Abstract
Research suggests that not only is marijuana use prevalent among women of reproductive age, but a significant number of women continue to use marijuana and its derivatives throughout pregnancy. Many studies have shown, in both humans and animals, that marijuana exposure during adolescence and adulthood is detrimental to normal cognition and memory. In this study, we examined the effects of daily intravenous injections of 0.15mg/kg Δ9-tetrahydrocannabinol (THC), given to pregnant dams throughout gestation, on cognitive function in the offspring. Offspring were exposed to three tests: a passive avoidance test at postnatal day (PND) 22, an active place avoidance test at PND 45, and an attention task at PND 60, which assessed learning and long-term memory, spatial working memory and prediction, and attention, respectively. Other offspring were also given a 1mg/kg amphetamine challenge at PND 60. Passive avoidance testing showed that prenatal THC had no effect on acquisition but interfered with consolidation during retention testing. The active place avoidance task showed no treatment-related effects on acquisition but a significant treatment effect was observed in reversal performance in males. The attention task showed that a smaller percentage of THC-exposed rats completed the test, although the failure rate of both groups was quite high. Finally, THC exposed animals, both male and female, showed a dampened locomotor response to amphetamine, but females were more active than males overall. These results suggest that prenatal THC exposure has effects on certain aspects of cognitive function in rats from weaning to adulthood. These effects suggest that prenatal marijuana exposure could also alter cognitive function in humans and therefore have an impact on school performance and dampen responses to psychostimulants as well.
Keywords: cannabinoid, brain development, sex differences, avoidance learning, attention, amphetamine
1. Introduction
Although it has been shown in both human and animal studies that prenatal marijuana exposure poses significant threats to normal fetal development, behavior, executive and cognitive functioning later in life (Bernard et al., 2005);(Campolongo et al., 2011); (Fried and Smith, 2001); (Jutras-Aswad et al., 2009); (Schneider, 2009); (Richardson et al., 2002), marijuana remains the illicit substance most commonly abused by pregnant women in the western world. Estimates for the United States and United Kingdom range from 2–5% of pregnant women continue to use cannabis and its derivatives throughout their pregnancies (Jutras-Aswad et al., 2009).
Marijuana use during pregnancy is of utmost concern, as it is commonly thought that there are few, if any, severely detrimental effects of the drug on fetal development. This misconception undoubtedly contributes to the continued use of marijuana by young women during pregnancy (Fried and Smith, 2001). However, it has been shown that THC, the psychoactive component of marijuana, readily crosses the placenta and enters the fetal blood supply (Harbison and Mantilla-Plata, 1972); (Hutchings et al., 1989) where it could have detrimental effects on fetal development.
Studies in humans have shown that not all domains of executive and cognitive function are affected equally by cannabis exposure. The greatest adverse effects are seen in tasks requiring analysis and synthesis, planning, and integration in addition to basic visuoperceptual abilities (Fried and Smith, 2001). Sustained attention has also been shown to be altered in exposed children (Noland et al., 2005); (Fried and Watkinson, 2001). Increased child behavior problems and delinquency have also been reported in adolescents prenatally exposed to cannabinoids (Day et al., 2011); (Goldschmidt et al., 2000).
Previous studies in our lab have examined the effects of Δ9-tetrahydrocannabinol (THC) administration in rats during various stages of development such as preadolescence (Dow-Edwards and Zhao, 2008), early and late adolescence (Harte and Dow-Edwards, 2010). This study aims to examine the effects, specifically on components of cognitive and executive function, of THC when the exposure is prenatal. Since 200mg/kg of THC has been shown to cause in utero deaths and reduced body weight in mice (Harbison and Mantilla-Plata, 1972), we used a substantially lower dose, so as not to cause severe fetal effects. We wanted to mimic as closely as possible the blood plasma levels of a light daily marijuana user, so we chose to dose using an intravenous catheter, as IV administration most closely resembles the pharmacokinetics of inhaled marijuana in humans (Spano et al., 2007).
In order to assess different domains of cognitive and executive function we have chosen a set of behavioral tests: a passive avoidance test, an active place avoidance test, and an attention test. As each test utilizes a unique set of cognitive abilities, using them in conjunction will allow us to tease apart specifically which of the abilities are most affected by prenatal marijuana exposure in a rat model.
Trezza et al. (2008) have recently reviewed literature concerning the effects of cannabinoid exposure on developing brain and emphasized the role of endocannabinoids (the endogenous ligand for the cannabinoid receptor) in neuronal signaling and plasticity throughout the brain. For example, the endocannabinoid system has been shown to be involved in development of reciprocal connections of pyramidal neurons in the cortex (Wu et al., 2010). Additionally, prenatal cannabinoid treatment is associated with increased self-administration of opiates in adulthood (González et al., 2003); (Spano et al., 2007), changes in social and open field behavior (Newsom and Kelly, 2008), and changes in motor behavior and nigrostriatal dopaminergic activity (Navarro et al., 1994). Finally, perinatal and prenatal exposure to cannabinoids in rats is associated with changes in many neurotransmitter systems including DA, 5HT and GABA (Garcia-Gil et al., 1999); (Molina-Holgado et al., 1996);(Bonnin et al., 1994; Bonnin et al., 1995; Rodriguez de Fonseca et al., 1991). In order to determine if changes in DA and 5HT systems persisted into adulthood, we challenged exposed adults with amphetamine. Amphetamine is a drug that targets multiple neurotransmitter systems, including DA, NE and 5HT, and this broad spectrum of effects is easily quantified by examining motor behavior in an activity moniter. Therefore, changes in motor behavior may indicate persistent changes in the DA, NE and 5HT systems that could be caused by prenatal THC exposure.
2. Methods and materials
All procedures were carried out in accordance with NIH-approved standards under IACUC approval.
2.1. Subjects and drugs
Adult virgin female Sprague Dawley rats (Charles River, Wilmington, MA) were housed in a 12-hour light/dark cycle with lights off at 7:00 pm and ad libitum access to food and water. After 1 week of habituation to our housing conditions, a permanent intravenous catheter was surgically implanted and secured to the right jugular vein as described in (Spano et al., 2007). Beginning the day after surgery, the catheters were flushed daily with a Heparin solution (100U/ml in saline) for 6 days. Pain medication (children’s Tylenol 3cc/100ml) was mixed with the drinking water for 5 days. On day 7 post-surgery, the rats started receiving daily intravenous injections of THC (0.15 mg/kg) or vehicle (pluronic acid in saline). Females were allowed to mate with a male starting on day 9 post-surgery. A sperm positive smear was considered gestational day 1 (GD1). Pregnant rats continued to receive daily intravenous injections of 0.15 mg/kg THC from GD1 to GD21. Throughout the pregnancy and drug treatment, maternal weight was recorded as well as gestational length, total number of pups birthed, and male/female pup ratio.
Litters were culled to 10 pups, 5 males and 5 females, fostered on postnatal day (PND) 2 and were moved on PND 5 to a 12-hour reverse light/dark cycle with lights off at 11:00 am. Pups were weaned on PND 21 and housed in same-sex cages with ad libitum access to food and water. Each animal was tested only once.
2.2. Passive avoidance
Passive avoidance tests were carried out in a Coulburn Shuttle Box with two equally sized compartments divided by white Plexiglas and equipped with an electrified floor for foot shocks and an activity monitor. Two males and two females from each litter were tested on PND 22 in the task, which involved a 180-second habituation trial, 5 180-second training trials with intertrial intervals of 60 seconds, and a 1-hour retention test on the same day. The habituation trial was a 180-second trial with no shock, during which the animal could freely cross between the illuminated and dark sides of the compartment. During each of the 180-second trials, the half of the arena occupied by the animal was illuminated by a small light bulb. The animal would receive a mild (0.5 mA) foot shock upon crossing over to the dark half of the compartment. Latency to cross was measured for each of the 5 trials and used to measure the ability of the animals to learn the task. The animals were returned to their home cage. At 1 hr, the animals were tested for retention which consisted of a single trial with no shock again using latency to cross as a measure of performance. Retention trials were then run again at 24 hours and 1 week.
