Abstract
ATP is actively released into the extracellular environment from a variety of cell types in response to mechanical stimuli. This is particularly true in bone where mechanically induced ATP release leads to immediate early gene activation to regulate bone remodelling; however there is no consensus as to which mechanical stimuli stimulate osteoblasts the most. To elucidate which specific type(s) of mechanical stimuli induce ATP release and gene activation in human osteoblasts, we performed an array of experiments using different mechanical stimuli applied to both monolayer and 3D cultures of the same osteoblast cell type, SaOS-2. ATP release from osteoblasts cultured in monolayer significantly increased in response to turbulent fluid flow, laminar fluid flow and substrate strain. No significant change in ATP release could be detected in 3D osteoblast cultures in response to cyclic or static compressive loading of osteoblast-seeded scaffolds, whilst turbulent fluid flow increased ATP release from 3D cultures of osteoblasts to a greater degree than observed in monolayer cultures. Cox-2 expression quantified using real time PCR was significantly lower in cells subjected to turbulent fluid flow whereas c-fos expression was significantly higher in cells subjected to strain. Load-induced signalling via c-fos was further investigated using a SaOS-2 c-fos luciferase reporter cell line and increased in response to substrate strain and turbulent fluid flow in both monolayer and 3D, with no significant change in response to laminar fluid flow or 3D compressive loading. The results of this study demonstrate for the first time strain-induced ATP release from osteoblasts and that turbulent fluid flow in 3D up regulates the signals required for bone remodelling.
Abbreviations: AP-1, Activator protein-1; Cox-2, Cyclooxygenase-2; Egr-2, Early Growth Response 2; FBS, Foetal Bovine Serum; IEG, Immediate early gene; PTH, Parathyroid hormone
Keywords: ATP, Osteoblast, Immediate early gene, Mechanical stimuli, Mechanostat
1. Introduction
Mechanical loading placed upon bone creates substrate deformation and movement of interstitial fluid to engender mechanical stimuli that include substrate strain, compressive loading and fluid flow-induced shear stress (Ehrlich and Lanyon, 2002; Klein-Nulend et al., 2005). These mechanical stimuli are of interest as they may alter bone mineral density via adaptive remodelling (Frost, 1964) mediated by a combination of systemic factors such as parathyroid hormone (PTH) and localised signalling molecules including ATP (Bowler et al., 2001). In vitro studies have demonstrated that ATP release from human HOBIT osteoblastic cells is increased 4 fold in response to medium displacement (Romanello et al., 2001) and 10 fold in murine MC3T3-E1 preosteoblasts in response to laminar fluid flow within a parallel flow chamber (Genetos et al., 2005). ATP release from osteoblasts in response to substrate strain has not previously been investigated despite evidence that it induces increased ATP release from other cell types (Sauer et al., 2000).
Once ATP has been released in response to mechanical stimuli it may act back upon P2 receptors and trigger downstream signalling cascades to activate immediate early genes (IEG) such as c-fos (Wagstaff et al., 2000). The gene products c-Fos and c-Jun form the activator protein-1 (AP-1) complex which binds to the promoter regions of AP-1 recognition elements on genes important for osteogenic responses such as osteocalcin, alkaline phosphatase and collagen type I (Angel and Karin, 1991). This makes c-fos a critical transcriptional mediator in bone, as demonstrated by the phenotype of the c-fos−/− mice (Demiralp et al., 2002).
There have been several studies linking mechanical stimulation and c-fos activation in osteoblasts albeit in different cell types and using different methods of mechanical stimulation: murine MC3T3-E1 pre-osteoblastic cells subjected to laminar fluid flow exhibited increased c-fos mRNA along with increased nuclear immunostaining of c-fos protein (Pavalko et al., 1998), and substrate strain in combination with strain-induced fluid flow increased c-fos mRNA in MC3T3-E1 cells cultured on 3D porous collagen matrices (Tanaka et al., 2005). In addition to c-fos, several other important IEG have been shown to be regulated in osteoblasts by mechanical stimuli: Cyclooxygenase-2 (Cox-2) transcription in MC3T3-E1 cells is increased in response to laminar fluid flow (Wadhwa et al., 2002); Early growth response 2 (Egr-2) and c-jun are up regulated in response to equibiaxial, homogeneous tensile strain in MC3T3-E1 subclone 4 osteoblasts (Ott et al., 2009).
