Abstract
Objective
To determine the feasibility and effectiveness of in-clinic decision aid distribution using a care assistant.
Methods
We identified potentially eligible patients scheduled for upcoming appointments in our General Internal Medicine Clinic (n=1229). Patients were deemed eligible for two decision aids: prostate cancer screening and/or weight loss surgery. Patients were approached to view the decision aid in-clinic. Our primary measures were the proportion of decision aids distributed to eligible patients, and the proportion of decision aids viewed.
Results
Among 913 patients who attended their scheduled appointments, 58% (n=525) were approached and eligibility was assessed by the staff member. Among the 471 who remained eligible, 57% (n=268) viewed at least a portion of the target decision aid. The mean viewing time for patients who watched less than the complete decision aid was 13 minutes.
Conclusions
In clinic viewing of decision aids may be a feasible and effective distribution method in primary care.
Practice Implications
In clinic distribution requires an electronic health information system to identify potentially eligible patients, and a staff member dedicated to DA distribution. Brief decision aids (less than 10 minutes) are needed so patients can complete their use prior to the visit to facilitate patient-physician decision making.
Keywords: Decision making, Patient Education, Diffusion of Innovation
Introduction
Decision aids are tools designed to educate patients about personal medical decisions and to facilitate decision making. They have been shown to improve patient knowledge about medical decisions and increase participation during the doctor visit [1-2]. Although numerous studies have been performed to demonstrate the efficacy of decision aids, relatively few have focused on their implementation and uptake in clinical practice [2-8].
Important system barriers to decision aid implementation and uptake in primary care have been identified. Visits are short, and there is limited time for patients to engage in decision aid use in the clinic, prior to the visit. Patients being evaluated have an array of medical issues, which complicates the effort to target specific medical decisions or conditions [9]. Furthermore, at present, the costs of decision aid implementation are seldom reimbursed [10].
To facilitate implementation of decision aids in primary care practice and overcome some of these barriers, previous studies have evaluated mailings of decision aids to patients to avoid the barriers of in clinic implementation. This method has advantages, specifically, its ability to reach all eligible patients, and can be performed relatively inexpensively. However, as Glasgow and colleagues point out in the RE-AIM Framework [11], reach is just one aspect of successful interventions. Another key aspect is efficacy, or in the case of decision aids, patient uptake or use of decision aids provided. Using mailings, previous studies have found uptake ranging from 24-36% [4-6]. Therefore, other approaches, which encourage patient viewing, may have better overall efficacy if adequate reach can be maintained while increasing uptake.
The purpose of this project was to test the feasibility and effectiveness of an in-clinic distribution model using a designated clinical staff member to identify eligible patients and distribute decision aids to them. We hypothesized that a clinic-affiliated staff member identifying and delivering a decision aid before an office visit would increase the salience of the information to the patient and encourage decision aid use [11] compared to mailing decision aids to patients. We also evaluated the ability of one staff member to identify and approach patients prior to their visit and estimate the time and cost of such an approach.
METHODS
Setting
The project was performed in an academic, general internal medicine practice with a diverse patient population. The practice has approximately 11,000 active patients, and an average of 110 patient appointments per day. The practice has 33 exam rooms in two adjacent wings of an outpatient care facility (Figure 1).
Figure 1.
Decision Aids
Two decision aids were distributed as part of this project: Is A PSA Test Right For You? (PSA) and Weight Loss Surgery, Is It Right For You? (WLS). Both decision aids used in this study are produced by The Foundation for Informed Medical Decision Making [12]. The PSA decision aid is 32 minutes long, and the WLS decision aid is 37 minutes. The PSA decision aid was selected by the physicians in the practice, and the WLS decision aid was selected by the investigators. The rationale for these choices included a desire to have a contrast in both the complexity of the inclusion criteria to test ascertainment methods and in the acceptability of the decision aid content by both doctors and patients. As reviewed in a 2007 meta-analysis, the PSA decision aid’s efficacy had been demonstrated in randomized controlled trials and has been shown to increase knowledge as well as decrease PSA testing [13], however no known trials of the WLS surgery DA were available at the time of the study. Additionally, there was concern among physicians in our practice about the limited empirical data supporting WLS in general [14].
Target Population
Our intent was to perform an effectiveness study to mirror real world circumstances. Because the study was low risk and would have been impracticable with a full consent, we asked for and were granted a waiver of signed consent for participants by the University of North Carolina’s Biomedical Institutional Review Board. Therefore, all patients who met the eligibility for the targeted decision aids were included in this study. Additionally, to minimize response burden for participants in clinic, and to decrease the potential to interfere with patient flow, we limited the number of measures obtained from patients for this study.
