Abstract
Standard MRI cannot distinguish between radiation necrosis and tumor progression; however, this distinction is critical in the assessment of tumor response to therapy. In this study, one delayed radiation necrosis model (dose, 40 Gy; radiation field, 10 × 10 mm2; n = 13) and two orthotopic glioma models in rats (9L gliosarcoma, n = 8; human glioma xenografts, n = 5) were compared using multiple DTI indices. A visible isotropic apparent diffusion coefficient (ADC) pattern was observed in the lesion due to radiation necrosis, which consisted of a hypointense central zone and a hyperintense peripheral zone. There were significantly lower ADC, parallel diffusivity, and perpendicular diffusivity in the necrotic central zone than in the peripheral zone (all p < 0.001). When radiation-induced necrosis was compared with viable tumor, radiation necrosis had significantly lower ADC than 9L gliosarcoma and human glioma xenografts (both p < 0.01) in the central zone, and significantly lower FA than 9L gliosarcoma (p = 0.005) and human glioma xenografts (p = 0.012) in the peripheral zone. Histological analysis revealed parenchymal coagulative necrosis in the central zone, and damaged vessels and reactive astrogliosis in the peripheral zone. These data suggest that qualitative and quantitative analysis of the DTI maps can provide useful information by which to distinguish between radiation necrosis and viable glioma.
Keywords: radiation necrosis, glioma, tumor recurrence, MRI, DTI, rat
Introduction
Gliomas are the most common type of primary malignant brain tumors. Of these, glioblastoma multiforme (GBM) accounts for ~50% of cases, and has a dismal prognosis (the median survival time is approximately 14 months) [1]. The standard therapy for malignant gliomas includes surgery, radiation therapy, and concurrent chemotherapy [2]. It is well recognized that radiation can induce injury to brain tissue that potentially causes necrosis within months to years after treatment [3, 4]. Unlike patients with tumor recurrence, who need alternative therapies, patients with radiation necrosis (treatment response) require the continuation of current therapy. Therefore, determination of tumor recurrence versus radiation necrosis has immediate clinical implications.
The differential diagnosis of radiation necrosis versus tumor recurrence in the clinic has been challenging, because their conventional MRI features are very similar, including gadolinium enhancement on T1-weighted (T1w) images, heterogeneous hyperintensity on T2-weighted (T2w) images, and mass effect [3, 4]. Although some interesting MRI patterns have been observed in radiation necrosis, such as the “Swiss cheese appearance” or the “soap bubble pattern,” these characteristics cannot reliably differentiate between radiation necrosis and tumor recurrence [5, 6]. Advanced MRI techniques, such as MR spectroscopy [7], dynamic contrast-enhanced MRI [8, 9], and many others [10, 11], have been applied to distinguish between radiation necrosis and tumor recurrence. However, there is still no consensus about an MRI method that can clearly and reliably differentiate between these two entities.
Diffusion tensor imaging (DTI) measures the three-dimensional diffusion of water in vivo. Clinically, this technique has been widely used to detect neurological diseases [12, 13] and characterize brain development [14]. Generated from DTI-based eigenvalues, fractional anisotropy (FA), isotropic apparent diffusion coefficient (ADC), parallel diffusivity (λ//), and perpendicular diffusivity (λ⊥) have been shown to be sensitive markers for the characterization of radiation-induced white matter injury at as early as two weeks post-radiation [15], and for the detection of radiation-induced delayed white matter necrosis [16]. Additionally, these DTI indices have been demonstrated to reflect the growth patterns of brain tumors and longitudinal changes with time [17–20]. This study specifically addresses the differentiation between radiation necrosis and glioma by comparing their respective DTI features with histology in animal models. This study aims to: 1) characterize the diffusion properties of radiation necrosis and gliomas in animal models; 2) correlate these imaging features with histological evaluations; and 3) evaluate the diagnostic power of DTI to distinguish radiation necrosis from glioma.
Materials and Methods
Brain glioma animal model preparation
Two tumor cell lines were used in this study. The Mayo GBM22 cell line (provided by C. David James, Mayo Clinic) was established from resected glioblastoma multiforme (GBM) in humans, passaged serially as tumor xenografts, and cultured briefly prior to implantation into the rat brain [21]. The 9L gliosarcoma model was derived from nitrosourea-induced malignancies of glial origin. Compared to the 9L gliosarcoma model, the GBM22 tumor xenografts exhibited the essential characteristics of high invasiveness and necrosis [17, 22], as are typically found in patients with GBM. Adult nude and Fisher 344 rats (male; 200–250 g; n = 5 and 8, respectively) were anesthetized with an intraperitoneal injection of 0.2 ml/100 g body weight of 2.5 mg/ml of xylazine and 25 mg/ml of ketamine hydrochloride. Tumor cells (100,000 GBM22 cells in 2 µL for each nude rat; 25,000 9L gliosarcoma tumor cells for each Fisher rat) were stereotactically implanted into the right caudate-putamen area (3 mm lateral to the bregma and 4.5 mm deep from the skull). All experiments were approved by the Johns Hopkins Animal Care and Use Committee.
Radiation necrosis model preparation
Thirteen adult rats (Fischer 344; male; 250–300 g) were irradiated using a small animal radiation research platform [23]. The rats were anesthetized (4% isoflurane in O2 in a box for about 5 min for induction, followed by 2–2.5% isoflurane through a nose cone during radiation) and immobilized in a custom-made plastic cranial fixation device, which supported gas anesthesia. A single, well-collimated x-ray beam was delivered in a single dose of 40 Gy to a 10 × 10 mm2 region in the left hemisphere under planar fluoroscopic image guidance, and the right hemisphere received a negligible dose. The radiation dose (40 Gy) has been proven to produce late necrosis without deaths or gross neurologic deficits [24].
MRI data acquisition
Imaging experiments were performed on a 4.7T horizontal bore animal MR system (Bruker Biospin, Billerica, MA), with an actively decoupled cross-coil set-up of a 70 mm body coil for radiofrequency transmission and a 25 mm surface coil for signal reception. Briefly, rats were anesthetized with 5% isoflurane in a mixture of 75% air and 25% O2 in a box for 5 minutes for induction, followed by 1.5–2% isoflurane through a mask. Rats were placed in a specially designed animal holder, in which their heads and bodies were fixed to the cradle with tape and ear pins. Rats in the magnet were monitored visually online through a small-animal monitoring and gating system, connected by fiber optics, and their breathing rates were maintained at 35 ± 10 breaths/min by adjusting the concentration of isoflurane. The rats underwent MRI scanning at days 26–28 (GBM22) and days 9–11 (9L) post-implantation, when the tumors were 3–5 mm in diameter. For the radiation model, the rats were monitored with anatomical MR images monthly until radiation necrosis was detected.