Understanding which types of mechanical loading in human osteoblasts lead to purinergic signalling and the activation of key osteogenic genes is fundamental to bone tissue engineering. Therefore, given the disparity in species, cell types, loading mechanisms and gene products measured in the research on the effects of mechanical loading in osteoblasts, we undertook the current study to determine changes in ATP release and IEG expression in response to turbulent fluid flow, laminar fluid flow, substrate strain and 3D compressive loading in the same cell type, human SaOS-2 osteoblastic cells.
2. Materials and methods
2.1. Cell culture
SaOS-2 cultures were maintained as previously described (Bowler et al., 2001) (see also online supplementary material). Cells were plated at 5×104 per well in 24 well plates, 1×105 per slide coated with poly-l-lysine for laminar fluid flow or tissue culture coated plastic for 4-point bending. Polyurethane scaffolds (Caligen Foam Ltd, Accrington, UK) were seeded with 5×105 in 100 μl cell suspension and incubated for 1 h at 37 °C to ensure cell attachment prior to scaffolds being flooded with medium in 6 well plates (Sittichockechaiwut et al., 2009). Monolayer cultures were maintained for 24 h and 3D cultures were maintained for 7 day prior to loading.
2.2. Mechanical stimulation of cells
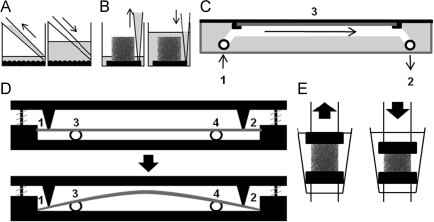
Prior to mechanical stimulation cultures were rinsed and starved in serum free medium overnight to minimise c-fos expression. Medium was replaced 1 h prior to loading to remove exogenous ATP. Eighty per cent of the medium in the wells was displaced at 2.4×10−4 L s−1 between 5 to 20 times in monolayer (Fig. 1A) and 5 times through a polyurethane foam base beneath each scaffold (Fig. 1B) to engender turbulent fluid flow. Laminar fluid flow was applied to cultures grown on poly-l-lysine coated slides inside a parallel flow chamber (Fig. 1C) powered by a peristaltic pump (You et al., 2001; Huesa et al., 2010) such that the resultant shear stress applied was up to 23 dyne/cm2. A custom made 4-point bending rig was used to apply quantifiable substrate strain (1 Hz, peak 3400 μstrain, 10 min) to cultures grown on plastic slides. Although this is not pure substrate strain as it will induce an unquantifiable amount of fluid flow, we will refer to it as substrate strain for brevity from here on in (Fig. 1D) (Armstrong et al., 2007). Static compression was applied to scaffolds placed between the platens of a Bose ElectroForce 3200 biodynamic chamber with an in house cone adaptation to reduce the volume of medium around the scaffold (0–20% compressive strain, single 2 min loading bout, Fig. 1E). Dynamic compressive loading was applied to additional scaffolds in the same apparatus (1 Hz, 5% compressive strain, 2 min loading bouts). For all experiments additional cultures were placed inside loading apparatus without loading to provide basal controls.
Fig. 1.
Mechanical stimulation of SaOS-2 cultures: (A) Turbulent fluid flow was applied in monolayer using a pipette to displace 80% of the medium; (B) Turbulent fluid flow was applied in 3D using a pipette to draw 80% of the medium through the scaffold; (C) Laminar fluid flow was applied to cells using a parallel flow chamber (1: Inlet; 2: Outlet; 3: Parallel plate chamber; inverted slide in grey)(Huesa et al., 2010); (D) Substrate strain was applied inside a custom made four point-bending chamber (1,2: Points on roof of chamber; 3,4: Points on base of chamber; slide in grey); (E) Compression was applied to scaffolds using a Bose ElectroForce 3200 biodynamic chamber.
2.3. ATP quantification
ATP was quantified from heat inactivated medium samples using the HS ViaLight Kit (Lonza, Slough, UK) and normalised to cell number (please refer to the online supplementary material for more details).
2.4. Real time PCR
One hour after loading cells were lysed in Tri reagent and RNA extracted according to the manufacturer's protocol. RNA concentration and purity were quantified using a Nanodrop ND-1000 spectrophotometer (Thermo Scientific). The quality of synthesised cDNA templates was tested using a polymerase chain reaction for the housekeeping gene GAPDH. TaqMan real time PCR (Applied Biosystems by Life Technologies, Warrington, UK) was carried out according to the manufacturer's instructions on an ABI 7900 platform with FAM detector.