Potentially eligible patients had scheduled visits at the general internal medicine practice during the time when the staff member was available in clinic to identify eligible patients deliver decision aids. Participants were eligible if they could speak English, and were not blind or deaf. Additional criteria for patients eligible for the PSA decision aid were (1) men between 49 and 70 years of age (2) without a previous diagnosis of prostate cancer. Those eligible for the WLS decision aid had (1) a body mass index (BMI) of 40 or greater and (2) had health insurance that would cover weight loss surgery, should the patient choose this option.
Identification Process of Eligible Patients
As identifying eligible patients is an important barrier to implementing decision aids in primary care, one of our goals was to evaluate the feasibility of several different methods for identifying potentially eligible patients. We tested three distinct methods in order to determine the most effective and efficient process of identification using only one staff member. The first method entailed identification of patients for the PSA decision aid only, using a manual review of medical records of age-eligible men with scheduled visits for the following clinic day. The second method entailed identification of patients for both the PSA and WLS decision aids, also using a manual review of records for patients scheduled the following day. Lastly, queries from a clinical administrative database or patient registry were used to electronically generate a list of potentially eligible patients for both the PSA and WLS decision aids from the scheduled patients for the following day. Unlike the previous two methods, this approach did not require manual medical record review.
Decision Aid Distribution Process
The staff member attempted to approach all eligible patients after clinic check-in and prior to their physician visit. If the staff member was not able to capture the patient in the waiting room, she attempted to capture them in the exam room before the providers started the visit. Patients were approached by the staff member who identified herself as part of the clinic staff. She explained the purpose of the project, the need and extent of the patient’s involvement and extended the option to view or decline the decision aid. If they agreed, she confirmed their eligibility for the decision aid verbally and eligible patients were encouraged to view the PSA or WLS decision aid on a portable DVD player while in the clinic. Patients viewed a decision aid before and/or after the clinic visit, keeping the DVD player as they progressed through their appointment. Following the patient’s visit, the DVD player was collected from the patient by the staff member in their exam room or in the hallway. If the patient had not completed the decision aid, they were offered the option of completing viewing in a designated decision support area of the practice.
Measures and Analyses
Our primary measures based on the RE-AIM framework were the proportion of decision aids distributed to eligible patients (reach), and the proportion of decision aids viewed (efficacy). Recognizing that one staff member’s reach would be limited by the in clinic approach, our goal for this pilot was to try to target patients and encourage viewing, hypothesizing that use or efficacy could be higher with this approach than mailing out decision aids. Ideally, patients would have adequate time to complete viewing prior to their visit. Given the length of the decision aids, however, we wanted to assess both full and partial viewing, as we were concerned about the time available for viewing prior to the patients visit. Additional outcomes included the location where the patient was approached by designated the staff member, and estimated cost of identification and delivery. To estimate the time and cost of the program, the staff member documented her time as she worked performing tasks to either identify potentially eligible patients (identification time) or delivering decision aids to patients in clinic (delivery time). To estimate costs for identification time she documented the time spent ascertaining and verifying eligibility via medical records and documenting tracking in administrative databases. She also documented encounter time by tracking the time spent in direct contact with patients.
We estimated the total cost of the program using the following formula:
To estimate the cost per decision aid viewed we divided the cost by the number of decisions partially or fully viewed. We estimated the hourly wage for the staff member was $12 per hour including benefits. Time spent on other tasks unrelated to the project was not counted. Additionally fixed costs associated with this project, such as computers and DVD players, were not included in cost calculations.
For our outcomes, we determined the number of patients scheduled while the staff member was available for this project. Then, we calculated the frequency of patients who attended their scheduled visit and whether the staff member was able to capture the patient and assess eligibility. We counted the number of patients who agree to watch the video. In addition, the time the patients spent watching the video was assessed from the time elapsed on the video players. We used chi squared tests to determine if there were differences in those who watched the complete video by topic. We used t-tests to determine whether the mean viewing time differed by topic.
RESULTS
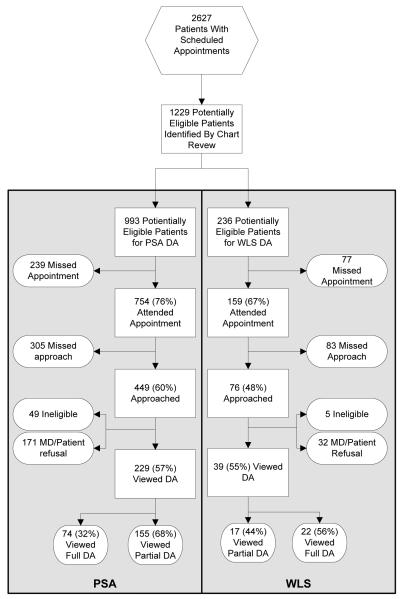
During a 5 months period from September 2007 through February 2008 the staff member spent a total of 446 hours (54 working days) on the project. During this time, 2627 patients had scheduled appointments (Figure 2). Of those, 1229 patients were identified as potentially eligible for one of the two decision aids. Of the 913 potentially eligible patients who attended their appointment, the decision support staff member was able to approach 525 (58%). Four-hundred and seventy-one remained eligible after further review and subsequently, 268 (57%) patients were found to be eligible and agreed to view one of the DAs. Two hundred and twenty-two (83%) patients who watched the decision aids were captured at their first scheduled visit and the remaining 46 (17%) viewed them at either a second or third scheduled clinic visit. Viewers of the PSA DA had a mean age of 59.4 years, while the WLS viewers were, on average 52.7 years-old. No data on race/ethnicity were collected.
Figure 2.
Participant Flow
Decision aid distribution and viewing
Of the 268 patients who agreed to watch their target decision aid, 229 viewed PSA and 39 viewed WLS. Ninety-six patients (36%) viewed the full DA they were assigned. Complete viewing varied by decision aid topic. Thirty-two percent of patients who viewed the PSA DA watched the entire video, whereas 56% of patients who viewed the WLS DA watched the entire video (p<.01). For patients who viewed a portion of the videos, the mean time spent viewing the DA was 13 minutes, and did not differ by topic (PSA = 13; WLS = 14.1; p=0.44). There was also no age difference found among patients who agreed or disagreed to view either of the DAs, or between those who viewed in full versus those who only viewed a portion of the DAs (p =1.0).
Location of Decision Aid Delivery
The staff member was able to identify and approach the majority (of the patients 59%) in the waiting area. For those remaining patients (41%) the staff member was able to find them after they were already placed in the exam room. Patients who began viewing the DA before their clinic visit were no more likely to complete viewing than those who started watching at another point in their visit.
Identification methods
Patient identification time differed across the three identification methods tested. The first identification method, in which the care assistant ascertained patients for the PSA decision aid only by reviewing the medical records for male patients scheduled for the next clinic day, took approximately 40 minutes per day. This method was employed over 20 clinic days and totaled 13 hours of care assistant time. The second identification method, in which the care assistant ascertained patients for both the PSA and WLS decision aids by reviewing medical records for all patients with appointments scheduled for the next clinic day, took approximately 4.5 hours per day. This method was employed over 25 clinic days and totaled 112 hours. The care assistant was attempting to identify patients and deliver decision aids simultaneous to patient eligibility identification for the following day, which increased the potential for missing scheduled patients. The third identification method, in which the care assistant ascertained patients for both the PSA and WLS decision aids utilized an automated clinical algorithm to create a list of potentially eligible patients for the next clinic day and verified eligibility by reviewing those medical records, took approximately 1 hour per day. This latter method was employed over 9 clinic days. In the third phase, when queries of clinic databases were utilized, identification of eligible patients for both decision aid topics took 1 hour, which was spent reviewing the records of the list queried from clinical databases and totaled 9 hours.
Estimated Costs
Total identification time was calculated using the estimated daily identification time for each identification method. Therefore, total identification time was 135 hours (13 +112+9). Encounter time, or time the care assistant spent with patients, was documented to be 157 hours. Total time that the care assistant spent on both activities was 292 hours and the total cost was estimated to be $3,504 (292 X$12/hour). Two hundred sixty-eight patients viewed some portion of their assigned decision aid, so the cost per decision aid viewed, topics combined, was $13.
DISCUSSION AND CONCLUSION
We were able to reach the majority of patients who attended their schedule appointments using a dedicated clinic staff member to identify patients and deliver two decision aids in clinic. A significant proportion (56% WLS, 32% PSA) completed viewing of their assigned decision aid, each of which was greater than 30 minutes in length. The mean viewing time of approximately 13 minutes for those who watched less than the complete video reflects the average waiting time in our clinic. The need to target decision aids to specific eligibility criteria limits the efficiency of the clinic staff when a significant portion of time is spent reviewing patient records. We were able to improve the efficiency by using an automated system; however, neither the manual or automated system resulted in perfect fidelity, with 10% of approached patients reporting that they were not eligible. We estimated that the average cost to implement decision aids using a designated clinic staff is $13 per decision aid viewed.