2.3. Active place avoidance
Equipment used was a Bio-Signal Group (Denver, USA) active place avoidance monitor. The experiment was conducted in a small (92″ × 77″ × 93″), darkened room. Visual cues were located at 4 locations around the room which was covered by a black felt curtain. The testing took place in an arena, a metal disc (32.5″ diameter) that rotated at a rate of 1 cycle per minute. A tall Perspex wall to prevent the animal from escaping during testing enclosed the arena but still allowed the animal to see the cues located around the room. The rotating disc would carry a stationary animal into the shock zone which was a 60° wedge of the circular arena that was fixed with respect to the room. Upon entering the shock zone, the animal would receive a mild 0.3–0.5 mA footshock with duration of 500ms and an inter-shock interval of 1500ms. An infrared camera, which monitored an LED light clipped to the animal’s back, tracked the position of the animal on the arena. Animals were placed onto the arena on the side opposite the shock zone. There were three phases to this task.
2.3.1. Acquisition
On PND 45, one male and one female from each litter were given seven 10-minute acquisition trials (each separated by a 10-minute rest interval) in which to learn to avoid the shock zone. Total number of entries into the shock zone, total number of shocks received, and maximum time spent avoiding the shock zone were recorded and used as measures of performance.
2.3.2. Retention
After a 24-hour period, the animals were given a single 10-minute retention trial with the shock off. Total number of entries into the shock zone, total number of shocks, time to first entry, and maximum time spent avoiding the shock zone were recorded and used as measures of performance.
2.3.3. Reversal
After the retention trial, the animals were given a 10-minute rest period. They were then given a 20-minute shift trial, with shock on, in which the shock zone had been shifted 180° with respect to the room. Number of entries into the shock zone, total number of shocks, and maximum time spent avoiding the new shock zone were calculated for the first 10 minute interval and the second 10 minute interval of the 20-minute test as measures of performance. The data from each 10-minute interval were analyzed separately as the effects of reversal on performance are maximal during the initial reversal period.
2.4. Attention task
Beginning on PND 55, one male and one female from each litter were single housed, weighed and then food deprived, receiving approximately 2 food pellets/day with ad libitum access to water. Animals were weighed daily and food pellets adjusted to maintain 85% (±3g) for males and 90% (±3g) for females of their original PND 55 body weight. Once these target weights were attained, the rats were started on the attention task, maintaining the reduced body weight throughout the duration of the task. All testing occurred in the animal’s home cage, with the animal being held in an empty cage between trials. Wood boxes with removable lids were used to conceal a food reward (Honey Nut Cheerios®, General Mills, MN). The boxes were 9 L × 6 W × 4 H cm outside and 7.7 L × 4 W × 3.2 H cm inside. Two boxes were plain wood and 4 boxes were covered with black rubber, sandpaper, green paper or white rubber mesh. A thin layer of shavings from the home cage and ½ a crushed Cheerio were added to the bottom of each box to mask olfactory cues. There was one plain wood lid without weights (~9g) for pre-training. All other lids were made heavy (~20g) with weights. The heavy lids consisted of two plain wood lids, one black rubber covered lid, and one sandpaper covered lid. Each rat was tested for 6 consecutive trials per day with a maximum trial duration of 5 minutes. Total number of trials to either failure or successful completion was recorded. There were 4 phases involved in the attention task.
2.4.1. Habituation and pre-training
The rat was first removed from its home cage and placed in an empty holding cage. Then a small wooden box full of Cheerios was placed in the center of the home cage. The rat was returned to the cage and allowed to explore for 5 minutes. After the 5 minutes, the rat was removed, and then the box of Cheerios was removed and the rat returned to its home cage. After habituation, the animal had a 1-hour rest until pre-training was started. During pre-training, the rat learned how to remove the lid from the plain wooden box in order to receive ½ a Cheerio as a food reward. The rat was randomly placed either to the left or right of the box and the same sequence of placement was used for all rats being tested that day. The first combination was the plain wood box with a plain wood lid that was placed slightly ajar. Once the rat was able to remove the lid and put its whole head inside the box within 2 minutes or less, it was allowed to move on to the next phase. In the second phase, the plain wood box was paired with the plain wood lid, but the lid was no longer ajar. The criterion to move on was again to remove the lid and put the whole head inside the box within 2 minutes or less. The final phase of pre-training involved switching the plain wood lid to a heavy plain wood lid using the same criterion. Most rats were able to complete pre-training in fewer than 7 trials. Rats which did not meet criteria within 10 days (60 trials) were excluded from further testing.
2.4.2. Training
During the training phase, the rat learned to pay attention to cues on the boxes. One black rubber covered box with a heavy plain lid and 1 sandpaper covered box with a heavy plain lid were used. The ½ Cheerio reward was always placed in the black rubber box and the placement of the box, either to the right or left, was randomly determined by a coin flip and the same sequence was used for all rats being tested that day. The rat was placed in the middle of the two boxes and given up to 5 minutes to choose a box to open. Opening and putting its entire head in one of the boxes was considered a choice, and a correct choice was picking the box containing the food reward. The rat was allowed to move on to the next phase after making 12 correct choices within 15 consecutive trials. Rats who failed to achieve this criterion within 60 trials were excluded from continuing the testing.
2.4.3. Extra-dimensional Shift
In the shift phase, the rat learned to shift its attention to cues on the lids as opposed to the boxes. Two plain wood boxes were used with one heavy black rubber covered lid and one heavy sandpaper covered lid. Again, the food reward was always paired with the black rubber lid and placement on the left or right was randomly determined as described above. The procedure and criteria are the same as for the training phase. Again, rats that failed to reach criterion within 60 trials were excluded from continuing the testing.
2.4.4. Distraction phase
The final phase involved introducing new stimuli (distracters). Instead of the plain wooden boxes, one box was covered in green paper and one was covered in white rubber mesh. The rats had to learn to ignore these cues and continue to pay attention to the cues on the lids. The food reward, again, was always paired with the black rubber lid. The placement of the black rubber lid was randomly determined as described above. A separate coin flip randomly determined the placement of the white rubber mesh box. This ensured that the pairing of the black rubber lid with either the green paper or white rubber mesh box was random and therefore, the box could not provide any cues as to the location of the food reward. The procedure and criteria were the same as described above with rats that failed to reach criterion within 60 trials being excluded. Rats who did reach criterion were considered to have successfully passed the task.
2.5. Amphetamine challenge
One male and one female from each litter were given an amphetamine challenge at PND 60. Versamax activity monitors (VMRXYZ16; Accuscan instruments, Columbus, OH) were used to assess locomotor activity both before and after administration of amphetamine. A clear Plexiglas box was placed inside the Accuscan recording chamber, which recorded movement based on the interruption of photobeams. Animals were placed in the Plexiglas box inside the monitor and locomotor activity was recorded for 3 5-minute time blocks (15 minutes), constituting the baseline activity level. After the baseline 15 minutes, animals were removed from the chambers, injected with 1mg/kg amphetamine sulfate ip and returned to the chambers. Locomotor activity was then recorded for 12 5-minute time blocks (60 minutes).
2.6. Statistical analysis
Data for all tests were analyzed by SAS Statistical Software, v. 9.2 (SAS Institute Inc., Cary, NC). For all tests, a significance level of p≤0.05 was used.
2.6.1. Passive avoidance analysis
A mixed linear model was constructed, with power-transformed time to crossover as the dependent variable. About 15% of observations involved crossover not having been made within the allotted 180 sec. Due to the overall complexity of the analysis, these right-censored cases were treated simply as having values of 180s. Fixed effects were treatment, sex, time, and their interactions. Litter was a random effect. An unstructured intra-subject covariance matrix was modeled. Satterthwaite corrections were made to denominator degrees of freedom. Model residuals were examined for skew and for outliers. Three outlying observations (all with times <180s) were excluded from analysis. Paired t-tests were conducted to compare crossing latency in trials 2–5 with trial 1 latency.