2.5. c-fos reporter quantification
SaOS-2 reporter cells were serum starved overnight to minimise c-fos activity prior to loading. At the same time as loading occurred, additional cultures were treated with 10% FBS and 100 ng/ml PTH as a positive control. After loading, cultures were incubated for 5 h to allow accumulation of luciferase protein and treated with 100 μl of lysis buffer. Luciferase activity was measured with ONE-Glo luciferase substrate (Promega). Results were normalised to total protein.
2.6. Statistics
Statistical analysis was performed using Prism 5 (Graphpad) software, for specific tests please refer to online supplementary material.
3. Results
3.1. ATP release in response to mechanical stimulation
3.1.1. Turbulent fluid flow
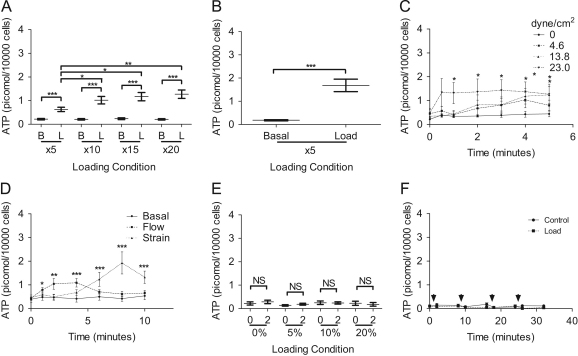
SaOS-2 cells were subjected to increasing amount of turbulent fluid flow in monolayer. ATP release increased 2.93 fold with 5 bouts of loading (basal 0.214 picomol/104 cells, load 0.628 picomol/104 cells, P<0.0001 cf basal), 4.84 fold with 10 bouts (basal 0.209 picomol/104 cells, load 1.020 picomol/104 cells, P<0.0001 cf basal, P<0.05 cf 5 loading bouts), 4.96 fold with 15 bouts (basal 0.236 picomol/104 cells, load 1.170 picomol/104 cells, P<0.0001 cf basal, P<0.05 cf 5 loading bouts) and 6.18 fold with 20 bouts (basal 0.206 picomol/104 cells, load 1.272 picomol/104 cells, P<0.0001 cf basal, P<0.01 cf 5 loading bouts, Fig. 2A). A greater increase in ATP release was observed with cells cultured in 3D as 5 bouts of loading increased ATP release 9.18 fold (basal 0.184 picomol/104 cells, load 1.685 picomol/104 cells, P<0.0001 Fig. 2B).
Fig. 2.
ATP release in response to mechanical stimuli: (A) SaOS-2 cultures were subject to turbulent fluid flow in monolayer; (B) 3D turbulent fluid flow; (C) laminar fluid flow; (D) substrate strain with fluid flow control; (E) 3D static compressive loading (F) 3D repeated bouts of cyclic compressive loading (arrowheads). Medium samples were collected for ATP measurements immediately before (Basal) and after fluid flow (Load) or at the time stated. N=2–6 samples per condition per experiment, 3–4 independent repeat experiments. ⁎P<0.05, ⁎⁎P<0.01, ⁎⁎⁎P<0.001 (t-test, paired t-test, Wilcoxon matched-pairs signed rank test, one-way ANOVA and Kruskal Wallis test).
3.1.2. Laminar fluid flow
We next subjected SaOS-2 cells to laminar fluid flow; no increase in ATP release was detected at flow-induced shear stresses of 4.6 dyne/cm2, but at 13.8 dyne/cm2 ATP release increased 3.44 fold after 4 min (basal 0.344 picomol/104 cells, load 1.184 picomol/104 cells, P<0.05), increasing to 3.6 fold after 5 min (1.239 picomol/104 cells, P<0.01). Laminar fluid flow-induced shear stress at 23 dyne/cm2 increased ATP release 2.88 fold after 1 min (basal 0.467 picomol/104 cells, load 1.343 picomol/104 cells, P<0.05).
3.1.3. Substrate strain
Similarly to laminar fluid flow, significant ATP release from SaOS-2 was only detected after substrate strain was applied over a period of time. Substrate strain at 3400 μStrain increased ATP release 2.70 fold after 6 min (basal 0.438 picomol/104 cells, load 1.181 picomol/104 cells after 6 min, P<0.001), increasing to 4.10 fold after 8 min (1.797 picomol/104 cells, P<0.0001) and decreasing to 2.86 fold after 10 min (1.252 picomol/104 cells, P<0.0001) In contrast, five bouts of turbulent fluid flow applied inside for the substrate strain rig generated an immediate 1.79 fold increase in ATP release (basal 0.439, load 0.789 picomol/104 cells, P<0.05 cf basal, Fig. 2D), 2.38 fold increase after 2 min (1.042 picomol/104 cells, P<0.001) and 2.48 fold after 4 min (1.09 picomol/104 cells, P<0.0001) which was hydrolysed within 10 min (Fig. 2D).
