Abstract
Objective
This review examines generic preference-based measures and their ability to reflect health-related quality of life in patients with visual disorders.
Methods
A systematic search was undertaken to identify clinical studies of patients with visual disorders where health state utility values were measured and reported. Data were extracted to assess the validity and responsiveness of the measures. A narrative synthesis of the data was undertaken due to the heterogeneity between different studies.
Results
There was considerable heterogeneity in the 31 studies identified in terms of patient characteristics, visual disorders, and outcomes reported. Vision loss was associated with a reduction in scores across the preference-based measure, but the evidence on validity and responsiveness was mixed. The EQ-5D health-related assessment instrument's performance differed according to condition, with poor performance in age-related macular degeneration (AMD) and diabetic retinopathy. The more limited evidence on the HUI-3 instrument found it performed best in differentiating between severity groups of patients with glaucoma, AMD, cataracts, and diabetic retinopathy. One study reported data on the SF-6D instrument and showed it was able to differentiate between patients with AMD.
Conclusions
The performance of the EQ-5D in visual disorders was mixed. The HUI-3 seemed to perform better in some conditions, but the evidence on this and SF-6D is limited. More head to head comparisons of these three measures are required. The new five-level version of EQ-5D may do better at the milder end of visual function.
Keywords: Health-related quality of life, quality of life, QALYs, utilities, vision
Introduction
Health state utility values (HSUVs) [1] are key parameters for economic evaluations that use a cost-utility analysis framework. Cost-utility analyses return a cost effectiveness estimate for a new intervention in terms of the incremental cost per quality adjusted life year gained. Reimbursement agencies such as the National Institute for Health and Clinical Excellence in the United Kingdom, as well as other similar organizations worldwide, routinely undertake cost-utility analyses for new health care interventions.
HSUVs are most frequently derived from a description of a health state by a patient using a standardized generic instrument such as the EQ-5D, the SF-6D, or the HUI-3. A patient's description is valued using a set of values obtained for each possible health state taken from a general population sample. These values are elicited using standard health state valuation techniques such as visual analogue scaling (VAS), standard gamble, or time trade off (TTO).
It has been shown that the different instruments for obtaining HSUVs produce different values [2–4]. It is important to assess the validity of any health outcome instrument, including those used to obtain HSUVs, in the particular condition of interest. Three widely used measures are EQ-5D, SF-6D, and HUI-3. EQ-5D is a five-dimension instrument, and measures the influence of a health state on mobility, self-care, usual activities, pain/discomfort, and depression/anxiety. Each dimension is given one of three levels of severity, which results in 243 unique health states (although a five-level version of the descriptive system has recently been developed). The SF-6D (a classification system for the SF-36 and SF-12 health questionnaires) has six dimensions and either four or six severity levels. This results in 18,000 possible unique health states. The HUI-3 has eight dimensions and either five or six severity levels, resulting in 972,000 possible unique health states. The additional dimensions and levels may increase the instruments responsiveness to particular changes in health status; however, this comes at the cost of increased patient burden and completion time, as well as requiring more health state valuations and/or increased uncertainty around these values. These instruments differ in the dimensions described, the number of levels, and the severity range covered, which may have implications for the appropriateness of an instrument to describe particular conditions. The fewer dimensions and levels in the EQ-5D compared to other instruments has been seen by some as a limitation. However the EQ-5D has been validated in many clinical areas and has shown that it has construct validity and is responsive to change [3].
There has been concern about the validity of the EQ-5D instrument [3,5] and SF-6D [5] in some visual disorders. Visual disorders are a broad set of conditions that can affect a patient in a range of ways. Certain conditions are painful, can affect central or peripheral vision, and can affect one or both eyes therefore affecting a patient's health-related quality of life (HRQoL). The aim of this review is to examine the appropriateness of the EQ-5D, SF-6D, and HUI-3 in patients with visual disorders due to the different ways particular conditions affect HRQoL.
Methods
Search strategy and data identification
The objective of the literature review was to identify relevant journal articles reporting evidence of the performance of EQ-5D, SF-6D, and HUI-3 in patients with visual disorders.
A broad search was conducted to identify studies reporting preference-based utility instruments that were used to examine the HRQoL of patients with a visual disorder. BIOSIS, CINAHL, EMBASE, MEDLINE, PsychINFO, Web of Science electronic databases, and the Euroqol Website were searched. A search strategy was developed following consultation with experts in information resources and health economics. The search included both free text and controlled terms. Free text words included “visual disorder,” “euroqol,” “hui3,” and “sf6d” (all with alternative spellings). Specific visual disorders were also searched, including “cataracts,” “retinopathy,” and “macular degeneration.” The criteria for inclusion was that patients had a visual disorder, the study reported at least one from the EQ-5D, SF-6D, or HUI-3 and reported another measure of quality of life (generic or condition-specific) or a measure of clinical severity. Valuations of vignettes and current health were not included because the focus was on generic preference-based measures of HRQoL. Vignettes are not measures of HRQoL, they are scenarios constructed from interviews with patients, professionals, and other experts intended to reflect the influence of a condition. Valuations of current health are preference based, but are not part of the class of generic preference-based measures based on generic health state classification with values coming from members of the general population [6]. There was no restriction relating to the type of study. Due to resource limitations only English language studies were reviewed.
Analytic strategy
Data extraction. Data were extracted from the studies using a standardized set of forms developed for this study after reviewing forms used for similar studies in other disease areas [7,8]. Data extracted included:
-
•
Study characteristics – country, type of visual disorder, disease or treatment stage, any treatment given, and study type;
-
•
Participant characteristics – number of participants, age, sex, ethnicity, and proportion of missing data;
-
•
Instrument used – EQ-5D/SF-6D/HUI-3, other health-related utility measures, other generic measures of HRQoL, or condition-specific HRQoL measures and clinical measures of disease severity;
-
•
Health state utility values – index mean, scoring algorithm, and VAS mean;
-
•
Construct and convergent validity; and
-
•
Responsiveness
Assessment of quality. The assessment of quality of included studies requires a different methodology from the conventional approach required for reviewing clinical evidence. Of most importance was the relevance of the study in terms of the patient population. For studies that included a mixed population of patients (i.e., with various chronic conditions), then studies were only included if they reported HSUV's for subgroups of patients with a specific visual disorder.
Also important are response or completion rates, which may have some implications for generalizability and provide evidence on the acceptability of the questionnaire to patients.
