Abstract
Doublecortin (DCX) is a microtubule associated protein that is critical for neuronal migration and the development of the cerebral cortex. In the adult, it is expressed in newborn neurons in the subventricular and subgranular zones but not in the mature neurons of the cerebral cortex. By contrast, neurogenesis and neuronal migration of cells in the cerebellum continue into early postnatal life; migration of one class of cerebellar interneuron, unipolar brush cells (UBCs), may continue into adulthood. To explore the possibility of continued neuronal migration in the adult cerebellum, closely spaced sections through the brainstem and cerebellum of adult (3–16 months old) Sprague Dawley rats were immunolabeled for DCX. Neurons immunoreactive (ir) to DCX were present in the granular cell layer of the vestibulocerebellum, most densely in the transition zone (tz), the region between the flocculus (FL) and ventral paraflocculus (PFL), as well as in the dorsal cochlear nucleus (DCN). These DCX-ir cells had the morphological appearance of unipolar brush cells (UBCs) with oval somata and a single dendrite ending in a “brush.” There were many examples of colocalization of DCX with Eps8 or calretinin, UBC markers. We also identified DCX-ir elements along the fourth ventricle and its lateral recess that had labeled somata but lacked the dendritic structure characteristic of UBCs. Labeled UBCs were seen in nearby white matter. These results suggest that there may be continued neurogenesis and/or migration of UBCs in the adult. Another possibility is that UBCs maintain DCX expression even after migration and maturation, reflecting a role of DCX in adult neuronal plasticity in addition to a developmental role in migration.
Keywords: cerebellar cortex, granule cells, mossy fibers, neurogenesis, plasticity, vestibulocerebellum
1.0. INTRODUCTION
Many studies in both humans and animals have shown that the protein doublecortin (DCX) is essential for the normal development of the cerebral cortex (des Portes et al., 1998, Gleeson et al., 1999, Bai et al., 2003). DCX plays a critical role in the regulation of microtubule dynamics during neuronal migration (Tanaka et al., 2004); it is highly expressed in postmitotic, migrating neurons (Francis et al., 1999, Gleeson et al., 1999, Tanaka et al., 2004). While initial reports suggested that DCX expression is downregulated to undetectable levels in the adult (Gleeson et al., 1999), subsequent studies have shown DCX expression in postmitotic cells in regions of adult neurogenesis, the subventricular zone (SVZ) and the subgranular zone (SGZ), as well as in migrating neuroblasts in the rostral migratory stream (RMS; Nacher et al., 2001, Brown et al., 2003b, Rao and Shetty, 2004, Couillard-Despres et al., 2005, Ming and Song, 2005, Gutierrez-Mecinas et al., 2007, Zhao et al., 2008).
The time course of neurogenesis and neuronal migration in the cerebellum is quite different from that in the cortex; cortical neurogenesis occurs prenatally but several cerebellar interneuron populations are born postnatally (Caviness and Sidman, 1973, Carletti and Rossi, 2008). In the mouse, granule cell neurogenesis is not complete until postnatal day 21 (Carletti and Rossi, 2008). An intriguing observation in the cat suggests that neuronal migration may continue for several months postnatally for one class of cerebellar interneuron, the unipolar brush cell (UBC; Takács et al., 2000). The adult distribution of UBCs was not established until postnatal day 132; apparently migrating UBCs could be found in white matter up until that age. This observation was quite surprising since other studies have suggested that neurogenesis in the cat cerebellum is complete by about 3–4 weeks postnatally (Anderson and Stromberg, 1977b, a). Takács et al. (2000) suggested that there might be continued UBC neurogenesis and migration in the adult. To investigate this possibility we used immunohistochemistry to look at expression of DCX in the adult rat cerebellum since there have been many studies of the UBC population in this species (Floris et al., 1994, Mugnaini and Floris, 1994, Jaarsma et al., 1995, Morin et al., 2001, Sekerkova et al., 2004, Sekerkova et al., 2007, Diño and Mugnaini, 2008, Russo et al., 2008, Birnstiel et al., 2009, Mugnaini et al., 2011). We consistently found DCX expression in UBCs of defined regions of the vestibulocerebellum and dorsal cochlear nucleus (DCN) in adult rats. We also saw DCX-immunoreactive cells around the fourth ventricle and its lateral recess that had the morphology of neuroblasts.
Some of these results have been presented as abstracts (Baizer et al., 2011, Manohar et al., 2011, Paolone et al., 2011).
2.0. EXPERIMENTAL PROCEDURES
2.1 Animals
We used adult (ages 3–16 months) male, albino SASCO Sprague-Dawley rats from Charles River Laboratories (Wilmington, MA), and archival sections from one Wistar and one Fisher344 rat. We followed the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) revised 1996, and all animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the University at Buffalo.
Animals were housed individually and had ad lib. access to water and standard laboratory rodent chow. They were maintained on a 12 hour light-dark cycle.
2.2. Tissue preparation
Rats were deeply anesthetized with 86 mg/kg i.p. of Fatal-plus (Vortech, Pharmaceutical Ltd.) and perfused through the heart with 0.1M phosphate buffered saline (PBS) followed by 10% formalin or 4% paraformaldehyde in PBS. The brains were removed, post-fixed for 24 hrs-1 week, and then cryoprotected in 15% sucrose and then 30% sucrose in PBS. Forty μm thick coronal sections were cut on a cryostat and stored in tissue culture wells in a cryoprotection solution of 30% ethylene glycol and 30% glycerol in PB at −20°C.
2.3. Immunohistochemistry
All processing was on free-floating sections. Sections were removed from the cryoprotectant and rinsed in PBS. Non-specific binding of primary antibodies was blocked by incubating the sections in a solution of 1% bovine serum albumin (BSA, Sigma), 1.5 % normal horse serum (NHS, Vector laboratories) and 0.1–0.2% TritonX-100 (TX) in PBS for 30 min. For single- label immunohistochemistry, the primary antibody was added and the sections incubated overnight at 4°C. Table 1 shows the primary antibodies and dilutions that we used. Sections were then rinsed and incubated in the appropriate biotinylated secondary antibody (Vector Laboratories, Burlingame CA, following manufacturer’s instructions); further processing was with the Vector ABC method using a Vectastain kit. Immunoreactivity was visualized using the glucose oxidase (GO) modification of the diaminobenzidine (DAB) method (Shu et al., 1988, Van Der Gucht et al., 2006).
TABLE 1.
Primary antibodies and dilutions.
| Antigen | Manufacturer, Catalogue number | species, type | Dilution |
|---|---|---|---|
| calretinin | Millipore/Chemicon, AB5054 | rb, polyclonal | 1:5000 |
| doublecortin (DCX) | SCBT, sc-8066 C-18 | gt, polyclonal | 1:250–1:500 |
| Eps8 | BD Transduction Laboratories, 610143 | ms, monoclonal | 1:200 |
| mGluR1 | BD Transduction Laboratories, 610965 | ms, monoclonal | 1:1000 |
| PLCβ4 (C-18) | SCBT, sc-404 | rb, polyclonal | 1:100 |
| PSA-NCAM | Millipore, MAB 5324 | ms, monoclonal | 1:500 |
For double-label immunofluorescence, sections were first incubated in a cocktail of PBS, 1% normal serum, 1% BSA and 0.1–0.2% TX and combinations of two primary antibodies (DCX+Eps8; DCX+ CR; DCX+ mGluR1; DCX+ PLCβ4) overnight, rinsed, and then incubated with the appropriate fluorescent secondary antibody, (donkey anti-rabbit Alexa 546; donkey-anti mouse Alexa 555 or chicken anti-goat Alexa 488). Secondary fluorescent antibodies were used at a dilution of 2 μg/ml in a solution of PBS, normal serum, 1% BSA and 0.1–0.2% TX. Sections were rinsed and mounted on Fisher “Superfrost” polarized slides (Fisher Scientific, Pittsburgh, PA). The DAB-GO slides were dehydrated in ethanol, cleared in xylene or Histosol (National Diagnostics, Atlanta, Georgia) and coverslipped with Depex (Electron Microscopy Sciences, Hatfield, PA) or Permount (Fisher Scientific). The immunofluorescence slides were coverslipped with Prolong Antifade mounting medium (Invitrogen, Carlsbad, CA) or Vector Vectashield Hard Mount mounting medium. For the preadsorption control, 0.4 μg of DCX antibody (sc-8066, Santa Cruz, 1:500) was diluted in 1 ml of a solution of PBS, 1.5% NHS, 1% BSA and 1% TX. This was then divided into two aliquots of 500 μl each. Two μg of the immunizing peptide (sc-8066 P, Santa Cruz, 1:100) was added to one aliquot, and both aliquots incubated for 30 min at RT. Sections were then processed by standard procedures using either the preadsorbed antibody or the standard antibody, and DAB-GO visualization.
2.4. Data analysis and microscopy
The DAB-GO sections were examined with a Leitz Dialux 20 light microscope, and digital images captured with a SPOT Insight Color Mosaic camera (1200 × 1600 pixels). Brightness and contrast of images were adjusted with Zeiss software or with Adobe Photoshop, and plates assembled using Adobe Photoshop. Immunofluorescence was visualized with a Zeiss AxioImager Z1 Microscope, used both with and without the Axioimager optics. Digital images were captured with Zeiss software and brightness and contrast adjusted with that software.
We used Accustage (Shoreview, MN) stage position encoders and MDplot software to plot the distribution of labeled cells on selected sections. The stage position encoders were mounted on a Leitz Dialux 20 microscope and the data sent to a Dell computer. We first drew an outline of the brainstem and cerebellum and then marked the locations of labeled cells using the criterion of marking only those cells with a darkly stained soma and brush.
3.0. RESULTS
3.1. Antibody specificity
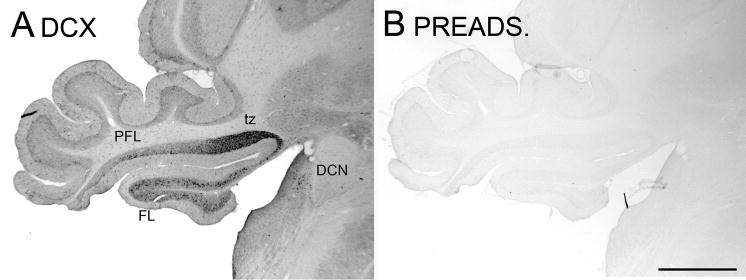
The DCX antibody recognizes a 40 kDa band on a Western Blot of rat brain tissue (Brown et al., 2003b). In order to verify that the antibody was specifically recognizing DCX in immunohistochemistry on fixed tissue sections, we performed a preadsorption control. Figure 1A shows the typical pattern of staining in the brainstem and cerebellum with this antibody and Figure 1B shows that immunostaining was eliminated completely in a control section in which the antibody was preadsorbed with the immunizing peptide.
Figure 1.
Antibody specificity. A. DCX staining in the PFL, FL and DCN. B. Adjacent section stained with preadsorbed antibody; there is no immunostaining. Abbreviations: DCN, dorsal cochlear nucleus; FL, flocculus; PFL, paraflocculus; tz, transition zone between ventral PFL and FL. Scale bar = 1 mm.
3.2. Distribution of DCX-ir elements
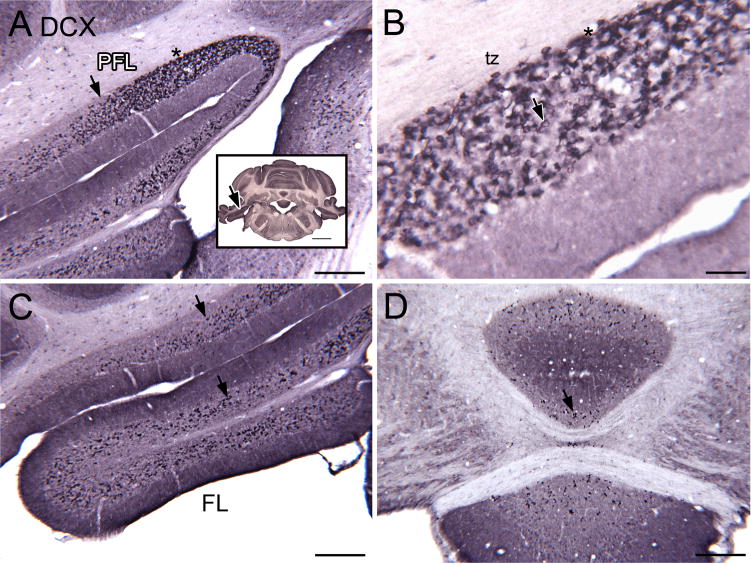
We examined the brainstem and cerebellum on closely-spaced single-label sections from the level of the caudal medulla to the midbrain. Inspection of these sections revealed unexpected but reliable and consistent DCX immunoreactivity in neurons in the vestibulocerebellum and DCN. The photomicrographs in Figure 2 illustrate this staining pattern. Figure 2A shows particularly dense labeling in the granular layer of cerebellar cortex in a region between the ventral paraflocculus (PFL) and flocculus (FL). This region has been called the “transition zone, tz” or transitional area (a small region transitional between the ventral PFL and FL) by Mugnaini and colleagues (Floris et al., 1994, Mugnaini et al., 1997, Sekerkova et al., 2004) who noted a very high density of UBCs in this region. The dense DCX label in the tz extended roughly 1.0 mm in the rostro-caudal direction, from about Bregma −11.6 mm at the caudal limit to Bregma −10.5 mm at the rostral limit (levels from the atlas of Paxinos and Watson, 1997). The DCX-ir cells in the tz are scattered over the width of, but restricted to, the granular layer. The maximum transverse width of the DCX-positive band in the PFL was approximately 750 μm. Figure 2B shows a higher magnification image of the staining in the tz. The density of stained cells decreases both laterally in the PFL (Fig. 2A, the arrow indicates the lateral border of the densely stained tz) and ventrally in the FL (Fig. 2C). Figure 2D shows a scattering of stained profiles in the vermis on the same section; the density of stained neurons in these regions was much lower than in the tz.
Figure 2.
A–D. DCX-ir neurons in vestibulocerebellum. A. Photomicrograph showing DCX-ir cells in the ventral PFL and FL. The arrow shows the lateral border of the tz and is an alignment point for the inset. The inset shows a photomicrograph of the section. The asterisk in the inset is an alignment point for the higher magnification image in B. Scale bars = 250 μm, inset = 2 mm. B. DCX-staining (examples at arrows) in the tz between the FL and PFL. This region has the highest density of stained cells. Scale bar = 50 μm. C. Stained cells in the FL (examples at arrows). Scale bar= 250 μm. D. There are also scattered stained neurons (example at arrow) in the vermis. Scale bar = 250 μm.
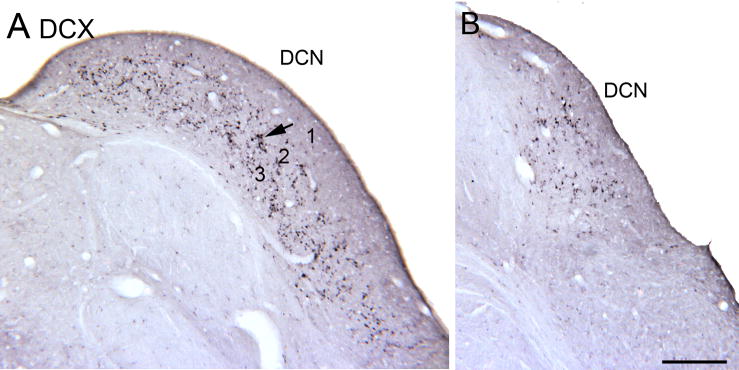
In the neighboring DCN, DCX-ir cells were seen in layers 2 and 3 but not in the molecular layer (layer 1). Figures 3A and B show the pattern of DCX labeling in the DCN on two sections about 500 μm apart.
Figure 3.
A, B. Immunostained neurons (examples at arrow) in the DCN. The section in B is about 0.5 mm rostral to the one in A and the dorsal-ventral extent of the stained neurons is smaller. Scale bar = 250 μm. 1, 2, 3, the layers of the DCN.
This pattern of label in the vestibulocerebellum and DCN was seen in 20 Sprague Dawley rats. While most of the animals were 3–4 months old at the time of sacrifice, we also processed sections from a few older animals (12–16 months) and saw a similar distribution of DCX-labeled cells. DCX-ir cells were seen in both formalin and paraformaldehyde fixed brains. We also saw DCX-labeled neurons in the same regions in sections from one Fisher344 and one Wistar rat, suggesting that this pattern is not peculiar to the Sprague Dawley rat.
3.3. DCX-ir elements are unipolar brush cells
The DCX- labeled cells all have the morphological characteristics of unipolar brush cells (UBCs), an excitatory cerebellar interneuron that has been extensively described by Mugnaini and colleagues as well as other investigators (Floris et al., 1994, Mugnaini and Floris, 1994, Jaarsma et al., 1995, Jaarsma et al., 1996, Mugnaini et al., 1997, Jaarsma et al., 1998, Diño et al., 1999, Diño et al., 2000a, Diño et al., 2000b, Diño et al., 2001, Morin et al., 2001, Nunzi et al., 2001, Nunzi et al., 2002, Nunzi et al., 2003, Sekerkova et al., 2004, Sekerkova et al., 2005, Russo et al., 2007, Sekerkova et al., 2007, Diño and Mugnaini, 2008, Russo et al., 2008, Birnstiel et al., 2009, Chung et al., 2009, Nunzi and Mugnaini, 2009, Mugnaini et al., 2011). Figure 4(A–F) shows higher magnification examples of DCX-labeled neurons from the DCN (A, D), FL, (B, E), PFL (C) and vermis (F). They all have oval somata (arrowheads) and a single, short, thick process (arrows). We used an antibody to Eps8, a marker for all UBCs (Sekerkova et al., 2007), to see if the DCX-labeled UBCs also expressed this protein. Figure 4(G–H) shows images of a section double-labeled for DCX (G) and Eps8 (H). Figure 4I shows colocalization of the two proteins, indicating that the DCX-labeled neurons have neurochemical characteristics of the UBCs.
Figure 4.

A–F. Photomicrographs of DCX- ir UBCs in the DCN (A, D) FL (B, E), PFL (C) and vermis (F). The morphology of the stained neurons is similar in all locations. G. DCX immunoreactivity on a section double labeled for DCX and Eps8. H. Eps8 label. I Merged image showing colocalization of the two proteins. Scale bars F= 20 μm; I= 10 μm.
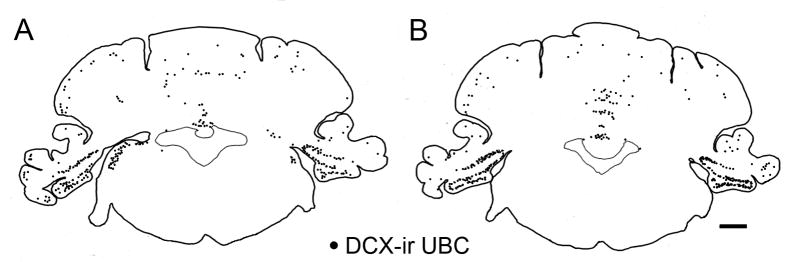
In contrast to the high density of labeled neurons in the tz, FL, ventral PFL and DCN, there was only a scattering of labeled cells in the rest of the cerebellar cortex. Figure 5(A, B) shows the distribution of labeled UBCs on drawings of two coronal sections from the animal illustrated in Figure 2. Figure 5A shows the UBCs in the DCN, ventral PFL and FL, and scattered stained cells elsewhere in cerebellar cortex. Figure 5B illustrates the distribution of stained UBCs on a section about 0.5 mm more rostral; the same staining pattern is seen in the PFL and FL with the highest density of stained neurons in the tz and a low density of stained UBCs elsewhere in vestibulocerebellum.
Figure 5.
A, B. Plots illustrating the relative densities of DCX-ir UBCs in different regions of the cerebellum and brainstem at the level of the tz. The section in A is about 0.5 mm caudal to the one in B. The section in B is illustrated in Figure 2. Each dot represents one labeled UBC; only neurons for which both soma and brush were both visible were marked. Scale bar = 1 mm.
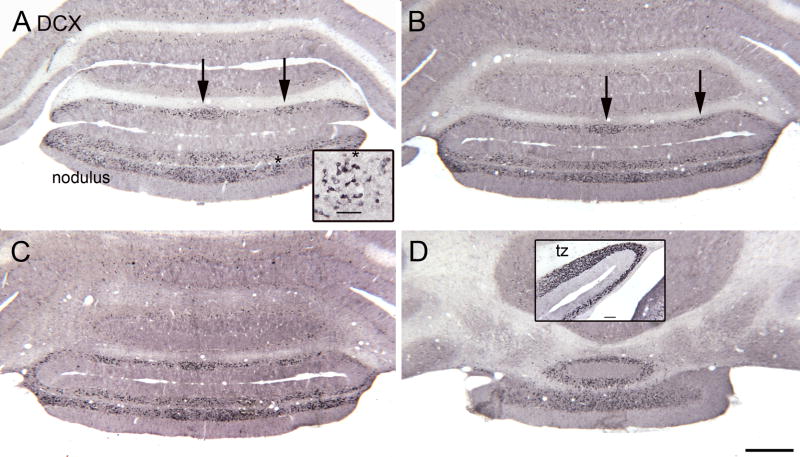
We also examined sections caudal and rostral to the tz. Figure 6(A–D) shows DCX-ir UBCs in four sections through the nodulus (lobule X) and uvula (lobule IX). The inset in A shows that the labeled cells are UBCs. While there are scattered cells across the medio-lateral extent of both lobules, the cells have a patchy distribution (Fig. 6A, B arrows). The inset in Figure 6D shows the higher density of DCX label in the tz compared to the nodulus and uvula from a more rostral section of the same rat. Figure 7(A, B, C) shows the pattern of DCX label more rostrally in the FL. Again, there are scattered DCX-ir UBCs (Fig. 7D) but the density of labeled cells is lower than in the tz (see inset in Fig. 6D).
Figure 6.
A–D. DCX staining in the nodulus. The sections are about 480 μm apart, A is the most caudal. A. The arrows indicate clusters of DCX-ir cells. The asterisk is an alignment point for the inset which shows that the DCX-ir neurons are UBCs. Scale bar in inset= 50 μm. D. Rostral nodulus. Scale bar= 500 μm. The inset shows the higher density of DCX-ir neurons in the tz found about 600 μm more rostral. Scale bar in inset= 100 μm.
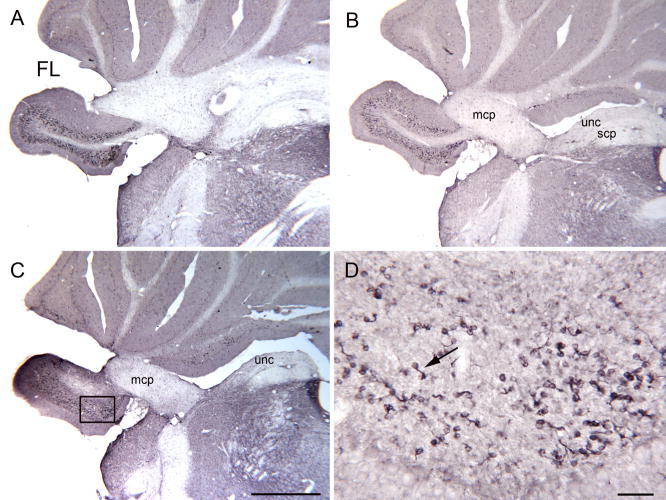
Figure 7.
A–C. DCX-ir UBCs in the rostral FL on three sections about 240 μm apart. C. The rectangle shows the location of the higher magnification image in D. Scale bar = 1 mm. D. Scattered DCX-ir UBCs (example at arrow). Scale bar= 50 μm. Abbreviations, scp, superior cerebellar peduncle; uc, uncinate tract
3.4 DCX expression and subtypes of UBCs
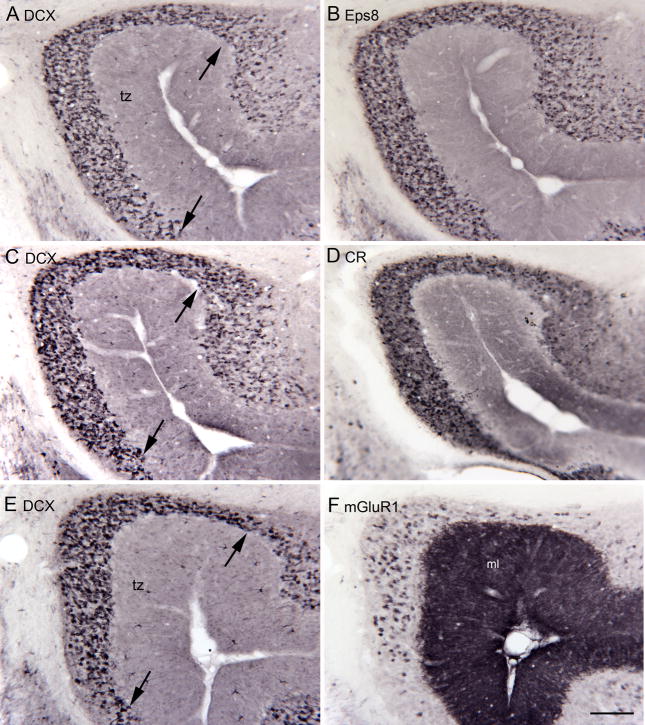
Several neurochemical subtypes of UBCs have been identified in both the DCN and cerebellar cortex of mice and rats. Initial reports found that UBCs express the calcium-binding protein calretinin (CR; Floris et al., 1994, Diño et al., 1999); subsequent studies recognized a second subtype of UBC that express the mGluR1α receptor (Nunzi et al., 2002, Diño and Mugnaini, 2008). Eps8 labels both the CR and the mGluR1 subsets (Diño and Mugnaini, 2008). A third subtype of UBC, described in mouse, expresses phospopholipase Cβ4 (PLCβ4; Chung et al., 2009). We asked if DCX expression was seen in all of these UBC classes or if it might be restricted to one of them. We first compared the distribution and density of DCX-ir UBCs in the tz with the distributions of populations immunoreactive to Eps8, CR and mGluR1. Since the UBC density varies so dramatically over very small distances we compared DCX and the other markers on pairs of adjacent sections. Figure 8(A–F) shows pairs of sections through the tz of two different animals (A–D are from one animal, E and F are from a second). The panels on the left (A, C and E) show DCX-immunoreactivity. The panels on the right show Eps8 (B), CR (D) and mGluR1 (F) immunoreactivity on adjacent sections. The distributions and density of DCX-labeled cells (A) and Eps8-labeled cells (B) appear very similar. For DCX (C) and CR (D) the distributions are again similar, with a suggestion of a lower density of CR than DCX labeled neurons. However, the expression of mGlur1 (F) was seen in far fewer neurons than DCX (E).
Figure 8.
Comparison of DCX expression with the expression of other markers for UBCs shown on adjacent pairs of sections. A, B. Similar distribution of DCX and Eps8-ir UBCs. A. DCX. The arrows show the borders of the tz, the region of the highest DCX expression. B. Eps8. C, D. Similar distributions of DCX and CR-ir UBCs in the tz. E, F. There are many more DCX- (E) than mGluR1-ir (F) UBCs. Note also the high levels of expression of mGluR1 in the molecular layer (ML). Scale bar = 100 μm.
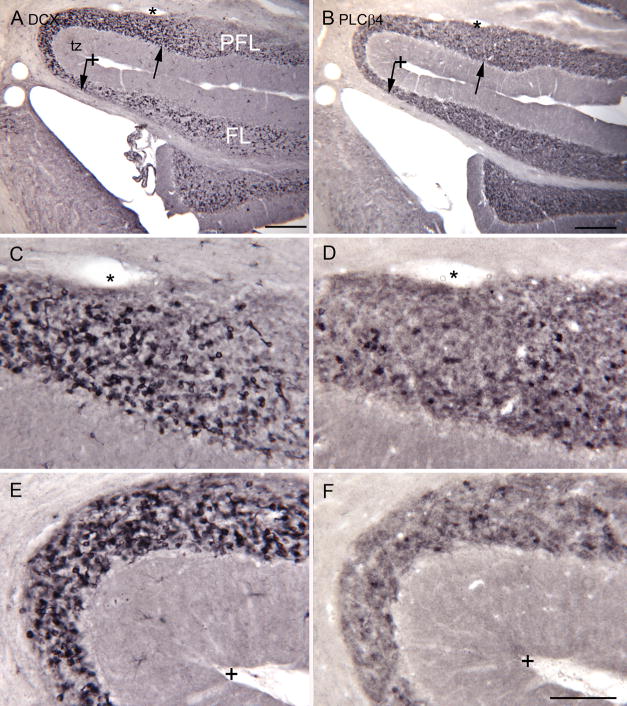
We also looked at the expression of PLCβ4 on sections through the tz. As in the mouse (Chung et al., 2009), we found some UBCs that were labeled with this protein, but these were scarce compared to cells labeled with the other UBC markers. The left and right columns in Figure 9B compare DCX and PLCβ4 immunoreactivity respectively through the PFL, FL and tz on adjacent sections. The density of PLCβ4 immunolabeled cells is much less than the density of DCX- labeled cells and this is especially dramatic in the tz. The density of PLCβ4 labeled cells is no higher in the tz than in PFL and FL, suggesting that this population is separate from the DCX-labeled cells.