3.1.4. Compressive loading in 3D
Unlike the other forms of loading, 2 min of up to 20% static compressive loading of 3D polyurethane scaffolds seeded with SaOS-2 cells caused no measurable increase in ATP release (Fig. 2E). Similarly, cyclic compressive loading of cell seeded scaffolds at 5% compression (1 Hz, 1 mm deformation per second, repeated 2 min loading bouts) caused no measurable increase in ATP release (Fig. 2F).
3.2. IEG mRNA expression in response to mechanical stimulation
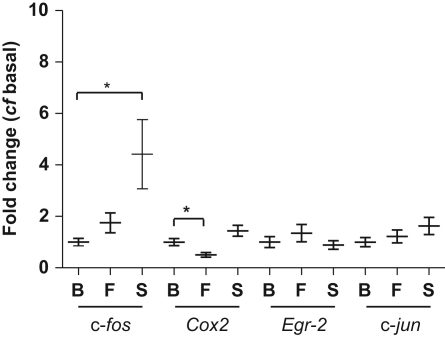
c-fos mRNA measured using real time PCR increased 0.75 fold in response to turbulent fluid flow (P<0.075, Fig. 3), and 3.4 fold in response to substrate strain (P<0.05, Fig. 3). Cox-2 mRNA expression in SaOS-2 cells decreased 0.6 fold in response to turbulent fluid flow (P<0.05, Fig. 3) whilst there was no significant change in Cox-2 mRNA expression in response to substrate strain. Neither turbulent flow nor substrate strain induced any changes in Egr-2 or c-jun mRNA.
Fig. 3.
IEG activation: SaOS-2 cells received basal (B), five bouts of turbulent fluid flow (F) or 10 min of substrate strain (S) prior to mRNA extraction and real time PCR for IEG. ⁎=P<0.05, (Mann Whitney test and Kruskal Wallis test). N=3–4 per condition per experiment, with 3 independent repeat experiments.
3.3. 3 c-fos promoter activation in response to mechanical stimulation
3.3.1. Turbulent fluid flow
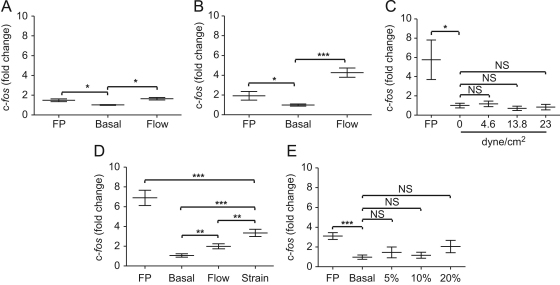
SaOS-2 reporter cells subjected to five bouts of turbulent fluid flow in monolayer gave a small but significant response, elevating luciferase activity by 0.6 fold above basal activity (P<0.05, Fig. 4A). As with ATP release, five bouts of turbulent fluid flow in 3D gave a robust and very significant 3.3 fold increase in luciferase activity (P<0.001, Fig. 4B).
Fig. 4.
c-fos activation in response to mechanical stimuli: SaOS-2 reporter cells were subjected to no loading (Basal), loading or treated with 10% FBS and 100 ng−1ml PTH (FP) as a positive control. Cultures were loaded by (A) turbulent fluid flow in monolayer; (B) 3D turbulent fluid flow; (C) laminar fluid flow; (D) substrate strain with fluid flow control; (E) or static compressive loading. N=2–8 per condition per experiment, 3 independent repeat experiments, ⁎P<0.05, ⁎⁎P<0.01, ⁎⁎⁎P<0.001 (t-test, paired t-test, Wilcoxon matched-pairs signed rank test, one-way ANOVA and Kruskal Wallis test).
3.3.2. Laminar fluid flow
Mechanical loading of SaOS-2 reporter cells in the form of laminar fluid flow-induced shear stress up to 23 dyne/cm2 for 5 min gave no significant increase in luciferase activity above basal levels (Fig. 4C). Treatment of cells cultured on the slides and inside the parallel flow chamber with FBS and PTH gave a somewhat variable, but significant 4.8 fold increase in luciferase activity above basal levels (P<0.05).