Assessment of validity. Validity is defined as how well an instrument measures what it was intended to measure. More specifically, for instruments constructed to measure HRQoL, if the dimensions adequately cover the key determinants of HRQoL. Criterion validity would be determined by comparing an instrument to an established gold standard. A gold standard for measuring health-related utility, however, has not been established, and so construct validity incorporates a range of tests to see if patterns in scores confirm prior constructs. These tests provide an indication of a measure's performance to a degree; however, the results are open to interpretation and opinion.
The most common test to identify construct validity is the known group method. This compares the results of a preference based measure between groups of patients that are expected to differ in the construct. If a study presents a population stratified on the basis of a clinical indicator, then one can investigate the ability of a preference-based measure to distinguish between these groups. It should be noted that the usefulness of these comparisons can be limited by sample size (especially because studies are usually not powered on a preference based measure), the appropriateness of the clinical groups defined, and exogenous factors that may influence quality of life. For instance, groups defined solely by the presence of a biomarker may not have a clinical difference that influences their HRQoL. Also, if patients have a number of comorbidities then these may have a greater influence on HRQoL than the condition of interest. Known groups can be defined using a case-control analysis. A more stringent test is to define known groups based on different levels of condition severity.
Another test is convergent validity. This is defined as the extent to which one measure correlates with another measure of the same concept. In this review, this would be the extent to which EQ-5D, SF-6D, or the HUI-3 correlate with each other and with measures of vision problems or quality of life.
A final test for construct validity is the responsiveness of a measure, which is the ability to measure a change in health status. A pre/postintervention study that reports EQ-5D, SF-6D, or the HUI-3 and another valid measure of health change would allow the responsiveness of a measure due to change in health status to be identified. As with the tests of validity, it is important to consider if the measures of health change that are being used for comparison are themselves valid. In addition, it is important to consider if other health changes not directly related to the condition could have influenced health-related utility (e.g., side effects of treatment).
Assessment of reliability. In addition to validity, reliability was assessed. Reliability of a measure is defined as its ability to reproduce results when measurements are repeated on an unchanged population [9]. Reliability can be measured by retesting and reporting either the correlation or difference between estimates.
Presentation and analysis. Tables presenting summaries of the study characteristics, analyses on the effects of visual acuity on HSUVs are presented. The analysis is broken down into particular visual disorders to allow for conclusions to be formed both on specific disorders as well as visual disorders as a whole. Heterogeneity in the studies reviewed, in terms of study design and patient populations, means that a formal meta-analysis would be inappropriate.
Results
Search results
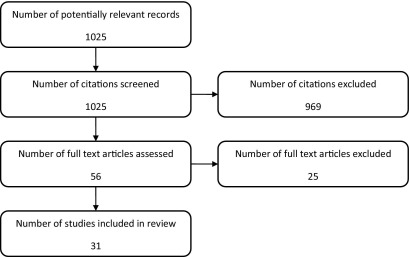
Bibliographic searching was completed in August 2010. A total of 1025 potentially relevant articles were identified. Abstracts and titles for all articles were screened to identify articles meeting the inclusion criteria. A total of 969 records were excluded, and full articles were ordered for the remaining 56 records. After reviewing the full articles, 25 were excluded and a total of 31 articles were included in the review. A flow chart of the study selection process is shown in Figure 1.
Fig. 1.
Flow chart of the study selection process.
The 31 studies identified from the bibliographic search are presented in Tables 1 and 2. Thirty of the 31 studies were observational studies, with the remaining study being a randomized controlled trial. The selected studies were conducted in different countries; three are multicountry studies, three are in the United States, five in Canada, and nine are from the United Kingdom.
Table 1.
Characteristics of included studies.
| Study reference (author, year) | Country | Disease/treatment stage | Study type (e.g., cross sectional, randomized controlled trial, cohort) |
|---|---|---|---|
| Glaucoma | |||
| Aspinall et al. 2008 [10] | UK | Glaucoma | Cross-sectional study |
| Kobelt et al. 2006 [11] | Sweden | Ocular hypertension or open-angle glaucoma | Cross-sectional study |
| Mittmann et al. 2001 [12] | Canada | Glaucoma | Cross-sectional study |
| Montemayor et al. 2001 [13] | Canada | COAG, normal-pressure glaucoma, or suspected glaucoma with treatment | Cross-sectional study |
| Thygesen et al. 2008 [14] | Multi-country | Late-stage primary open-angle glaucoma | Case review |
| Age-related macular degeneration | |||
| Cruess et al. 2007 [15] | Canada | Neovascular age-related macular degeneration | Cross-sectional observational study |
| Espallargues et al. 2005 [16] | UK | Wet or dry age-related macular degeneration | Cross-sectional study |
| Kim et al. 2010 [17] | Korea | - | Cohort study |
| Lotery et al. 2007 [18] | UK | Bilateral subfoveal neovascular age-related macular degeneration | Cross-sectional study |
| Payakachat et al. 2009 [19] | Multi-country | Wet age-related macular degeneration | Cross-sectional study |
| Ruiz-Moreno et al. 2008 [20] | Spain | Bilateral neovascular age-related macular degeneration | Prospective case-control study |
| Soubrane et al. 2007 [21] | Multi-country | Neovascular age-related macular degeneration | Cross-sectional study |
| Cataracts | |||
| Asakawa et al. 2008 [22] | Canada | ± other comorbidities | Cross-sectional study |
| Black et al. 2009 [23] | UK | First or second eye | Prospective cohort study |
| Conner-Spady et al. 2005 [24] | Canada | - | Cohort study |
| Datta et al. 2008 [25] | UK | Bilateral cataracts in over 70s | Secondary analysis of RCT |
| Jayamanne et al. 1999 [26] | UK | First eye | Prospective study |
| Polack et al. 2007 [27] | Kenya | - | Case-control study |
| Polack et al. 2008 [28] | Bangladesh | - | Case-control study |
| Polack et al. 2010 [29] | The Philippines | Over 50s | Case control study |
| Diabetic retinopathy | |||
| Lloyd et al. 2008 [30] | UK | Diabetic retinopathy | Cross-sectional study |
| Smith et al. 2008 [31] | US | Type 2 diabetes | Cross-sectional study |
| Conjuntivitis | |||
| Pitt et al. 2004 [32] | UK | - | Cohort study |
| Rajagopalan et al. 2005 [33] | Multi-country | Non-Sjogren's keratoconjunctivitis or Sjogren's Syndrome | Cross-sectional study |
| Smith et al. 2005 [34] | Spain | - | Cohort study |
| Other visual disorders | |||
| Boulton et al. 2006 [35] | UK | Vision impairment or blindness in children | Cross-sectional study |
| Clark et al. 2008 [36] | Australia | Post cataract surgery endophthalmitis | Cohort study |
| Kempen et al. 2003 [37] | US | Cytomegalovirus retinitis in patients with AIDS | Prospective cohort study |
| Langelaan et al. 2007 [38] | Netherlands | Low-vision patients | Cross-sectional study |
| Quinn et al. 2007 [39] | US | Retinopathy of prematurity | Cohort study |
| Van Nispen et al. 2009 [40] | Netherlands | Vision impairment in older people | Observational study |
Table 2.