In vivo multi-slice images of rat brains were acquired in the horizontal plane (5 slices; slice thickness 1.5 mm; field of view = 42 mm × 32 mm) and in the coronal plane (5 slices; slice thickness 1.5 mm; field of view = 32 mm × 32 mm). T2w images were acquired using a fast-spin-echo sequence (echo train length = 4; repetition time = 3 sec; echo time = 64 ms; number of averages = 2). Diffusion tensor images were acquired using a multiple-slice, multiple-spin-echo diffusion-weighted sequence (in the horizontal plane; repetition time = 2 sec; echo times = 26.3/36.7/47.1/57.5 ms; matrix = 128 × 96; resolution = 0.33 mm × 0.33 mm; number of averages = 2). Seven diffusion-weighted images with different b values were acquired (one image with b value = 50 sec/mm2 and the rest with b value = 625 sec/mm2). The images with the different echo times were averaged to improve the signal-to-noise ratio during data processing. Finally, T1w images (repetition time = 700 ms; echo time = 10 ms; number of average = 10) with and without gadolinium enhancement were acquired with the same geometry and location as the T2w images. The total image time for each animal was about 2 hours.
Image analysis
The FA, ADC, λ//, and λ⊥ maps were generated by the DTIStudio v2.30 [25]. The relationships between these DTI indices and diffusion tensor eigenvalues (λ1, λ2 and λ3) were defined according to the following equations:
| (1) |
| (2) |
| (3) |
| (4) |
The regions of interest (ROIs) were manually drawn for quantitative analysis. First, the ROIs were placed in the whole radiation necrotic lesions and the whole tumor lesions, based on the contrast enhancement on the conventional Gd-T1w images or the signal abnormalities on the T2w images (if Gd-T1w was not available). Further, we found that radiation necrosis consisted of a hypointense central zone and a hyperintense rim on the ADC map. Therefore, two ROIs, the central zone and the peripheral zone, were also drawn on the ADC map for quantitative analysis. This was used to compare with tumors, where two ROIs were placed in the high-FA tumor peripheral zone and the low-FA central zone on the FA map. These ROIs were transferred to identical sites on other DTI index maps for each rat. ImageJ 1.43n (National Institutes of Health, Bethesda, MD) was used to evaluate the quantitative indices in all DTI maps.
Histopathology evaluation
Rats were sacrificed for histological evaluation after MRI scanning. Brain specimens were processed using the standard histological protocol. Briefly, rats were perfused through the left cardiac ventricle, with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (PFA) in PBS. The rat brains were fixed in 4% PFA in PBS (pH 7.4) at 4 °C overnight. The brains were sectioned and histological sections (10 µm thick) were stained with hematoxylin and eosin (H&E). Histological specimens were then analyzed within the regions that corresponded to the quantitative MRI measurements. Histological images were acquired by digital photomicrography, using a light microscope under 10×–200× magnification.
Statistical analysis
All results were expressed as mean ± standard deviation. The paired t-test was applied to evaluate statistical differences between the peripheral zone and the central zone of the lesion (radiation necrosis and glioma). A one-way analysis of variance (ANOVA) test, followed by the Tukey test, was applied to analyze the statistical differences in DTI values between radiation necrosis and gliomas.
The efficacy of DTI indices (FA, ADC, λ// and λ⊥) in the central zone and peripheral zone for the classification of radiation necrosis and tumors was initially evaluated by the univariate logistic regression analysis method. Then, the receiver operating characteristic (ROC) curve was applied to determine the cut-off values of the selected DTI indices and areas under the curves (AUC) for predicting radiation necrosis versus tumors. The best cut-off values were calculated to be those with a maximized Youden index (sensitivity + specificity − 1). All statistical analysis procedures were performed using the SPSS for Windows statistical package (Version 18, Chicago, IL). A p-value of <0.05 was considered to be statistical significant.
Results
Conventional MRI features of radiation necrosis
Radiation-induced necrosis began to appear at approximately 22–24 weeks post-radiation in all rats (n = 13). Two to three weeks later, a large necrotic lesion (a few mm in size; Fig. 1), which was heterogeneous on the T2w images and showed gadolinium enhancement on the post-contrast T1w images, as seen in the clinic, could be observed in the ipsilateral hemisphere.
Fig. 1.
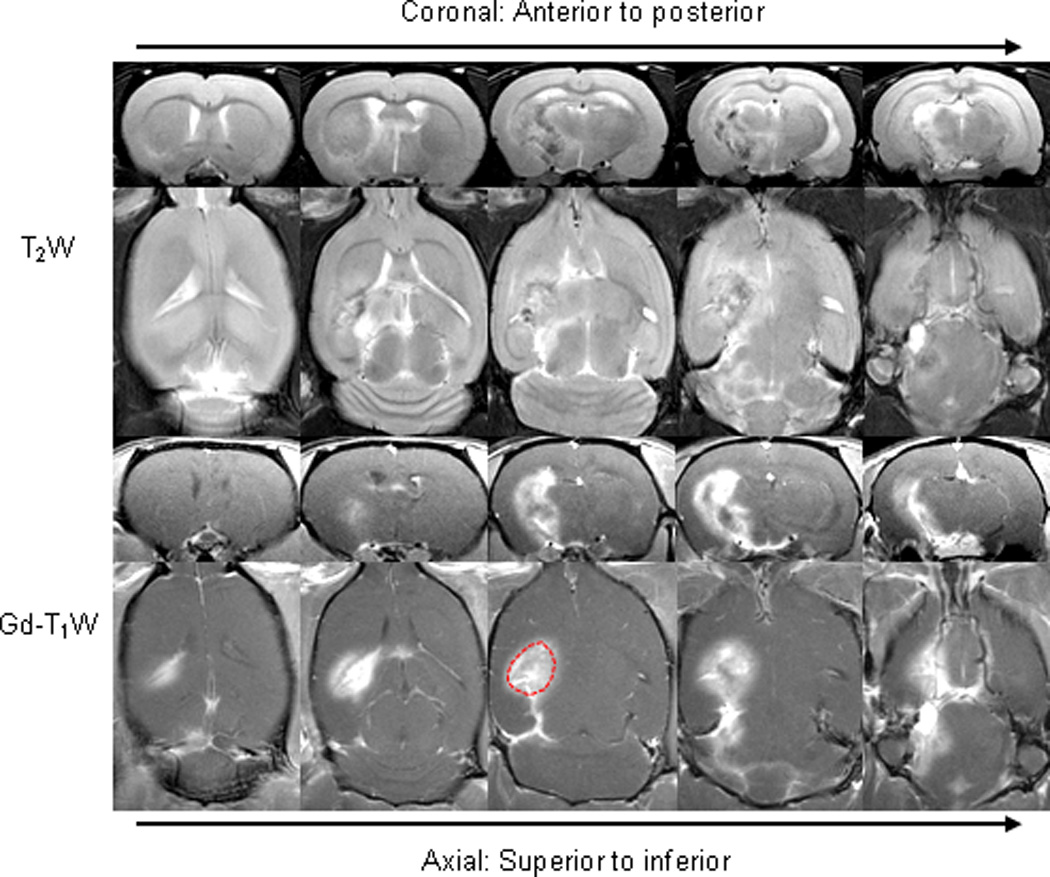
Conventional MR images of radiation necrosis (24 weeks post-radiation). The lesion was heterogeneous on the T2w image and showed gadolinium enhancement on the post-contrast T1w image. An example of ROIs for the quantitative analysis was drawn on Gd-T1w images.