3.3.3. Substrate strain
SaOS-2 reporter cells subject to substrate strain together with strain-induced fluid flow increased c-fos promoter activity 2.3 fold (P<0.001, Fig. 4D). When cells in the 4-point bending chamber were subject to fluid flow alone there was, as expected, a slight 1.0 fold increase in luciferase activity (P<0.01, Fig. 4D) but this was significantly lower than the level induced by strain and strain-induced fluid flow (P<0.01, Fig. 4D).
3.3.4. Compressive loading in 3D
As with ATP release, there was no significant increase in luciferase activity in response to up to 20% static compressive loading (Fig. 4E).
4. Discussion
The aims of this study were to investigate ATP release and IEG activation in response to turbulent fluid flow, laminar fluid flow, substrate strain, and 3D compressive loading in the same osteoblastic cell type, SaOS-2 cells (see Table 1 for summary of results). Our results show that with increasing amounts of loading the amount of ATP released also increased, suggesting that extracellular ATP may be an important primitive form of the “mechanostat” (Frost, 2003) in bone by which dose dependent ATP release could influence osteogenesis in vivo. Whilst monolayer cultures of osteoblasts are an extremely well used and extensively characterised model system for osteoblast behaviour, we wanted to mimic the in vivo situation better. Therefore we cultured SaOS-2 cells in a 3D scaffold culture system in which increased proliferation and extracellular matrix deposition occurs after compressive loading (Sittichockechaiwut et al., 2009), consistent with the effects of extracellular ATP (Shimegi, 1996; Nakano et al., 2007). Surprisingly, we could not detect any significant ATP release in 3D in response to up to 20% static compressive loading or 5% cyclic compressive loading despite this type of loading being osteogenic to MLO-A5 osteoblast cells (Sittichockechaiwut et al., 2009). This lack of ATP release may be attributable to specific mechanical properties of the polyurethane scaffolds used. Cells within polyurethane scaffolds will experience very different complex combinations of mechanical stimuli depending upon cell attachment to the scaffold and the direction of the loading force in relation to the individual scaffold struts (Sittichokechaiwut et al., 2010). Whilst we measured a global response, cells can receive substrate tension or compression or indeed neither stimulus. In the case of the cyclic compressive loading a certain amount of unquantifiable oscillatory fluid flow is generated, but clearly this is below the threshold level required to induce ATP release. We did however, detect significant levels of ATP release in response to turbulent fluid flow in 3D. The extent of the increase was more than that seen in monolayer, suggesting that osteoblast-like cells are more responsive to this type of fluid flow in 3D. Therefore the amount of ATP released in response to turbulent fluid flow in bone in vivo would have been underestimated based on previous work using monolayer cultures of osteoblasts in vitro. These findings are in consistent with the effects of fluid flow upon osteoblast cultures in 3D scaffolds which significantly increase the osteogenic response (Bancroft et al., 2002).
Table 1.
Summary of results.
| Loading type | ATP release | Gene expression | Number of samples |
|---|---|---|---|
| A: Turbulent fluid flow in monolayer | Rapid increase | Increased c-fos, decreased Cox-2 | 18 (3 independent experiments, 6 replicates per experiment) |
| B: Turbulent fluid flow in 3D | Large and rapid increase | Large increase in c-fos | 23 (5 independent experiments, 4 to 6 replicates per experiment) |
| C: Laminar fluid flow | Gradual increase | No change in c-fos | 6 (3 independent experiments, 2 replicates per experiment) |
| D: Substrate strain | Gradual increase | Large increase in c-fos | 18 to 24 (4 to 6 independent experiments with 4 replicates per experiment) |
| E: Static compressive loading | No change | No change in c-fos | 8 (4 independent experiments with 2 replicates per experiment) |
| F: Cyclic compressive loading | No change | NA | 8 (3 independent experiments with 2 to 3 replicates per experiment) |
Laminar fluid flow, in contrast to turbulent fluid flow, induced a time dependent “all or nothing” response to loading. There was no ATP release at shear stresses of 4.6 Dyne/cm2, but at 13.8 and 23 Dyne/cm2 there was significantly increased ATP release between 3 and 5 min of loading which peaked at 1.239 and 1.344 picomol/104 cells, equivalent to a concentration of 54 to 56 nM ATP for both levels of stimuli. The amount of ATP released in response to laminar fluid flow in this study is very similar to a previous study where 59.8 nM ATP was released from MC3T3-E1 osteoblasts subjected to laminar fluid flow-induced shear stresses of 12 dyne/cm2 for 10 min (Genetos et al., 2005). The fact that there is no more ATP released from SaOS-2 cells at 23 dyne/cm2 than at 13.8 dyne/cm2 and that approximately the same amount of ATP is released from MC3T3-E1 cells in response to 12 dyne/cm2 suggests that osteoblasts have a maximum response to laminar fluid flow, suggesting that shear stress-induced ATP release is part of the mechanostat regulation of bone remodelling in response to loading. Turbulent fluid flow engendered rapid ATP release whereas laminar fluid flow engenders gradual ATP release from SaOS-2 cells. This suggests that SaOS-2 cells can detect the differences between multidirectional and uniaxial fluid movement. Previously, immortalised human foetal osteoblast cells subjected to laminar fluid flow demonstrated increased intracellular Ca2+ ion concentration, however oscillating fluid flow had a less potent effect than either steady or pulsing flow (Jacobs et al., 1998). This is in contrast to the effects of these kinds of fluid movements upon ATP release and although calcium signalling often correlates to ATP release the two may be mutually exclusive. MC3T3-E1 osteoblasts cultured in ceramic scaffolds proliferated uniformly in response to oscillatory but not unidirectional flow (Du et al., 2009), which taken together with the results of this study further suggest that osteoblasts respond differently to different types of flow.
