Abstract
The polycystin family of transient receptor potential (TRP) channels form Ca2+ regulated cation channels with distinct subcellullar localizations and functions. As part of heteromultimeric channels and multi-protein complexes, polycystins control intracellular Ca2+ signals and more generally the translation of extracellular signals and stimuli to intracellular responses. Polycystin-2 channels have been cloned from retina, but their distribution and function in retinal ganglion cells (RGCs) have not yet been established. In the present study, we determined cellular and subcellular localization as well as functional properties of polycystin-2 channels in RGCs. Polycystin-2 expression and distribution in RGCs was assessed by immunohistochemistry on vertical cryostat section of mouse retina as well as primary cultured mouse RGCs, using fluorescence microscopy. Biophysical and pharmacological properties of polycystin-2 channels isolated from primary cultured RGCs were determined using planar lipid bilayer electrophysiology. We detected polycystin-2 immunoreactivity both in the ganglion cell layer as well as in primary cultured RGCs. Subcellular analysis revealed strong cytosolic localization pattern of polycystin-2. Polycystin-2 channel current was Ca2+ activated, had a maximum slope conductance of 114 pS and could be blocked in a dose-dependent manner by increasing concentrations of Mg2+. The cytosolic localization of polycystin-2 in RGCs is in accordance with its function as intracellular Ca2+ release channel. We conclude that polycystin-2 forms functional channels in RGCs, of which biophysical and pharmacological properties are similar to polycystin-2 channels reported for other tissues and organisms. Our data suggest a potential role for polycystin-2 in RGC Ca2+ signaling.
Keywords: polycystin, transient receptor potential channel, retinal ganglion cells, calcium, electrophysiology
Introduction
Calcium homeostasis and signaling are tightly regulated in excitable cells (Koulen and Thrower, 2001, Berridge et al., 2003), including neurons of the retina (Akopian and Witkovsky, 2002), and control a large number of intracellular mechanisms, such as gene expression, neurotransmitter and hormone secretion and apoptosis (Koulen and Thrower, 2001, Berridge et al., 2003, Duncan et al., 2010) through modulation of the intracellular free Ca2+ concentration.
Two pathways exist that permit Ca2+ entering the intracellular milieu: 1.) from the extracellular space through voltage-, ligand-, store- or second messenger-activated Ca2+ channels (Berridge et al., 2003), or 2.) from intracellular stores such as the endoplasmic reticulum (ER) (Berridge et al., 2003). Besides Ca2+ entering the cell through these pathways, Ca2+ uptake into intracellular stores or Ca2+ extrusion to the extracellular space are under cellular control and critically shape cellular Ca2+ transients and Ca2+ homeostasis (Koulen and Thrower, 2001, Berridge et al., 2003), however, their mechanisms are less well understood.
Three classes of intracellular Ca2+ channels exist, each containing several isoforms, which each display temporospatially distinct expression patterns, biophysical properties and physiological functions (Koulen and Thrower, 2001): inositol-1,4,5-trisphosphate receptors (IP3Rs), ryanodine receptors (RyRs) and polycystin-2 family transient receptor potential channels (TRPPs). The group of TRPPs comprises eight proteins, three of which have been shown to be cation channels (for review see (Gees et al., 2010)): polycystin-2, polycystin-L and polycystin-2L2.
The mammalian retina contains a large number of specialized cell types (Masland, 2001a, b). Retinal ganglion cells (RGCs) convert the synaptic input into spike output that transduces visual information to the brain for processing, and hence, are critical for visual function (Nassi and Callaway, 2009). Recent progress has been made in elucidating the signaling pathways in RGC dendrites involving voltage-gated Ca2+ channels (Margolis et al., 2010), however, surprisingly little is known about expression, distribution and function of intracellular Ca2+ channels in RGCs. We previously reported the differential distribution of IP3Rs in RGCs (Mafe et al., 2006), and polycystin-L and polycystin-2L2 were detected in retina (Nomura et al., 1998, Wu et al., 1998, Guo et al., 2000). The lack of knowledge on the role of polycystin-2 in the retina is surprising given the tentative association between polycystic kidney disease and macular defects in patients and experimental models (Narendran et al., 2004, Feng et al., 2009).
A better understanding of the expression, distribution and function of polycystin-2 will provide the basis for further elucidation of the intracellular Ca2+ signaling pathways in RGCs and their putative role in pathologies such as glaucoma, in which loss of RGCs can ultimately lead to blindness (Fan and Wiggs, 2010).
Here, we report the subcellular distribution, expression and functional characterization of polycystin-2 channels in mouse RGCs.
Materials and Methods
Animals
Male adult albino Swiss-Webster and C57/B6 mice (4–6 weeks of age) were euthanized by CO2 overexposure. All experiments described herein were in compliance with the guidelines for the welfare of experimental animals issued by the National Institutes of Health, in accordance with institutional guidelines and the ARVO statement for the use of animals in ophthalmic and vision research.
Materials and antibodies
Cell culture reagents were obtained from Gibco® (Invitrogen, Carlsbad, CA). Unless otherwise indicated, all other reagents were obtained from Sigma (St. Louis, MO).
The mouse anti-CD90 (Thy1.2) was obtained from Caltag (Burlingame, CA) and used at 1:200 for immunohistochemistry and immunocytochemistry. Rabbit anti-neurofilament (NFM) 68 kDA (1:1,000 for immunocytochemistry), mouse anti-OX42 (IgG2a; 1:200), mouse anti-Brn3a (clone 5A3.2; 1:200 for immunocytochemistry) and rabbit anti-PC-2 (#AB9088; 1:1,000 for immunohistochemistry and immunocytochemistry) were purchased from Millipore (Temecula, CA).
Fluorescently conjugated secondary antibodies (AlexaFluor® 488 goat anti-rabbit IgG and AlexaFluor® 594 goat anti-mouse IgG; Invitrogen, Carlsbad, CA) were used at 1:2,000. ProLong® Antifade (Invitrogen, Carlsbad, CA) was used for mounting and nuclear staining of cultured RGCs. 4′,6-diamidino-2-phenylindole (DAPI; EMD Chemicals, Gibbstown, NJ; 1:50,000 dilution) and Aqua-Poly/Mount (Polysciences Inc., Warrington, PA) were used for nuclear staining and mounting of retina sections.