Instruments used.
| Study reference (author, year) | Generic preference based |
Direct valuation |
Rating scale |
Condition specific HRQoL instruments and measures of clinical severity |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EQ-5D | SF-6D | HUI-3 | TTO | VAS | VFQ (20/25) | VF (14/4D) | RQLQ | VFA | IDEEL | |
| Glaucoma | ||||||||||
| Aspinall et al. 2008 [10] | ✓ | ✓ | ||||||||
| Kobelt et al. 2006 [11] | ✓ | ✓ | ||||||||
| Mittmann et al. 2001 [12] | ✓ | |||||||||
| Montemayor et al. 2001 [13] | ✓ | ✓ | ||||||||
| Thygesen et al. 2008 [14] | ✓ | |||||||||
| Age-related macular degeneration | ||||||||||
| Cruess et al. 2007 [15] | ✓ | ✓ | ||||||||
| Espallargues et al. 2005 [16] | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Kim et al. 2010 [17] | ✓ | ✓ | ||||||||
| Lotery et al. 2007 (18) | ✓ | ✓ | ||||||||
| Payakachat et al. 2009 [19] | ✓ | ✓ | ||||||||
| Ruiz-Moreno et al. 2008 [20] | ✓ | ✓ | ||||||||
| Soubrane et al. 2007 [21] | ✓ | ✓ | ||||||||
| Cataracts | ||||||||||
| Asakawa et al. 2008 [22] | ✓ | |||||||||
| Black et al. 2009 [23] | ✓ | ✓ | ||||||||
| Conner-Spady et al. 2005 [24] | ✓ | ✓ | ✓ | |||||||
| Datta et al. 2008 [25] | ✓ | ✓ | ||||||||
| Jayamanne et al. 1999 [26] | ✓ | ✓ | ||||||||
| Polack et al. 2007 [27] | ✓ | ✓ | ||||||||
| Polack et al. 2008 [28] | ✓ | ✓ | ||||||||
| Polack et al. 2010 [29] | ✓ | ✓ | ||||||||
| Diabetic retinopathy | ||||||||||
| Lloyd et al. 2008 [30] | ✓ | ✓ | ✓ | |||||||
| Smith et al. 2008 [31] | ✓ | |||||||||
| Conjunctivitis | ||||||||||
| Pitt et al. 2004 [21] | ✓ | ✓ | ✓ | ✓ | ||||||
| Rajagopalan et al. 2005 [33] | ✓ | ✓ | ✓ | |||||||
| Smith et al. 2005 [34] | ✓ | ✓ | ✓ | ✓ | ||||||
| Other visual disorders | ||||||||||
| Boulton et al. 2006 [35] | ✓ | |||||||||
| Clark et al. 2008 [36] | ✓ | ✓ | ✓ | |||||||
| Kempen et al. 2003 [37] | ✓ | ✓ | ||||||||
| Langelaan et al. 2007 [38] | ✓ | |||||||||
| Quinn et al. 2007 [39] | ✓ | |||||||||
| Van Nispen et al. 2009 [40] | ✓ | |||||||||
HRQoL, health-related quality of life; IDEEI, Impact of Dry Eyes on Everyday Life questionnaire; RQLQ, Rhinoconjunctivitis Quality of Life Questionnaire; VFA, Visual Function Assessment; VF-14/4D, Visual Functional Questionnaire 14/4 dimension; VFQ20/25, Visual Function Questionnaire 20/25.
Quality of studies
A judgment regarding the risk of bias of each study was determined by reviewing the methods of patient recruitment, and noting any missing data reported (either study drop-outs or incomplete questionnaires). A range of recruitment procedures was seen in the review, and this was usually determined by the study design. Some involved a retrospective analysis of datasets [12,22] with a predetermined inclusion criteria, a number of studies were case-controlled analyses [27–29], but the majority were cross-sectional observational studies. The randomized controlled trial reviewed had well-defined inclusion criteria [25]. As is typical, in the longitudinal studies some patients dropped out before the end of the study. The general implication of patients dropping out was that no measurements were taken. Response rates for questionnaires range from 33% to 96%, with completion rates of longitudinal studies above 85% in all but one study (range 52%–98.1%).
Patient characteristics
The studies identified were in a wide range of visual disorders. Nine studies were in patients with cataract [22–29]. Seven studies were in patients with age related macular degeneration (AMD) [15–21], five studies were in patients with glaucoma [10–14]. Three studies were in patients with conjunctivitis [32–34], two studies were in patients with diabetic retinopathy [30,31], and the remaining six studies were in populations with various other visual disorders [35–40].
The inclusion criteria varied across the studies reviewed within each of the specific conditions. This was due to the study design, the study methodology, and also in some studies the inclusion criteria was unclear. Some studies reported that patients were identified through case notes, but no more details are provided. It was noted if AMD was bilateral or unilateral and wet or dry, if cataracts were first or second eye, and if glaucoma was primary or multiple.
Reliability
No tests of reliability were performed on the generic preference based measures.
Construct validity
Twenty-one of the 31 studies allow a known group analysis to be performed, 17 for the EQ-5D, four for the HUI-3, but no studies for the SF-6D. In six of the studies, groups were defined by visual acuity (VA), or by contrast sensitivity (CS). These were clearly defined groups with mean estimates of utility provided for each group [14,16,21,30,34,38]. The remaining 25 studies had either a case-control design, had differing conditions, or did not define levels of severity.
The differences in clinical definition of groups, conditions, characteristics of patients, and study designs do not allow for a direct comparison of the utility values, or a meta-analysis.