Similarly to laminar fluid flow, our substrate strain model (which will also induced unquantifiable amounts of fluid flow) induced ATP release from SaOS-2 cells which was time dependent. Substrate strain at 3400 μStrain increased ATP release 4.1 fold after 8 min. This suggests that substrate strain, in combination with fluid flow, is important in inducing ATP release from osteoblasts in bone that is subjected to longer periods of loading. In contrast, five bouts of turbulent fluid flow inside the rig without any substrate strain applied generated an immediate 1.79 fold increase which then became hydrolysed within 10 min. In an in vivo system this could be useful for maintaining localised autocrine/paracrine signalling at discrete foci (Bowler et al., 2001). The transient nature of this signal emphasises the need for longer periods of oscillating fluid flow to generate osteogenic responses throughout 3D scaffolds for tissue engineering (Du et al., 2009).
In order to determine which type of mechanical loading would lead to activation of downstream signalling cascades to activate immediate early genes we performed real time PCR on cells subjected to turbulent fluid flow and substrate strain. c-fos mRNA increased 3.4 fold in response to substrate strain, but in contrast to a previous study using MC3T3-E1 cells (Wadhwa et al., 2002), Cox-2 mRNA expression in SaOS-2 cells decreased 0.6 fold in response to turbulent fluid flow. Previous studies have shown that over expression of Cox-2 caused decreased proliferation and increased apoptosis (Xu et al., 2006), so perhaps a decrease in Cox-2 expression could represent the early stages of increased SaOS-2 cell proliferation and survival. The lack of any change in Cox-2 in response to substrate strain suggests that this is a turbulent fluid flow mediated response and not necessarily ATP dependent as substrate strain also induced ATP release. Surprisingly there were no changes in Egr-2 or c-jun; this might be explained by the species difference of the cells used as changes in both these genes previously reported were from a mouse derived cell line (You et al., 2001; Ott et al., 2009) unlike human derived SaOS-2 cells (Rodan et al., 1987) used in this study.
To determine if the increased c-fos mRNA was due to transcriptional activation rather than stabilisation of the mRNA, we measured c-fos promoter activation using a c-fos luciferase report SaOS-2 cell line. Although turbulent fluid flow did not significantly increase c-fos mRNA, it did increase c-fos promoter activation both in monolayer, and as with ATP release, to a more significant level in 3D. This suggests that SaOS-2 cells are more responsive to turbulent fluid flow in a 3D scaffold that better replicates the bone microenvironment.
The lack of c-fos activation in SaOS-2 cells subjected to laminar fluid flow-induced shear stresses of up to 23 dyne/cm2 for 5 min coupled with a previous study that demonstrated increased c-fos activation in osteoblasts in response to laminar fluid flow-induced shear stress at 12 dyne/cm2 applied for 60 min (Pavalko et al., 1998) would suggest that c-fos transcriptional activation in response to laminar fluid flow requires prolonged flow application.