Figure 9.
Comparison of the expression of PLCβ4 and DCX in the tz, PFL and FL. A. DCX. Scale bar = 250 μm. B. PLCβ4. The arrows in A and B show the limits of the tz, with a high density of DCX- ir cells. The asterisk is an alignment points for the images in C and D. The + is an alignment point for the images in E and F. Scale bar = 250 μm. The density of DCX expression is much higher in the tz, and dense overall whereas the expression of PLCβ4 is much lower and more uniform. C, D. Higher magnification images of DCX (C) and PLCβ4 in the dorsal tz; E, F DCX and PLCβ4 in the curve of the tz. Scale bar = 100 μm.
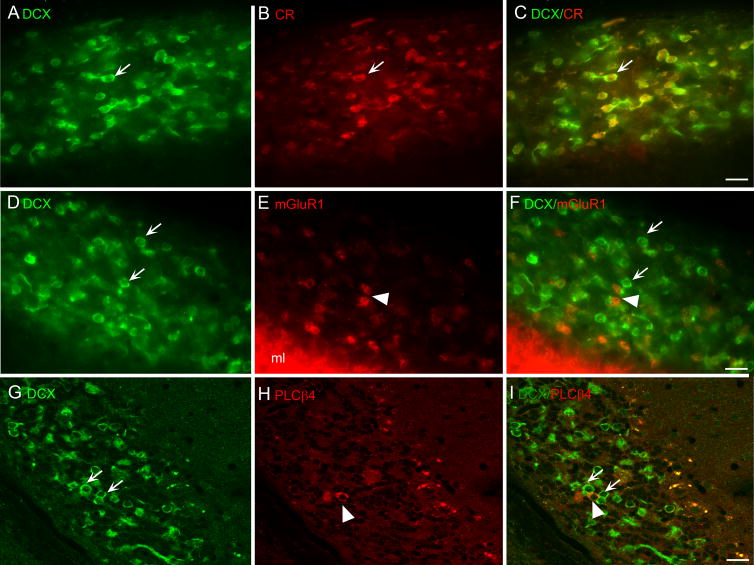
We also examined the labeling pattern in the tz on sections double-labeled for DCX and CR, mGluR1, or PLCβ4. We found that most or all cells expressing DCX also express Eps8. We also found many examples of cells colocalizing DCX and CR and no examples of cells that expressed CR and not DCX. Figure 10A–C shows images of a section double labeled for DCX and CR. The arrow shows an example of a neuron that expresses DCX (A) and CR (B). The merged image (Fig. 10C) shows colocalization in that neuron as well as in several other neurons. Figure 10D–E show a section double labeled for DCX and mGluR1. There are many DCX-labeled cells (Fig. 10D, examples at arrows) and a few mGluR1 labeled cells (Fig. 10E, examples at arrowhead). There were no clear and convincing examples of colocalization of DCX with mGluR1 (Fig. 10F). There were even fewer cells labeled for PLCβ4 (Fig. 10H, examples at arrowhead) than for DCX (Fig. 10G, examples at arrows) and, again no examples of colocalization of the two proteins (merged image in Fig. 10I).
Figure 10.
A, B, C. Colocalization of DCX (A) and CR (B) The merged images shows neurons (example at arrow) with CR in the soma surrounded by DCX. D, E, F. Expression of DCX (D, examples at arrows) and mGluR1 (E, arrowhead) in separate UBCs. The merged image (F) shows many cells immunoreactive for DCX (examples at arrows) and only a few immunoreactive to mGluR1 (examples at arrowheads) with an absence of colocalization. G, H, I. Many cells are immunoreactive for DCX (G, examples at arrows with only a few immunoreactive for PLCβ4 (H, arrowhead) and no colocalization (I). G, H, I were acquired using the “Axioimager” feature of the microscope.
3.5. PSA-NCAM in UBCs
DCX is a protein typically expressed in migrating and newly-born neurons (Francis et al., 1999, Gleeson et al., 1999, Brown et al., 2003b). To see if the DCX-ir UBCs expressed any other proteins typically associated with new neurons we looked at expression of polysialylated neural cell adhesion molecule (PSA-NCAM). PSA-NCAM is expressed in new neurons of the SGZ and SVZ over about the same time period as DCX (see timelines of expression of different proteins in Fig. 4, Zhao et al., 2008). Figure 11 shows PSA-NCAM-ir neurons (arrows) in the PFL-FL region; the inset shows an example of a PSA-NCAM-labeled neuron with UBC morphology from a 16 month old rat.
Figure 11.
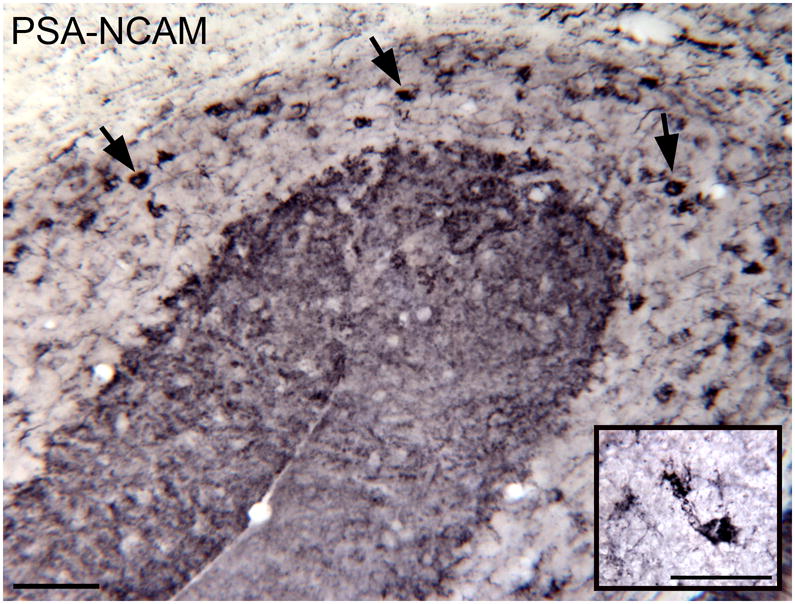
PSA-NCAM immunoreactivity in UBCs (examples at arrows) of the FL and PFL in a four month old rat. The inset shows a higher magnification image of an PSA-NCAM-ir UBC from a 16 month old rat. Scale bars = 50 μm.
3.6. DCX ir elements along the fourth ventricle and its lateral recess
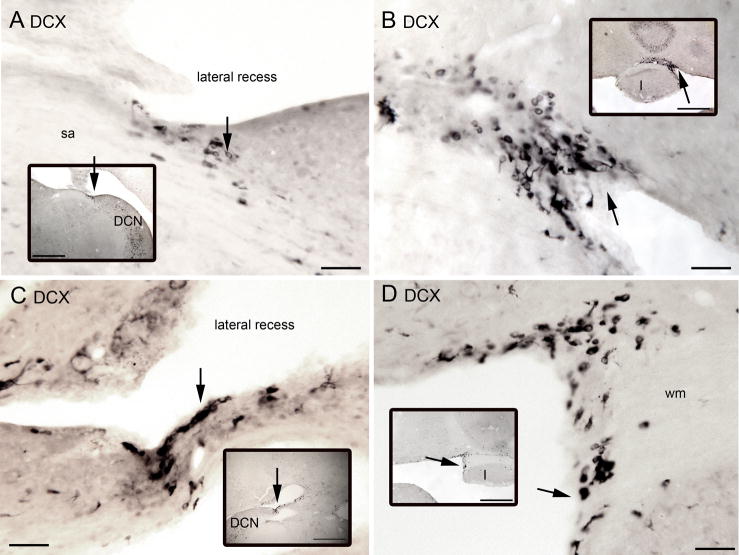
In addition to labeled neurons with UBC morphology in the DCN, FL and PFL we also saw DCX-ir cells medially around the fourth ventricle and in the lateral recess of the fourth ventricle. These neurons typically had oval cell bodies and one or two processes emerging from either end of the soma, but did not have the “brush” structure characteristic of mature UBCs. Examples are illustrated in Figure 12(A–D). These cells were found in two sites. The first was in the lateral recess of the fourth ventricle at about the level (in coronal sections going from caudal to rostral) at which the cerebellum and brainstem are first attached (examples in Fig. 12A, C). The second region was medially along the enclosed ventricle adjacent to cerebellar lobule I (Fig. 12B, D). Near these regions we saw DCX-ir cells with the morphology of UBCs in the white matter; examples from two different animals are shown in Figure 13.
Figure 12.
A–D. DCX-ir neurons around the fourth ventricle on four sections from two different animals. The insets in each panel show the location of the labeled cells on lower-magnification images. The arrows in each pair are alignment points. A. Arrow shows cluster of DCX-ir cells along the lateral recess of the fourth ventricle B. DCX-ir cells (arrows) along the midline in the fourth ventricle just adjacent to cerebellar lobule I. C. DCX-ir cells (arrows) along the lateral recess of the fourth ventricle on another section. D. DCX-ir cells (arrows) along the midline of the fourth ventricle adjacent to white matter (wm) just dorsal to lobule I. Scale bars, main panels = 50 μm, insets= 500 μm. Abbreviation sa, stria acustica.
Figure 13.
DCX-ir UBCs in white matter. A. The arrow indicates a DCX-ir UBC in the white matter near the PFL and FL. Wistar rat. Scale bar = 50 μm. B. Several DCX-ir UBCs (arrows) in white matter near the lateral recess of the fourth ventricle. Sprague Dawley rat. Scale bar= 50 μm. The arrow in the inset shows the location of the labeled cells. Scale bar in inset = 500 μm.
4.0. DISCUSSION
DCX is heavily expressed in newborn and migrating neurons, and was previously thought to disappear from the adult brain except in the SVG and SGZ, sites of continued neurogenesis. Here we show robust, consistent and reliable DCX expression in neurons in the tz, the PFL and the FL as well as in the DCN, a nucleus of the auditory brainstem. The DCX-ir neurons have the morphological characteristics of UBCs. UBCs are excitatory glutamatergic interneurons that receive mossy fiber inputs; their axons synapse on the dendrites of other UBCs and granule cells (Diño et al., 1999, Diño et al., 2000a, Diño et al., 2000b, Diño et al., 2001, Morin et al., 2001, Birnstiel et al., 2009, Mugnaini et al., 2011). UBCs are found in both the DCN and in limited regions of cerebellar cortex (Diño and Mugnaini, 2008, Mugnaini et al., 2011). The embryonic origin of both populations is the rhombic lip (Englund et al., 2006). UBCs are unique among cerebellar interneurons in that they are not evenly distributed throughout the cerebellum but are most numerous in the vestibulocerebellum and scarce in the cerebellar hemispheres (Floris et al., 1994, Mugnaini and Floris, 1994, Diño et al., 1999). The region of the highest density of cerebellar UBCs is the “tz”, a small region “transitional” between the ventral PFL and the FL (see Figure 1B in Sekerkova et al., 2004). This region also had the highest density of DCX-ir UBCs.
The finding of DCX expression in UBCs in the adult is surprising, since DCX was thought to be expressed in newly born and migrating neurons and indeed is critical for neuronal migration (Francis et al., 1999, Gleeson et al., 1999, Bai et al., 2003, Brown et al., 2003b, Tanaka et al., 2004, Couillard-Despres et al., 2005). We consider two interpretations of these results. One possibility is that the DCX expression reflects ongoing neurogenesis and migration of UBCs in the adult. A second possibility is that DCX expression in UBCs continues long past both neurogenesis and neuronal migration, and this protein is mediating functions other than migration.
4.1. Does DCX expression reflect adult neurogenesis of UBCs?
Could the DCX-ir UBCs be newly born neurons? While neurogenesis is solidly established for the SGZ and SVZ (reviews and references in Gage, 2002, Gould, 2007b), there are reports of adult neurogenesis elsewhere in the brain, including cerebral cortex and brainstem, both in normal animals and as a response to injury (Magavi et al., 2000, Gould, 2007a, Tighilet et al., 2007, Dutheil et al., 2009, Guo et al., 2010, Ohira et al., 2010). The present understanding of the origins of UBCs is that they are generated before or at the time of birth. UBC neurogenesis has been studied in mouse and rat using injections of bromodeoxyuridine (BrdU), a thymidine analogue, which is taken up in dividing cells and later immunohistochemically visualized in their nuclei (Gould, 2007a, Zhao et al., 2008, Leuner et al., 2009). In the mouse, BrdU injections showed that UBCs are generated in the rhombic lip at around E14.5 – E19.5 and migrate through cerebellar white matter to the DCN or vestibulocerebellum, with migration largely complete by P10 (Englund et al., 2006). In rat, similar experiments showed that UBCs are born around E16.5 – E22.5 (Sekerkova et al., 2004). However, in neither study was the possibility of later UBC neurogenesis examined. In the rat, the latest age for BrdU injections was E21 (Sekerkova et al., 2004) and in the mouse, Englund et al. (2006) used BrdU injections at ages E15.5–E17.5. Adult-born cells would therefore not have been seen in either study. A previous study of UBC maturation did not see evidence of immature UBCs after P60 (Morin et al., 2001), however that study used sections from the vermis, where there are relatively few DCX-UBCs, compared to the tz, where there are many. The possibility of cerebellar neurogenesis is supported by studies that have isolated neuronal stem cells from postnatal mouse cerebellum (Lee et al., 2005, Alcock et al., 2007). In our sections, we saw DCX-labeled elements around the fourth ventricle that did not have the typical UBC morphology and resemble migrating neuroblasts (Fig. 12). We also saw DCX-ir UBCs in nearby white matter (Fig. 13). These observations are consistent with neurogenesis occurring in very limited zones around the fourth ventricle with subsequent differentiation and migration of UBCs through white matter. Takács et al. (2000) also observed “ectopic” UBCs and proposed that UBCs might continue to be born in the adult. The presence of PSA-NCAM in UBCs is also consistent with the view of adult neurogenesis of neurons that migrate to the vestibulocerebellum and DCN. None of the earlier data about the origins of UBCs specifically exclude adult neurogenesis of these neurons.
4.2. Does DCX expression reflect plasticity of UBC circuits?
Another possibility consistent with our results is that the labeled UBCs have long since finished migration but nonetheless continue to express DCX. This is not the typical pattern of DCX expression. In the adult hippocampus, DCX expression is at its peak in new neurons between days 4 and 7 after cell birth, is found in only 2% of 1 month old cells and disappears completely by 2 months after neurogenesis (Brown et al., 2003b). For the RMS, the time of migration from SVZ to olfactory bulb is estimated at 4–10 days and with DCX expression decreasing to very low levels by one month after neuronal birth (Brown et al., 2003b). However, there are some data suggesting a role for DCX in plastic processes in adult neurons. Nacher et al. (2001) found cells expressing DCX in nonneurogenic regions and suggested that its role was to mediate microtubule reorganization necessary for processes like neurite outgrowth or synaptogenesis. Continued DCX expression might then confer a special role for UBCs in vestibular or auditory plasticity. Another possibility is that DCX expression plays a role in the establishment of the brush, a process shown to take several weeks (Morin et al., 2001).
4.3 DCX expression and neurochemical UBC subtype
We saw colocalization of DCX with two markers that have been shown to label UBCs, Eps8 and CR, and did not see colocalization of DCX and either mGluR1 or PLCβ4. There are two possible interpretations of these observations. The first is that there is only one DCX-expressing subtype of UBC, those that also express CR, and that the other two UBC types do not. However, the division of UBCs into neurochemical subtypes was based on the assumption that the labeled UBCs are all adult neurons with stable neurochemical properties. If, however, there is adult neurogenesis of UBCs then the interpretation of these data is more complex, and depends on knowing the time of expression of these different proteins relative to the time of cell birth. The only protein which was studied in other regions of neurogenesis is CR, and its expression begins close to the time of cell birth, and overlaps that of DCX (Zhao et al., 2008). It is possible that Eps8 is also expressed soon after cell birth since it is an actin-associated protein that may be involved in synapse formation or axonal growth (Offenhauser et al., 2004, Menna et al., 2009). In mouse PLCβ4 expression is not seen until P12, while UBCs are generated as early as E15 in that species (Chung et al., 2009), suggesting that its expression may be characteristic of mature neurons. It is possible that, despite our double-label data, new UBCs of all classes are being generated in the adult but that PLCβ4 and mGlur1α are not expressed in new neurons until after DCX expression has decreased or disappeared.
4.4 Total UBC distribution relative to DCX-expressing population
Our analysis focused on the population of UBCs in which we saw DCX expression, and especially on the tz. The total distribution of UBCs in the cerebellum has been described for rat as well as several other species (Floris et al., 1994, Diño et al., 1999). Using CR as the marker, in rat UBCs were found in vestibulocerebellum, including ventral vermal lobules IX (uvula) and X (nodulus), ventral PFL, and FL with a high concentration of UBCs in the tz. We found DCX-ir UBCs most densely in the tz, PFL and FL with a lower density in the nodulus and uvula and scattered cells in other regions of cerebellar cortex. We also saw a patchy distribution of DCX-ir UBCs in lobules IX and X. These patches were reminiscent of the parasagittal compartments defined by connections with the inferior olive and immunoreactivity for Aldolase C/Zebrin (Hawkes and Leclerc, 1987, Ruigrok, 2003, Pijpers et al., 2005).
Diño and Mugnaini (2008) saw “ectopic” UBCs elsewhere in the brainstem including the trapezoid body, the spinal and principal spinal trigeminal nuclei, the cerebellar peduncles, ventral cochlear nuclei, vestibular nuclei, and pedunculopontine regions. We did not see DCX-labeled UBCs in any of these regions with the exception of an occasional cell in the vestibular nerve root.
4.5. Regulation of UBC neurogenesis and cell death
Does the continued putative neurogenesis of UBCs imply an ever-increasing number of these cells or is cell birth balanced by cell death? A similar question has been addressed for other regions of adult neurogenesis, the subgranular zone of the hippocampus and the subventricular zone. Some, (Bayer, 1982, Bayer et al., 1982, Kaplan et al., 1985) but not all (Boss et al., 1985), reports suggest that the number of granule cells in hippocampus and olfactory lobe does continue to increase throughout life. However, there is also evidence that a large percentage of the newborn neurons do not survive. Early studies noted large numbers of pyknotic cells in the dentate gyrus (Gould et al., 1990, Gould and McEwen, 1993). The rates of cell birth, survival and death for granule cells of the hippocampus have been extensively studied. In many studies, BrdU is injected into living animals which are allowed to survive for various times; the animals are subsequently sacrificed and the brains processed for BrdU histochemistry in order to trace the migration and development of these cells. These studies have shown that the rates of cell birth and survival are influenced by many behavioral, environmental and pharmacological factors (Gould and Cameron, 1996, Gould et al., 1997, Gould et al., 1998, Tanapat et al., 1998, Gould et al., 1999, Gould and Tanapat, 1999, Cameron and McKay, 2001, Lee et al., 2001, Brown et al., 2003a, Dayer et al., 2003, Santarelli et al., 2003, Leuner et al., 2004, Couillard-Despres et al., 2005, Kraus et al., 2010). That work suggests useful experimental strategies for the vestibulocerebellum and DCN to confirm neurogenesis and to assess factors influencing the rates of neurogenesis and survival of the new neurons.
4.6. PSA-NCAM, plasticity, and neurogenesis
We also saw UBCs immunoreactive to PSA-NCAM. Like DCX, this protein is expressed in newly-born neurons, and its expression continues until about one month of cell age (Zhao et al., 2008). However, as for DCX, a role in adult plasticity has been suggested for PSA-NCAM (Nacher et al., 2004), so that our data are consistent with both the neurogenesis and prolonged expression of plasticity markers hypotheses.
4.7. Possible role of UBCs in cerebellar plasticity
Regardless of the time of neurogenesis, what might the DCX-labeled UBCs do? There is evidence that the cerebellum and DCN subserve plastic functions for the vestibular and auditory systems respectively. The PFL and FL play a critical role in behavioral and neural recovery from unilateral vestibular deafferentation, a process known as vestibular compensation (reviews and references in Courjon et al., 1982, Goto et al., 1997, Kitahara et al., 1997, Babalian and Vidal, 2000, Kitahara et al., 2000, Balaban, 2001, Johnston et al., 2002, Kaufman et al., 2003, Gliddon et al., 2004, Horii et al., 2004, Newlands et al., 2005, Fukasawa et al., 2009). UBCs receive vestibular input (Jaarsma et al., 1996, Diño et al., 2000a, Diño et al., 2001) and might participate in these plastic processes. UBCs express c-fos under vestibular stimulation conditions that are known to invoke changes in gene expression (Lai et al., 2004, Sekerkova et al., 2005), further evidence for a role in vestibular plasticity.
4.8. Possible role of UBCs in auditory plasticity
In the DCN, the UBCs are geographically distributed with the granule cell system (GCS, Diño and Mugnaini, 2008). UBCs are thought to form excitatory synapses on granule cells as well as other UBCs (Diño and Mugnaini, 2008) and may therefore participate in the integration of auditory information with information from other modalities including somatosensory (Zhou and Shore, 2004, Shore et al., 2007) and vestibular systems; such information is distributed to the GCS (review and references in Diño and Mugnaini, 2008). Another possible role for UBCs is in mediating the plasticity in DCN circuitry triggered by exposure to loud sounds or deafferentation (Kaltenbach et al., 2000, Coad et al., 2001, Biggs and Ramsden, 2002, Kaltenbach et al., 2002). The delayed time course of these changes (Kaltenbach et al., 2000) is compatible with the idea that it is mediated by integration of new neurons into existing circuits. Many studies suggest that DCN plasticity may correlate with and even contribute to the development of tinnitus (Levine, 1999, Kaltenbach and Afman, 2000, Brozoski et al., 2002, Kaltenbach et al., 2002, Rachel et al., 2002, Zacharek et al., 2002, Wang et al., 2009, Middleton et al., 2011). A role of the DCN is especially indicated in forms of tinnitus that are modulated by somatosensory input (e.g. Shore et al., 2007), since the anatomical substrate for interaction of the two modalities is present. Understanding the role of the DCX-ir UBCs of the DCN in the plastic changes underlying tinnitus might then have significant clinical implications.
4.9. Summary
We have shown that there is expression of the neuronal migration protein DCX in UBCs of the vestibulocerebellum and DCN of adult rats. The density of labeled UBCs varies over the FL and ventral PFL and is greatest in the region between them called the transition zone. Many UBCs also colocalize DCX and CR but not DCX with either mGluR1 or PLCβ4. There is also expression of PSA-NCAM in some UBCs. These data, along with the finding of DCX- labeled neuroblasts along the fourth ventricle, support the idea that there is continued adult neurogenesis of the UBC cell population for a very restricted region of vestibulocerebellum and the DCN.
Highlights.
We studied the expression of doublecortin (DCX) in adult rat cerebellum and brainstem
Many unipolar brush cells in cerebellum and dorsal cochlear nucleus were DCX-ir
These also expressed Eps8 and calretinin
A few cells along the fourth ventricle and in its lateral recess were DCX-ir
Results suggest the possibility of adult neurogenesis of UBCs
Acknowledgments
Supported by NIH grants R01DC0090910 and R01DC009219-01, RJS.
We acknowledge the assistance of the Confocal Microscope and Flow Cytometry Facility in the School of Medicine and Biomedical Sciences, University at Buffalo.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Senthilvelan Manohar, Email: smanohar@buffalo.edu.
Nicholas A. Paolone, Email: npaolone@buffalo.edu.
Marni Bleichfeld, Email: marnibleichfeld@gmail.com.
Sarah Hayes, Email: shhayes@buffalo.edu.
Richard J. Salvi, Email: salvi@buffalo.edu.
References
- Alcock J, Scotting P, Sottile V. Bergmann glia as putative stem cells of the mature cerebellum. Med Hypotheses. 2007;69:341–345. doi: 10.1016/j.mehy.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Anderson WJ, Stromberg MW. Effects of low-level x-irradiation on cat cerebella at different postnatal intervals. I. Quantitative evaluation of morphological changes. J Comp Neurol. 1977a;171:17–37. doi: 10.1002/cne.901710103. [DOI] [PubMed] [Google Scholar]
- Anderson WJ, Stromberg MW. Effects of low-level x-irradiation on cat cerebella at different postnatal intervals. II. Changes in Purkinje cell morphology. J Comp Neurol. 1977b;171:39–50. doi: 10.1002/cne.901710104. [DOI] [PubMed] [Google Scholar]
- Babalian AL, Vidal PP. Floccular modulation of vestibuloocular pathways and cerebellum-related plasticity: An in vitro whole brain study. J Neurophysiol. 2000;84:2514–2528. doi: 10.1152/jn.2000.84.5.2514. [DOI] [PubMed] [Google Scholar]
- Bai J, Ramos RL, Ackman JB, Thomas AM, Lee RV, LoTurco JJ. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat Neurosci. 2003;6:1277–1283. doi: 10.1038/nn1153. [DOI] [PubMed] [Google Scholar]
- Baizer JS, Manohar S, Paolone N, Hayes S, Salvi R. Expression of double cortin in unipolar brush cells of the ventral paraflocculus, flocculus and dorsal cochlear nucleus of the adult rat: evidence for adult neurogenesis? Neural Control of Movement San Juan, Puerto Rico 2011 [Google Scholar]
- Balaban CD. Role of gene regulation during vestibular compensation: an integrative approach. Ann N Y Acad Sci. 2001;942:52–64. doi: 10.1111/j.1749-6632.2001.tb03735.x. [DOI] [PubMed] [Google Scholar]
- Bayer SA. Changes in the total number of dentate granule cells in juvenile and adult rats: a correlated volumetric and 3H-thymidine autoradiographic study. Exp Brain Res. 1982;46:315–323. doi: 10.1007/BF00238626. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Yackel JW, Puri PS. Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science. 1982;216:890–892. doi: 10.1126/science.7079742. [DOI] [PubMed] [Google Scholar]
- Biggs ND, Ramsden RT. Gaze-evoked tinnitus following acoustic neuroma resection: a de-afferentation plasticity phenomenon? Clin Otolaryngol Allied Sci. 2002;27:338–343. doi: 10.1046/j.1365-2273.2002.00591.x. [DOI] [PubMed] [Google Scholar]
- Birnstiel S, Slater NT, McCrimmon DR, Mugnaini E, Hartell NA. Voltage-dependent calcium signaling in rat cerebellar unipolar brush cells. Neuroscience. 2009;162:702–712. doi: 10.1016/j.neuroscience.2009.01.051. [DOI] [PubMed] [Google Scholar]
- Boss BD, Peterson GM, Cowan WM. On the number of neurons in the dentate gyrus of the rat. Brain Res. 1985;338:144–150. doi: 10.1016/0006-8993(85)90257-4. [DOI] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003a;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003b;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Bauer CA, Caspary DM. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J Neurosci. 2002;22:2383–2390. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Carletti B, Rossi F. Neurogenesis in the cerebellum. Neuroscientist. 2008;14:91–100. doi: 10.1177/1073858407304629. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Sidman RL. Time of origin or corresponding cell classes in the cerebral cortex of normal and reeler mutant mice: an autoradiographic analysis. The Journal of comparative neurology. 1973;148:141–151. doi: 10.1002/cne.901480202. [DOI] [PubMed] [Google Scholar]
- Chung S-H, Marzban H, Watanabe M, Hawkes R. Phospholipase Cbeta4 expression identifies a novel subset of unipolar brush cells in the adult mouse cerebellum. Cerebellum. 2009;8:267–276. doi: 10.1007/s12311-009-0092-x. [DOI] [PubMed] [Google Scholar]
- Coad ML, Lockwood A, Salvi R, Burkard R. Characteristics of patients with gaze-evoked tinnitus. Otol Neurotol. 2001;22:650–654. doi: 10.1097/00129492-200109000-00016. [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- Courjon JH, Flandrin JM, Jeannerod M, Schmid R. The role of the flocculus in vestibular compensation after hemilabyrinthectomy. Brain Res. 1982;239:251–257. doi: 10.1016/0006-8993(82)90847-2. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- des Portes V, Pinard JM, Billuart P, Vinet MC, Koulakoff A, Carrie A, Gelot A, Dupuis E, Motte J, Berwald-Netter Y, Catala M, Kahn A, Beldjord C, Chelly J. A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell. 1998;92:51–61. doi: 10.1016/s0092-8674(00)80898-3. [DOI] [PubMed] [Google Scholar]
- Diño MR, Mugnaini E. Distribution and phenotypes of unipolar brush cells in relation to the granule cell system of the rat cochlear nucleus. Neuroscience. 2008;154:29–50. doi: 10.1016/j.neuroscience.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diño MR, Nunzi MG, Anelli R, Mugnaini E. Unipolar brush cells of the vestibulocerebellum: afferents and targets. Prog Brain Res. 2000a;124:123–137. doi: 10.1016/S0079-6123(00)24013-2. [DOI] [PubMed] [Google Scholar]
- Diño MR, Perachio AA, Mugnaini E. Cerebellar unipolar brush cells are targets of primary vestibular afferents: an experimental study in the gerbil. Exp Brain Res. 2001;140:162–170. doi: 10.1007/s002210100790. [DOI] [PubMed] [Google Scholar]
- Diño MR, Schuerger RJ, Liu Y, Slater NT, Mugnaini E. Unipolar brush cell: a potential feedforward excitatory interneuron of the cerebellum. Neuroscience. 2000b;98:625–636. doi: 10.1016/s0306-4522(00)00123-8. [DOI] [PubMed] [Google Scholar]
- Diño MR, Willard FH, Mugnaini E. Distribution of unipolar brush cells and other calretinin immunoreactive components in the mammalian cerebellar cortex. J Neurocytol. 1999;28:99–123. doi: 10.1023/a:1007072105919. [DOI] [PubMed] [Google Scholar]
- Dutheil S, Brezun JM, Leonard J, Lacour M, Tighilet B. Neurogenesis and astrogenesis contribution to recovery of vestibular functions in the adult cat following unilateral vestibular neurectomy: cellular and behavioral evidence. Neuroscience. 2009;164:1444–1456. doi: 10.1016/j.neuroscience.2009.09.048. [DOI] [PubMed] [Google Scholar]
- Englund C, Kowalczyk T, Daza RA, Dagan A, Lau C, Rose MF, Hevner RF. Unipolar brush cells of the cerebellum are produced in the rhombic lip and migrate through developing white matter. J Neurosci. 2006;26:9184–9195. doi: 10.1523/JNEUROSCI.1610-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floris A, Diño M, Jacobowitz DM, Mugnaini E. The unipolar brush cells of the rat cerebellar cortex and cochlear nucleus are calretinin-positive: a study by light and electron microscopic immunocytochemistry. Anat Embryol (Berl) 1994;189:495–520. doi: 10.1007/BF00186824. [DOI] [PubMed] [Google Scholar]
- Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet MC, Friocourt G, McDonnell N, Reiner O, Kahn A, McConnell SK, Berwald-Netter Y, Denoulet P, Chelly J. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23:247–256. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- Fukasawa M, Okamoto K, Nakamura M, Mikami K, Shimada S, Tanaka Y, Nagai K, Arito M, Kurokawa MS, Masuko K, Suematsu N, Koizuka I, Kato T. Proteomic analysis of the rat cerebellar flocculus during vestibular compensation. J Vestib Res. 2009;19:83–94. doi: 10.3233/VES-2009-0356. [DOI] [PubMed] [Google Scholar]
- Gage FH. Neurogenesis in the adult brain. J Neurosci. 2002;22:612–613. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Gliddon CM, Darlington CL, Smith PF. Rapid vestibular compensation in guinea pig even with prolonged anesthesia. Neurosci Lett. 2004;371:138–141. doi: 10.1016/j.neulet.2004.08.056. [DOI] [PubMed] [Google Scholar]
- Goto MM, Romero GG, Balaban CD. Transient changes in flocculonodular lobe protein kinase C expression during vestibular compensation. J Neurosci. 1997;17:4367–4381. doi: 10.1523/JNEUROSCI.17-11-04367.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007a;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007b;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Gould E, Cameron HA. Regulation of neuronal birth, migration and death in the rat dentate gyrus. Dev Neurosci. 1996;18:22–35. doi: 10.1159/000111392. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS. Neuronal birth and death. Curr Opin Neurobiol. 1993;3:676–682. doi: 10.1016/0959-4388(93)90138-o. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Stress and hippocampal neurogenesis. Biol Psychiatry. 1999;46:1472–1479. doi: 10.1016/s0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolley CS, McEwen BS. Short-term glucocorticoid manipulations affect neuronal morphology and survival in the adult dentate gyrus. Neuroscience. 1990;37:367–375. doi: 10.1016/0306-4522(90)90407-u. [DOI] [PubMed] [Google Scholar]
- Guo F, Maeda Y, Ma J, Xu J, Horiuchi M, Miers L, Vaccarino F, Pleasure D. Pyramidal neurons are generated from oligodendroglial progenitor cells in adult piriform cortex. J Neurosci. 2010;30:12036–12049. doi: 10.1523/JNEUROSCI.1360-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Mecinas M, Crespo C, Blasco-Ibanez JM, Nacher J, Varea E, Martinez-Guijarro FJ. Migrating neuroblasts of the rostral migratory stream are putative targets for the action of nitric oxide. The European journal of neuroscience. 2007;26:392–402. doi: 10.1111/j.1460-9568.2007.05672.x. [DOI] [PubMed] [Google Scholar]
- Hawkes R, Leclerc N. Antigenic map of the rat cerebellar cortex: the distribution of parasagittal bands as revealed by monoclonal anti-Purkinje cell antibody mabQ113. J Comp Neurol. 1987;256:29–41. doi: 10.1002/cne.902560104. [DOI] [PubMed] [Google Scholar]
- Horii A, Masumura C, Smith PF, Darlington CL, Kitahara T, Uno A, Mitani K, Kubo T. Microarray analysis of gene expression in the rat vestibular nucleus complex following unilateral vestibular deafferentation. J Neurochem. 2004;91:975–982. doi: 10.1111/j.1471-4159.2004.02781.x. [DOI] [PubMed] [Google Scholar]
- Jaarsma D, Diño MR, Cozzari C, Mugnaini E. Cerebellar choline acetyltransferase positive mossy fibres and their granule and unipolar brush cell targets: a model for central cholinergic nicotinic neurotransmission. J Neurocytol. 1996;25:829–842. doi: 10.1007/BF02284845. [DOI] [PubMed] [Google Scholar]
- Jaarsma D, Diño MR, Ohishi H, Shigemoto R, Mugnaini E. Metabotropic glutamate receptors are associated with non-synaptic appendages of unipolar brush cells in rat cerebellar cortex and cochlear nuclear complex. J Neurocytol. 1998;27:303–327. doi: 10.1023/a:1006982023657. [DOI] [PubMed] [Google Scholar]
- Jaarsma D, Wenthold RJ, Mugnaini E. Glutamate receptor subunits at mossy fiber-unipolar brush cell synapses: light and electron microscopic immunocytochemical study in cerebellar cortex of rat and cat. J Comp Neurol. 1995;357:145–160. doi: 10.1002/cne.903570113. [DOI] [PubMed] [Google Scholar]
- Johnston AR, Seckl JR, Dutia MB. Role of the flocculus in mediating vestibular nucleus neuron plasticity during vestibular compensation in the rat. J Physiol. 2002;545:903–911. doi: 10.1113/jphysiol.2002.024281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach JA, Afman CE. Hyperactivity in the dorsal cochlear nucleus after intense sound exposure and its resemblance to tone-evoked activity: a physiological model for tinnitus. Hear Res. 2000;140:165–172. doi: 10.1016/s0378-5955(99)00197-5. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Rachel JD, Mathog TA, Zhang J, Falzarano PR, Lewandowski M. Cisplatin-induced hyperactivity in the dorsal cochlear nucleus and its relation to outer hair cell loss: relevance to tinnitus. J Neurophysiol. 2002;88:699–714. doi: 10.1152/jn.2002.88.2.699. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zhang J, Afman CE. Plasticity of spontaneous neural activity in the dorsal cochlear nucleus after intense sound exposure. Hear Res. 2000;147:282–292. doi: 10.1016/s0378-5955(00)00138-6. [DOI] [PubMed] [Google Scholar]
- Kaplan MS, McNelly NA, Hinds JW. Population dynamics of adult-formed granule neurons of the rat olfactory bulb. J Comp Neurol. 1985;239:117–125. doi: 10.1002/cne.902390110. [DOI] [PubMed] [Google Scholar]
- Kaufman GDMR, Shinder ME. Microarray analysis of vestibular compensation in the gerbil. Neuro Sci Abs #70312 2003 [Google Scholar]
- Kitahara T, Fukushima M, Takeda N, Saika T, Kubo T. Effects of pre-flocculectomy on Fos expression and NMDA receptor-mediated neural circuits in the central vestibular system after unilateral labyrinthectomy. Acta Otolaryngol. 2000;120:866–871. doi: 10.1080/000164800750061741. [DOI] [PubMed] [Google Scholar]
- Kitahara T, Takeda N, Saika T, Kubo T, Kiyama H. Role of the flocculus in the development of vestibular compensation: immunohistochemical studies with retrograde tracing and flocculectomy using Fos expression as a marker in the rat brainstem. Neuroscience. 1997;76:571–580. doi: 10.1016/s0306-4522(96)00374-0. [DOI] [PubMed] [Google Scholar]
- Kraus KS, Mitra S, Jimenez Z, Hinduja S, Ding D, Jiang H, Gray L, Lobarinas E, Sun W, Salvi RJ. Noise trauma impairs neurogenesis in the rat hippocampus. Neuroscience. 2010;167:1216–1226. doi: 10.1016/j.neuroscience.2010.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CH, Tse YC, Shum DK, Yung KK, Chan YS. Fos expression in otolith-related brainstem neurons of postnatal rats following off-vertical axis rotation. J Comp Neurol. 2004;470:282–296. doi: 10.1002/cne.11048. [DOI] [PubMed] [Google Scholar]
- Lee A, Kessler JD, Read TA, Kaiser C, Corbeil D, Huttner WB, Johnson JE, Wechsler-Reya RJ. Isolation of neural stem cells from the postnatal cerebellum. Nat Neurosci. 2005;8:723–729. doi: 10.1038/nn1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Kim JW, Yim SV, Kim MJ, Kim SA, Kim YJ, Kim CJ, Chung JH. Fluoxetine enhances cell proliferation and prevents apoptosis in dentate gyrus of maternally separated rats. Mol Psychiatry. 2001;6:610, 725–618. doi: 10.1038/sj.mp.4000954. [DOI] [PubMed] [Google Scholar]
- Leuner B, Glasper ER, Gould E. Thymidine analog methods for studies of adult neurogenesis are not equally sensitive. The Journal of comparative neurology. 2009;517:123–133. doi: 10.1002/cne.22107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, Samburg D, Gould E, Shors TJ. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J Neurosci. 2004;24:7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RA. Somatic (craniocervical) tinnitus and the dorsal cochlear nucleus hypothesis. Am J Otolaryngol. 1999;20:351–362. doi: 10.1016/s0196-0709(99)90074-1. [DOI] [PubMed] [Google Scholar]
- Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- Manohar S, Hayes S, Salvi R, Baizer JS. Unipolar brush cells express DCX in the dorsal cochlear nucleus, paraflocculus and flocculus of adult rat. ARO; Baltimore, Maryland: 2011. p. 431. [Google Scholar]
- Menna E, Disanza A, Cagnoli C, Schenk U, Gelsomino G, Frittoli E, Hertzog M, Offenhauser N, Sawallisch C, Kreienkamp H-J, Gertler FB, Di Fiore PP, Scita G, Matteoli M. Eps8 regulates axonal filopodia in hippocampal neurons in response to brain-derived neurotrophic factor (BDNF) PLoS Biol. 2009;7:e1000138. doi: 10.1371/journal.pbio.1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton JW, Kiritani T, Pedersen C, Turner JG, Shepherd GMG, Tzounopoulos T. Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7601–7606. doi: 10.1073/pnas.1100223108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Morin F, Dino MR, Mugnaini E. Postnatal differentiation of unipolar brush cells and mossy fiber-unipolar brush cell synapses in rat cerebellum. Neuroscience. 2001;104:1127–1139. doi: 10.1016/s0306-4522(01)00144-0. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Diño MR, Jaarsma D. The unipolar brush cells of the mammalian cerebellum and cochlear nucleus: cytology and microcircuitry. Prog Brain Res. 1997;114:131–150. doi: 10.1016/s0079-6123(08)63362-2. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Floris A. The unipolar brush cell: a neglected neuron of the mammalian cerebellar cortex. J Comp Neurol. 1994;339:174–180. doi: 10.1002/cne.903390203. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Sekerkova G, Martina M. The unipolar brush cell: a remarkable neuron finally receiving deserved attention. Brain Res Rev. 2011;66:220–245. doi: 10.1016/j.brainresrev.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacher J, Crespo C, McEwen BS. Doublecortin expression in the adult rat telencephalon. Eur J Neurosci. 2001;14:629–644. doi: 10.1046/j.0953-816x.2001.01683.x. [DOI] [PubMed] [Google Scholar]
- Nacher J, Pham K, Gil-Fernandez V, McEwen BS. Chronic restraint stress and chronic corticosterone treatment modulate differentially the expression of molecules related to structural plasticity in the adult rat piriform cortex. Neuroscience. 2004;126:503–509. doi: 10.1016/j.neuroscience.2004.03.038. [DOI] [PubMed] [Google Scholar]
- Newlands SD, Dara S, Kaufman GD. Relationship of static and dynamic mechanisms in vestibuloocular reflex compensation. Laryngoscope. 2005;115:191–204. doi: 10.1097/01.mlg.0000154718.80594.2e. [DOI] [PubMed] [Google Scholar]
- Nunzi MG, Birnstiel S, Bhattacharyya BJ, Slater NT, Mugnaini E. Unipolar brush cells form a glutamatergic projection system within the mouse cerebellar cortex. J Comp Neurol. 2001;434:329–341. doi: 10.1002/cne.1180. [DOI] [PubMed] [Google Scholar]
- Nunzi MG, Mugnaini E. Aspects of the neuroendocrine cerebellum: expression of secretogranin II, chromogranin A and chromogranin B in mouse cerebellar unipolar brush cells. Neuroscience. 2009;162:673–687. doi: 10.1016/j.neuroscience.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Nunzi MG, Russo M, Mugnaini E. Vesicular glutamate transporters VGLUT1 and VGLUT2 define two subsets of unipolar brush cells in organotypic cultures of mouse vestibulocerebellum. Neuroscience. 2003;122:359–371. doi: 10.1016/s0306-4522(03)00568-2. [DOI] [PubMed] [Google Scholar]
- Nunzi MG, Shigemoto R, Mugnaini E. Differential expression of calretinin and metabotropic glutamate receptor mGluR1alpha defines subsets of unipolar brush cells in mouse cerebellum. J Comp Neurol. 2002;451:189–199. doi: 10.1002/cne.10344. [DOI] [PubMed] [Google Scholar]
- Offenhauser N, Borgonovo A, Disanza A, Romano P, Ponzanelli I, Iannolo G, Di Fiore PP, Scita G. The eps8 family of proteins links growth factor stimulation to actin reorganization generating functional redundancy in the Ras/Rac pathway. Mol Biol Cell. 2004;15:91–98. doi: 10.1091/mbc.E03-06-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohira K, Furuta T, Hioki H, Nakamura KC, Kuramoto E, Tanaka Y, Funatsu N, Shimizu K, Oishi T, Hayashi M, Miyakawa T, Kaneko T, Nakamura S. Ischemia-induced neurogenesis of neocortical layer 1 progenitor cells. Nat Neurosci. 2010;13:173–179. doi: 10.1038/nn.2473. [DOI] [PubMed] [Google Scholar]
- Paolone N, Manohar S, Hayes S, Baizer JS, Salvi R. Neurosci Abs. 491.06/RR9. Washington, DC: 2011. Expression of a neuronal migratory protein in unipolar brush cells of the adult rat dorsal cochlear nucleus, paraflocculus, and flocculus. [Google Scholar]
- Paxinos G, Watson C. The rat brain, in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Pijpers A, Voogd J, Ruigrok TJ. Topography of olivo-cortico-nuclear modules in the intermediate cerebellum of the rat. J Comp Neurol. 2005;492:193–213. doi: 10.1002/cne.20707. [DOI] [PubMed] [Google Scholar]
- Rachel JD, Kaltenbach JA, Janisse J. Increases in spontaneous neural activity in the hamster dorsal cochlear nucleus following cisplatin treatment: a possible basis for cisplatin-induced tinnitus. Hear Res. 2002;164:206–214. doi: 10.1016/s0378-5955(02)00287-3. [DOI] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19:234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- Ruigrok TJ. Collateralization of climbing and mossy fibers projecting to the nodulus and flocculus of the rat cerebellum. J Comp Neurol. 2003;466:278–298. doi: 10.1002/cne.10889. [DOI] [PubMed] [Google Scholar]
- Russo MJ, Mugnaini E, Martina M. Intrinsic properties and mechanisms of spontaneous firing in mouse cerebellar unipolar brush cells. J Physiol. 2007;581:709–724. doi: 10.1113/jphysiol.2007.129106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo MJ, Yau HJ, Nunzi MG, Mugnaini E, Martina M. Dynamic metabotropic control of intrinsic firing in cerebellar unipolar brush cells. J Neurophysiol. 2008;100:3351–3360. doi: 10.1152/jn.90533.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sekerkova G, Dino MR, Ilijic E, Russo M, Zheng L, Bartles JR, Mugnaini E. Postsynaptic enrichment of Eps8 at dendritic shaft synapses of unipolar brush cells in rat cerebellum. Neuroscience. 2007;145:116–129. doi: 10.1016/j.neuroscience.2006.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekerkova G, Ilijic E, Mugnaini E. Time of origin of unipolar brush cells in the rat cerebellum as observed by prenatal bromodeoxyuridine labeling. Neuroscience. 2004;127:845–858. doi: 10.1016/j.neuroscience.2004.05.050. [DOI] [PubMed] [Google Scholar]
- Sekerkova G, Ilijic E, Mugnaini E, Baker JF. Otolith organ or semicircular canal stimulation induces c-fos expression in unipolar brush cells and granule cells of cat and squirrel monkey. Exp Brain Res. 2005;164:286–300. doi: 10.1007/s00221-005-2252-7. [DOI] [PubMed] [Google Scholar]
- Shore S, Zhou J, Koehler S. Neural mechanisms underlying somatic tinnitus. Progress in brain research. 2007;166:107–123. doi: 10.1016/S0079-6123(07)66010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu SY, Ju G, Fan LZ. The glucose oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett. 1988;85:169–171. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- Takács J, Borostyankoi ZA, Veisenberger E, Vastagh C, Vig J, Gorcs TJ, Hamori J. Postnatal development of unipolar brush cells in the cerebellar cortex of cat. J Neurosci Res. 2000;61:107–115. doi: 10.1002/1097-4547(20000701)61:1<107::AID-JNR13>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Serneo FF, Tseng HC, Kulkarni AB, Tsai LH, Gleeson JG. Cdk5 phosphorylation of doublecortin ser297 regulates its effect on neuronal migration. Neuron. 2004;41:215–227. doi: 10.1016/s0896-6273(03)00852-3. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Galea LA, Gould E. Stress inhibits the proliferation of granule cell precursors in the developing dentate gyrus. Int J Dev Neurosci. 1998;16:235–239. doi: 10.1016/s0736-5748(98)00029-x. [DOI] [PubMed] [Google Scholar]
- Tighilet B, Brezun JM, Sylvie GD, Gaubert C, Lacour M. New neurons in the vestibular nuclei complex after unilateral vestibular neurectomy in the adult cat. Eur J Neurosci. 2007;25:47–58. doi: 10.1111/j.1460-9568.2006.05267.x. [DOI] [PubMed] [Google Scholar]
- Van Der Gucht E, Youakim M, Arckens L, Hof PR, Baizer JS. Variations in the structure of the prelunate gyrus in Old World monkeys. The anatomical record. 2006;288:753–775. doi: 10.1002/ar.a.20350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience. 2009;164:747–759. doi: 10.1016/j.neuroscience.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharek MA, Kaltenbach JA, Mathog TA, Zhang J. Effects of cochlear ablation on noise induced hyperactivity in the hamster dorsal cochlear nucleus: implications for the origin of noise induced tinnitus. Hear Res. 2002;172:137–143. doi: 10.1016/s0378-5955(02)00575-0. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhou J, Shore S. Projections from the trigeminal nuclear complex to the cochlear nuclei: a retrograde and anterograde tracing study in the guinea pig. J Neurosci Res. 2004;78:901–907. doi: 10.1002/jnr.20343. [DOI] [PubMed] [Google Scholar]