Abstract
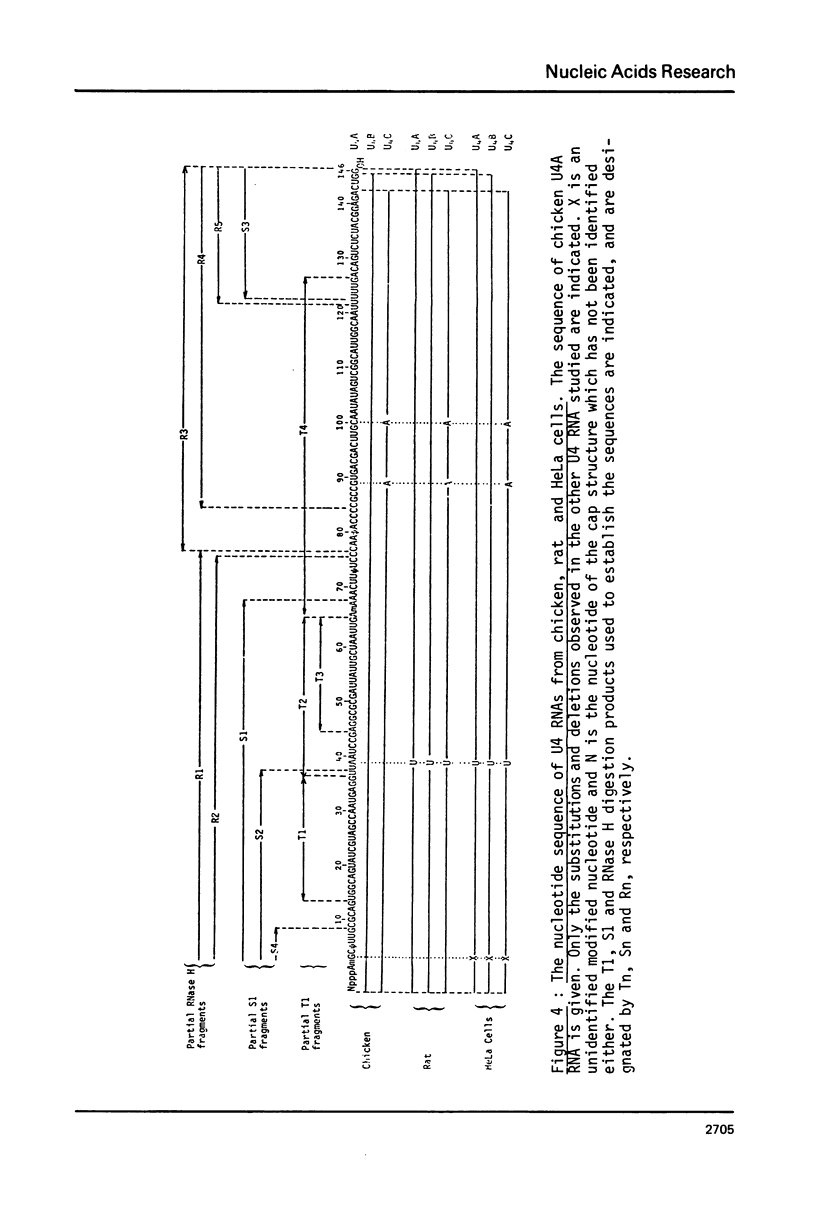