Substrate strain together with strain-induced fluid flow activated the c-fos promoter more than fluid flow alone. This suggests that the c-fos promoter is sensitive to multiple types of mechanical stimuli and where there is more than one type of loading present there is a cumulative effect upon c-fos promoter activation. This is similar to a previous study in MC3T3-E1 cells which demonstrated the combined effects of substrate strain in combination and fluid flow had a greater effect on c-fos activation than substrate strain alone (Tanaka et al., 2005). There were no significant changes in ATP release or c-fos promoter activation in SaOS-2 cells in response to static compression (Figs.2E and 4E), suggesting that this type of loading in SaOS-2 osteoblasts does not induce the immediate release of ATP nor activation of IEGs. Taken together these findings suggest that scaffolding materials in tissue engineering need to be porous enough to allow fluid flow and rigid enough to conduct strain to provide the mechanical stimuli necessary to stimulate the signals associated with bone formation.
This is the first study to record the effects of turbulent fluid flow, laminar fluid flow, substrate strain and compressive loading upon one cell type. The results demonstrate for the first time ATP release from osteoblasts in response to substrate strain in monolayer and turbulent fluid flow in 3D. The increased response to turbulent fluid flow in 3D demonstrates enhanced sensitivity of SaOS-2 cells to flow in the 3D environment. In contrast, the lack of response to loading via compression of the scaffold in which they are contained in suggests this type of substrate strain does not play a role in mechanically induced signalling mechanisms in the short term. Overall, the findings of this study demonstrate the concentration of ATP released in response to mechanical loading varied in a time, direction and strain dependent manner that may represent a local mechanostat in bone that may influence osteogenesis.
Conflict of interest statement
None declared.
Acknowledgements
We would like to thank Arthritis Research UK [Grant no. 17538] and Bone Research Society for funding; Carmen Huesa for the design for the ALIFFC and Professor Jim Gallagher for its construction; Profs. Price and Lanyon for hosting RR whilst using the substrate strain apparatus. This study was supported in part by the European Commission under the 7th Framework Programme (proposal #202231) performed as a collaborative project among the members of the ATPBone Consortium (Copenhagen University, University College London, University of Maastricht, University of Ferrara, University of Liverpool, University of Sheffield (AG), and Université Libre de Bruxelles), and is a sub study under the main study “Fighting osteoporosis by blocking nucleotides: purinergic signalling in bone formation and homoeostasis”.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jbiomech.2011.11.036.
Appendix A. Supplementary materials
Supplementary materials
References
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochimica et Biophysica Acta. 1991;1072(2–3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Armstrong V.J., Muzylak M. Wnt/beta-catenin signaling is a component of osteoblastic bone cell early responses to load-bearing and requires estrogen receptor alpha. J Biological Chemistry. 2007;282(28):20715–20727. doi: 10.1074/jbc.M703224200. [DOI] [PubMed] [Google Scholar]
- Bancroft G.N., Sikavitsas V.I. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proceedings of the National Academy of Sciences U S A. 2002;99(20):12600–12605. doi: 10.1073/pnas.202296599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler W.B., Buckley K.A. Extracellular nucleotide signaling: a mechanism for integrating local and systemic responses in the activation of bone remodeling. Bone. 2001;28(5):507–512. doi: 10.1016/s8756-3282(01)00430-6. [DOI] [PubMed] [Google Scholar]
- Demiralp B., Chen H.L. Anabolic actions of parathyroid hormone during bone growth are dependent on c-fos. Endocrinology. 2002;143(10):4038–4047. doi: 10.1210/en.2002-220221. [DOI] [PubMed] [Google Scholar]
- Du D., Furukawa K.S. 3D culture of osteoblast-like cells by unidirectional or oscillatory flow for bone tissue engineering. Biotechnology and Bioengineering. 2009;102(6):1670–1678. doi: 10.1002/bit.22214. [DOI] [PubMed] [Google Scholar]
- Ehrlich P.J., Lanyon L.E. Mechanical strain and bone cell function: a review. Osteoporos International. 2002;13(9):688–700. doi: 10.1007/s001980200095. [DOI] [PubMed] [Google Scholar]