Preparation of retinal cultures
RGCs were prepared from 4–6 weeks old male albino Swiss Webster mice (6 retinae from three different animals per preparation) and cultured as described by us previously (Mafe et al., 2006). Briefly, eyes were enucleated and dissected in Neurobasal A medium (Invitrogen, Carlsbad, CA). Retinae were enzymatically dissociated by incubation in papain solution (34 U/ml papain, 3.3 mM DL-cysteine, 0.4 mg/ml bovine serum albumin in Neurobasal A medium for 25 min at 37 °C), washing three times in culture medium and mechanical trituration. Cells were plated onto 12 mm glass coverslips coated with poly-D-lysin/laminin (BD Biocoat™, BD Biosciences, Bedford, MA) and maintained for 14 days in a humidified atmosphere of 95% air and 5% CO2at 37 °C. Cells were grown in culture medium (Neurobasal A supplemented with 1 × B27, 100 U/ml penicillin, 100 μg/ml streptomycin, 110 mg/ml pyruvate, 292 ng/ml glutamine, 1% fetal bovine serum, 5 ng/ml insulin, 100 μg/ml transferrin, 100 μg/ml crystalline bovine serum albumin (BSA), 60 ng/ml progesterone, 16 μg/ml putrescine, 40 ng/ml sodium selenite, 40 ng/ml thyroxine, 40 ng/ml tri-iodothyronin, 50 ng/ml, brain derived neurotrophic factor, 10 ng/ml ciliary neurotrophic factor, 2 mg/ml Forskolin, and 10 ng/ml fibroblast growth factor b).
Immunocytochemistry
Immunocytochemistry was carried out as described by us previously for RGCs (Mafe et al., 2006). Briefly, after 7–14 days of culture, RGCs were fixed using 4% paraformaldehyde in 10 mM phosphate buffered saline pH 7.4 (PBS) for 30 min. Cells were permeabilized in 3% normal goat serum (NGS), 1% BSA and 0.05%, Triton X-100 following a 1 hr block in PBS containing 10% NGS, 1% BSA and 0.05% Triton X-100. Primary antibody was applied and coverslips incubated overnight at 4 °C in a humidified chamber, protected from light. Following wash with PBS, secondary antibody was applied for 1 hr at RT, in a humidified chamber and protected from light. After washing, coverslips were mounted on slides using ProLong® Antifade (Invitrogen, Carlsbad CA). Negative controls consisted of the omission of either primary or secondary antibody from the incubation steps and were performed at the same time (data not shown). Immunofluorescence labeling was examined and recorded using an Olympus IX70 microscope (Olympus Corp., Center Valley, PA). We performed immunocytochemistry on four separate cultures of RGCs (n=4).
Immunohistochemistry
Immunohistochemistry was carried out essentially as previously described (Koulen and Brandstatter, 2002, Kaja et al., 2003, Koulen et al., 2005a, Mafe et al., 2006). Eye cups were immersion fixed in 4% paraformaldehyde in PBS for 20 min. Tissue was cryoprotected by infusion with 30% sucrose, 0.05% sodium azide in PBS. Vertical cryosections (12 μm) were cut on a cryostat (Leica Microsystems, Bannockburn, IL). Sections were collected on silane coated slides (Mercedes Medical, Sarasota, FL) and dried for 30 min at RT. Immunofluorescence labeling was examined, recorded and analyzed using a Leica SP5X white light laser, laser scanning microscope (Leica Microsystems, Bannockburn, IL) and using the Leica Application Suite Advanced Fluorescence Software v2.4.1 (Leica Microsystems, Bannockburn, IL). Negative controls consisted of the omission of either primary or secondary antibody from the incubation steps and were performed at the same time (data not shown). Furthermore, specificity of the antibody was confirmed by pre-incubation with 100– fold excess of the peptide used for antibody generation (#AG565, Millipore, Temecula, CA). Immunoreactivity was measured in three sections each for a total of 4 eyes (each obtained from a different mouse).
Single channel electrophysiology
ER membrane-enriched vesicles from immunopanned RGCs (obtained from retinae of 12 animals) were used in planar bilayer lipid membrane (BLM) electrophysiology as described previously (Koulen and Ehrlich, 2000, Koulen et al., 2001, Koulen et al., 2002, Westhoff et al., 2003, Koulen et al., 2005a, Koulen et al., 2005b, Hayrapetyan et al., 2008, Rybalchenko et al., 2008, Rybalchenko et al., 2009, Kaja et al., 2011b) using a Warner Instruments BLM workstation (Warner Instruments, LLC, Hamden, CT).
Single channel activity was determined with BLM cis solution corresponding to the cytosol on the cytosolic side of the channel [250 mM HEPES-Tris, pH 7.35] and BLM trans-solution corresponding to the ER lumen on the ER luminal side of the channel [250 mM HEPES, 55 mM Ba(OH)2, pH 7.35] under voltage-clamp conditions. Other cationic conductances were measured by substituting the appropriate hydroxide salt for Ba(OH)2. Ryanodine receptor activity was employed to identify the proper orientation during incorporation of ER membrane proteins into the BLM (Koulen et al., 2002). Purity of the endoplasmic reticulum membrane preparation was determined using standard markers of cellular sub-compartments, calnexin, NHE3, EGFR and the Golgi 58K protein, as described previously (Koulen et al., 2002). Addition to the cis-side of the BLM, i.e. the cytosolic side of the ER, was used for pharmacological agents and physiological modulators. Addition to the trans side of the BLM, corresponding to the ER lumen, resulted in no change in channel activity. Known pharmacological modulators of intracellular calcium channels (cyclic adenosine diphosphate ribose [cADPR], caffeine, ryanodine, ruthenium red, IP3, Xestospongin C, heparin approximate average Mr, 6000) and all other reagents were obtained from Sigma-Aldrich (St. Louis, MO). Electrophysiological recordings were performed and analyzed using Clampex version 8.1.0.12 (Axon Instruments, Burlingame, CA).
Results
Polycystin-2 is localized to the cytosol in mouse retinal ganglion cells
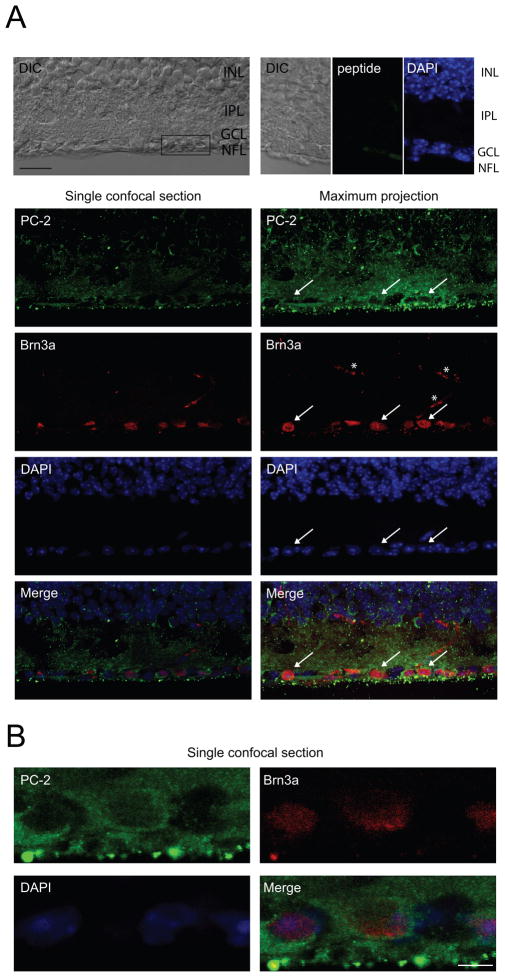
In order to investigate the expression and localization of polycystin-2 in RGCs, we performed immunohistochemistry on vertical whole retina sections. Polycystin-2-specific immunoreactivity was strong in the cytosol of RGCs (Fig. 1), in accordance with its role as intracellular Ca2+ channel (Koulen et al., 2002). In order to identify retinal ganglion cells, we double-labeled retina sections with a marker for RGCs, brain-specific homeobox/POU domain protein 3A (Brn3a) (Nadal-Nicolas et al., 2009). Specificity of the antibody was confirmed by performing control experiments, in which primary antibody was pre-incubated with the peptide used for antibody generation (Fig. 1). Furthermore, specificity has previously shown by us and others (Tran et al., 2010, Kaja et al., 2011b).
Figure 1. Polycystin-2 is localized to the cytosol of adult mouse RGCs.
A. Immunohistochemical analysis of vertical cryostat sections showing polycystin-2 immunoreactivity in adult mouse RGCs. Brn3a was used as nuclear RGC marker. Representative images are shown. A single confocal section acquired with differential interference contrast (DIC) is shown, indicating the retinal layers (INL, inner nuclear layer; GCL, ganglion cell layer; NFL, nerve fiber layer). The box shows the location of the high-magnification image shown in B. No significant immunoreactivity was obtained when pre-incubating with 100-fold excess of blocking peptide, confirming the specificity of the antibody. A single confocal section (252 nm thickness) and a maximum projection of a z-stack (8.82 μm thickness) are shown. Strong immunoreactivity was found in the cytosol of RGCs (indicated by white arrows) and throughout the inner plexiform layer (IPL). Scale bar, 20 μm. White asterisks indicate non-specific blood vessel staining by the AlexaFluor™ 594 goat anti-mouse secondary antibody. B. Single high magnification confocal section through the RGC layer. Polycystin-2 staining is confined to the cytosol, whereas Brn3a and DAPI are nuclear markers. Scale bar, 5 μm.
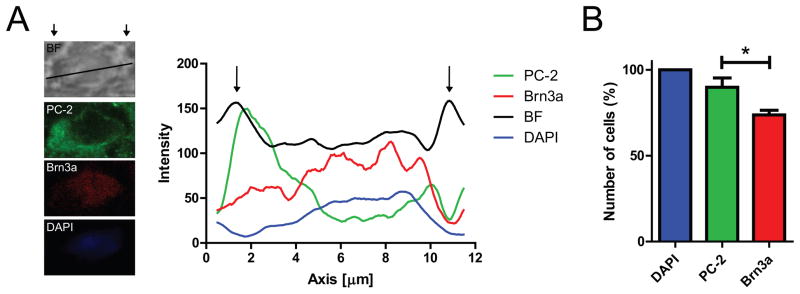
In order to further identify the subcellular expression in RGCs, we performed intensity line scans on high resolution single confocal section images, as indicated in Fig. 2A. Plotting the intensity of immunoreactivity over distance reveals distinct peaks for the bright field (BF) signal, defining the borders of the cell (Fig. 2A; indicated by arrows). Furthermore, intensities for the nuclear markers Brn3a and DAPI overlap, whereas polycystin-2 immunoreactivity shows distinct cytosolic peaks indicative of ER localization.
Figure 2. Subcellular analysis of polycystin-2 immunoreactivity in adult mouse RGCs.
A. Representative intensity analysis along a region of interest (ROI) line indicated in the bright field (BF) image. Intensity of immunoreactivity is plotted as 8 point moving average over distance (in μm). Arrows define the borders of the RGC as identified as peaks in BF intensity. Maximum intensity of polycystin-2 (PC-2) is in the cytoplasm, whereas Brn3a immunoreactivity co-localizes with the nuclear stain DAPI. B. A total of 89.8 ± 5.4% of cells in the RGC layer (defined based on positive DAPI staining) were positive for polycystin-2, whereas only 73.7 ± 2.8% of cells were positive for Brn3a (n=4, P<0.05). Quantification is based on analysis of maximum projection images of z-stacks (7–10 μm thickness) obtained from four different retinae.
Lastly, we quantified the number of polycystin-2 and Brn3a positive cells in the RGC layer. 89.9 ± 5.4% of cells in the RGC layer showed polycystin-2 immunoreactivity, when normalized to the total number of cells in the RGC layer with DAPI staining (Fig. 2B). In contrast, 73.8 ± 2.8% of cells were immunoreactive for Brn3a (Fig. 2B).
Taken together our data suggest that polycystin-2 is expressed in cytosol of RGCs in the murine retina.
Polycystin-2 is expressed in primary cultures of RGCs
Given that primary cultures of retinal cells are an important model system for studying physiological and pathophysiological mechanisms, including calcium signaling (Koulen et al., 2005b, Koulen et al., 2008), we next investigated whether polycystin-2 expression is maintained in primary cultures of RGCs.
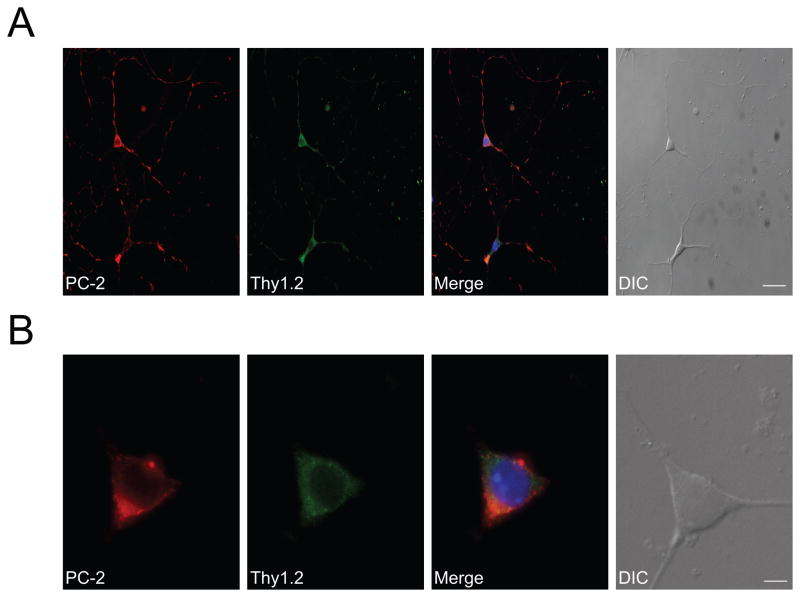
We identified polycystin-2 immunoreactivity in essentially all RGCs, as identified based on their morphology as well as the RGC-specific marker CD90/Thy1.2 (Barnstable and Drager, 1984, Morris and Grosveld, 1989) (Fig. 3). The expression and distribution of polycystin-2 was confined to the cytosol and neurites of murine RGCs in culture. In order to ensure that neural properties were not lost during culturing, we used NFM 68 kDa immunoreactivity as neuronal marker and detected strong immunoreactivity in Thy1.2-positive RGCs (data not shown). Omission of primary antibodies resulted in complete lack of immunoreactivity for polycystin-2, NFM and Thy1.2 (data not shown).
Figure 3. Polycystin-2 localizes to the cytosol of primary cultured RGCs.
A. Polycystin-2 is expressed in primary cultured RGCs. Representative images are shown. Identity of cultured cells was confirmed with the RGC marker Thy 1.2. In Thy 1.2-positive cells, polycystin-2 immunoreactivity was punctate and cytosolic, indicative of intracellular localization. Prominent polycystin-2 immunoreactivity was also observed in neurites. Thy1.2 was used as a cellular marker for positively identifying RGCs. The merged image also shows DAPI staining, positively identifying the nucleus. Scale bar, 25 μm. B. High magnification images showing that polycystin-2 immunoreactivity is confined to the cytosol. Scale bar, 5 μm.
Biophysical and pharmacological properties of polycystin-2 isolated from RGCs
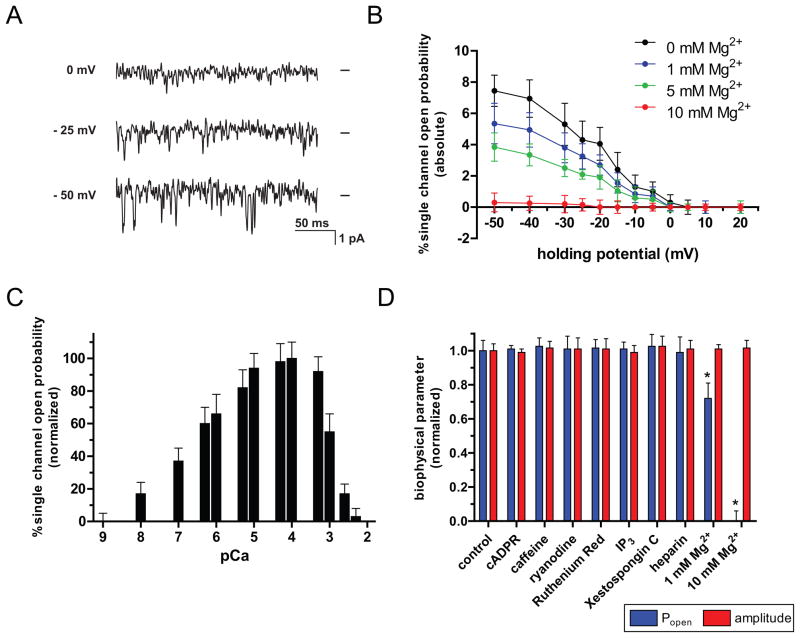
In order to identify the functional role of polycystin-2 expression in RGCs, we tested whether polycystin-2 forms intracellular Ca2+ channels in RGCs. To this end, we determined the biophysical and pharmacological properties of native polycystin-2 channels isolated in ER microsomes from immunopurified RGCs using single channel electrophysiology (Fig. 4). Polycystin-2 in ER vesicles purified from isolated RGCs was reconstituted into planar bilayer lipid membranes. In the absence of IP3 and in the presence of 20 μM ruthenium red and 10 μM ryanodine to exclude activity from IP3 receptors and ryanodine receptors, respectively, polycystin-2 single channel activity was recorded. As reported for kidney- and lacrimal gland-derived polycystin-2 channels (Koulen et al., 2002, Kaja et al., 2011b), polycystin-2 from RGCs also displayed a distinct voltage-dependent activity with increasing negative holding potentials leading to further activation that could be dose-dependently inhibited by increasing amounts of cytoplasmic Mg2+ (Fig. 4A, B). The slope conductance for polycystin-2 channels obtained from immunopanned RGCs was 114 pS, when using Ba2+ as the current carrier. This is identical to the reported values for kidney-derived polycystin-2 and similar to the C. elegans polycystin-2 properties (104 pS; (Koulen et al., 2005a)). Ion selectivity was determined at 90%, 85%, 78% of the Ba2+ conductance for the ions Sr2+, Ca2+ and Mg2+, respectively (data not shown). Because of the resulting high single channel currents and the lack of potential modulation of intracellular calcium channels, Ba2+ was chosen as the current carrier. Polycystin-2 showed well-defined calcium dependence with activation by physiological concentrations of cytosolic Ca2+ (100nM – 1mM, i.e. pCa 7-3) and inactivation at higher cytosolic Ca2+ levels (Fig. 4C). The channel could be blocked with La3+ and Gd3+. Pharmacological modulators IP3 and ryanodine receptors did not influence single channel activity (measured as open probability; Fig. 4D) or biophysical properties (measured as single channel amplitude; Fig. 4D). However, while cytosolic Mg2+ had also no effect on single channel current, conductance and amplitude-voltage relationship, channel open probability was dose-dependent on the cytosolic Mg2+ concentration with complete channel inactivation at 10 mM (Fig. 4A–C).
Figure 4. Biophysical and pharmacological characteristics of single channel polycystin-2 isolated from RGCs.
(A) Single channel currents of RGC polycystin-2 were recorded at different holding potentials. Representative traces of a typical experiment with native channels isolated from ER microsomes and incorporated into bilayer lipid membranes are shown. Single channel activity was recorded at a cytoplasmic Ca2+ concentration of 0.1 μM. Zero current levels are indicated by bars at the right of each trace and downward deflections indicate channel openings. Channels display a pronounced voltage-dependence and activation by negative holding potentials. (B) The single-channel open probability of native channels isolated from RGC ER microsomes was measured in the presence of 0.1 μM cytoplasmic Ca2+ as a function of the membrane holding potential. Addition of cytosolic Mg2+ reduced single-channel open probability in a dose-dependent manner. Data is shown as mean ± s.e.m. (n=4). (C) The normalized single-channel open probability of native polycystin-2 isolated from RGC ER microsomes was measured in the presence of different cytosolic Ca2+ concentrations plotted as pCa. Both increased single-channel mean open time and opening frequency contributed to elevated single-channel open probability in the physiological range of cytosolic Ca2+ concentrations. Data is shown as mean ± s.e.m. (n=4). (D) Dependence of biophysical characteristics of single channel polycystin-2 isolated from RGCs on pharmacological modulators of intracellular calcium channels. Properties of native channels isolated from RGC ER microsomes were measured in the presence of 0.1 μM cytoplasmic Ca2+ at a sub-maximal holding potential of −10mV. Compounds were added to the cytoplasmic side of the channel at concentrations that had been established previously to modulate other intracellular Ca2+ channels (cADPR: 10 μM; caffeine: 10 mM; ryanodine: 10 μM; ruthenium red: 10 μM; IP3: 1 μM, Xestospongine C: 1 μM; heparin: 50 μg/ml) and single-channel open probability and amplitude were monitored. Neither one of the two biophysical parameters were affected by these compounds. Addition of cytosolic Mg2+, however, reduced channel activity significantly at 1 mM and blocked activity a cytosolic concentration of 10 mM while single-channel amplitude was not affected by cytosolic Mg2+. Data is shown as mean ± s.e.m. (n=4). * P<0.05.
Discussion
We here provide novel insight into the expression, subcellular localization and biophysical and pharmacological properties of polycystin-2, an important intracellular Ca2+ channel, in mouse RGCs.
Evidence for expression of polycystin-2 in mouse RGCs
In the present study, we investigated polycystin-2 expression in mouse RGCs. The specificity of the polyclonal antibody has previously been confirmed by us (Stokely et al., 2006), and we have recently described the expression and localization of polycystin-2 in the mouse lacrimal gland (Kaja et al., 2011b). The transcription factor Brn3a was used as a nuclear marker to positively identify RGCs in vertical cryostat sections (Quina et al., 2005, Nadal-Nicolas et al., 2009, Badea and Nathans, 2011). Our quantification revealed that 89.9% of all cells in the RGC layer, as assessed by DAPI fluorescence, stained positive for polycystin-2, whereas 73.7% were stained positive for Brn3a. Brn3a labels only a subset of RGCs, and Brn3a expression in RGCs is age- and strain-dependent (Nadal-Nicolas et al., 2009). Our data suggest that most or all RGCs express polycystin-2. 10.1% of all DAPI labeled cells in RGC layer did not show polycystin-2 immunoreactivity. This cell population likely consists of displaced amacrine cells, which constitute a significant number of somata in the RGC layer, however, their number can differ widely depending on species, strain, age and eccentricity (Perry and Walker, 1980, Vaney et al., 1981, Jeon et al., 1998, Masland, 2001a, b, Raymond et al., 2009). We, therefore, cannot exclude the possibility that some displaced amacrine cells also express polycystin-2. Furthermore, the subcellular localization analysis for polycystin-2 in vertical retina sections revealed strong cytosolic immunoreactivity (Fig. 2), which is in accordance with the proposed function of polycystin-2 as an intracellular Ca2+ release channel (Koulen et al., 2002, Koulen et al., 2005a, Stokely et al., 2006, Kaja et al., 2011b). This localization was retained in primary cultures of mouse RGCs, where cytosolic polycystin-2 immunoreactivity co-localized with staining for Thy1.2, a cytosolic marker for RGCs (Mafe et al., 2006). As such, primary cultures of RGCs represent a suitable in vitro model to further investigate the contributions of polycystin-2 to intracellular Ca2+ signaling. Our finding is in accordance with one report showing polycystin-2 immunoreactivity in rat RGCs (Gallagher et al., 2006).
Role of polycystin-2 in RGC Ca2+ signaling
Polycystin-2 is an intracellular Ca2+ channel (Koulen et al., 2002) that amplifies Ca2+ signals through voltage-activation and mechanisms of Ca2+-induced Ca2+ release (CICR). In microdomains, co-localization of polycystin-2 and intracellular Ca2+ release channels such as IP3Rs is essential for CICR (Koulen et al., 2005a, Li et al., 2005, Sammels et al., 2010). It is the disruption of this interaction that is the assumed cause for the pathophysiology of autosomal dominant polycystic kidney disease (Koulen et al., 2002, Koulen et al., 2005a, Sammels et al., 2010). Similarly, in cardiac myocytes polycystin-2 regulates RyR-mediated Ca2+ signaling (Anyatonwu et al., 2007). We have recently shown the presence of functional polycystin-2 channels in the murine lacrimal gland and proposed that polycystin-2 is likely involved in regulation of exocrine activity (Kaja et al., 2011b). Based on these findings, it is likely that polycystin-2 channels are modulators of intracellular Ca2+ signaling in RGCs.
In the mammalian retina, one of the highly specialized functions of RGCs is to convert the synaptic input into spike output that transduces visual information to the brain for processing. Like other specialized neurons, RGCs critically rely on tightly controlled mechanisms of Ca2+ signaling and homeostasis (Nassi and Callaway, 2009). Recent progress has been made in elucidating the signaling pathways in RGC dendrites involving voltage-gated Ca2+ channels (Margolis et al., 2010). IP3R isotypes are expressed in mouse RGCs, as described by us previously (Mafe et al., 2006), little however is known regarding the expression of RyRs in the murine retina. In tiger salamander, type 2 RyRs were not detected in RGCs (Krizaj et al., 2004), whereas strong functional RyR expression was shown in bovine RGCs (Shoshan-Barmatz et al., 2005). Early functional studies on CICR in the turtle retina showed strong expression of both IP3Rs and RyRs in the ganglion cell layer (Akopian et al., 1998). Functional expression of these intracellular Ca2+ release channels is critical for the presumed cellular function of polycystin-2 channels requiring both Ca2+- and voltage activation (Koulen et al., 2002, Koulen et al., 2005a, Kaja et al., 2011b). Furthermore, polycystin-1 signaling (Hanaoka et al., 2000) may play a role in polycystin-2 activation in RGCs, however, further studies are needed to test for the existence of a functional polycystin-1/polycystin-2 interaction in the retina or RGCs.
Implications of polycystin-2 signaling in the pathophysiology of eye diseases
Intriguingly, there are reports of a tentative association between polycystic kidney disease and retinal defects in patients and experimental models of eye disease (Berkley, 1951, Narendran et al., 2004, Gallagher et al., 2006, Feng et al., 2009). In a rare syndrome, neonates were diagnosed with diabetes, congenital hypothyroidism, glaucoma, hepatic fibrosis and polycystic kidneys (Senee et al., 2006). In an Australian pedigree, a missense mutation in PKD2, the gene encoding polycystin-2, manifests as age-related macular degeneration (Narendran et al., 2004). Disturbance of intracellular Ca2+ signaling through elevated levels of reactive oxygen species (ROS) is the pathophysiological mechanism underlying a number of degenerative disorders (Duncan et al., 2010, Kaja et al., 2011a, Zundorf and Reiser, 2011). Given the known modulation of polycystin-2 by oxidative stress (Montalbetti et al., 2008), polycystin-2 channels might potentially contribute to the etiology of degenerative eye diseases involving RGCs.
Further studies are necessary to identify mechanisms of action underlying the role of polycystin-2 channels in Ca2+ handling in RGCs under physiological and pathological conditions. Data from these studies will allow the assessment of the potential benefit and feasibility of targeting polycystin-2 channels as novel pharmaceutical intervention approaches for diseases involving RGC degeneration, such as macular degeneration and glaucoma.
Highlights.
Polycystin-2 shows cytosolic immunoreactivty in mouse retinal ganglion cells.
Polycystin-2 expression is retained in cultured mouse retinal ganglion cells.
Polycystin-2 forms functional calcium channels in mouse retinal ganglion cells.
Polycystin-2 channels likely play a role in retinal ganglion cell calcium signaling.
Acknowledgments
Grant Support/Acknowledgements:
This study was supported in part by a Fight for Sight grant-in-aid (S.K.) and grants EY014227 from NIH/NEI, RR022570, RR027093 from NIH/NCRR and AG010485, AG022550 and AG027956 from NIH/NIA, the Felix and Carmen Sabates Missouri Endowed Chair in Vision Research and the Vision Research Foundation of Kansas City (P.K.). We thank Margaret, Richard and Sara Koulen for generous support and encouragement.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akopian A, Gabriel R, Witkovsky P. Calcium released from intracellular stores inhibits GABAA-mediated currents in ganglion cells of the turtle retina. J Neurophysiol. 1998;80:1105–1115. doi: 10.1152/jn.1998.80.3.1105. [DOI] [PubMed] [Google Scholar]
- Akopian A, Witkovsky P. Calcium and retinal function. Mol Neurobiol. 2002;25:113–132. doi: 10.1385/MN:25:2:113. [DOI] [PubMed] [Google Scholar]
- Anyatonwu GI, Estrada M, Tian X, Somlo S, Ehrlich BE. Regulation of ryanodine receptor-dependent calcium signaling by polycystin-2. Proc Natl Acad Sci U S A. 2007;104:6454–6459. doi: 10.1073/pnas.0610324104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea TC, Nathans J. Morphologies of mouse retinal ganglion cells expressing transcription factors Brn3a, Brn3b, and Brn3c: analysis of wild type and mutant cells using genetically-directed sparse labeling. Vision Res. 2011;51:269–279. doi: 10.1016/j.visres.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstable CJ, Drager UC. Thy-1 antigen: a ganglion cell specific marker in rodent retina. Neuroscience. 1984;11:847–855. doi: 10.1016/0306-4522(84)90195-7. [DOI] [PubMed] [Google Scholar]