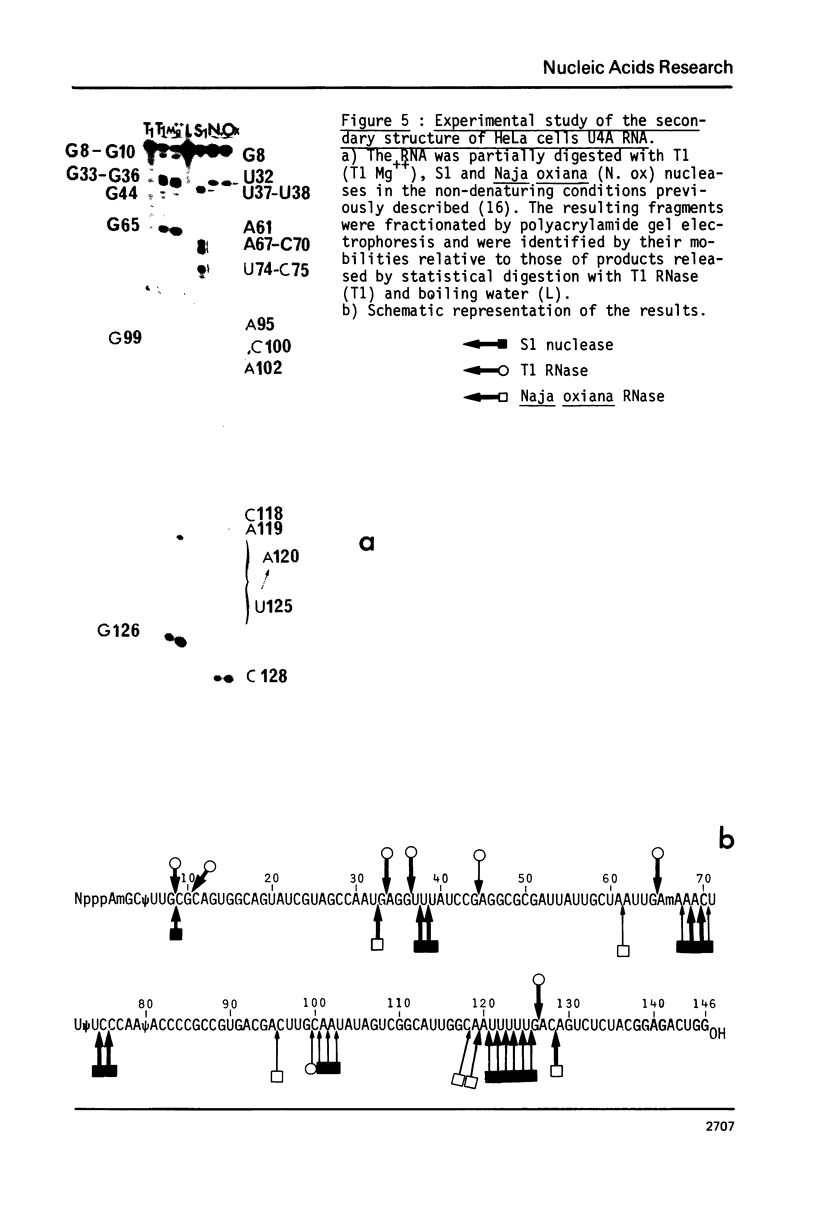