Convergent validity
Nine of the 31 studies reviewed provide evidence on correlation or regression between generic measure of HRQoL with either each other or with visual measures. Eight studies report evidence of convergent validity in the EQ-5D compared to a visual measure, with Espallargues et al. [16] also reporting correlations across EQ-5D, SF-6D, and HUI-3.
Glaucoma
Construct validity. Three studies of the EQ-5D allowed an analysis of groups defined by severity of vision problems in glaucoma patients (see Table 3). Aspinall et al. [10] present EQ-5D utility values stratified by mild, moderate, and severe visual field loss. The values decrease appropriately but are not statistically significant. Kobelt et al. [11] saw the EQ-5D decrease with increasing glaucomatous damage, but the difference between groups was not statistically significant when controlling for co-morbidity, except for the most severe group. The study by Thygesen et al. [14] defined three groups on the basis of the Snellen score, and saw consistent ordering of mean utility values.
Table 3.
Utility, visual acuity (VA) and visual analogue scale (VAS) values.
| Study reference (author, year) | Instrument | Index (mean (SD)) | VA (logMAR mean (SD) unless specified) | VAS (mean (SD)) |
|---|---|---|---|---|
| Glaucoma | ||||
| Aspinall et al. 2008 [10] | EQ-5D | 0.76 (0.19) | Median group 6/12 or better in both eyes | |
| Kobelt et al. 2006 [11] | EQ-5D | 0.80 (0.23) | (right/left) 0.76/0.74 (0.30/0.29) | 74.7 (18.2) |
| Mittmann et al. 2001 [12] | HUI-3 | 0.924 (0.086) | ||
| Montemayor et al. 2001 [13] | EQ-5D | 0.89 (range −0.08 to 1.00) | −0.10 (0.17) | |
| Thygesen et al. 2008 [14] | EQ-5D | 0.65 (0.28) | Best/worst eye 0.28(0.26) / 0.14(0.18) | |
| Age-related macular degeneration | ||||
| Cruess et al. 2007 [15] | EQ-5D | 0.64 (0.52,0.76) | 0.66 (0.64) | |
| Espallargues et al. 2005 [16] | EQ-5D | 0.72 (0.22) |
|
65.0 |
| SF-6D | 0.66 (0.14) | |||
| HUI-3 | 0.34 (0.28) | |||
| Kim et al. 2010 [17] | EQ-5D | Pre-treatment 0.729 (0.236) Post-treatment 0.793 (0.222) | ||
| Lotery et al. 2007 [18] | EQ-5D | 0.67 | 0.26 (0.19) | |
| Payakachat et al. 2009 [19] | EQ-5D | 0.7711 (0.21) | Median group for Better and worse eye groups: 20/80 to 20/160 | |
| Ruiz-Moreno et al. 2008 [20] | EQ-5D | 0.68 (0.62,0.74) 95% CI | ||
| Soubrane et al. 2007 [21] | EQ-5D | 0.65 | 0.6 (0.7) | |
| Cataracts | ||||
| Asakawa et al. 2008 [22] | HUI-3 | - | ||
| Black et al. 2009 [23] | EQ-5D | 0.81 (0.23) | ||
| Conner-Spady et al. 2005 [24] | EQ-5D |
|
First eye, second eye VA op 0.58 (0.30), 0.52 (0.27) VA non-op 0.43 (0.28), 0.29 (0.21) |
|
| Datta et al. 2008 [25] | EQ-5D | Median 0.73 (0.26) | 0.28 [0.16;0.42] | |
| Jayamanne et al. 1999 [26] | EQ-5D | - | 90% had VA 6/18-6/60 | |
| Polack et al. 2007 [27] | EQ-5D | - | Median group < 6/60 > 3/60 | |
| Polack et al. 2008 [28] | EQ-5D | - | Median group < 3/60 > PL | |
| Polack et al. 2010 [29] | EQ-5D | - | Median group < 3/60 > PL | |
| Diabetic retinopathy | ||||
| Lloyd et al. 2008 (30) | EQ-5D, HUI-3 |
|
|
|
| Smith et al. 2008 (31) | EQ-5D | 0.8 (0.18) | Median group >20/20 | |
| Conjunctivitis | ||||
| Pitt et al. 2004 (32) | EQ-5D | - |
|
|
| Rajagopalan et al. 2005 (33) | EQ-5D |
|
|
|
| Smith et al. 2005 (34) | EQ-5D | - |
|
|
| Other visual disorders | ||||
| Boulton et al. 2006 (35) | HUI-3 | 0.34 (0.43) | ||
| Clark et al. 2008 (36) | EQ-5D |
|
||
| Kempen et al. 2003 (37) | EQ-5D |
|
|
|
| Langelaan et al. 2007 (38) | EQ-5D | 0.73 (0.22) | Functional Acuity Score 38.61 (26.5) | |
| Quinn et al. 2007 (39) | HUI-3 |
|
|
|
| Van Nispen et al. 2009 (40) | EQ-5D |
|
|
|
CMV, cytomegalovirus retinitis; DR, diabetic retinopathy; non-SS KCS, non-SS or keratoconjunctivitis; PL, perception of light; ROP, retinopathy of prematurity; SAC, seasonal allergic conjunctivitus; SS, Sjogren's Syndrome.
No such data were available for HUI-3 or SF-6D. However, one article reported the use of HUI-3 in a case-control study that showed a significant and appropriate difference in HUI-3 between cases and controls [12].
Convergent validity. Three studies report correlation statistics for EQ-5D with VA in patients with glaucoma [10,13,14]. Aspinall et al. [10] found moderate and statistically significant correlations for the mobility, self-care, and anxiety dimensions, along with the summed index score. Montemayor et al. [13] did not find significant correlations for EQ-5D with VA. The study by Thygesen et al. [14] showed a significant correlation between VA and EQ-5D.
Responsiveness. No studies reported responsiveness in patients with glaucoma.
AMD
Construct validity. Of the seven articles, six allowed an assessment of construct validity of the EQ-5D in people with AMD. Of these, three differentiated between groups based on severity of vision disorder and four included assessments of cases against controls (one of which also grouped by severity).
Of the case-control studies, three found that EQ-5D showed an appropriate and statistically significant reduction in HRQoL for people with AMD compared to general population controls [18,20,21]. One reported a difference that was not statistically significant, but the difference was in the appropriate direction [15].