- Berkley WL. Glaucoma associated with polycystic kidney disease. Am J Ophthalmol. 1951;34:1539–1542. doi: 10.1016/0002-9394(51)90158-4. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Duncan RS, Goad DL, Grillo MA, Kaja S, Payne AJ, Koulen P. Control of intracellular calcium signaling as a neuroprotective strategy. Molecules. 2010;15:1168–1195. doi: 10.3390/molecules15031168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan BJ, Wiggs JL. Glaucoma: genes, phenotypes, and new directions for therapy. J Clin Invest. 2010;120:3064–3072. doi: 10.1172/JCI43085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Wang Y, Stock O, Pfister F, Tanimoto N, Seeliger MW, Hillebrands JL, Hoffmann S, Wolburg H, Gretz N, Hammes HP. Vasoregression linked to neuronal damage in the rat with defect of polycystin-2. PLoS One. 2009;4:e7328. doi: 10.1371/journal.pone.0007328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher AR, Hoffmann S, Brown N, Cedzich A, Meruvu S, Podlich D, Feng Y, Konecke V, de Vries U, Hammes HP, Gretz N, Witzgall R. A truncated polycystin-2 protein causes polycystic kidney disease and retinal degeneration in transgenic rats. J Am Soc Nephrol. 2006;17:2719–2730. doi: 10.1681/ASN.2005090979. [DOI] [PubMed] [Google Scholar]
- Gees M, Colsoul B, Nilius B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb Perspect Biol. 2010;2:a003962. doi: 10.1101/cshperspect.a003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Schreiber TH, Weremowicz S, Morton CC, Lee C, Zhou J. Identification and characterization of a novel polycystin family member, polycystin-L2, in mouse and human: sequence, expression, alternative splicing, and chromosomal localization. Genomics. 2000;64:241–251. doi: 10.1006/geno.2000.6131. [DOI] [PubMed] [Google Scholar]
- Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature. 2000;408:990–994. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- Hayrapetyan V, Rybalchenko V, Rybalchenko N, Koulen P. The N-terminus of presenilin-2 increases single channel activity of brain ryanodine receptors through direct protein-protein interaction. Cell Calcium. 2008;44:507–518. doi: 10.1016/j.ceca.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998;18:8936–8946. doi: 10.1523/JNEUROSCI.18-21-08936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaja S, Duncan RS, Longoria S, Hilgenberg JD, Payne AJ, Desai NM, Parikh RA, Burroughs SL, Gregg EV, Goad DL, Koulen P. Novel mechanism of increased Ca(2+) release following oxidative stress in neuronal cells involves type 2 inositol-1,4,5-trisphosphate receptors. Neuroscience. 2011a;175:281–291. doi: 10.1016/j.neuroscience.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaja S, Hilgenberg JD, Rybalchenko V, Medina-Ortiz WE, Gregg EV, Koulen P. Polycystin-2 expression and function in adult mouse lacrimal acinar cells. Invest Ophthalmol Vis Sci. 2011b;52:5605–5611. doi: 10.1167/iovs.10-7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaja S, Yang SH, Wei J, Fujitani K, Liu R, Brun-Zinkernagel AM, Simpkins JW, Inokuchi K, Koulen P. Estrogen protects the inner retina from apoptosis and ischemia-induced loss of Vesl-1L/Homer 1c immunoreactive synaptic connections. Invest Ophthalmol Vis Sci. 2003;44:3155–3162. doi: 10.1167/iovs.02-1204. [DOI] [PubMed] [Google Scholar]
- Koulen P, Brandstatter JH. Pre- and Postsynaptic Sites of Action of mGluR8a in the mammalian retina. Invest Ophthalmol Vis Sci. 2002;43:1933–1940. [PubMed] [Google Scholar]
- Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol. 2002;4:191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- Koulen P, Duncan RS, Liu J, Cohen NE, Yannazzo JA, McClung N, Lockhart CL, Branden M, Buechner M. Polycystin-2 accelerates Ca2+ release from intracellular stores in Caenorhabditis elegans. Cell Calcium. 2005a;37:593–601. doi: 10.1016/j.ceca.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Koulen P, Ehrlich BE. Reversible block of the calcium release channel/ryanodine receptor by protamine, a heparin antidote. Mol Biol Cell. 2000;11:2213–2219. doi: 10.1091/mbc.11.7.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulen P, Janowitz T, Johenning FW, Ehrlich BE. Characterization of the calcium-release channel/ryanodine receptor from zebrafish skeletal muscle. J Membr Biol. 2001;183:155–163. doi: 10.1007/s00232-001-0063-8. [DOI] [PubMed] [Google Scholar]
- Koulen P, Madry C, Duncan RS, Hwang JY, Nixon E, McClung N, Gregg EV, Singh M. Progesterone potentiates IP(3)-mediated calcium signaling through Akt/PKB. Cell Physiol Biochem. 2008;21:161–172. doi: 10.1159/000113758. [DOI] [PubMed] [Google Scholar]
- Koulen P, Thrower EC. Pharmacological modulation of intracellular Ca(2+) channels at the single-channel level. Mol Neurobiol. 2001;24:65–86. doi: 10.1385/MN:24:1-3:065. [DOI] [PubMed] [Google Scholar]
- Koulen P, Wei J, Madry C, Liu J, Nixon E. Differentially distributed IP3 receptors and Ca2+ signaling in rod bipolar cells. Invest Ophthalmol Vis Sci. 2005b;46:292–298. doi: 10.1167/iovs.04-0939. [DOI] [PubMed] [Google Scholar]
- Krizaj D, Liu X, Copenhagen DR. Expression of calcium transporters in the retina of the tiger salamander (Ambystoma tigrinum) J Comp Neurol. 2004;475:463–480. doi: 10.1002/cne.20170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wright JM, Qian F, Germino GG, Guggino WB. Polycystin 2 interacts with type I inositol 1,4,5-trisphosphate receptor to modulate intracellular Ca2+ signaling. J Biol Chem. 2005;280:41298–41306. doi: 10.1074/jbc.M510082200. [DOI] [PubMed] [Google Scholar]
- Mafe OA, Gregg EV, Medina-Ortiz WE, Koulen P. Localization of inositol 1,4,5-trisphosphate receptors in mouse retinal ganglion cells. J Neurosci Res. 2006;84:1750–1758. doi: 10.1002/jnr.21090. [DOI] [PubMed] [Google Scholar]
- Margolis DJ, Gartland AJ, Euler T, Detwiler PB. Dendritic calcium signaling in ON and OFF mouse retinal ganglion cells. J Neurosci. 2010;30:7127–7138. doi: 10.1523/JNEUROSCI.5694-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001a;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- Masland RH. Neuronal diversity in the retina. Curr Opin Neurobiol. 2001b;11:431–436. doi: 10.1016/s0959-4388(00)00230-0. [DOI] [PubMed] [Google Scholar]
- Montalbetti N, Cantero MR, Dalghi MG, Cantiello HF. Reactive oxygen species inhibit polycystin-2 (TRPP2) cation channel activity in term human syncytiotrophoblast. Placenta. 2008;29:510–518. doi: 10.1016/j.placenta.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Morris R, Grosveld F. Expression of Thy-1 in the nervous system of the rat and mouse. Immunol Ser. 1989;45:121–148. [PubMed] [Google Scholar]
- Nadal-Nicolas FM, Jimenez-Lopez M, Sobrado-Calvo P, Nieto-Lopez L, Canovas-Martinez I, Salinas-Navarro M, Vidal-Sanz M, Agudo M. Brn3a as a marker of retinal ganglion cells: qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Invest Ophthalmol Vis Sci. 2009;50:3860–3868. doi: 10.1167/iovs.08-3267. [DOI] [PubMed] [Google Scholar]
- Narendran N, Guymer RH, Cain M, Baird PN. Identification of a mutation in the PKD2 gene in a family with age-related macular degeneration. Am J Med Genet A. 2004;127A:208–210. doi: 10.1002/ajmg.a.20673. [DOI] [PubMed] [Google Scholar]
- Nassi JJ, Callaway EM. Parallel processing strategies of the primate visual system. Nat Rev Neurosci. 2009;10:360–372. doi: 10.1038/nrn2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura H, Turco AE, Pei Y, Kalaydjieva L, Schiavello T, Weremowicz S, Ji W, Morton CC, Meisler M, Reeders ST, Zhou J. Identification of PKDL, a novel polycystic kidney disease 2-like gene whose murine homologue is deleted in mice with kidney and retinal defects. J Biol Chem. 1998;273:25967–25973. doi: 10.1074/jbc.273.40.25967. [DOI] [PubMed] [Google Scholar]
- Perry VH, Walker M. Amacrine cells, displaced amacrine cells and interplexiform cells in the retina of the rat. Proc R Soc Lond B Biol Sci. 1980;208:415–431. doi: 10.1098/rspb.1980.0060. [DOI] [PubMed] [Google Scholar]
- Quina LA, Pak W, Lanier J, Banwait P, Gratwick K, Liu Y, Velasquez T, O’Leary DD, Goulding M, Turner EE. Brn3a-expressing retinal ganglion cells project specifically to thalamocortical and collicular visual pathways. J Neurosci. 2005;25:11595–11604. doi: 10.1523/JNEUROSCI.2837-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond ID, Pool AL, Vila A, Brecha NC. A Thy1-CFP DBA/2J mouse line with cyan fluorescent protein expression in retinal ganglion cells. Vis Neurosci. 2009;26:453–465. doi: 10.1017/S095252380999023X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybalchenko V, Grillo MA, Gastinger MJ, Rybalchenko N, Payne AJ, Koulen P. The unliganded long isoform of estrogen receptor beta stimulates brain ryanodine receptor single channel activity alongside with cytosolic Ca2+ J Recept Signal Transduct Res. 2009;29:326–341. doi: 10.3109/10799890903295168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybalchenko V, Hwang SY, Rybalchenko N, Koulen P. The cytosolic N-terminus of presenilin-1 potentiates mouse ryanodine receptor single channel activity. Int J Biochem Cell Biol. 2008;40:84–97. doi: 10.1016/j.biocel.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Sammels E, Devogelaere B, Mekahli D, Bultynck G, Missiaen L, Parys JB, Cai Y, Somlo S, De Smedt H. Polycystin-2 activation by inositol 1,4,5-trisphosphate-induced Ca2+ release requires its direct association with the inositol 1,4,5-trisphosphate receptor in a signaling microdomain. J Biol Chem. 2010;285:18794–18805. doi: 10.1074/jbc.M109.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senee V, Chelala C, Duchatelet S, Feng D, Blanc H, Cossec JC, Charon C, Nicolino M, Boileau P, Cavener DR, Bougneres P, Taha D, Julier C. Mutations in GLIS3 are responsible for a rare syndrome with neonatal diabetes mellitus and congenital hypothyroidism. Nat Genet. 2006;38:682–687. doi: 10.1038/ng1802. [DOI] [PubMed] [Google Scholar]
- Shoshan-Barmatz V, Orr I, Martin C, Vardi N. Novel ryanodine-binding properties in mammalian retina. Int J Biochem Cell Biol. 2005;37:1681–1695. doi: 10.1016/j.biocel.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Stokely ME, Hwang SY, Hwang JY, Fan B, King MA, Inokuchi K, Koulen P. Polycystin-1 can interact with homer 1/Vesl-1 in postnatal hippocampal neurons. J Neurosci Res. 2006;84:1727–1737. doi: 10.1002/jnr.21076. [DOI] [PubMed] [Google Scholar]
- Tran U, Zakin L, Schweickert A, Agrawal R, Doger R, Blum M, De Robertis EM, Wessely O. The RNA-binding protein bicaudal C regulates polycystin 2 in the kidney by antagonizing miR-17 activity. Development. 2010;137:1107–1116. doi: 10.1242/dev.046045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaney DI, Peichi L, Boycott BB. Matching populations of amacrine cells in the inner nuclear and ganglion cell layers of the rabbit retina. J Comp Neurol. 1981;199:373–391. doi: 10.1002/cne.901990305. [DOI] [PubMed] [Google Scholar]
- Westhoff JH, Hwang SY, Duncan RS, Ozawa F, Volpe P, Inokuchi K, Koulen P. Vesl/Homer proteins regulate ryanodine receptor type 2 function and intracellular calcium signaling. Cell Calcium. 2003;34:261–269. doi: 10.1016/s0143-4160(03)00112-x. [DOI] [PubMed] [Google Scholar]
- Wu G, Hayashi T, Park JH, Dixit M, Reynolds DM, Li L, Maeda Y, Cai Y, Coca-Prados M, Somlo S. Identification of PKD2L, a human PKD2-related gene: tissue-specific expression and mapping to chromosome 10q25. Genomics. 1998;54:564–568. doi: 10.1006/geno.1998.5618. [DOI] [PubMed] [Google Scholar]
- Zundorf G, Reiser G. Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid Redox Signal. 2011;14:1275–1288. doi: 10.1089/ars.2010.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]