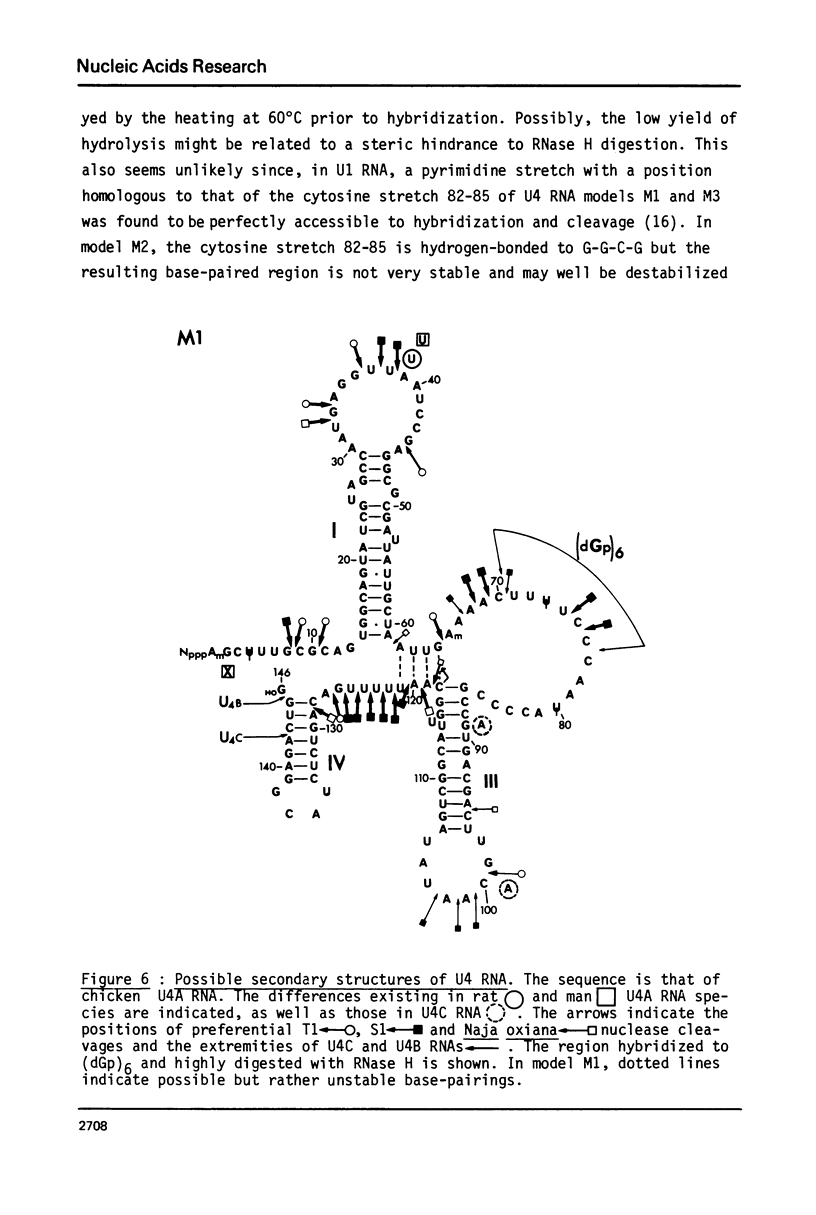
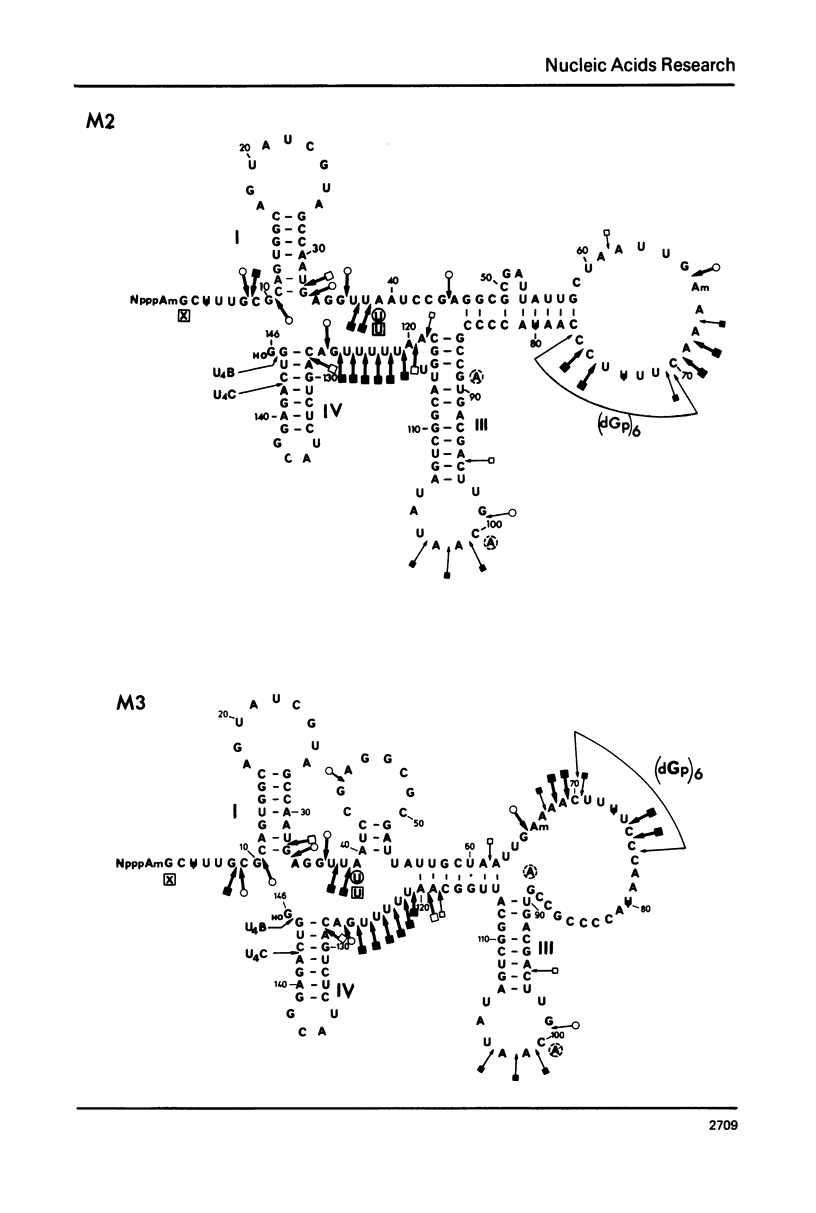