DTI patterns of radiation necrosis and glioma
In all cases of radiation necrosis, the ADC signals were hypointense in the central zone of the lesion, with respect to the contralateral brain (Fig. 2). The hypointense regions covered several ipsilateral white matter tracts, such as the external capsule, internal capsule, cerebral peduncle, and fornix. On the contrary, the ADC signals were hyperintense in the peripheral zone of radiation necrosis, compared to the contralateral brain. The hyperintense regions mainly included to the surrounding gray matter, such as the cortex, caudate putamen, and hypothalamus. On the FA map, a diffused hypointense lesion was observed on the ipsilateral brain, with respect to the contralateral brain. The ipsilateral white matter tracts seemed thinner and more hypointense than contralateral white matter tracts.
Fig. 2.
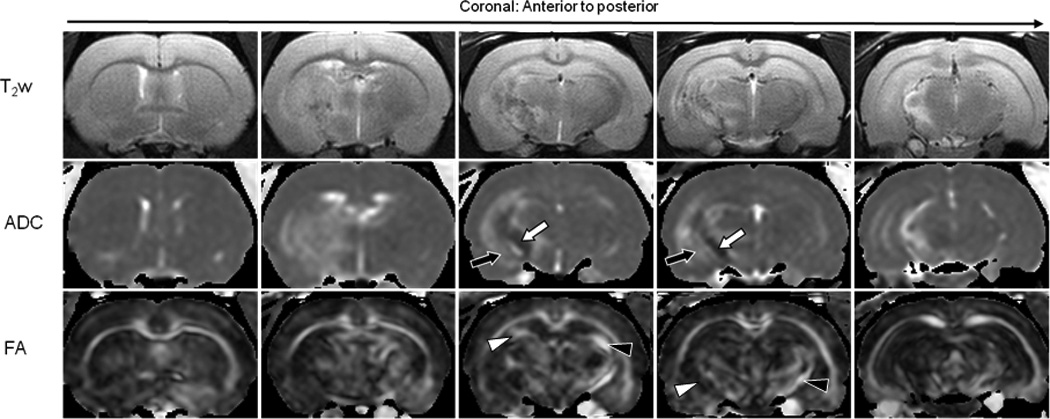
MRI features of radiation necrosis (25 weeks post-radiation). T2w images show heterogeneity in the lesion. On the ADC map, radiation necrosis is hypointense in the central zone (white arrow) and hyperintense in the peripheral zone (black arrow). Radiation necrosis shows low diffusion anisotropy on the FA map. Ipsilateral white matter tracts appear thinner and show a decreased intensity (white arrowhead), compared to the contralateral side (black arrowhead).
In comparison, both GBM22 and 9L tumors were hyperintense and relatively homogeneous on the ADC maps (Fig. 3). The FA maps showed that these tumors appeared as lesions of a central zone with low FA signal and a peripheral zone with high FA, as previously reported [17]. Consequently, on the DTI index maps, the diffusion patterns within radiation necrosis differed from those within gliomas.
Fig. 3.
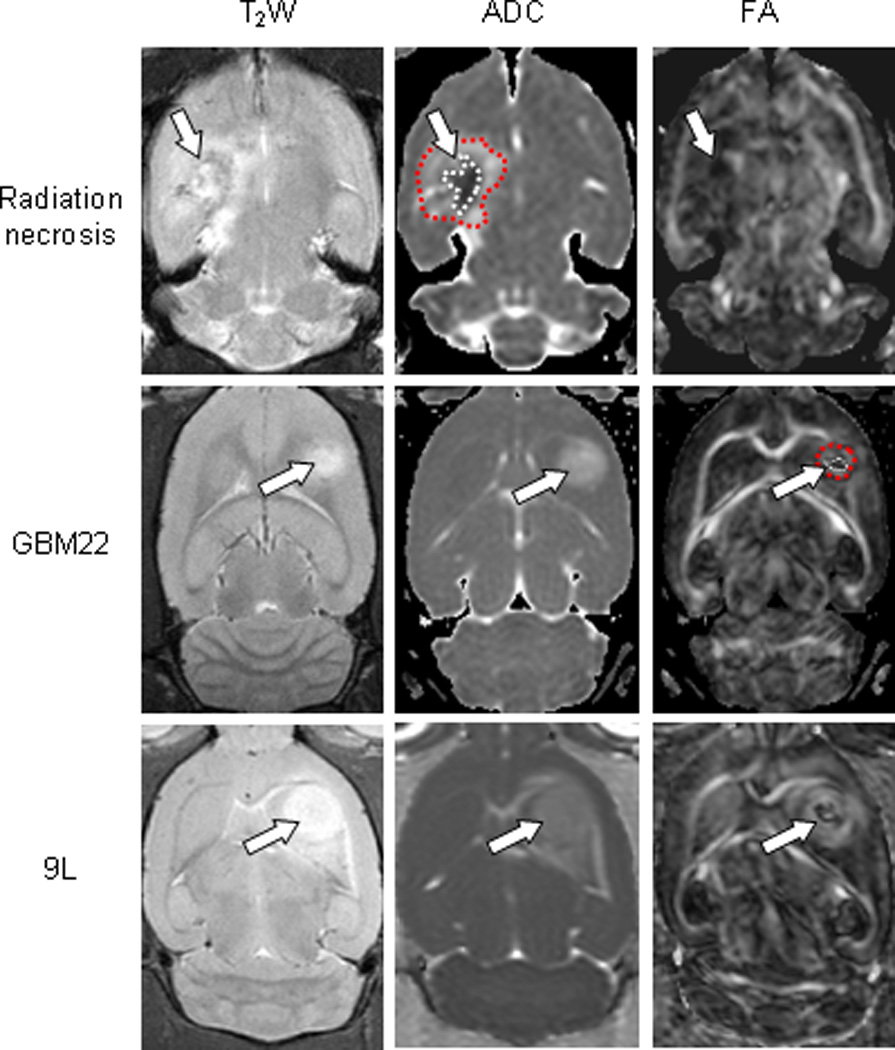
Comparison of the MRI features of radiation necrosis (26 weeks post-radiation), GBM22 (3 weeks post-implantation), and 9L gliosarcoma (9 days post-implantation). Radiation necrosis: On the ADC map, radiation necrosis is hypointense in the central zone and hyperintense in the peripheral zone. The lesion shows low diffusion anisotropy on the FA map. GBM22: The tumor is hyperintense on the ADC map. On the FA map, there is high diffusion anisotropy in the peripheral zone, with low diffusion anisotropy in the central zone. 9L gliosarcoma: The lesion is hyperintense on the ADC map. On FA map, it shows high contrasts and various degrees of high-level diffusion anisotropy within the tumor. Examples of ROIs, the central zone (1) and the peripheral zone (2), were drawn on ADC maps (for the radiation necrosis model), and on FA maps (for the GBM22 and 9L models). These ROIs were transferred to identical sites on other DTI index maps.
A quantitative comparison between the DTI indices in the peripheral zone and the central zone for both radiation necrosis and viable gliomas is shown in Fig. 4. In the case of radiation necrosis, there were significantly lower ADC, λ//, and λ⊥ values in the central zone than in the peripheral zone (all p < 0.001); however, no significant difference was found for the FA values, as seen from the FA maps. In contrast, in the GBM22 tumor, the FA and λ// values were significantly lower in the central zone than in the peripheral zone (p < 0.001, p = 0.03, respectively). In the case of 9L gliosarcomas, the tumor central zone had significantly lower FA values (p = 0.047) and significantly higher λ⊥ values (p = 0.01) than the tumor peripheral zone. No significant differences were found for the other DTI indices for these two tumor models.
Fig. 4.
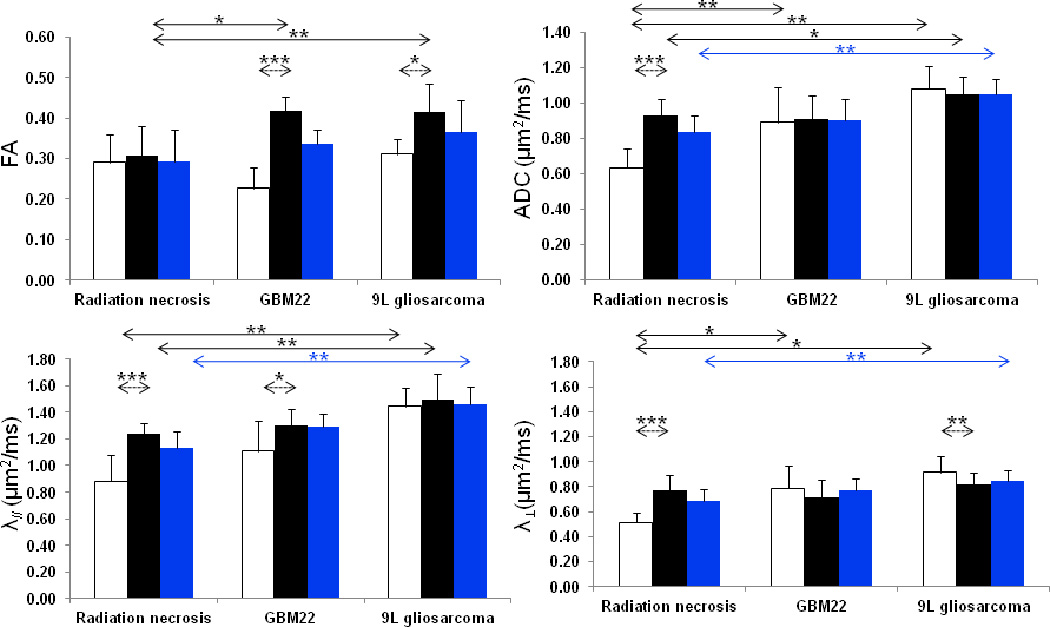
Quantitative analysis of DTI indices from radiation necrosis (n = 13), GBM22 tumors (n = 6), and 9L gliosarcomas (n = 8). White bar = central zone; black bar = peripheral zone; blue bar = whole lesion. For examples of ROIs selected, see Figs. 1 and 3. Solid line indicates significant differences in DTI indices between radiation necrosis and tumor; dashed line indicates significant differences between the central zone and the peripheral zone. * < 0.05; ** < 0.01; *** < 0.001.
DTI indices to distinguish between radiation necrosis and glioma
When the entire necrotic lesion was compared with the entire tumor lesion, the values of ADC, λ// and λ⊥ were significantly lower in radiation necrosis than in the 9L gliosarcoma (all p < 0.01; Fig. 4). No significant difference was found for FA (p = 0.083). However, there were no significant differences between the entire lesions of radiation necrosis and GBM22 tumors for all DTI indices, due to the fact that GBM22 was highly necrotic (spontaneous necrosis), as observed below in histology.
Radiation-induced necrosis was further compared with viable tumor in the central and peripheral zones (Fig. 4). Radiation necrosis had a significantly lower FA than 9L gliosarcoma (0.31 ± 0.07 vs. 0.40 ± 0.08, p = 0.005) and GBM22 (0.31 ± 0.07 vs. 0.39 ± 0.06, p = 0.012) in the peripheral zone. For the ADC measure, radiation necrosis had a significantly lower value than both 9L gliosarcoma (0.64 ± 0.11 µm2/ms vs. 1.08 ± 0.12 µm2/ms, p < 0.01) and GBM22 (0.64 ± 0.11 µm2/ms vs. 0.89 ± 0.19 µm2/ms, p < 0.01) in the central zone. In addition, radiation necrosis had a slightly lower value than 9L gliosarcoma in the peripheral zone (0.93 ± 0.10 µm2/ms vs. 1.05 ± 0.10 µm2/ms, p = 0.048). Furthermore, λ// was significantly lower in radiation necrosis than in 9L gliosarcoma in both the central zone and the peripheral zone (both p < 0.01). λ⊥ was significantly lower in radiation necrosis than in 9L gliosarcoma and GBM22 in the central zone (both p < 0.05). There were no significant differences for other comparisons between radiation necrosis and 9L gliosarcoma or GBM22. These results indicate that radiation necrosis had decreased water diffusion properties, compared to viable gliomas, particularly the 9L gliosarcoma. Note that the unique DTI patterns within radiation necrosis and tumors were not observed on the conventional T2w and Gd-T1w images. No other reference images could be used to draw the ROIs. Thus, the obtained results using DTI maps to define ROIs may be somewhat biased.