- Frost H. Dynamics of bone remodelling. In: Frost H., editor. Bone Biodynamics. Little, Brown and Co; Boston: 1964. pp. 315–333. [Google Scholar]
- Frost H.M. Bone's mechanostat: a 2003 update. Anatomy Recap A Discovery of Molecular, Cellular and Evolutionary Biology. 2003;275(2):1081–1101. doi: 10.1002/ar.a.10119. [DOI] [PubMed] [Google Scholar]
- Genetos D.C., Geist D.J. Fluid shear-induced ATP secretion mediates prostaglandin release in MC3T3-E1 osteoblasts. Journal of Bone and Mineral Research. 2005;20(1):41–49. doi: 10.1359/JBMR.041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huesa C., Helfrich M.H. Parallel-plate fluid flow systems for bone cell stimulation. Journal of Biomechanics. 2010;43(6):1182–1189. doi: 10.1016/j.jbiomech.2009.11.029. [DOI] [PubMed] [Google Scholar]
- Jacobs C.R., Yellowley C.E. Differential effect of steady versus oscillating flow on bone cells. Journal of Biomechanics. 1998;31(11):969–976. doi: 10.1016/s0021-9290(98)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Nulend J., Bacabac R.G. Mechanobiology of bone tissue. Pathological Biology (Paris) 2005;53(10):576–580. doi: 10.1016/j.patbio.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Nakano Y., Addison W.N. ATP-mediated mineralization of MC3T3-E1 osteoblast cultures. Bone. 2007;41(4):549–561. doi: 10.1016/j.bone.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Ott C.E., Bauer S. Promiscuous and depolarization-induced immediate-early response genes are induced by mechanical strain of osteoblasts. Journal of Bone and Mineral Research. 2009;24(7):1247–1262. doi: 10.1359/jbmr.090206. [DOI] [PubMed] [Google Scholar]
- Pavalko F.M., Chen N.X. Fluid shear-induced mechanical signaling in MC3T3-E1 osteoblasts requires cytoskeleton-integrin interactions. American Journal of Physiology. 1998;275(6 Pt 1):C1591–1601. [PubMed] [Google Scholar]
- Rodan S.B., Imai Y. Characterization of a human osteosarcoma cell line (Saos-2) with osteoblastic properties. Cancer Research. 1987;47(18):4961–4966. [PubMed] [Google Scholar]
- Romanello M., Pani B. Mechanically induced ATP release from human osteoblastic cells. Biochemical and Biophysical Research Communications. 2001;289(5):1275–1281. doi: 10.1006/bbrc.2001.6124. [DOI] [PubMed] [Google Scholar]
- Sauer H., Hescheler J. Mechanical strain-induced Ca(2+) waves are propagated via ATP release and purinergic receptor activation. American Journal of Physiology: Cell Physiology. 2000;279(2):C295–307. doi: 10.1152/ajpcell.2000.279.2.C295. [DOI] [PubMed] [Google Scholar]
- Shimegi S. ATP and adenosine act as a mitogen for osteoblast-like cells (MC3T3-E1) Calcified Tissue International. 1996;58(2):109–113. doi: 10.1007/BF02529732. [DOI] [PubMed] [Google Scholar]
- Sittichockechaiwut A., Scutt A.M. Use of rapidly mineralising osteoblasts and short periods of mechanical loading to accelerate matrix maturation in 3D scaffolds. Bone. 2009;44(5):822–829. doi: 10.1016/j.bone.2008.12.027. [DOI] [PubMed] [Google Scholar]
- Sittichokechaiwut A., Edwards J.H. Short bouts of mechanical loading are as effective as dexamethasone at inducing matrix production by human bone marrow mesenchymal stem cell. European Cell Materials. 2010;20:45–57. doi: 10.22203/ecm.v020a05. [DOI] [PubMed] [Google Scholar]
- Tanaka S.M., Sun H.B. Osteoblast responses one hour after load-induced fluid flow in a three-dimensional porous matrix. Calcified Tissue International. 2005;76(4):261–271. doi: 10.1007/s00223-004-0238-2. [DOI] [PubMed] [Google Scholar]
- Wadhwa S., Choudhary S. Fluid flow induces COX-2 expression in MC3T3-E1 osteoblasts via a PKA signaling pathway. Biochemical and Biophysical Research Communications. 2002;297(1):46–51. doi: 10.1016/s0006-291x(02)02124-1. [DOI] [PubMed] [Google Scholar]
- Wagstaff S.C., Bowler W.B. Extracellular ATP activates multiple signalling pathways and potentiates growth factor-induced c-fos gene expression in MCF-7 breast cancer cells. Carcinogenesis. 2000;21(12):2175–2181. doi: 10.1093/carcin/21.12.2175. [DOI] [PubMed] [Google Scholar]
- Xu Z., Choudhary S. Overexpression of COX-2 in human osteosarcoma cells decreases proliferation and increases apoptosis. Cancer Research. 2006;66(13):6657–6664. doi: 10.1158/0008-5472.CAN-05-3624. [DOI] [PubMed] [Google Scholar]
- You J., Reilly G.C. Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. Journal of Biological Chemistry. 2001;276(16):13365–13371. doi: 10.1074/jbc.M009846200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials