AM, Justice AC, Rollman BL, McGinnis KA, Rabeneck L, Weissman S, et al. Clinical importance of HIV and depressive symptoms among veterans with HIV infection. J Gen Intern Med. 2002;17:512–20. doi: 10.1046/j.1525-1497.2002.10803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- [34].Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- [35].Radloff LS. The CES-D scale: A self report depression scale for research in the general population. Applied Psychological Measurement. 1977:1. [Google Scholar]
- [36].Huba GJ, Melchior LA, Staff of The Measurement Group and HRSA/HAB’s SPNS Cooperative Agreement Steering Committee . Module 46: Social Supports Form. The Measurement Group; Culver City, California: 1996. [Google Scholar]
- [37].Anderson LA, Dedrick RF. Development of the Trust in Physician scale: a measure to assess interpersonal trust in patient-physician relationships. Psychol Rep. 1990;67:1091–100. doi: 10.2466/pr0.1990.67.3f.1091. [DOI] [PubMed] [Google Scholar]
- [38].Berger BE, Ferrans CE, Lashley FR. Measuring stigma in people with HIV: psychometric assessment of the HIV stigma scale. Res Nurs Health. 2001;24:518–29. doi: 10.1002/nur.10011. [DOI] [PubMed] [Google Scholar]
- [39].Rattan G, Strom DA, Dean RS. The efficacy of a neuropsychological symptom inventory in the differential diagnosis of neurological, depressed, and normal patients. Arch Clin Neuropsychol. 1987;2:257–64. [PubMed] [Google Scholar]
- [40].McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- [41].Fisher JD, Fisher WA, Cornman DH, Amico RK, Altice FL, Bryan A, et al. Clinician-delivered intervention during routine clinical care reduces unprotected sexual behavior among HIV-infected patients. J Acquir Immune Defic Syndr. 2006;41:44–52. doi: 10.1097/01.qai.0000192000.15777.5c. [DOI] [PubMed] [Google Scholar]
- [42].Giordano TP, Guzman D, Clark R, Charlebois ED, Bangsberg DR. Measuring adherence to antiretroviral therapy in a diverse population using a visual analogue scale. HIV Clin Trials. 2004;5:74–9. doi: 10.1310/JFXH-G3X2-EYM6-D6UG. [DOI] [PubMed] [Google Scholar]
- [43].Maru DS, Bruce RD, Walton M, Springer SA, Altice FL. Persistence of virological benefits following directly administered antiretroviral therapy among drug users: results from a randomized controlled trial. J Acquir Immune Defic Syndr. 2009;50:176–81. doi: 10.1097/QAI.0b013e3181938e7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gross R, Tierney C, Andrade A, Lalama C, Rosenkranz S, Eshleman SH, et al. Modified directly observed antiretroviral therapy compared with self-administered therapy in treatment-naive HIV-1-infected patients: a randomized trial. Arch Intern Med. 2009;169:1224–32. doi: 10.1001/archinternmed.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychology. 2006;25:462–73. doi: 10.1037/0278-6133.25.4.462. [DOI] [PubMed] [Google Scholar]
- [46].Wong IY-Ze. The Development & Assessment of an Innovative Video to Introduce Concepts of Adherence. Yale University School of Medicine; Soweto, South Africa: 2004. [Google Scholar]
- [47].Munk RJ. In: BLOCKING THE MUTANT INVASION. Concepts VH, editor. Visionary Health Concepts; NYC: 2007. [Google Scholar]
- [48].Crosland C, Poshkus M, Rich JD. Treating prisoners with HIV/AIDS: the importance of early identification, effective treatment, and community follow-up. AIDS Clin Care. 2002;14:67–71. 6. [PubMed] [Google Scholar]
- [49].Batki SL. Treatment of intravenous drug users with AIDS: the role of methadone maintenance. J Psychoactive Drugs. 1988;20:213–6. doi: 10.1080/02791072.1988.10524497. [DOI] [PubMed] [Google Scholar]
- [50].O’Connor PG, Molde S, Henry S, Shockcor WT, Schottenfeld RS. Human immunodeficiency virus infection in intravenous drug users: a model for primary care. Am J Med. 1992;93:382–6. doi: 10.1016/0002-9343(92)90166-9. [DOI] [PubMed] [Google Scholar]
- [51].Lincoln T, Kennedy S, Tuthill R, Roberts C, Conklin TJ, Hammett TM. Facilitators and barriers to continuing healthcare after jail: a community-integrated program. J Ambul Care Manage. 2006;29:2–16. doi: 10.1097/00004479-200601000-00002. [DOI] [PubMed] [Google Scholar]
- [52].Conklin TJ, Lincoln T, Flanigan TP. A public health model to connect correctional health care with communities. Am J Public Health. 1998;88:1249–50. [PubMed] [Google Scholar]
- [53].Stephenson BL, Wohl DA, McKaig R, Golin CE, Shain L, Adamian M, et al. Sexual behaviours of HIV-seropositive men and women following release from prison. Int J STD AIDS. 2006;17:103–8. doi: 10.1258/095646206775455775. [DOI] [PubMed] [Google Scholar]
- [54].MacGowan RJ, Margolis A, Gaiter J, Morrow K, Zack B, Askew J, et al. Predictors of risky sex of young men after release from prison. Int J STD AIDS. 2003;14:519–23. doi: 10.1258/095646203767869110. [DOI] [PubMed] [Google Scholar]
- [55].Baillargeon J, Borucki MJ, Zepeda S, Jenson HB, Leach CT. Antiretroviral prescribing patterns in the Texas prison system. Clin Infect Dis. 2000;31:1476–81. doi: 10.1086/317478. [DOI] [PubMed] [Google Scholar]
- [56].Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- [57].Mimiaga MJ, Safren SA, Dvoryak S, Reisner SL, Needle R, Woody G. “We fear the police, and the police fear us”: structural and individual barriers and facilitators to HIV medication adherence among injection drug users in Kiev, Ukraine. AIDS Care. 2010;22:1305–13. doi: 10.1080/09540121003758515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Springer SA, Spaulding AC, Meyer JP, Altice FL. Public health implications for adequate transitional care for HIV-infected prisoners: five essential components. Clin Infect Dis. 2011;53:469–79. doi: 10.1093/cid/cir446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bae JW, Guyer W, Grimm K, Altice FL. Medication persistence in the treatment of HIV infection: a review of the literature and implications for future clinical care and research. AIDS. 2011;25:279–90. doi: 10.1097/QAD.0b013e328340feb0. [DOI] [PubMed] [Google Scholar]
- [60].World Health Organization . Treatment of tuberculosis. WHO; Geneva: 2003. Global Tuberculosis Programme. Geneva 2003. [Google Scholar]
- [61].Lucas GM, Flexner CW, Moore RD. Directly administered antiretroviral therapy in the treatment of HIV infection: benefit or burden? AIDS Patient Care STDS. 2002;16:527–35. doi: 10.1089/108729102761041083. [DOI] [PubMed] [Google Scholar]
- [62].Conway B, Prasad J, Reynolds R, Farley J, Jones M, Jutha S, et al. Directly observed therapy for the management of HIV-infected patients in a methadone program. Clin Infect Dis. 2004;38(Suppl 5):S402–8. doi: 10.1086/421404. [DOI] [PubMed] [Google Scholar]
- [63].Lucas GM, Weidle PJ, Hader S, Moore RD. Directly administered antiretroviral therapy in an urban methadone maintenance clinic: a nonrandomized comparative study. Clin Infect Dis. 2004;38(Suppl 5):S409–13. doi: 10.1086/421405. [DOI] [PubMed] [Google Scholar]
- [64].Mitty JA, Macalino GE, Bazerman LB, Loewenthal HG, Hogan JW, MacLeod CJ, et al. The use of community-based modified directly observed therapy for the treatment of HIV-infected persons. J Acquir Immune Defic Syndr. 2005;39:545–50. [PubMed] [Google Scholar]
- [65].Behforouz HL, Kalmus A, Scherz CS, Kahn JS, Kadakia MB, Farmer PE. Directly observed therapy for HIV antiretroviral therapy in an urban US setting. J Acquir Immune Defic Syndr. 2004;36:642–5. doi: 10.1097/00126334-200405010-00016. [DOI] [PubMed] [Google Scholar]
- [66].Tinoco I, Giron-Gonzalez JA, Gonzalez-Gonzalez MT, Vergara de Campos A, Rodriguez-Felix L, Serrano A, et al. Efficacy of directly observed treatment of HIV infection: experience in AIDS welfare homes. Eur J Clin Microbiol Infect Dis. 2004;23:331–5. doi: 10.1007/s10096-003-1099-8. [DOI] [PubMed] [Google Scholar]
- [67].Greenberg B, Berkman A, Thomas R, Hoos D, Finkelstein R, Astemborski J, et al. Evaluating supervised HAART in late-stage HIV among drug users: a preliminary report. J Urban Health. 1999;76:468–80. doi: 10.1007/BF02351504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Berg KM, Litwin A, Li X, Heo M, Arnsten JH. Directly observed antiretroviral therapy improves adherence and viral load in drug users attending methadone maintenance clinics: A randomized controlled trial. Drug Alcohol Depend. 2010 doi: 10.1016/j.drugalcdep.2010.07.025. In Press (Epub Sep 14, 2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Brust JC, Litwin AH, Berg KM, Li X, Heo M, Arnsten JH. Directly observed antiretroviral therapy in substance abusers receiving methadone maintenance therapy does not cause increased drug resistance. AIDS Res Hum Retroviruses. 2011;27:535–41. doi: 10.1089/aid.2010.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ford N, Nachega JB, Engel ME, Mills EJ. Directly observed antiretroviral therapy: a systematic review and meta-analysis of randomised clinical trials. Lancet. 2009;374:2064–71. doi: 10.1016/S0140-6736(09)61671-8. [DOI] [PubMed] [Google Scholar]
- [71].Hart JE, Jeon CY, Ivers LC, Behforouz HL, Caldas A, Drobac PC, et al. Effect of directly observed therapy for highly active antiretroviral therapy on virologic, immunologic, and adherence outcomes: a meta-analysis and systematic review. J Acquir Immune Defic Syndr. 2010;54:167–79. doi: 10.1097/QAI.0b013e3181d9a330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Altice FL, Springer SA. DAART for human immunodeficiency virus-infected patients: Studying subjects not at risk for nonadherence and use of untested interventions. Arch Intern Med. 2010;170:109–10. doi: 10.1001/archinternmed.2009.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Kinlock TW, Battjes RJ, Schwartz RP. A novel opioid maintenance program for prisoners: preliminary findings. J Subst Abuse Treat. 2002;22:141–7. doi: 10.1016/s0740-5472(02)00226-x. [DOI] [PubMed] [Google Scholar]
- [74].Kinlock TW, Gordon MS, Schwartz RP, O’Grady KE. A Study of Methadone Maintenance For Male Prisoners: 3-Month Postrelease Outcomes. Crim Justice Behav. 2008;35:34–47. doi: 10.1177/0093854807309111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17:377–81. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Halkitis PN, Kutnick AH, Slater S. The social realities of adherence to protease inhibitor regimens: substance use, health care and psychological states. J Health Psychol. 2005;10:545–58. doi: 10.1177/1359105305053422. [DOI] [PubMed] [Google Scholar]
- [77].Gatt S, Sammut R. An exploratory study of predictors of self-care behaviour in persons with type 2 diabetes. Int J Nurs Stud. 2008;45:1525–33. doi: 10.1016/j.ijnurstu.2008.02.006. [DOI] [PubMed] [Google Scholar]
- [78].Azar MM, Springer SA, Meyer JP, Altice FL. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend. 2010;112:178–93. doi: 10.1016/j.drugalcdep.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Springer SA, Azar MM, Altice FL. HIV, alcohol dependence, and the criminal justice system: a review and call for evidence-based treatment for released prisoners. Am J Drug Alcohol Abuse. 2011;37:12–21. doi: 10.3109/00952990.2010.540280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Amato L, Minozzi S, Davoli M, Vecchi S, Ferri MM, Mayet S. Psychosocial and pharmacological treatments versus pharmacological treatments for opioid detoxification. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD005031. CD005031. [DOI] [PubMed] [Google Scholar]
- [81].WHO . Joint position paper on substitution maintenance therapy in the management of opioid dependence and HIV/AIDS prevention. World Health Organization, United Nations Office On Drugs and Crime, and the Joint United Nations Programme on HIV/AIDS; Geneva: 2004. [Google Scholar]
- [82].Krupitsky E, Nunes EV, Ling W, Illeperuma A, Gastfriend DR, Silverman BL. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377:1506–13. doi: 10.1016/S0140-6736(11)60358-9. [DOI] [PubMed] [Google Scholar]
- [83].Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet. 2010;376:59–79. doi: 10.1016/S0140-6736(10)60829-X. [DOI] [PMC free article] [PubMed] [Google Scholar]