2.6.2. Active place avoidance analysis
2.6.2.1. Acquisition
A generalized mixed linear model was constructed, with number of entries as the dependent variable; a negative-binomial distribution was found to fit this variable. Treatment, sex, trial and interactions among these terms were introduced as fixed factors; litter as a random factor. A first-order autoregressive structure with homogeneous variance was found to fit the intra-subject covariance matrix. Satterthwaite corrections were made to denominator degrees of freedom. Model residuals were examined, and a single outlying score excluded from analysis. A paired t-test was conducted to compare number of entries in trial 7 with trial 1 entries.
2.6.2.2. Retention
Time to first entry into the shock zone was used as the dependent variable. Since 9 of the 39 animals did not reach this endpoint within the allotted 10 min, a positive stable frailty model was used to account for this right-censoring problem. Fixed effects were treatment, sex, and their interaction. Litter was a random factor.
2.6.2.3. Reversal
A generalized mixed linear model was constructed, with number of entries as the dependent variable; a Poisson distribution was found to fit this variable. Treatment, sex, trial and interactions among these terms were introduced as fixed factors; litter as a random factor. A first-order autoregressive structure with homogeneous variance was found to fit the intra-subject covariance matrix. Satterthwaite corrections were made to denominator degrees of freedom. Two-sample t-tests were performed to assess differences between treatment groups with respect to mean number of entries into the shock zone for each time period (0–10min and 10–20min).
2.6.3. Attention task analysis
For each animal, the phase at which failure occurred was recorded as 1 (pre-training), 2 (training), 3 (shift), 4 (distract) or 5 (successful completion of all phases). One animal was excluded from analysis for failing to habituate to the task. A generalized mixed linear model was constructed, with failure phase modeled as a negative-binomially distributed outcome; fixed factors were sex, treatment and their interaction; litter was a random factor.
For the animals that successfully completed the task, a mixed linear model was constructed with total number of trials as the dependent variable. Treatment was a fixed factor and litter was a random factor. Finally, since the number of animals that successfully completed the test was small, a Kruskal-Wallis non-parametric analysis of the distribution of scores was also performed.
2.6.4. Amphetamine challenge analysis
A mixed linear model was constructed, with distance traveled as the dependent variable. Separate analyses were conducted for pre- and post-challenge conditions. Fixed effects were treatment, sex, and their interaction. Litter was a random effect. Satterthwaite corrections were made to denominator degrees of freedom. Model residuals were examined for skew and outliers. Two-sample t-tests were performed to assess differences in treatment groups.
3. Results
3.1. Physical data
Dams were weighed weekly from gestational day (GD) 1 until GD 22. The difference in weight gain (GD 22 – GD1) was analyzed with a student’s t-test. There was no significant difference (t[19]=−0.381, p=0.707) in maternal weight gain between the vehicle and THC treated dams. Additionally, there were no significant differences between treatment groups in the birth weights of male pups (t[19]= 0.041, p=0.967) or female pups (t[19]= 0.013, p=0.989), total number of pups per litter (t[19]= 1.267, p=0.220), or the percent of pups that were male (t[19]=0.009, p=0.993). (Table 1).
Table 1.
| Treatment | N | Maternal Weight Gain (g) | Male Birth Weight (g) | Female Birth Weight (g) | Total Pups per Litter | Percent Male Pups per Litter |
|---|---|---|---|---|---|---|
| Vehicle | 12 | 162.09 (7.831)a | 6.91 (0.214) | 6.60 (0.168) | 14.67 (0.432) | 49.71 (3.363) |
| THC | 9 | 157.98 (6.746) | 6.90 (0.184) | 6.59 (0.180) | 13.44 (0.959) | 49.76 (3.901) |
Data expressed as mean (S.E.M.)
3.2. Passive avoidance results
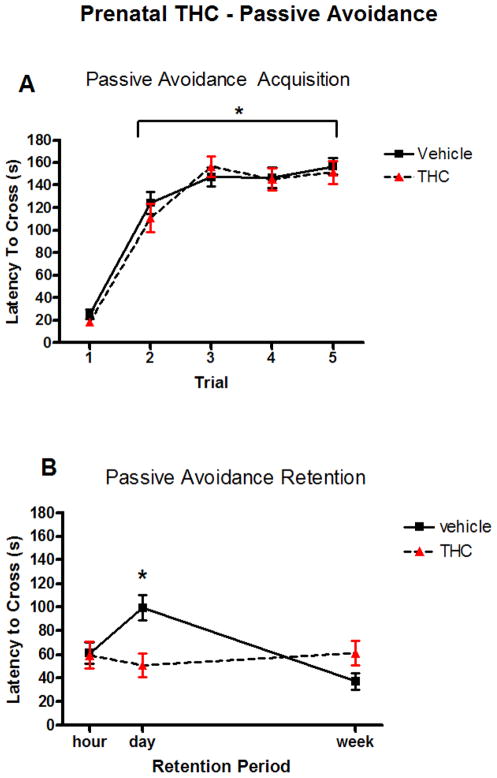
Passive avoidance was assessed using 82 animals. The vehicle pre-treated group contained 24 males and 24 females from 12 litters and the THC pre-treated group contained 18 males and 16 females from 9 litters. All animals were able to learn to avoid the shock zone within the 5-trial period (Figure 1A). Latency to crossing, in seconds, was analyzed for each of the retention time periods (1h, 1d, 7d) and compared across time accounting for sex and treatment. There was a significant treatment by time interaction; simple effects analysis showed change over time in the vehicle pre-treated group (F[2,78]=17.32, p<0.001) but not in the THC pre-treated group (F[2,75]=0.65, p=0.527). Tukey adjusted pair-wise comparisons among time points for the vehicle group showed a significant difference between latency to crossing during the 1d and the 7d retention test (t[78]=5.87, p<0.001), but not between 1h and 1d or 1h and 7d. The vehicle group showed a significantly increased latency to cross during the 1d retention test when compared to the 7d retention test (Figure 1B). The THC treated rats showed no change in latency across any of the retention trials. There was no significant sex (F[1,56.8]=0.07, p=0.791) or litter effect (Z=0.62, p=0.268).
Fig. 1.
Latency in seconds to cross to the dark chamber during each of the 5 acquisition trials (A) and during the three retention tests (B). PND 22 rats received either prenatal vehicle or daily THC exposure. In A, * indicates a significant difference from trial 1 latency assessed with paired t-tests, all with p<0.001. In B * indicates a significant difference from latency at one week (t[45]=5.265, p<0.001). There were no differences in latency across the retention tests for the THC-exposed rats.
3.3. Active place avoidance results
Active place avoidance performance was assessed using 39 animals at PND 45. The vehicle pre- treated group contained 11 males and 11 females and the THC pretreated group contained 9 males and 8 females from 11 and 9 litters respectively.
3.3.1. Acquisition
All animals were able to learn the task so that by the 7th trial they made fewer than 5 entries into the shock zone (Figure 2A). No significant treatment effect (F[1,1]=0.14, p=0.773), sex effect (F[1,154]=0.03, p=0.865), or sex by treatment interaction (F[1,154]=0.30, p=0.585) was seen for number of entries. There was only a main effect of trial (F[6,194]=6.60, p<0.0001), with mean number of entries decreasing monotonically over time.
Fig. 2.