Previous studies have evaluated models of decision aid implementation in primary care practices. In general, the use of the materials when mailed has been limited. Previously, we found that 25% of respondents viewed a decision aid about Colorectal Cancer (CRC) screening when it was mailed unrelated to an office visit [5]. Brackett and colleagues using questionnaire returns as a proxy for the number of decision aids viewed estimated that between 24-36% of patients viewed decision aids when mailed or offered to be mailed a decision aid before an office visit [4]. Frosch and colleagues demonstrated a 41% viewing rate in private practices in underserved areas using similar methodology as we used in this study [3]. Although we found a higher proportion of patients viewed the targeted decision aids than in our previous study, viewing differed by topic. It should also be noted that we did not directly compare the in clinic delivery strategy with mailing out decision aids; therefore, within the RE-AIM framework [11], questions remain in regards to the reach and efficacy of these two models and additional research is needed to make this direct comparison.
In this study, we assessed both full and partial viewing of the decision aids. An ideal approach would provide sufficient time to complete viewing of decision aids so that patients would have the full benefit of the decision aid while interacting with their provider. While no formal data was collected on the reason for incomplete viewing, time was a clear barrier to identification and delivery in the current study. As the mean viewing time for partial completions was considerably shorter than the duration of the decision aid, it may be beneficial for the length of the decision aids to be condensed to a viewing time of 10-15 minutes. Limiting decision aid length to 10 to 15 minutes would likely have less effect on visit length. Our practice has worked diligently to decrease patient wait times, but found an increase of approximately 3-5 minutes over the duration of this project, which may be attributable to decision aid use. Efforts to modify patient expectations about decision support may be one approach to address time barriers and patient satisfaction. Interestingly, a greater proportion of the WLS decision aid population viewed the complete decision aid, despite it being the longer of the two videos, leading us to believe that topic may influence complete viewing. Patients in the present study may have been more willing to complete viewing of the weight loss surgery videos for several reasons including, but not limited to, lack of previous exposure to the information provided in the DA, and/or perhaps they found the information in the WLS DA more salient. Previously, Tingen, et. al, showed that, dependent on demographic factors, some men bypass education on PSA screening based on their previous screening practices and known familial risk factors [13].
An additional barrier to implementation was the time invested in patient identification. This process of targeting identification and delivery to only those meeting specific eligibility criteria limits distribution efficiency; though it is necessary for specific decisions such as weight loss surgery. This study illustrates the variability in efficiency when comparing a chart review method to an automated medical records system. For clinics with limited resources or without electronic medical records, manual review of medical records is feasible with one staff member, if a singular decision aid is being distributed in-clinic. An automated system may improve efficiency when implementing multiple decision aids. The goal of this study was to test the feasibility of a staff member identifying and delivering targeted decision aids in a primary care clinic. We did not want to impede patient flow or viewing of decision aids with extensive data collection, which could have influenced our results. Consequently, desirable measures are lacking with this pragmatic approach which could be pursued in future studies. For example, we were not able to explore patient attributes or attitudes that may have been associated with complete viewing of DAs. In addition to patient attributes, we did not collect information on confounding variables of patient flow such as the type of patient visit, MD patient load, scheduling punctuality, patient wait time, appointment conflicts, etc. Although we did not formally assess physicians’ attitudes, we had almost complete provider participation from the 16 full time equivalents. Out of our 85 part time providers, one physician refused to allow his patients to participate. Anecdotally, the physicians concurred that the decision aids were worth a slight increase in patient wait time that occurred during while this project was ongoing.
The project was designed to test the feasibility of decision aid identification and delivery by a designated staff member for patients visiting an academic internal medicine clinic. Dependent on the identification and delivery method selected, the results may not be generalizable to other practice sites, given the diversity of primary care practices. For example, smaller practices may have less difficulty with this model based on structural design pragmatics of their clinical space. Furthermore, the time estimated to identify potentially eligible patients may not be relevant for other medical records systems.
Conclusions
Our findings suggest that using a dedicated clinic staff member to facilitate in-clinic viewing of decision aids may be feasible for identification and delivery of one or two decision aids in primary practice. Manual review of medical records may limit the efficiency of decision aid identification and delivery for targeted populations unless an automated identification system is used to improve efficiency. Average viewing time suggests that 10 to 15 minutes may be the maximum time available for in-clinic decision aids use in primary care practices.
Practice Implications
In clinic distribution requires an electronic health information system to efficiently identify potentially eligible patients and a staff member dedicated to distribution. To facilitate in clinic viewing and reap the benefit of decision aid use prior to the clinic visit, brief decision aids (less than 10 minutes) are needed. Practices may want to consider coupling decision aid delivery with health coaching in order to increase the efficiency of the model using overlapping staff.
Acknowledgements
The project was supported by the Foundation for Informed Medical Decision Making. Dr. Lewis was also supported by a K07 Mentored Career Development Award (5K07CA104128) from the National Cancer Institute. Dr Pignone was supported by a National Cancer Institute Established Investigator Award (K05 CA129166).
The funding source has no role in the design, conduct, or analysis of this project or in the decision to submit the manuscript for publication.
References
- 1.Leatherman S, Warrick L. Effectiveness of decision aids: A review of the evidence. Medical Care Research & Review. 2008;65(6S):79S–116S. doi: 10.1177/1077558708324234. [DOI] [PubMed] [Google Scholar]
- 2.O’Connor AM, Bennett CL, Stacey D, Barry M, Col NF, Eden KB, Entwistle VA, Fiset V, Holmes-Rovner M, Khangura S, Llewellyn-Thomas H, Rovner D. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews. 2009;(3) doi: 10.1002/14651858.CD001431.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Frosch DL, Legare F, Mangione CM. Using decision aids in community-based primary care: A theory-driven evaluation with ethnically diverse patients. Patient Educ Couns. 2008;73(3):490–6. doi: 10.1016/j.pec.2008.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brackett C, Kearing S, Cochran N, Tosteson AN, Blair BW. Strategies for distributing cancer screening decision aids in primary care. Patient Educ Couns. 2010;78:166–8. doi: 10.1016/j.pec.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Lewis CL, Brenner AT, Griffith JM, Pignone MP. The uptake and effect of a mailed multi-modal colon cancer screening intervention: A pilot controlled trial. Implement Sci. 2008;3:32. doi: 10.1186/1748-5908-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zapka JG, Lemon SC, Puleo E, Estabrook B, Luckmann R, Erban S. Patient education for colon cancer screening: a randomized trial of a video mailed before a physical examination. Ann Intern Med. 2004;141:683–92. doi: 10.7326/0003-4819-141-9-200411020-00009. [DOI] [PubMed] [Google Scholar]
- 7.Wandersman A, Duffy J, Flaspohler P, et al. Bridging the gap between prevention research and practice: The Interactive Systems Framework for Dissemination and Implementation. American Journal of Community Psychology. 2008;41:171–81. doi: 10.1007/s10464-008-9174-z. [DOI] [PubMed] [Google Scholar]
- 8.Volk RJ, Hawley ST, Kneuper S, et al. Trials of Decision Aids for Prostate Cancer Screening: A Systematic Review. American Journal of Preventive Medicine. 2007;33:42. doi: 10.1016/j.amepre.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 9.Woolf S, Chan E, Tunis S, et al. Promoting informed choice: Transforming health care to dispense knowledge for decision making. Annals of Internal Medicine. 2005 August 16;143(4):293–W-81. doi: 10.7326/0003-4819-143-4-200508160-00010. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor AM, Llewellyn-Thomas HA, Flood AB. Modifying unwarranted variations in health care: shared decision making using patient decision aids. Health Aff (Millwood) 2004 doi: 10.1377/hlthaff.var.63. Suppl Web Exclusives: VAR63-72. [DOI] [PubMed] [Google Scholar]
- 11.Glasgow RE, Vogt TM, Boles SM. Evaluating the Public Health Impact of Health Promotion Interventions: The RE-AIM Framework. American Journal of Public Health. 1999;89:13. doi: 10.2105/ajph.89.9.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foundation for Informed Medical Decision Making Website http://www.fimdm.org/
- 13.Tingen MS, Weinrich SP, Heydt DD, Boyd MD, Weinrich MC. Perceived benefits: a predictor of participation in prostate cancer screening. Cancer Nurs. 1998;21:349–57. doi: 10.1097/00002820-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric Surgery: A Systematic Review and Meta-analysis. JAMA: Journal of the American Medical Association. 2004 October 13;292(14):1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]