Three studies differentiated in terms of severity: one in terms of levels of visual acuity and the other based on if they had unilateral or bilateral AMD. Soubrane et al. [21] showed inconsistency with the mean estimates, with normal VA (> 20/40) having a worse mean utility (0.69) on the EQ-5D when compared to mild, moderate, severe, and near blind utility values. This inconsistency was not seen in the VFQ-25; however, the HADS anxiety dimension was also inconsistent between the normal and mild VA groups. The study did show a significant difference between those with neovascular AMD (NV-AMD) and the control group. The study was relatively large (N=401 NV-AMD patients), however proportions in each group are not provided. Kim et al. [17] found a statistically significant difference in EQ-5D values between those with unilateral and bilateral AMD [17]. Espallargues et al. [16] found a consistent relationship between VA and CS with HUI-3, SF-6D, TTO, and VAS but not the EQ-5D [16].
Convergent validity. Only Espallargues et al. [16] provided correlation statistics between generic and visual measures in patients with AMD. They found that the VAS, TTO, HUI-3, and SF-6D were all significantly correlated with both VA and CS. They did not, however, find significant correlations for EQ-5D with VA or CS.
Responsiveness. Kim et al. [17] reported a statistically significant improvement in both the VF-4D and the EQ-5D after photodynamic therapy in patients with AMD.
Cataracts
Construct validity. Five of the seven studies in people with cataracts allowed an assessment of the construct validity of the EQ-5D. Conner-Spady et al. [24] identified an appropriate but nonsignificant change in EQ-5D between first and second eye surgery groups. Three case-control studies conducted in different countries by Polack et al. in 2007 [27], 2008 [28], and 2010 [29] found that there were significant differences in EQ-5D between cases and controls, and found that cases were likely to report a significant difference across all dimensions (except pain dimension in Polack et al. [28]). There was no strong evidence to support a significant and consistent association between the degree of VA and EQ-5D. Polack et al. [29] reports an inconsistent association between EQ-5D and VA level.
One study reported HUI-3 values for cases and control [22], and identified statistically significant and appropriate difference between the two groups.
Convergent validity. Three studies provide evidence of the convergent validity of the EQ-5D with VA. Polack et al. in 2007 [27] and 2008 [28] tested associations between EQ-5D and VA, with one finding that poorer VA was associated with higher odds of reporting any problem with all EQ-5D dimensions apart from anxiety in the Polack et al. study [28]. The other study found no significant associations between VA and EQ-5D dimensions, apart from a borderline association with self-care (P = 0.05) [27]. Datta et al. [25] did not find significant correlations for EQ-5D with VA.
Responsiveness. Black et al. [23] reported a statistically significant improvement in both the VF-14 and the EQ-5D post cataract surgery, although the later was relatively small. Conner-Spady et al. [24] reported a statistically significant improvement in the Visual Function Assessment (VFA) and VA post cataract surgery, but the subsequent mean improvements in EQ-VAS and EQ-5D were small and not statistically significant. This may suggest that the EQ-5D is not responsive in this population; however, it should be recognized that the study was not initially powered to identify statistically significant changes, and a mean improvement was identified. Also, the VAS did not change pre- to post-treatment, so it could be that the treatment did not significantly influence HRQoL.
Diabetic retinopathy
Construct validity. Two studies reported the EQ-5D identifying a statistically significant difference between the two extreme groups; however, the differences between neighbouring groups were not significant, and frequently inconsistent [30,31]. In the study by Lloyd et al. [30] the inconsistencies were also shown in VAS ratings of patients' own health and the HUI-3. This may be due to small sample sizes or the authors speculate that it may be due to a loss of independence of the participants when they reach that level of severity.
Convergent validity. Smith et al. [31] fitted a linear regression and found visual angle to be a predictor of EQ-5D utility values. They also fitted a nonparametric ordinal logistic regression and this estimated that any degree of visual impairment would see an increased likelihood of reporting nonperfect utility values.
Responsiveness. No articles reported the responsiveness of the measures in patients with diabetic retinopathy.
Conjunctivitis
Construct validity. All three studies allowed an assessment of construct validity of the EQ-5D in people with conjunctivitis. All three were case-control studies and showed a statistically significant difference between cases and controls [32–34]. Within the dimensions of the EQ-5D, The study by Pitt et al. [32] found the pain dimension to be the only dimension to show a statistical difference. The Smith et al. [34] study saw a significant difference across all dimensions except mobility. No studies provide evidence on the construct validity of the HUI-3 or SF-6D.
Convergent validity and responsiveness. No articles reported on convergent validity or the responsiveness of the measures in patients with conjunctivitis.
Other visual conditions
Construct validity. The remaining five studies were in unique visual conditions. Three of these studies allow an assessment of the construct validity of the EQ-5D, and two of the HUI-3.
Clark et al. [36] and Kempen et al. [37] reported an appropriate but nonsignificant difference in the EQ-5D between the control group and those with endophthalmitis and cytomegalovirus, respectively. Langelaan et al. [38] undertook a study in visually impaired patients, and identified an appropriate but nonsignificant difference in the EQ-5D between low and high visual field groups, but an inconsistent and nonsignificant difference in the EQ-5D between low and high VA groups.
Boulton et al. [35] and Quinn et al. [39] found the HUI-3 identified statistically significant and appropriate differences between groups of patients with unspecified blindness/visual impairment.
Convergent validity. van Nispen et al. [40] undertook a multivariate regression analysis in older patients with a visual impairment. They found that worsening VA was a significant risk factor for a lower EQ-5D value.
Responsiveness. No articles reported data on the responsiveness of the measures.
Discussion
The 31 studies found in this review show a worsening of utility values as visual impairment increases in many though not all studies. The magnitude and statistical significance of the association varied between different generic preference-based measures of HRQoL.
The largest amount of evidence was found for the EQ-5D compared to the other generic measures and the results were mixed. Nearly all studies showed significant differences between patients with the condition and a control group without it. This is a very crude test, however, of construct validity and furthermore, many were not well controlled for age and other conditions add so their conclusions may be limited. Studies comparing EQ-5D scores across severity groups were more mixed, with most showing little or no difference between groups defined by clinical measures of visual impairment. There was limited evidence on responsiveness, only in the form of before and after an intervention. The few studies identified changes consistent with an effective intervention, but differences were statistically significant in only two of three studies. The assessment of convergent validity was more concerning, with several studies not demonstrating a statistically significant correlation with clinical measures. Although there was less evidence for the HUI-3, all but one study demonstrated good validity; no studies assessed responsiveness. There was very limited evidence on the SF-6D in patients with visual impairment.
The results can also be grouped by visual disorder to examine the performance of each generic measure. A summary of the overall performance by visual disorder is provided in Table 4. The EQ-5D performs well in patients with conjunctivitis; however, the evidence is limited to case-control studies and no comparison is made to either generic HRQoL or clinical measures. In patients with diabetic retinopathy, both the EQ-5D and the HUI-3 distinguished between patients with and without the condition; however, some evidence showed that both instruments were unable to distinguish between severity levels. The EQ-5D was found to correlate with clinical measures in patients with diabetic retinopathy. In patients with AMD, the EQ-5D distinguished between patients with and without the condition; it was unable to distinguish between severity levels and did not correlate well with other measures. The HUI-3 and the SF-6D did distinguish according to severity and correlated well with other measures. Case-control evidence supports the EQ-5D and HUI-3 in patients with cataracts. One study of the EQ-5D in people with cataracts showed a nonsignificant trend reflecting severity; however, the association of EQ-5D dimensions with other measures of severity was mixed.
Table 4.
Overall performance by visual disorder.
| EQ-5D | HUI-3 | SF-6D | |
|---|---|---|---|
| Glaucoma | |||
| Severity |
  ✓ ✓ |
· | · |
| Case-control | · | ✓ | · |
| Correlation | ✓×✓ | · | · |
| Responsiveness | · | · | · |
| AMD | |||
| Severity | ✓×× | ✓ | |
| Case-control | ✓✓✓
|
· | · |
| Correlation | × | ✓ | ✓ |
| Responsiveness | ✓ | · | · |
| Cataracts | |||
| Severity | ✓✓×× | · | · |
| Case-control | ✓✓✓ | ✓ | · |
| Correlation | ×✓× | · | · |
| Responsiveness | ✓
|
· | · |
| Diabetic Retinopathy | |||
| Severity | ×× | × | · |
| Case-control | ✓✓ | ✓ | · |
| Correlation | · | · | |
| Responsiveness | · | · | |
| Conjunctivitus | |||
| Severity | · | · | · |
| Case-control | ✓✓✓ | · | · |
| Correlation | · | · | · |
| Responsiveness | · | · | · |
| Other | |||
| Severity | ? | ✓✓ | · |
| Case-control |
 
|
· | · |
| Correlation | ✓ | · | · |
| Responsiveness | · | · | · |
KEY
✓statistically significant.
 trend meeting prior expectation but difference not statistically significant.
trend meeting prior expectation but difference not statistically significant.
x Inconsistent or nonsignificant correlation.
?mixed.
No evidence.
In patients with glaucoma the EQ-5D distinguished between different levels of severity although this was not always statistically significant. Two of three studies in this condition showed that it correlated well with other measures but the third study failed to demonstrate a relationship. The HUI-3 distinguished between cases and controls in patients with glaucoma. The EQ-5D distinguished between people with and without conjunctivitis. In the “other” category, the EQ-5D evidence is mixed, but the HUI-3 is supportive.
Although there are 31 studies providing evidence on the validity and responsiveness of EQ-5D, HUI3, and SF-6D, overall the evidence base is weak. Much of the evidence is limited to comparisons with general population scores and this is rather crude. Many of the studies had small numbers, so some of the inconsistent findings and lack of statistical significance could be due to small numbers. Furthermore, this often did not control for age or comorbidities that may correlate with visual impairment. Finally, there were very few head to head studies that really enable a true comparison of performance.
This review has used psychometric tests to evaluate the ability of the EQ-5D, HUI3, and SF-6D to reflect the effects of visual impairment on HRQoL. The results of these tests allow an assessment of how well an instrument seems to capture the impact of a condition or treatment. The overall appropriateness of a measure extends further than its psychometric or statistical properties. It is important to consider that different audiences may have specific requirements, such as the preferred method of valuation or about whose preferences the measure should include. For some national policy makers such as National Institute for Health and Clinical Excellence in the United Kingdom, consistency in methods may be important and consequently the use of one specific outcome measure may be recommended [41]. Therefore, identifying a more appropriate measure, purely in terms of its psychometric properties, is not sufficient because it will not be suitable for economic evaluation. Instead such evidence should drive research to continue to refine and improve generic measures of HRQoL, or in some circumstances, the development of condition specific measures.
One might expect quality of life to decrease as the clinical severity of the visual disorder increases and therefore a relationship between generic instruments and clinical indicators, such as VA, may be expected. The clinical indicators are not themselves measures of quality of life and may only give a narrow representation of the disease. Furthermore, different conditions may affect clinical disorders differently, for example some affect on visual field whereas others affect on VA. A condition specific measure of HRQoL, such as the VFQ-25, might provide a better measure to examine convergent validity, but such measures do not usually reflect preferences, although recent research has investigated the development of condition-specific measures of HRQoL [42].
A new version of the EQ-5D has been developed with the number of levels increased to five rather than three [43]. It is possible that this could improve the EQ-5D's ability to demonstrate differences in utility between people with milder severities of visual impairment. Further research should be conducted on the validity of the five-level version in people with visual impairment. There is also interest in adding dimensions to EQ-5D to make it more relevant for certain conditions and there is currently a study being undertaken to look at the effects of developing an add-on dimension to pick up the influence of visual impairment on HRQoL. Another solution would be to develop a preference-based condition specific index using a widely used vision-specific instrument such as the VFQ-25 [44], although there are concerns that condition specific preference-based measures may not be comparable across different medical conditions [45].
Conclusions
This review has provided a narrative analysis of preference-based measures in visual disorders. The broad range of values, and the differing levels of performance in terms of construct validity, convergent validity, and responsiveness reflects the systematic variance attributable to different types of visual disorder, to different patient populations, and to study design. The number of studies investigating the EQ-5D is much larger than for HUI-3 or SF-6D. The results of this review show generally consistent results on the validity of the HUI-3 in people with visual impairment with the exception of diabetic retinopathy, the results for EQ-5D were mixed, and there was little evidence on the use of the SF-6D. Responsiveness was only assessed in the EQ-5D and this was found to be consistent, but not always statistically significant. More head to head comparisons are required of these measures across visual conditions.
Acknowledgments
Source of financial support: This project was funded by the Medical Research Council as part of the Medical Research Council-National Institute for Health Research Methodology Research programme (G0901486).
References
- 1.Torrance G.W. Measurement of health state utilities for economic appraisal: a review. J Health Econ. 1986;5:1–30. doi: 10.1016/0167-6296(86)90020-2. [DOI] [PubMed] [Google Scholar]
- 2.Longworth L., Bryan S. An empirical comparison of EQ-5D and SF-6D in liver transplant patients. Health Econ. 2003;12:1061–1067. doi: 10.1002/hec.787. [DOI] [PubMed] [Google Scholar]
- 3.Brazier J., Roberts J., Tsuchiya A., Busschbach J. A comparison of the EQ-5D and SF-6D across seven patient groups. Health Econ. 2004;13:873–884. doi: 10.1002/hec.866. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien B.J., Spath M., Blackhouse G. A view from the bridge: agreement between the SF 6D utility algorithm and the Health Utilities Index. Health Econ. 2003;12:975–981. doi: 10.1002/hec.789. [DOI] [PubMed] [Google Scholar]
- 5.Browne J., Jamieson L., Lewsey J. Patient Reported Outcome Measures (PROMs) in elective surgery: report to the Department of Health. Health Services Research Unit, London School of Hygiene & Tropical Medicine & Clinical Effectiveness Unit, Royal College of Surgeons of England; London: 2007. [Google Scholar]
- 6.Brazier J., Ratcliffe J., Tsuchiya A. Oxford University Press; New York: 2007. Measuring and valuing health benefits for economic evaluation. [Google Scholar]
- 7.Papaioannou D., Brazier J., Parry G. How valid and responsive are generic health status measures, such as EQ-5D and SF-36, in Schizophrenia?: A systematic review. Value Health. 2011;14:907–920. doi: 10.1016/j.jval.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pickard A.S., Wilke C.T., Lin H.W., Lloyd A. Health utilities using the EQ-5D in studies of cancer. Pharmacoeconomics. 2007;25:365–384. doi: 10.2165/00019053-200725050-00002. [DOI] [PubMed] [Google Scholar]
- 9.Brazier J., Deverill M. A checklist for judging preference-based measures of health related quality of life: learning from psychometrics. Health Econ. 1999;8:41–51. doi: 10.1002/(sici)1099-1050(199902)8:1<41::aid-hec395>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Aspinall P.A., Johnson Z.K., Azuara-Blanco A. Evaluation of quality of life and priorities of patients with glaucoma. Invest Ophthalmol Vis Sci. 2008;49:1907–1915. doi: 10.1167/iovs.07-0559. [DOI] [PubMed] [Google Scholar]
- 11.Kobelt G., Jonsson B., Bergstrom A. Cost-effectiveness analysis in glaucoma: what drives utility?: Results from a pilot study in Sweden. Acta Ophthalmologica Scandinavica. 2006;84:363–371. doi: 10.1111/j.1600-0420.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 12.Mittmann N., Chan D., Trakas K., Risebrough N. Health utility attributes for chronic conditions. Disease Manage Health Outcomes. 2001;9:11–21. [Google Scholar]
- 13.Montemayor F., Sibley L.M., Courtright P., Mikelberg F.S. Contribution of multiple glaucoma medications to visual function and quality of life in patients with glaucoma. Can J Ophthalmol. 2001;36:385–390. doi: 10.1016/s0008-4182(01)80082-x. [DOI] [PubMed] [Google Scholar]
- 14.Thygesen J., Aagren M., Arnavielle S., Walt J.G. Late-stage, primary open-angle glaucoma in Europe: social and health care maintenance costs and quality of life of patients from 4 countries. Curr Med Res Opin. 2008;24:1763–1770. doi: 10.1185/03007990802111068. [DOI] [PubMed] [Google Scholar]
- 15.Cruess A., Zlateva G., Xu X., Rochon S. Burden of illness of neovascular age-related macular degeneration in Canada. Can J Ophthalmol. 2007;42:836–843. doi: 10.3129/i07-153. [DOI] [PubMed] [Google Scholar]
- 16.Espallargues M., Czoski-Murray C.J., Bansback N.J. The impact of age-related macular degeneration on health status utility values. Invest Ophthalmol Vis Sci. 2005;46:4016–4023. doi: 10.1167/iovs.05-0072. [DOI] [PubMed] [Google Scholar]
- 17.Kim J., Kwak H.W., Lee W.K., Kim H.K. Impact of photodynamic therapy on quality of life of patients with age-related macular degeneration in Korea. Jpn J Ophthalmol. 2010;54:325–330. doi: 10.1007/s10384-010-0825-x. [DOI] [PubMed] [Google Scholar]
- 18.Lotery A., Xu X., Zlatava G., Loftus J. Burden of illness, visual impairment and health resource utilisation of patients with neovascular age-related macular degeneration: results from the UK cohort of a five-country cross-sectional study. Br J Ophthalmol. 2007;91:1303–1307. doi: 10.1136/bjo.2007.116939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Payakachat N., Summers K.H., Pleil A.M. Predicting EQ-5D utility scores from the 25-item National Eye Institute Vision Function Questionnaire (NEI-VFQ 25) in patients with age-related macular degeneration. Qual Life Res. 2009;18:801–813. doi: 10.1007/s11136-009-9499-6. [DOI] [PubMed] [Google Scholar]
- 20.Ruiz-Moreno J.M., Coco R.M., Garcia-Arumi J. G. Burden of illness of bilateral neovascular age-related macular degeneration in Spain. Curr Med Res Opin. 2008;24:2103–2111. doi: 10.1185/03007990802214300. [DOI] [PubMed] [Google Scholar]
- 21.Soubrane G., Cruess A., Lotery A. Burden and health care resource utilization in neovascular age-related macular degeneration: findings of a multicountry study. Arch Ophthalmol. 2007;125:1249–1254. doi: 10.1001/archopht.125.9.1249. [DOI] [PubMed] [Google Scholar]
- 22.Asakawa K., Rolfson D., Senthilselvan A. Health Utilities Index Mark 3 showed valid in Alzheimer disease, arthritis, and cataracts. J Clin Epidemiol. 2008;61:733–739. doi: 10.1016/j.jclinepi.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Black N., Browne J., van der Meulen J. Is there overutilisation of cataract surgery in England? Br J Ophthalmol. 2009;93:13–17. doi: 10.1136/bjo.2007.136150. [DOI] [PubMed] [Google Scholar]
- 24.Conner-Spady B.L., Sanmugasunderam S., Courtright P. The prioritization of patients on waiting lists for cataract surgery: validation of the Western Canada waiting list project cataract priority criteria tool. Ophthalmic Epidemiol. 2005;12:81–90. doi: 10.1080/09286580590932770. [DOI] [PubMed] [Google Scholar]
- 25.Datta S., Foss A.J., Grainge M.J. The importance of acuity, stereopsis, and contrast sensitivity for health-related quality of life in elderly women with cataracts. Invest Ophthalmol Vis Sci. 2008;49:1–6. doi: 10.1167/iovs.06-1073. [DOI] [PubMed] [Google Scholar]
- 26.Jayamanne D.G., Allen E.D., Wood C.M., Currie S. Correlation between early, measurable improvement in quality of life and speed of visual rehabilitation after phacoemulsification. J Cataract Refract Surg. 1999;25:1135–1139. doi: 10.1016/s0886-3350(99)00138-8. [DOI] [PubMed] [Google Scholar]
- 27.Polack S., Kuper H., Mathenge W. Cataract visual impairment and quality of life in a Kenyan population. Br J Ophthalmol. 2007;91:927–932. doi: 10.1136/bjo.2006.110973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polack S., Kuper H., Wadud Z. A. Quality of life and visual impairment from cataract in Satkhira district, Bangladesh. Br J Ophthalmol. 2008;92:1026–1030. doi: 10.1136/bjo.2007.134791. [DOI] [PubMed] [Google Scholar]
- 29.Polack S., Eusebio C., Fletcher A. Visual impairment from cataract and health related quality of life: results from a case-control study in the Philippines. Ophthalmic Epidemiol. 2010;17:152–159. doi: 10.3109/09286581003731536. [DOI] [PubMed] [Google Scholar]
- 30.Lloyd A., Nafees B., Gavriel S. Health utility values associated with diabetic retinopathy. Diabet Med. 2008;25:618–624. doi: 10.1111/j.1464-5491.2008.02430.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith D.H., Johnson E.S., Russell A. Lower visual acuity predicts worse utility values among patients with type 2 diabetes. Qual Life Res. 2008;17:1277–1284. doi: 10.1007/s11136-008-9399-1. [DOI] [PubMed] [Google Scholar]
- 32.Pitt A.D., Smith A.F., Lindsell L. Economic and quality-of-life impact of seasonal allergic conjunctivitis in Oxfordshire. Ophthalmic Epidemiol. 2004;11:17–33. doi: 10.1076/opep.11.1.17.26437. [DOI] [PubMed] [Google Scholar]
- 33.Rajagopalan K., Abetz L., Mertzanis P. Comparing the discriminative validity of two generic and one disease-specific health-related quality of life measures in a sample of patients with dry eye. Value Health. 2005;8:168–174. doi: 10.1111/j.1524-4733.2005.03074.x. [DOI] [PubMed] [Google Scholar]
- 34.Smith A.F., Pitt A.D., Rodruiguez A.E. The economic and quality of life impact of seasonal allergic conjunctivitis in a Spanish setting. Ophthalmic Epidemiol. 2005;12:233–242. doi: 10.1080/09286580590967781. [DOI] [PubMed] [Google Scholar]
- 35.Boulton M., Haines L., Smyth D., Fielder A. Health-related quality of life of children with vision impairment or blindness. Dev Med Child Neurol. 2006;48:656–661. doi: 10.1017/S0012162206001381. [DOI] [PubMed] [Google Scholar]
- 36.Clark A., Ng J.Q., Morlet N. Quality of life after postoperative endophthalmitis. Clin Experiment Ophthalmol. 2008;36:526–531. doi: 10.1111/j.1442-9071.2008.01827.x. [DOI] [PubMed] [Google Scholar]
- 37.Kempen J.H., Martin B.K., Wu A.W. The effect of cytomegalovirus retinitis on the quality of life of patients with AIDS in the era of highly active antiretroviral therapy. Ophthalmol. 2003;110:987–995. doi: 10.1016/S0161-6420(03)00089-7. [DOI] [PubMed] [Google Scholar]
- 38.Langelaan M., de Boer M.R., van Nispen R.M. Impact of visual impairment on quality of life: a comparison with quality of life in the general population and with other chronic conditions. Ophthalmic Epidemiol. 2007;14:119–126. doi: 10.1080/09286580601139212. [DOI] [PubMed] [Google Scholar]
- 39.Quinn G.E., Dobson V., Saigal S. Health-related quality of life at age 10 years in very low-birth-weight children with and without threshold retinopathy of prematurity. Archives Ophthalmol. 2004;122:1659–1666. doi: 10.1001/archopht.122.11.1659. [DOI] [PubMed] [Google Scholar]
- 40.van Nispen R.M., de Boer M.R., Hoeijmakers J.G. Co-morbidity and visual acuity are risk factors for health-related quality of life decline: five-month follow-up EQ-5D data of visually impaired older patients. Health Qual Life Outcomes. 2009;7:18. doi: 10.1186/1477-7525-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Longworth L., Longson C. NICE methodology for technology appraisals: cutting edge or tried and trusted? Pharmacoeconomics. 2008;26:729–732. doi: 10.2165/00019053-200826090-00003. [DOI] [PubMed] [Google Scholar]
- 42.Brazier J., Czoski-Murray C., Roberts J. Estimation of a preference-based index from a condition-specific measure: the King's Health Questionnaire. Med Decis Making. 2008;28:113. doi: 10.1177/0272989X07301820. [DOI] [PubMed] [Google Scholar]
- 43.Herdman M., Gudex C., Lloyd A. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011:1–10. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mangione C.M., Lee P.P., Gutierrez P.R. Development of the 25-item national eye institute visual function questionnaire. Archives Ophthalmol. 2001;119:1050. doi: 10.1001/archopht.119.7.1050. [DOI] [PubMed] [Google Scholar]
- 45.Brazier J., Tsuchiya A. Preference-based condition specific measures of health: what happens to cross programme comparability. Health Econ. 2010;19:125–129. doi: 10.1002/hec.1580. [DOI] [PubMed] [Google Scholar]