Univariate logistic regression analysis and ROC analysis were performed for all DTI indices to determine their predictive powers in distinguishing between radiation necrosis and glioma. It was found that FA values in the peripheral zone (p = 0.008), ADC and λ⊥ values in the central zone (p = 0.035 and 0.044), and λ// in both the peripheral zone and the central zone (p = 0.024 and 0.004) were significant predictors of radiation necrosis versus glioma. Table 1 summarizes the results of the ROC analysis for these selected DTI indices. The best parameter for the classification was λ⊥ in the central zone (AUC = 0.98, cut-off value = 0.61µm2/ms), followed by the ADC in the central zone (AUC = 0.96, cut-off value = 2.51 µm2/ms), and λ// in the central zone (AUC = 0.91, cut-off value = 0.91 µm2/ms). The results indicate that the DTI indices in the central zone had a higher predictive power than the DTI indices in the peripheral zone.
Table 1.
ROC analysis of DTI indices in the central zone or the peripheral zone to predict radiation necrosis and glioma
| DTI index | Location | Cut-off value | Sensitivity | Specificity | AUC | p |
|---|---|---|---|---|---|---|
| FA | Peripheral zone | 0.37 | 0.85 | 0.85 | 0.88 | 0.001 |
| ADC (µm2/ms) | Central zone | 0.84 | 0.77 | 1.00 | 0.96 | <0.001 |
| λ// (µm2/ms) | Central zone | 0.91 | 0.92 | 0.77 | 0.91 | <0.001 |
| Peripheral zone | 1.39 | 0.54 | 1.00 | 0.80 | 0.01 | |
| λ⊥ (µm2/ms) | Central zone | 0.61 | 1.00 | 0.92 | 0.98 | <0.001 |
Histological evaluations
It was observed that radiation caused considerable morphological changes, including the parenchymal coagulative necrosis, which was the cardinal characteristic of radiation necrosis, in the irradiated brain tissue (Fig. 5). These necrotic changes were found mainly in the white matter regions of the fornix, the external capsule, the internal capsule, and the cerebral peduncle. High-magnification histology showed the loss of normal brain tissue components, with vacuolation in the central zone of necrosis. In the peripheral zone of necrosis, there were randomly distributed necrotic cells, damaged vessels, hemorrhage, and reactive astrogliosis.
Fig. 5.
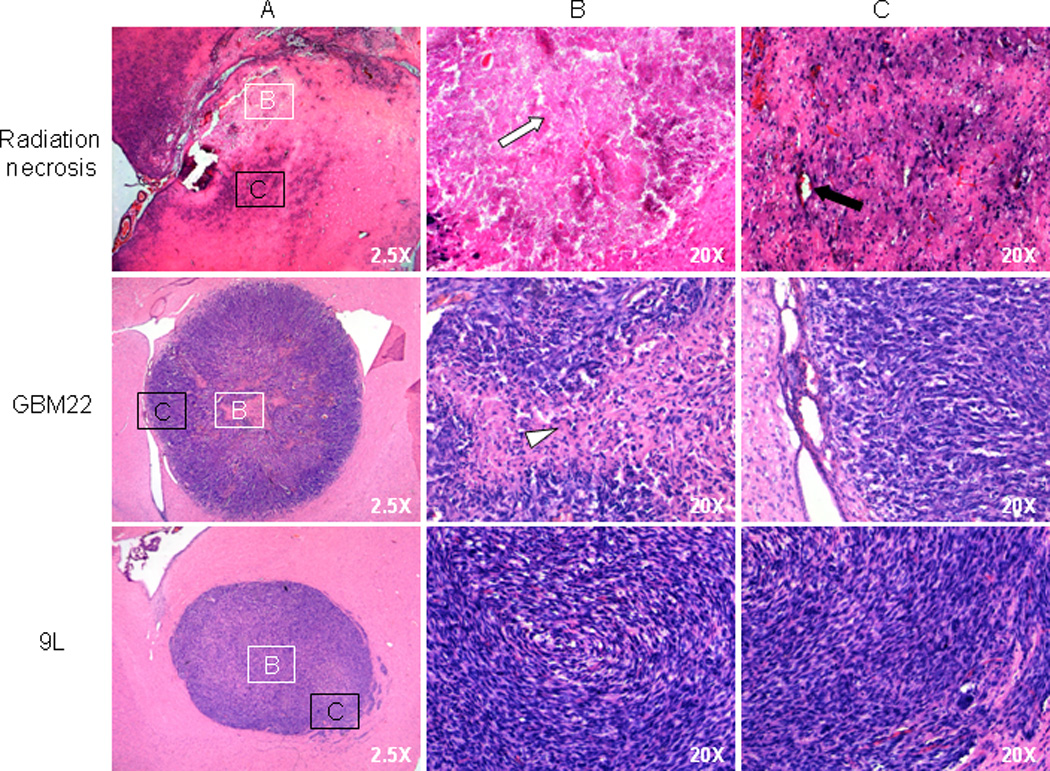
Histological features of radiation necrosis (25 weeks post-radiation), GBM22 (3 weeks post-implantation), and 9L gliosarcomas (10 days post-implantation). Panel A (2.5X) shows the whole view of lesions; Panels B and C show histological features of the central zones (white box) and the peripheral zones (black box) at higher magnification (20X). Radiation necrosis shows parenchymal coagulation necrosis, with fibrotic changes in the central zone (white arrow), as well as isolated necrotic cells, reactive astrogliosis, and dilated damaged vessels (black arrow) in the peripheral zone. GBM22 xenografts consist of a necrotic core (white arrowhead) with randomly distributed, low-density tumor cells and a peripheral zone of high-density tumor cells that organize in a radial pattern. The 9L gliosarcoma is relatively homogeneous from the central zone to the periphery, with a circular growth pattern of tumor cells in the peripheral zone.
In the GBM22 tumor (Fig. 5), there were high-density tumor cells in the tumor peripheral zone. It can be seen clearly that these high-density malignant cells were organized in a radial pattern (along a radius). As observed in high-grade brain tumors in the clinic, the GBM22 tumor central zone showed necrotic areas (spontaneous necrosis), corresponding to low FA values. On the other hand, the 9L tumor was composed of high-density tumor cells with round or fusiform shapes, high nuclear-to-cytoplasmic ratios, and deformed nuclei. In contrast to the GBM22 tumor, the 9L gliosarcoma displayed no spontaneous necrosis, and the entire tumor appeared to be solid high-density tumor cells. A circular organization of tumor cells was observed in its peripheral zone.
Discussion
Qualitative and quantitative DTI image analysis, as presented in this study, has clearly shown the distinctive imaging features of radiation-induced necrosis and viable glioma in rat models. Moreover, there is a close correlation between the DTI indices and the histological characteristics for these two pathologies.
It has been recognized that organized microstructures with high FA exist in viable glioma, where the brain parenchyma has been destroyed, due to tumor cells proliferating rapidly and packing closely in a coherent way. This is supported by some early pathology experiments in humans [26] as well as several recent DTI observations, both in animals [17–20] and in humans [27–29]. Interestingly, the FA maps reveal specific tumor cell arrangements within the peripheral zones of 9L and GBM22 xenografts (circular versus radial, respectively). On the other hand, radiation necrosis is associated with random microstructures, which reflects brain tissue necrosis and reactive reflection. Our results imply that FA has the potential to differentiate radiation necrosis from glioma with higher diffusion anisotropy.
The ADC is another important DTI index to measure the magnitude of water diffusion. Our preclinical results have indicated that the central zone of radiation necrosis is slightly hypointense (Figs. 2 and 3). The ADC values in the central zone were significantly lower in radiation necrosis than in either 9L gliosarcoma (with high density of tumor cells) or GBM22 (with spontaneous necrosis). This is because coagulative necrosis with fibrosis deposit (Fig. 5) is a major pathological change in the central zone of radiation necrosis. In this condition, the diffusion of water molecules is restricted in all directions due to decreased water content and obstacles from fibrosis deposit. On the contrary, the necrotic periphery shows a hyperintensity on the ADC map, which is associated with various complicated pathological changes, such as endothelial thickening, vascular dilation, and vasogenic edema [15, 16, 30]. Unlike radiation necrosis, there are consistently high ADC values in the entire glioma regions (Fig. 3), which have been attributed to the increase in perivascular space and micro-necrosis in the tumor [31].
λ// and λ⊥ can provide additional information about the directionality of water diffusion. These indices have been used to characterize myelin and axonal damage in white matter diseases [31, 32]. In radiation necrosis, λ// and λ⊥ were significantly decreased in the central zone, compared to the peripheral zone, but the FA values were not different for these two regions. Our ROC analysis results showed that λ// in the central zone and peripheral zone, as well as λ⊥ in the central zone, all provide satisfactory diagnostic powers to classify radiation necrosis or gliomas. Their diagnostic powers are approximately equal to ADC’s and higher than FA’s. It seems that, rather than the FA, the use of the ADC, λ//, and λ⊥, is better for distinguishing between radiation necrosis and glioma, although this needs to be validated further in patients who have more complex and histopathologically heterogeneous lesions.
When comparing radiation necrosis with glioma, it should be noted that the presence of spontaneous necrosis, commonly observed in high-grade gliomas, may be a confounder for radiation necrosis, because spontaneous necrosis would also be associated with low FA values, as observed in the central zone of the GBM22 tumor. Our results have shown that there are also different diffusion characteristics for the 9L and the GBM22 tumors, probably because of the spontaneous necrosis that leads to a very low FA value in the GBM22 central zone.
It is important to note that there are currently contradictory reports on the differentiation of radiation necrosis from tumor recurrence in patients using the DTI indices. For example, Sundgren et al. reported significantly decreased ADC, λ//, and λ⊥ values in radiation necrosis, compared to tumor recurrence, while no difference was found for FA [31]. However, several other researchers reported increased ADC and decreased FA values in radiation necrosis, compared to tumor recurrence [33–35]. This disagreement may be attributed to several factors. First, the quantitative analysis in the clinic is usually done in new contrast-enhancing regions, where delayed radiation injury, irradiated tumor, and tumor recurrence may coexist. The disruption of the blood-brain barrier for these pathologies is indistinguishable using standard MRI. Moreover, it is always very difficult to have the exact correspondence between the ROIs for the DTI analysis and tissue sampling for pathology. Based on our findings (Fig. 4), delayed radiation-induced necrosis consists of an ADC-hypointense central zone and an ADC-hyperintense peripheral zone, and increased and decreased ADC values can both be observed in the lesion, depending on the localization of the ROIs. In addition, it is know that the ADC values in brain tumors increase following treatment and drop to baseline when the tumors re-grow [36]. Therefore, the clinic results may differ between labs, as pointed out previously [31]. Second, clinical studies have reported that FA values differ within GBM, anaplastic astrocytoma, or pilocytic astrocytoma [12]. In particular, significant differences in FA were observed between grade-1 (mean FA: 0.150 ± 0.017) or grade-2 (0.159 ± 0.018), and grade-3 (0.230 ± 0.033) or grade-4 (0.229 ± 0.033) gliomas [37]. Obviously, when these gliomas consisting of various grades (thus various DTI indices) are compared as a whole with radiation necrosis, the predictive powers of the DTI indices in the differentiation between these two lesions will be reduced, and the results will be varied in different labs.
Care should be taken when translating our pre-clinical results to clinical practices. First, in the current pre-clinical study, we compared “pure” radiation necrosis with “pure” gliomas and further divided the lesions into the central and peripheral regions. However, "pure" radiation necrosis does not typically manifest itself in patients with gliomas because there is always an element of tumor cells left behind. Additionally, the relevant clinical question is typically "tumor recurrence" and not "pure" untreated gliomas. Moreover, radiation necrosis and recurrent tumor may often co-exist in patients. Second, radiation necrosis and gliomas are more heterogeneous in clinical conditions than in animal models. The strict periphery and central region observed in radiation necrosis are likely due to the well-controlled, focused radiation field in the current study. These characteristics are consistent with some previous reports of human cases [31], but not always observed in lesions thought to be radiation necrosis [33]. In spite of such complicity in real clinical situations, our pre-clinical results could provide a clear, reliable reference for numerous ongoing investigations into DTI’s ability to assess more heterogeneous radiation necrosis and gliomas in humans. In addition, our findings would be useful for the diagnosis of radiation-induced white matter necrosis in patients with nasopharyngeal carcinoma who have received radiation therapy [38].
Finally, there are several technical limitations in this study. A limitation is that the diffusion-weighted images were acquired only in six gradient directions. It is known that errors in FA decreased with the number of diffusion gradient directions [39, 40], so six was relatively small. Another one is that we reconstructed DTI maps by averaging the images over the different echo times to improve the signal-to-noise ratio [41, 42]. However, each echo technically has slightly different diffusion weighting due to the small extra contributions from crushers around 180°, read gradients, etc. These limitations may affect the precision and accuracy of DTI measurements.
In conclusion, our study suggests that delayed radiation-induced necrosis and viable glioma in rat models exhibit different DTI features. Particularly, the magnitudes and directionality of water diffusion are decreased in the central zone of radiation necrosis. DTI indices provide useful diagnostic information to distinguish between these two pathologies. These results may influence the clinical formulation of a treatment plan in the clinic.
Acknowledgments
The authors thank Dr. Hangyi Jiang for helpful discussion and Ms. Mary McAllister for editorial assistance. This work was supported in part by grants from NIH (EB009112 and EB009731) and the Dana Foundation.
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Mullins ME, Barest GD, Schaefer PW, Hochberg FH, Gonzalez RG, Lev MH. Radiation necrosis versus glioma recurrence: conventional MR imaging clues to diagnosis. AJNR Am J Neuroradiol. 2005;26:1967–1972. [PMC free article] [PubMed] [Google Scholar]
- 4.Butowski NA, Sneed PK, Chang S. Diagnosis and treatment of recurrent high-grade astrocytoma. J Clin Onc. 2006;24:1273–1280. doi: 10.1200/JCO.2005.04.7522. [DOI] [PubMed] [Google Scholar]
- 5.Jain R, Narang J, Sundgren PM, Hearshen D, Saksena S, Rock JP, Gutierrez J, Mikkelsen T. Treatment induced necrosis versus recurrent/progressing brain tumor: going beyond the boundaries of conventional morphologic imaging. J Neuro-Onc. 2010;100:17–29. doi: 10.1007/s11060-010-0139-3. [DOI] [PubMed] [Google Scholar]
- 6.Rogers LR, Gutierrez J, Scarpace L, Schultz L, Ryu S, Lord B, Movsas B, Honsowetz J, Jain R. Morphologic magnetic resonance imaging features of therapy-induced cerebral necrosis. J Neuro-Onc. 2011;101:25–32. doi: 10.1007/s11060-010-0222-9. [DOI] [PubMed] [Google Scholar]
- 7.Graves EE, Nelson SJ, Vigneron DB, Verhey L, McDermott M, Larson D, Chang S, Prados MD, Dillon WP. Serial proton MR spectroscopic imaging of recurrent malignant gliomas after gamma knife radiosurgery. AJNR. 2001;22:613–624. [PMC free article] [PubMed] [Google Scholar]
- 8.Barajas RF, Chang JS, Sneed PK, Segal MR, McDermott MW, Cha S. Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging AJNR. Am J Neuroradiol. 2009;30:367–372. doi: 10.3174/ajnr.A1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitsuya K, Nakasu Y, Horiguchi S, Harada H, Nishimura T, Bando E, Okawa H, Furukawa Y, Hirai T, Endo M. Perfusion weighted magnetic resonance imaging to distinguish the recurrence of metastatic brain tumors from radiation necrosis after stereotactic radiosurgery. J Neuro-Onc. 2010;99:81–88. doi: 10.1007/s11060-009-0106-z. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Tryggestad E, Wen Z, Lal B, Zhou T, Grossman R, Wang S, Yan K, Fu DX, Ford E, Tyler B, Blakeley J, Laterra J, van Zijl PC. Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nat Med. 2011;17:130–134. doi: 10.1038/nm.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arbab AS, Janic B, Jafari-Khouzani K, Iskander AS, Kumar S, Varma NR, Knight RA, Soltanian-Zadeh H, Brown SL, Frank JA. Differentiation of glioma and radiation injury in rats using in vitro produce magnetically labeled cytotoxic T-cells and MRI. PLoS One. 2010;5:e9365. doi: 10.1371/journal.pone.0009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinha S, Bastin ME, Whittle IR, Wardlaw JM. Diffusion tensor MR imaging of high-grade cerebral gliomas. AJNR. 2002;23:520–527. [PMC free article] [PubMed] [Google Scholar]
- 13.Mori S, Frederiksen K, van Zijl PC, Stieltjes B, Kraut MA, Solaiyappan M, Pomper MG. Brain white matter anatomy of tumor patients evaluated with diffusion tensor imaging. Ann Neurol. 2002;51:377–380. doi: 10.1002/ana.10137. [DOI] [PubMed] [Google Scholar]
- 14.Huang H, Zhang J, Wakana S, Zhang W, Ren T, Richards LJ, Yarowsky P, Donohue P, Graham E, van Zijl PCM, Mori S. White and gray matter development in human fetal, newborn and pediatric brains. NeuroImage. 2006;33:27–38. doi: 10.1016/j.neuroimage.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Wang SL, Wu EX, Qiu DQ, Leung LHT, Lau HF, Khong PL. Longitudinal diffusion tensor magnetic resonance imaging study of radiation-induced white matter damage in a rat model. Cancer Research. 2009;69:1190–1198. doi: 10.1158/0008-5472.CAN-08-2661. [DOI] [PubMed] [Google Scholar]
- 16.Chan KC, Khong PL, Cheung MM, Wang SL, Cai KX, Wu EX. MRI of late microstructural and metabolic alterations in radiation-induced brain injuries. J Magn Reson Imaging. 2009;29:1013–1020. doi: 10.1002/jmri.21736. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, van Zijl PCM, Laterra J, Salhotra A, Lal B, Mori S, Zhou J. Unique patterns of diffusion directionality in rat brain tumors revealed by high-resolution diffusion tensor MRI. Magn Reson Med. 2007;58:454–462. doi: 10.1002/mrm.21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S, Pickup S, Hsu O, Poptani H. Diffusion tensor MRI in rat models of invasion and well-demarcated brain tumors. NMR Biomed. 2008;21:208–216. doi: 10.1002/nbm.1183. [DOI] [PubMed] [Google Scholar]
- 19.Asanuma T, Doblas S, Tesiram YA, Saunders D, Cranford R, Pearson J, Abbott A, Smith N, Towner RA. Diffusion tensor imaging and fiber tractography of C6 rat glioma. J Magn Reson Imag. 2008;28:566–573. doi: 10.1002/jmri.21473. [DOI] [PubMed] [Google Scholar]
- 20.Lope-Piedrafita S, Garcia-Martin ML, Galons J-P, Gillies RJ, Trouard TP. Longitudinal diffusion tensor imaging in a rat brain glioma model. NMR Biomed. 2008;21:799–808. doi: 10.1002/nbm.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarkaria JN, Carlson BL, Schroeder MA, Grogan P, Brown PD, Giannini C, Ballman KV, Kitange GJ, Guha A, Pandita A, James CD. Use of an orthotopic xenograft model for assessing the effect of epidermal growth factor receptor amplification on glioblastoma radiation response. Clin Cancer Res. 2006;12:2264–2271. doi: 10.1158/1078-0432.CCR-05-2510. [DOI] [PubMed] [Google Scholar]
- 22.Salhotra A, Lal B, Laterra J, Sun PZ, van Zijl PCM, Zhou J. Amide proton transfer imaging of 9L gliosarcoma and human glioblastoma xenografts. NMR Biomed. 2008;21:489–497. doi: 10.1002/nbm.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong J, Armour E, Kazanzides P, Iordachita U, Tryggestad E, Deng H, Matinfar M, Kennedy C, Liu Z, Chan T, Gray O, Verhaegen F, McNutt T, Ford E, DeWeese TL. High-resolution, small animal radiation research platform with X-ray tomographic guidance capabilities. Int J Rad Oncol Bio Phys. 2008;71:1591–1599. doi: 10.1016/j.ijrobp.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy AS, Archambeau JO, Archambeau M-H, Holshouser B, Thompson J, Moyers M, Hinshaw D, Slater JM. Magnetic resonance imaging as a monitor of changes in the irradiated rat brain. An aid in determining the time course of events in a histologic study. Invest Radiol. 1995;30:214–220. doi: 10.1097/00004424-199504000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S. DtiStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed. 2006;81:106–116. doi: 10.1016/j.cmpb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Burger PC, Dubois PJ, Schold SCJ, Smith KRJ, Odom GL, Crafts DC, Giangaspero F. Computerized tomographic and pathologic studies of the untreated, quiescent, and recurrent glioblastoma multiforme. J Neurosurg. 1983;58:159–169. doi: 10.3171/jns.1983.58.2.0159. [DOI] [PubMed] [Google Scholar]
- 27.Beppu T, Inoue T, Shibata T, Kurose AHA, Ogasawara K, Ogawa A, Nakamura S, Kabasawa H. Measurement of fractional anisotropy using diffusion tensor MRI in supratentorial astrocytic tumors. J Neuro-Oncol. 2003;63:109–116. doi: 10.1023/a:1023977520909. [DOI] [PubMed] [Google Scholar]
- 28.Beppu T, Inoue T, Shibata Y, Yamada N, Kurose A, Ogasawara K, Ogawa A, Kabasawa H. Fractional anisotropy value by diffusion tensor magnetic resonance imaging as a predictor of cell density and proliferation activity of glioblastomas. Surg Neur. 2005;63:56–61. doi: 10.1016/j.surneu.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 29.Kinoshita M, Hashimoto N, Goto T, Kagawa N, Kishima H, Izumoto S, Tanaka H, Fujita N, Yoshimine T. Fractional anisotropy and tumor cell density of the tumor core show positive correlation in diffusion tensor magnetic resonance imaging of malignant brain tumors. NeuroImage. 2008;43:29–35. doi: 10.1016/j.neuroimage.2008.06.041. [DOI] [PubMed] [Google Scholar]
- 30.Yang I, Aghi MK. New advances that enable identification of glioblastoma recurrence. Nat Rev Clin Oncol. 2009;6:648–657. doi: 10.1038/nrclinonc.2009.150. [DOI] [PubMed] [Google Scholar]
- 31.Sundgren PC, Fan X, Weybright P, Welsh RC, Carlos RC, Petrou M, McKeever PE, Chenevert TL. Differentiation of recurrent brain tumor versus radiation injury using diffusion tensor imaging in patients with new contrast-enhancing lesions. Magn Reson Imag. 2006;24:1131–1142. doi: 10.1016/j.mri.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Wang SL, Wu EX, Tam CN, Lau HF, Cheung PT, Khong PL. Characterization of white matter injury in a hypoxic-ischemic neonatal rat model by diffusion tensor MRI. Stroke. 2008;39:2348–2353. doi: 10.1161/STROKEAHA.107.509927. [DOI] [PubMed] [Google Scholar]
- 33.Hein PA, Eskey CJ, Dunn JF, Hug EB. Diffusion-weighted imaging in the follow-up of treated high-grade gliomas: tumor recurrence versus radiation injury. AJNR Am J Neuroradiol. 2004;25:201–209. [PMC free article] [PubMed] [Google Scholar]
- 34.Asao C, Korogi Y, Kitajima M, Hirai T, Baba Y, Makino K, Kochi M, Morishita S, Yamashita Y. Diffusion-weighted imaging of radiation-induced brain injury for differentiation from tumor recurrence. AJNR Am J Neuroradiol. 2005;26:1455–1460. [PMC free article] [PubMed] [Google Scholar]
- 35.Kashimura H, Inoue T, Beppu T, Ogasawara K, Ogawa A. Diffusion tensor imaging for differentiation of recurrent brain tumor and radiation necrosis after radiotherapy -- three case reports. Clin Neurol Neuros. 2007;109:106–110. doi: 10.1016/j.clineuro.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Chenevert TL, McKeever PE, Ross BD. Monitoring early response of experimental brain tumors to therapy using diffusion magnetic resonance imaging. Clin Cancer Res. 1997;3:1457–1466. [PubMed] [Google Scholar]
- 37.Inoue T, Ogasawara K, Beppu T, Ogawa A, Kabasawa H. Diffusion tensor imaging for preoperative evaluation of tumor grade in gliomas. Clin Neurol Neurosurg. 2005;107:174–180. doi: 10.1016/j.clineuro.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Wang YX, King AD, Zhou H, Leung SF, Abrigo J, Chan YL, Hu CW, Yeung DK, Ahuja AT. Evolution of radiation-induced brain injury: MR imaging-based study. Radiology. 2010;254:210–218. doi: 10.1148/radiol.09090428. [DOI] [PubMed] [Google Scholar]
- 39.Poonawalla AH, Zhou XJ. Analytical error propagation in diffusion anisotropy calculations. J Magn Reson Imaging. 2004;19:489–498. doi: 10.1002/jmri.20020. [DOI] [PubMed] [Google Scholar]
- 40.Ni H, Kavcic V, Zhu T, Ekholm S, Zhong J. Effects of number of diffusion gradient directions on derived diffusion tensor imaging indices in human brain. AJNR Am J Neuroradiol. 2006;27:1776–1781. [PMC free article] [PubMed] [Google Scholar]
- 41.Gulani V, Weber T, Neuberger T. Improved time efficiency and accuracy in diffusion tensor microimaging with multiple-echo acquisition. J Magn Reson. 2005;177:329–335. doi: 10.1016/j.jmr.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Nana R, Zhao T, Hu X. Single-shot multiecho parallel echo-planar imaging (EPI) for diffusion tensor imaging (DTI) with improved signal-to-noise ratio (SNR) and reduced distortion. Magn Reson Med. 2008;60:1512–1517. doi: 10.1002/mrm.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]