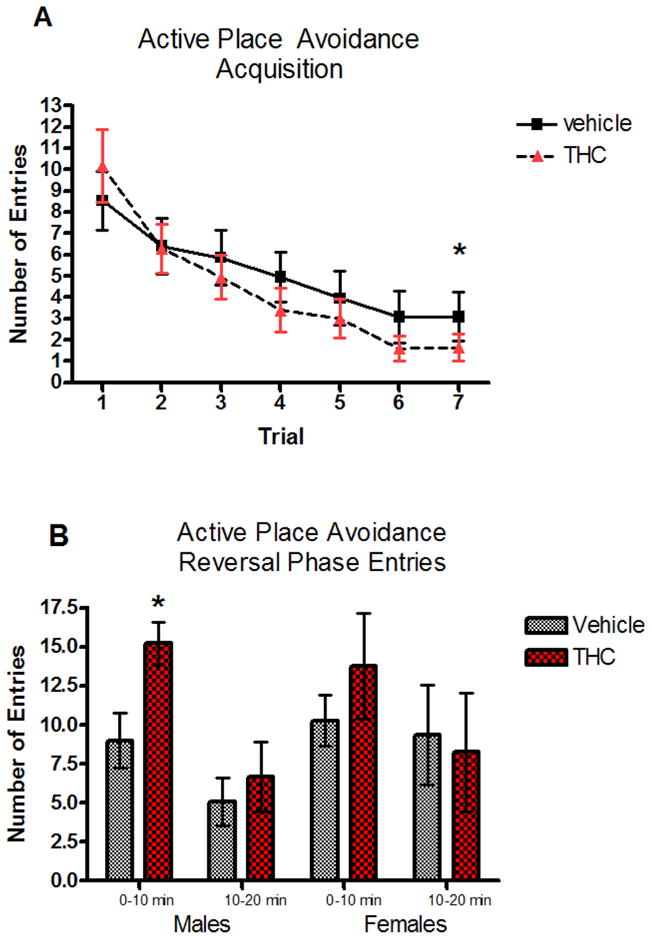
Number of entries into the shock zone during active place avoidance acquisition assessed in PND 45 vehicle or THC pre-exposed rats (A) * indicates a significant difference in number of entries on trial 7 versus trial 1 (t[38]=6.210, p<0.001). (B) Number of entries in the shock zone on the reversal trial (first 10 min and second 10 min) for males and females in the vehicle and THC-exposed groups. * indicates a significant difference from control (t[18]=2.705, p=0.015).
3.3.2. Retention
There were no significant treatment (p=0.336), sex (p=0.190), or treatment by sex interaction (p=0.478) effects for time to first entry and so the data are not shown. There were also no significant treatment-related differences in the maximum time avoided on the last training trial and time to first entry on the retention test.
3.3.3. Reversal
For both time intervals, there were no significant effects of treatment (F[1,32]=0.94, p=0.339), sex (F[1,25]=0.86 p=0.368), or treatment by sex interaction (F[1,25]=0.47, p=0.498). There was only a significant main effect of time (F[1,35]=10.16, p=0.003), with mean numbers of entries decreasing after 10 minutes for both sexes and treatment groups. However, a two-sample t-test showed that THC pre-treated males made significantly (t[18]=2.705, p=0.015) more entries into the shock zone during the first 10 minutes when compared to saline males (Figure 2B).
3.4. Attention task results
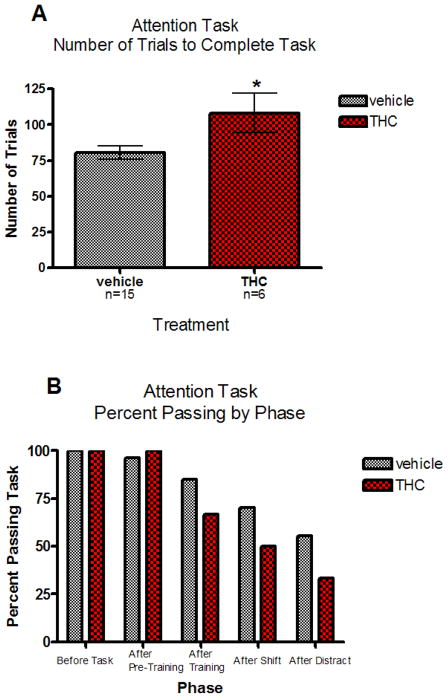
A total of 46 animals was used in the task. The vehicle pre-treated group contained 14 males and14 females, and the THC pre-treated group contained 9 males and 9 females from 14 vehicle and 9 THC-treated litters respectively. An analysis of all the animals in the task based on their point of failure yielded no significant sex by treatment interaction (F[1,21]=0.01, p=0.094). There were also no significant treatment (F[1,18]=3.04, p=0.099) or sex (F[1,22]=0.22, p=0.644) main effects. For the animals that did successfully complete the task, further analysis was done comparing the total number of trials to completion between the vehicle and THC pre-treated groups. The mixed linear model analysis showed no significant litter effect (Z=0.75, p=0.227) and no significant treatment effect (F[1,11]=4.03, p=0.071). However, these results suggest that there may be subtle differences between the vehicle and THC pre-treated groups, with THC-pretreated animals tending to take more trials to complete the task (Figure 3A). A Kruskal-Wallis analysis showed that the distribution of scores was significantly different between the two treatment groups (p=0.039) with the THC exposed rats performing more poorly than the controls. The percentage of animals completing each phase of the attention test also suggested that the THC treatment impaired performance on the attention task (Figure 3B).
Fig. 3.
(A) Total number of trials to completion for rats that successfully completed the attention task for vehicle and THC pre-exposed rats.* indicates a significant difference between the groups (p=0.039, Kruskal-Wallis) (B) Percent of each group passing each phase of the attention task. Following the distractor phase, greater than 50% of the controls passed the test while only 30% of the prenatal THC treated rats successfully completed this last phase of the test.
3.5. Amphetamine challenge results
A total of 35 animals was used for the amphetamine challenge. The vehicle pre-treated group contained 11 males and 8 females and the THC pre-treated group contained 9 males and 7 females.
The amphetamine challenge was analyzed in two separate time blocks: baseline (minutes 0–15) and post-challenge (minutes 15–75). For the baseline measurements, there was no significant litter effect (Z=1.26, p=0.104). There was a significant main effect of sex (F[1,17.3]=7.81, p=0.012), with females traveling farther than males overall but no significant effect of treatment on baseline locomotor activity. Post-challenge, there was a significant litter effect (Z=2.08, p=0.019). There was also a significant sex effect (F[1,16.5]=28.30, p<0.001), with females traveling farther than males. Finally there was a main effect of treatment (F[1,16.4]=5.23, p=0.036) indicating that pre-treatment with THC dampened the locomotor response to amphetamine (Figure 4A &B). There was however, no sex by treatment interaction, which suggests that although females traveled more than males overall, the locomotor activity of both sexes was diminished by pre-treatment with THC.
Fig. 4.
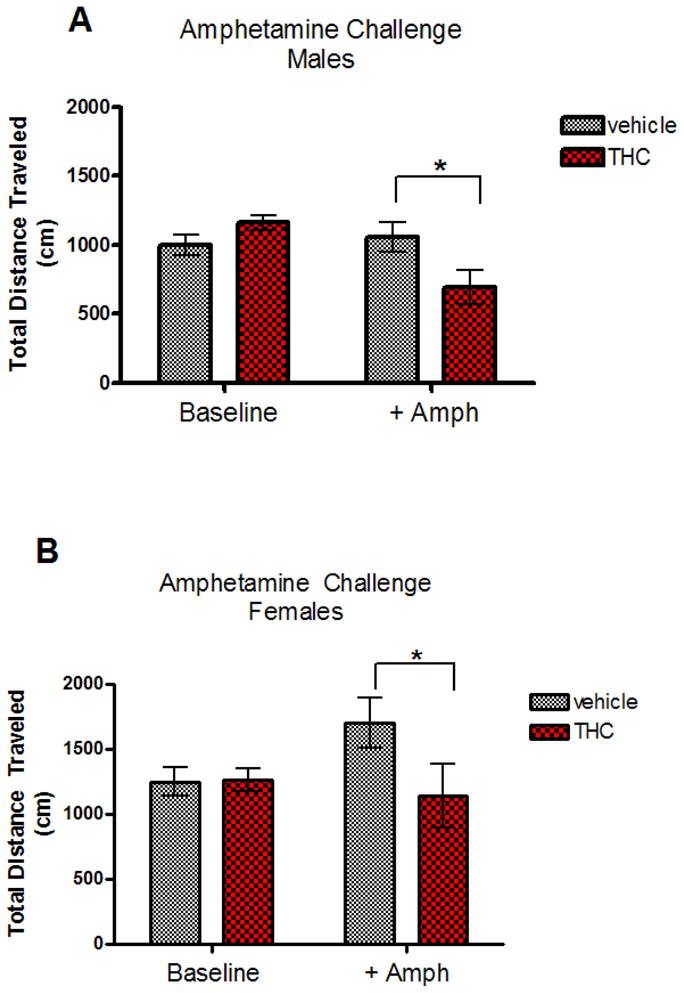
Locomotor activity (total distance traveled, in cm) during pre- and post-amphetamine challenge for male (A) and female (B) vehicle or THC pre-exposed rats. *Denotes significant difference between treatment groups (t[78]=5.87, p<0.001).
4. Discussion
Since multiple studies have shown deficits in cognitive function in both animals and humans exposed to THC during perinatal periods (Campolongo et al., 2011); (Fried and Smith, 2001); (Jutras-Aswad et al., 2009); (Schneider, 2009), we examined the effects of prenatal THC exposure on 3 measures of cognitive function in male and female offspring: passive avoidance (acquisition, consolidation, extinction), active place avoidance (acquisition, retention and learning flexibility) as well as a complex attention test with distracters. We also examined the effects of prenatal THC exposure on locomotor activity in response to an amphetamine challenge in adulthood. Generally the prenatal THC treatment did not impair acquisition of avoidance behaviors but did impair consolidation and sustained attention. Also, prenatal THC produced dampened motor responses to amphetamine, a response reminiscent of the responses to psychostimulants in children with ADHD.
4.1. Passive avoidance
A passive avoidance task measuring long-term memory function was examined from PND 22–29, a time corresponding to pre-adolescence in humans (Spear, 2000). The passive avoidance task is a common test of associative learning, and is also appropriate for use at this age (Ehman and Moser, 2006). Animals that had been prenatally exposed to THC showed normal rates of acquisition of the task but no change in latency to cross into the dark chamber across the three retention tests. Controls, on the other hand, showed a significantly increased latency to cross on the 1-day retention test, i.e., they exhibited consolidation of the fear-based contextual memory, which subsequently extinguished during the 7-day retention test. This was not seen in the THC-treated rats (Figure 1).
The passive avoidance task tests acquisition of an avoidance response and the long-term memory of the fear association. All animals were able to learn to avoid the shock (noxious stimulus) as seen by the increased latency to cross during the 5 trials for both treatment groups. This indicates that prenatal THC exposure does not significantly affect acquisition of an avoidance behavior and supports the fact that the shock stimulus used was sufficiently noxious to elicit the avoidance response. Others have reported that developmental manipulation of the cannabinoid system does not affect performance of similar spatial learning tasks. For example, Varvel and Lichtman (2002) reported that CB1 knock-out mice learned the Morris water maze as well as control mice, but showed impairments in reversal learning. We also found THC-induced differences in behavior only following the acquisition phase (i.e., during retention testing). While the vehicle treated rats showed an increased latency to cross into the dark chamber on the 1-day retention test when compared with the 1hr and 7-day retention test, there was no significant change in latency to cross during any of the retention trials in the THC treated group. Returning the animal to the chamber where the avoidance training had taken place should produce an avoidance behavior similar to that seen during acquisition. Interestingly, this was not seen in either group at the 1 hour retention test (Figure 1B) perhaps due to limited consolidation of the fear response soon after acquisition. On the other hand, at the 1 day retention test, the controls showed an increase in latency to cross which suggests that they exhibited consolidation of the fear response, a phenomena which usually requires 24 hr and benefits by a period of intervening sleep (Chang et al., 2009). THC-treated rats did not show this increase in latency on the day 1 test suggesting that the prenatal THC treatment disrupted the normal function of the circuits that mediate consolidation.
There is ample evidence for the involvement of the cannabinoid system in several phases of learning and memory (see review by (Lutz, 2007). CB1 receptors and endocannabinoids mediate short and long term modulation of synaptic transmission and have been shown to be involved in neural plasticity mechanisms related to processing of fear memories in the amygdala (Marsicano et al., 2002); (Azad et al., 2004); (Chevaleyre et al., 2007) and hippocampus (Chevaleyre et al., 2007); (Jutras-Aswad et al., 2009); (Lin et al., 2009); (Belue et al., 1994). Both fear memories and extinction memories are acquired, stored and retrieved in these brain areas but utilize distinct neuronal circuits (Herry et al., 2010). Consolidation specifically has been shown to be enhanced by intra-hippocampal administration of anandamide and impaired by intra-hippocampal injection of AM251, a cannabinoid antagonist, in an inhibitory avoidance paradigm (Herry et al., 2010). However, intrabasolateral amygdaloid administration of a cannabinoid agonist decreased consolidation of a similar inhibitory avoidance task (Campolongo et al., 2009). Therefore, cannabinoid receptors in both the hippocampus and the basolateral amygdala seem to be involved in memory consolidation and failure to consolidate may suggest a relative decrease in the effectiveness of the endogenous cannabinoid system (akin to an antagonist injection) in one or both of these brain regions.
Also, glutamatergic projections from the infralimbic region of the prefrontal cortex have been associated with the consolidation of context-based memories (Kaplan et al., 2011); (Millan et al., 2011); (Quirk and Mueller, 2008). Since prenatal activation of the cannabinoid receptor CB1, has been shown to have adverse effects on glutamatergic neurotransmission (Antonelli et al., 2005), it is possible that abnormal development of this glutamatergic projection from the prefrontal cortex may contribute to the disruption of consolidation of contextual memories. Therefore, disruption of the endocannabinoid control of synaptic activity in the key brain regions involved in consolidation of fear memories may have been manifested in our study.
Failure to show consolidation (and extinction) in the THC-treated rats may also be related to a delay in maturation of the response since normal PND 17 rats do not show long-term extinction (24 hours following the initial extinction) while normal PND 24 rats do show this response (Kim and Richardson, 2010). If the prenatal treatment induced a delay in the maturation of the brain regions involved in consolidation and extinction, then we may expect to see abnormal patterns. However, since the brain regions involved in acquisition of the association of the context with the fear are intermingled with those regions involved in consolidation and extinction learning, it is unlikely that a simple developmental delay could account for the results obtained. Since none of our animals received postnatal cannabinoid treatment, our results suggest that a developmental difference in the endocannabinoid system due to prenatal exposure to THC could have persistent effects after birth and thus alter both the consolidation of the fear memory and the subsequent extinction of this effect. A more detailed assessment using classical methodologies across the life span should clarify this potentially significant effect of prenatal THC exposure. Since strategies which enhance extinction have been used clinically to treat post-traumatic brain disorders and chronic anxiety, further understanding of the role of the endocannabinoid system and its disruption by developmental cannabinoid exposure in consolidation and extinction is warranted.
4.2. Active place avoidance
Active place avoidance, which measures spatial working memory and predictive ability, was tested on PND 45 and 46. Cimadevilla et al. (2001) showed that females were slower to reach optimal performance than males. However, these sex differences disappear by PND 45, making active place avoidance an appropriate test of cognitive function at this age. Active place avoidance (APA) tests the ability of the animal to use prediction, timing, spatial navigation and working memory to avoid receiving a shock. Retention tests for long-term memory and reversal tests for flexibility in learning and the ability to ignore a previously learned strategy and establish a new one. Although there was some suggestion that treated rats performed slightly better than controls during the acquisition phase, there was no significant difference between the groups and both groups learned to avoid the shock zone (Figure 2A). There was no treatment effect on the retention trial (data not shown). On the contrary, Mereu et al. (2003) found that prenatal cannabinoid agonist, WIN 55,212-2 produced memory deficits following passive avoidance training at PND 40, a finding which is not consistent with ours perhaps due to differences in the amount and potency of the cannabinoid agonist given as well as the age of testing. Surprisingly, we also did not see a treatment-related effect on consolidation of the active place avoidance test since the relationship between the maximum time avoided on the last training trial and the time to first entry on the retention trial (24 hours later) was similar in the controls and the THC exposed rats. However, since consolidation of the avoidance response did not occur in the controls (perhaps due to increased impulsivity at this age) we were unable to see any treatment-related effects on this measure. On the reversal trial in our APA test, treated males performed more poorly than the control males (Figure 2B). Studies with both humans and animals exposed to cannabinoids prenatally and/or perinatally have shown deficits in these abilities (Fried and Smith, 2001); (Moreno et al., 2003); (Schneider, 2009). Previous findings in our lab using the APA paradigm have also shown that rats exposed to THC during early adolescence demonstrated significant impairments compared to controls in the reversal phase of the APA task but adolescent exposure impaired performance in both male and female exposed rats (Harte and Dow-Edwards, 2010). A number of conditions could have contributed to this difference.
Firstly, the age at which the THC exposure occurred was different in our 2 studies. The present study utilized a prenatal exposure while the previous study utilized a periadolescent (PND 22–40) exposure. Cannabinoid receptors are undergoing modification in brain regions critical to learning and decision making, such as the hippocampus and cortex, throughout postnatal development (Belue et al., 1994). Additionally, since adolescence is a period of refinement in functional connections, drug administration during this period can have different and/or greater effects than prenatal exposure particularly if the drug interacts with the refinement process. Thus, THC exposure may be more effective in altering connections in hippocampus and cortex necessary for the reversal learning of the APA task in adolescent females compared to the prenatal period. We also found that females were more sensitive to the locomotor altering effects of THC compared to adolescent males (Harte and Dow-Edwards, 2010).
Secondly, the current study tested at PND 45, late-adolescence (Spear, 2000), while our earlier work (Harte and Dow-Edwards, 2010) tested at PND 70, adulthood. The age of testing could be a possible factor in the differences we found in the rats’ ability to perform the reversal task. The findings of Cha et al. (2006) suggest that spatial and non-spatial learning are more affected in adolescents than adult male rats by acute THC exposure, however, chronic THC exposure during either age group showed no significant impairment of either type of learning. However, Cha et al. (2006) do point out that adolescence is a time of dynamic changes in the brain. Pruning, or the reduction of the connections between neurons, occurs throughout the brain during adolescence and since our rats were still developing during testing, we might see different effects in adulthood. It would be advantageous to repeat the experiment at different testing ages to examine this possibility.
4.3. Attention task
The third test was the attention task, which utilized young adult rats (~ PND 60) (Spear, 2000) prenatally exposed to THC or vehicle. The attention task is designed to test sustained attention through a complex series of phases, during each of which an animal must learn to pay attention to one set of cues but not another in order to receive a food reward. At each phase, the cues change, the only consistent factor being the pairing of the reward with the black rubber stimulus, whether this be on the box containing the reward or on the lid of the box. Because the rats must undergo multiple trials over multiple days, they must not only learn and remember the rules of the task, but also pay attention to the location of the cue in order to receive a reward. While performance of all treatment groups was poor (many did not complete the task), our data suggest that prenatal THC produces attention deficits as shown by the increased number of trials to successfully complete the attention task compared to the controls as well as a reduction in the percent passing each phase of the task (Figure 3).
Disorders of attention and inattention in children prenatally exposed to marijuana have been described (Fried and Smith, 2001); (Jutras-Aswad et al., 2009). Our results support the idea that prenatal THC exposure, independent of polydrug and environmental factors, alters the development of the circuits that mediate attention including the prefrontal cortex. We found that our prenatal exposure impaired performance in this test since relatively more treated rats failed early in the testing and the percent completing the entire task was lower (Figure 3). One of the setbacks in this study was the relatively small number of animals that completed the entire task. Our earlier experience with this task using adolescent animals suggested that the task is relatively easy for younger rats (Harte and Dow-Edwards, 2010). Since it is impossible to be sure an animal will successfully complete the task, it would be advantageous to repeat the study with younger animals which seem to perform better overall on the task.
4.4. Amphetamine challenge
Finally, we tested the locomotor responses of both treatment groups to an amphetamine challenge at PND 60. The amphetamine challenge is not a test of cognitive function. However, because numerous studies show abnormal development of the dopaminergic system due to prenatal treatment with THC in animals (Garcia et al., 1996); (Moreno et al., 2003); (Navarro et al., 1994); (Rodriguez de Fonseca et al., 1991) and humans (Wang et al., 2004), as well as the potential clinical use of psychostimulants in the treatment of behavior disorders, it becomes important to test the function of the dopaminergic system in animals exposed to THC prenatally. While we found no alteration in baseline locomotor activity in exposed adults, we did see first that females overall were significantly more active than males in response to the amphetamine, and secondly that both males and females that had been exposed to THC showed dampened locomotor responses to amphetamine when compared with their vehicle exposed counterparts (Figure 4). Others have found that perinatal exposure to cannabinoids alters the normal development of dopaminergic neurons in the nigrostriatal, mesolimbic, and tuberoinfundibular pathways (Rodriguez de Fonseca et al., 1991). Since dopaminergic neurons, especially in the nigrostriatal and mesolimbic pathways, are involved with reward, motivation and the motor response to psychostimulants, prenatal disruption of the normal development of these neurons could be the cause of the motor differences we observed in adulthood. Perinatal exposure to oral THC also has been reported to alter the behavioral responses to D1 and D2 agonists. That is, Moreno et al. (2003) reported that oral THC throughout pregnancy dampened the locomotor responses to apomorphine, a direct DA agonist although responses of DA autoreceptors were found to be enhanced (Newsom and Kelly, 2008).
CB1 receptors are often co-localized with dopamine receptors on GABA and glutamate containing neurons especially in the striatum (Herkenham et al., 1991) and the mesolimbic pathway (Szabo et al., 2002), suggesting that actions at both of these receptors are involved in both locomotor and reward responses. Although chronic THC treatment has been shown to cause a loss of cannabinoid receptors in the adult rat (Breivogel et al., 1999), our data are consistent with the report of Castelli et al. (2007) who found enhanced functional responsivity of CB1 receptors in striatum in adult males exposed to the cannabinoid agonist WIN 55,212-2 prenatally. Activation of CB1 receptors can dampen the increase in amphetamine-induced dopaminergic output in the nigrostriatal pathway, and thus attenuate the locomotor response (Gorriti et al., 1999). Giuffrida et al. (1999) showed that endocannabinoids may be important in homeostatic responses to psychostimulants since cocaine increased the endogenous cannabinoid anandamide in the striatum which subsequently dampened the response to cocaine. Although not thoroughly understood, there are multiple examples of reduced motor activity following cannabinoid administration (see review by (Giuffrida et al., 1999) supporting the suggestion that an enhanced responsivity of basal ganglia CB1 receptors following prenatal THC exposure results in a relative increase in the effects of the endocannabinoid system and a decrease in the response to psychostimulants. The enhanced responsivity of endogenous cannabinoids produced by prenatal THC could be the mechanism for the dampened locomotor response to the amphetamine challenge we saw in adulthood.
Our findings also show that the time of exposure to THC can affect the behavioral response to amphetamine. A study of rats exposed to THC or a CB1 agonist during adolescence, the most common age of initiation of marijuana use in humans, showed no differences in behavioral response to amphetamine between treated and untreated groups in either adolescence or adulthood (Ellgren et al., 2004). This suggests that the effects of cannabinoids on the development of the dopaminergic system during the prenatal period is unique (effects differ from exposures at older ages) and may have an impact on behavioral responses later in life as shown by the dampened response to the amphetamine challenge.
5. Conclusions
The results of these studies demonstrate that prenatal THC exposure has little effect on acquisition of avoidance behaviors but rather impairs consolidation and reversal learning during the juvenile and adolescent periods. The results also suggest that performance on an attention test was impaired in adulthood, although the effect was subtle, perhaps due to the age of the subjects at the time of testing. There was a robust decrease in the motor response to amphetamine challenge in the prenatal THC exposed groups tested in adulthood. Further testing at younger ages would be helpful in providing translational information relating to ADHD. Clearly, the results indicate that prenatal exposure to relatively low doses of THC is detrimental to the neurobehavioral development of the offspring and clinicians should advise women to avoid marijuana consumption during pregnancy.
Highlights.
Prenatal THC administration using a clinically-relevant rate of drug delivery has long term effects on components of executive function in the rat offspring
Juvenile rats exposed to THC prenatally show normal acquisition of avoidance behaviors but impaired consolidation of this response
Adolescent rats exposed to THC show normal acquisition of avoidance behaviors but impaired ability in task reversal
Adult rats exposed to THC prenatally show impaired attention throughout many phases of the task (acquisition, reversal and distraction)
Adult rats exposed to THC prenatally show normal behavioral responses to novelty but dampened behavioral responses to amphetamine challenge
Acknowledgments
The authors would like to thank Dr. Jeremy Weedon for his statistical analysis; Stacy Stephenson, Maiko Iijima, and April Jackson for their technical assistance and Dothlyn Dunkley, MS for careful preparation of the manuscript. This work was supported by NIDA grant DA019348.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lindsay Silva, Email: lindsay.silva@downstate.edu.
Ning Zhao, Email: ning.zhao@downstate.edu.
Susanna Popp, Email: susanna.popp@downstate.edu.
Diana Dow-Edwards, Email: diana.dow-edwards@downstate.edu.
Reference List
- Antonelli T, Tomasini MC, Tattoli M, Cassano T, Tanganelli S, Finetti S, Mazzoni E, Trabace L, Steardo L, Cuomo V, Ferraro L. Prenatal exposure to the CB1 receptor agonist WIN 55,212-2 causes learning disruption associated with impaired cortical NMDA receptor function and emotional reactivity changes in rat offspring. Cerebral Cortex. 2005;15:2013–2020. doi: 10.1093/cercor/bhi076. [DOI] [PubMed] [Google Scholar]
- Azad SC, Monory K, Marsicano G, Cravatt BF, Lutz B, Zieglgänsberger W, Rammes G. Circuitry for associative plasticity in the amygdala involves endocannabinoid signaling. Neurosci. 2004;24:9953–9961. doi: 10.1523/JNEUROSCI.2134-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belue RC, Howlett AC, Westlake TM, Hutchings DE. The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxicology and Teratology. 1994;17:25–30. doi: 10.1016/0892-0362(94)00053-g. [DOI] [PubMed] [Google Scholar]
- Bernard C, Milh M, Morozov YM, Ben-Ari Y, Freund TF, Gozlan G. Altering cannabinoid signaling during development disrupts neuronal activity. PNAS. 2005;102:9388–9393. doi: 10.1073/pnas.0409641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnin A, de Miguel R, Hernández ML, Ramos JA, Fernández-Ruiz JJ. The prenatal exposure to delta 9-tetrahydrocannabinol affects the gene expression and the activity of tyrosine hydroxylase during early brain development. Life Sci. 1995;56:2177–2184. doi: 10.1016/0024-3205(95)00205-k. [DOI] [PubMed] [Google Scholar]
- Bonnin A, de Miguel R, Rodríguez-Manzaneque JC, Fernández-Ruiz JJ, Santos A, Ramos JA. Changes in tyrosine hydroxylase gene expression in mesencephalic catecholaminergic neurons of immature and adult male rats perinatally exposed to cannabinoids. Brain Res Dev Brain Res. 1994;81:147–150. doi: 10.1016/0165-3806(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ. Chronic Delta9-tetrahydrocannabinol Treatment Produces a Time-Dependent Loss of Cannabinoid Receptors and Cannabinoid Receptor-Activated G Proteins in Rat Brain. Journal of Neurochemistry. 1999;73:2447–2459. doi: 10.1046/j.1471-4159.1999.0732447.x. [DOI] [PubMed] [Google Scholar]
- Campolongo P, Roozendaal B, Trezza V, Hauer D, Schelling G, McGaugh JL, Cuomo V. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc Natl Acad Sci U S A. 2009;106:4888–4893. doi: 10.1073/pnas.0900835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campolongo P, Trezza V, Ratano P, Palmery M, Cuomo V. Developmental consequences of perinatal cannabis exposure: behavioral and neuroendocrine effects in adult rodents. Psychopharmacology (Berl) 2011;214:5–15. doi: 10.1007/s00213-010-1892-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli MP, Piras AP, D’Agostino A, Pibiri F, Perra S, Gessa GL, Maccarrone M, Pistis M. Dysregulation of the endogenous cannabinoid system in adult rats prenatally treated with the cannabinoid agonist WIN 55,212–2. European Journal of Pharmacology. 2007;573:11–19. doi: 10.1016/j.ejphar.2007.06.047. [DOI] [PubMed] [Google Scholar]
- Cha YM, White AM, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of delta9-THC on learning in adolescent and adult rats. Pharmacol Biochem Behav. 2006;83:448–455. doi: 10.1016/j.pbb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Chang CH, Knapska E, Orsini CA, Rabinak CA, Zimmerman JM, Maren S. Fear extinction in rodents. Unit8.23. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Heifets BD, Kaeser PS, Südhof TC, Castillo PE. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1alpha. Neuron. 2007;54:801–812. doi: 10.1016/j.neuron.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimadevilla JM, Fenton AA, Bures J. Transient sex differences in the between-sessions but not in the within-session memory underlying an active place avoidance task in weanling rats. Behav Neurosci. 2001;115:695–703. doi: 10.1037//0735-7044.115.3.695. [DOI] [PubMed] [Google Scholar]
- Day NL, Leech SL, Goldschmidt L. The effects of prenatal marijuana exposure on delinquent behaviors are mediated by measures of neurocognitive functioning. Neurotoxicol Teratol. 2011;33:129–136. doi: 10.1016/j.ntt.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow-Edwards D, Zhao N. Oral THC produces minimal behavioral alterations in preadolescent rats. Neurotoxicol Teratol. 2008;30:385–389. doi: 10.1016/j.ntt.2008.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehman KD, Moser VC. Evaluation of cognitive function in weanling rats: a review of methods suitable for chemical screening. Neurotoxicol Teratol. 2006;28:144–161. doi: 10.1016/j.ntt.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ellgren M, Hurd YL, Franck J. Amphetamine effects on dopamine levels and behavior following cannabinoid exposure during adolescence. European Journal of Pharmacology. 2004;497:205–213. doi: 10.1016/j.ejphar.2004.06.048. [DOI] [PubMed] [Google Scholar]
- Fried PA, Smith AM. A literature review of the consequences of prenatal marihuana exposure. An emerging theme of a deficiency in aspects of executive function. Neurotoxicol Teratol. 2001;23:1–11. doi: 10.1016/s0892-0362(00)00119-7. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B. Differential effects on facets of attention in adolescents prenatally exposed to cigarettes and marihuana. Neurotoxicol Teratol. 2001;23:421–430. doi: 10.1016/s0892-0362(01)00160-x. [DOI] [PubMed] [Google Scholar]
- Garcia L, de Miguel R, Ramos JA, Fernandez-Ruiz JJ. Perinatal delta (9)-tetrahydrocannabinol exposure in rats modifies the responsiveness of midbrain dopaminergic neurons in adulthood to a variety of challenges with dopaminergic drugs. Drug and Alcohol Dependence. 1996;42:155–166. doi: 10.1016/s0376-8716(96)01276-8. [DOI] [PubMed] [Google Scholar]
- Garcia-Gil L, de Miguel R, Romero J, Perez A, Ramos JA, Fernández-Ruiz JJ. Perinatal delta9-tetrahydrocannabinol exposure augmented the magnitude of motor inhibition caused by GABA(B), but not GABA(A), receptor agonists in adult rats. Neurotoxicol Teratol. 1999;21:277–283. doi: 10.1016/s0892-0362(98)00058-0. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nature Neuroscience. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Goldschmidt L, Day NL, Richardson GA. Effects of prenatal marijuana exposure on child behavior problems at age 10. Neurotoxicol Teratol. 2000;22:325–336. doi: 10.1016/s0892-0362(00)00066-0. [DOI] [PubMed] [Google Scholar]
- González B, de Miguel R, Martín S, Pérez-Rosado A, Romero J, García-Lecumberri C, Fernández-Ruiz J, Ramos JA, Ambrosio E. Effects of perinatal exposure to delta 9-tetrahydrocannabinol on operant morphine-reinforced behavior. Pharmacol Biochem Behav. 2003;75:577–584. doi: 10.1016/s0091-3057(03)00115-1. [DOI] [PubMed] [Google Scholar]
- Gorriti MA, Rodríguez de Fonseca F, Navarro M, Palomo T. Chronic (−)-delta9-tetrahydrocannabinol treatment induces sensitization to the psychomotor effects of amphetamine in rats. European Journal of Pharmacology. 1999;365:133–142. doi: 10.1016/s0014-2999(98)00851-6. [DOI] [PubMed] [Google Scholar]
- Harbison RD, Mantilla-Plata B. Prenatal toxicity, maternal distribution and placental transfer of tetrahydrocannabinol. Journal of Pharmacology and Experimental Therapeutics. 1972;180:446–452. [PubMed] [Google Scholar]
- Harte LC, Dow-Edwards D. Sexually dimorphic alterations in locomotion and reversal learning after adolescent tetrahydrocannabinol exposure in the rat. Neurotoxicology and Teratology. 2010;32:515–524. doi: 10.1016/j.ntt.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, De Costa BR, Rice KC. Characterization and Localization of Cannabinoid Receptors in Rat Brain: A Quantitative in vitro Autoradiographic Study. The Journal of Neuroscience. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Lüthi A. Neuronal circuits of fear extinction. Eur J Neurosci. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- Hutchings DE, Martin BR, Gamagaris Z, Miller N, Fico T. Plasma concentrations of delta-9-tetrahydrocannabinol in dams and fetuses following acute or multiple prenatal dosing in rats. Life Sciences. 1989;44:697–701. doi: 10.1016/0024-3205(89)90380-9. [DOI] [PubMed] [Google Scholar]
- Jutras-Aswad D, DiNieri JA, Harkany T, Hurd YL. Neurobiological consequences of maternal cannabis on human fetal development and its neuropsychiatric outcome. Eur Arch Psychiatry Clin Neurosci. 2009;259:395–412. doi: 10.1007/s00406-009-0027-z. [DOI] [PubMed] [Google Scholar]
- Kaplan GP, Heinrichs SC, Carey RJ. Treatment of addiction and anxiety using extinction approaches: Neural mechanisms and their treatment implications. Pharmacology, Biochemistry and Behavior. 2011;97:619–625. doi: 10.1016/j.pbb.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. New findings on extinction of conditioned fear early in development: theoretical and clinical implications. Biol Psychiatry. 2010;67:297–303. doi: 10.1016/j.biopsych.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Lin H, Mao S, Su C, Gean P. The role of prefrontal cortex CB1 receptors in the modulation of fear memory. Cerebral Cortex. 2009;19:165–175. doi: 10.1093/cercor/bhn075. [DOI] [PubMed] [Google Scholar]
- Lutz B. The endocannabinoid system and extinction learning. Mol Neurobiol. 2007;36:92–101. doi: 10.1007/s12035-007-8004-x. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Moosmann B, Hermann H, Lutz B, Behl C. Neuroprotective properties of cannabinoids against oxidative stress: role of the cannabinoid receptor CB1. J Neurochem. 2002;80:448–456. doi: 10.1046/j.0022-3042.2001.00716.x. [DOI] [PubMed] [Google Scholar]
- Mereu G, Fà M, Ferraro L, Cagiano R, Antonelli T, Tattoli M, Ghiglieri V, Tanganelli S, Gessa GL, Cuomo V. Prenatal exposure to a cannabinoid agonist produces memory deficits linked to dysfunction in hippocampal long-term potentiation and glutamate release. Proc Natl Acad Sci U S A. 2003;100:4915–4920. doi: 10.1073/pnas.0537849100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan EZ, Marchant NJ, McNally GP. Extinction of drug seeking. Behavioural Brain Research. 2011;217:454–462. doi: 10.1016/j.bbr.2010.10.037. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado F, Amaro A, González MI, Alvarez FJ, Leret ML. Effect of maternal delta 9-tetrahydrocannabinol on developing serotonergic system. Eur J Pharmacol. 1996;316:39–42. doi: 10.1016/s0014-2999(96)00753-4. [DOI] [PubMed] [Google Scholar]
- Moreno M, Trigo JM, Escuredo L, Rodriguez de Fonseca F, Navarro M. Perinatal exposure to delta-9-tetrahydrocannabinol increases presynaptic dopamine D2 receptor sensitivity: a behavioral study in rats. Pharmacology, Biochemistry and Behavior. 2003;75:565–575. doi: 10.1016/s0091-3057(03)00117-5. [DOI] [PubMed] [Google Scholar]
- Navarro M, Rodriguez de Fonseca F, Hernandez Ml, Ramos JA, Fernandez-Ruiz JJ. Motor behavior and nigrostriatal dopaminergic activity in adult rats perinatally exposed to cannabinoids. Pharmacology Biochemistry and Behavior. 1994;47:47–58. doi: 10.1016/0091-3057(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Newsom RJ, Kelly SJ. Perinatal delta-9-tetrahydrocannabiol exposure disrupts social and open field behavior in adult male rats. Neurotoxicology and Teratology. 2008;30:213–219. doi: 10.1016/j.ntt.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noland JS, Singer LT, Short EJ, Minnes S, Arendt RE, Kirchner HL, Bearer C. Prenatal drug exposure and selective attention in preschoolers. Neurotoxicol Teratol. 2005;27:429–438. doi: 10.1016/j.ntt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural Mechanisms of Extinction Learning and Retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GA, Ryan C, Willford J, Day NL, Goldschmidt L. Prenatal alcohol and marijuana exposure: effects on neuropsychological outcomes at 10 years. Neurotoxicol Teratol. 2002;24:309–320. doi: 10.1016/s0892-0362(02)00193-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Cebeira M, Fernandez-Ruiz JJ, Navarro M, Ramos JA. Effects of pre- and perinatal exposure to hashish extracts on the ontogeny of brain dopaminergic neurons. Neuroscience. 1991;43:713–723. doi: 10.1016/0306-4522(91)90329-m. [DOI] [PubMed] [Google Scholar]
- Schneider M. Cannabis use in pregnancy and early life and its consequences: animal models. Eur Arch Psychiatry Clin Neurosci. 2009;259:383–393. doi: 10.1007/s00406-009-0026-0. [DOI] [PubMed] [Google Scholar]
- Spano MS, Ellgren M, Wang X, Hurd YL. Prenatal cannabis exposure increases heroin seeking with allostatic changes in limbic enkephalin systems in adulthood. Biol Psychiatry. 2007;61:554–563. doi: 10.1016/j.biopsych.2006.03.073. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Szabo B, Siemes S, Wallmichrath I. Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. European Journal of Neuroscience. 2002;15:2057–2061. doi: 10.1046/j.1460-9568.2002.02041.x. [DOI] [PubMed] [Google Scholar]
- Trezza V, Cuomo V, Vanderschuren LJ. Cannabis and the developing brain: insights from behavior. Eur J Pharmacol. 2008;585:441–452. doi: 10.1016/j.ejphar.2008.01.058. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Lichtman AH. Evaluation of CB1 receptor knockout mice in the Morris water maze. J Pharmacol Exp Ther. 2002;301:915–924. doi: 10.1124/jpet.301.3.915. [DOI] [PubMed] [Google Scholar]
- Wang X, Dow-Edwards D, Anderson V, Minkoff H, Hurd YL. In utero marijuana exposure associated with abnormal amygdala dopamine D2 gene expression in the human fetus. Biol Psychiatry. 2004;56:909–915. doi: 10.1016/j.biopsych.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Wu CS, Zhu J, Wager-Miller J, Wang S, O’Leary D, Monory K, Lutz B, Mackie K, Lu HC. Requirement of cannabinoid CB(1) receptors in cortical pyramidal neurons for appropriate development of corticothalamic and thalamocortical projections. Eur J Neurosci. 2010;32:693–706. doi: 10.1111/j.1460-9568.2010.07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]