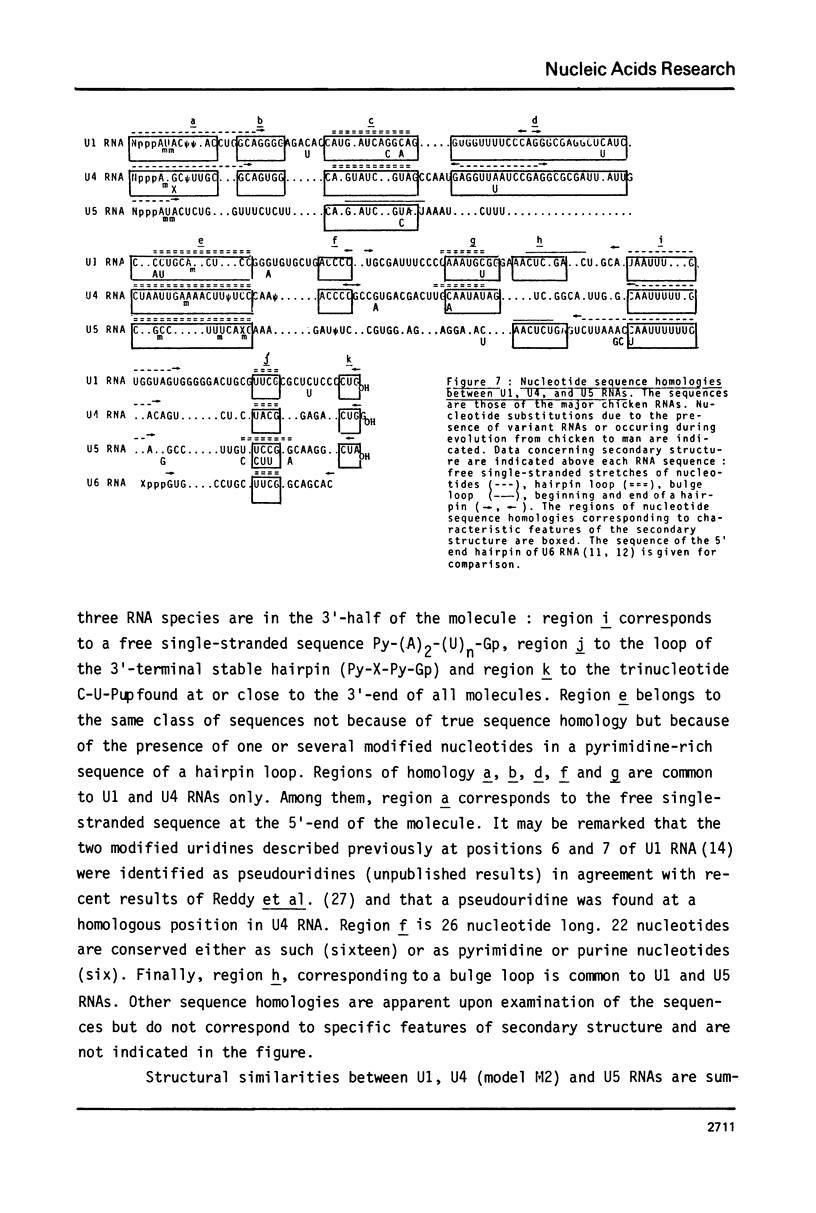
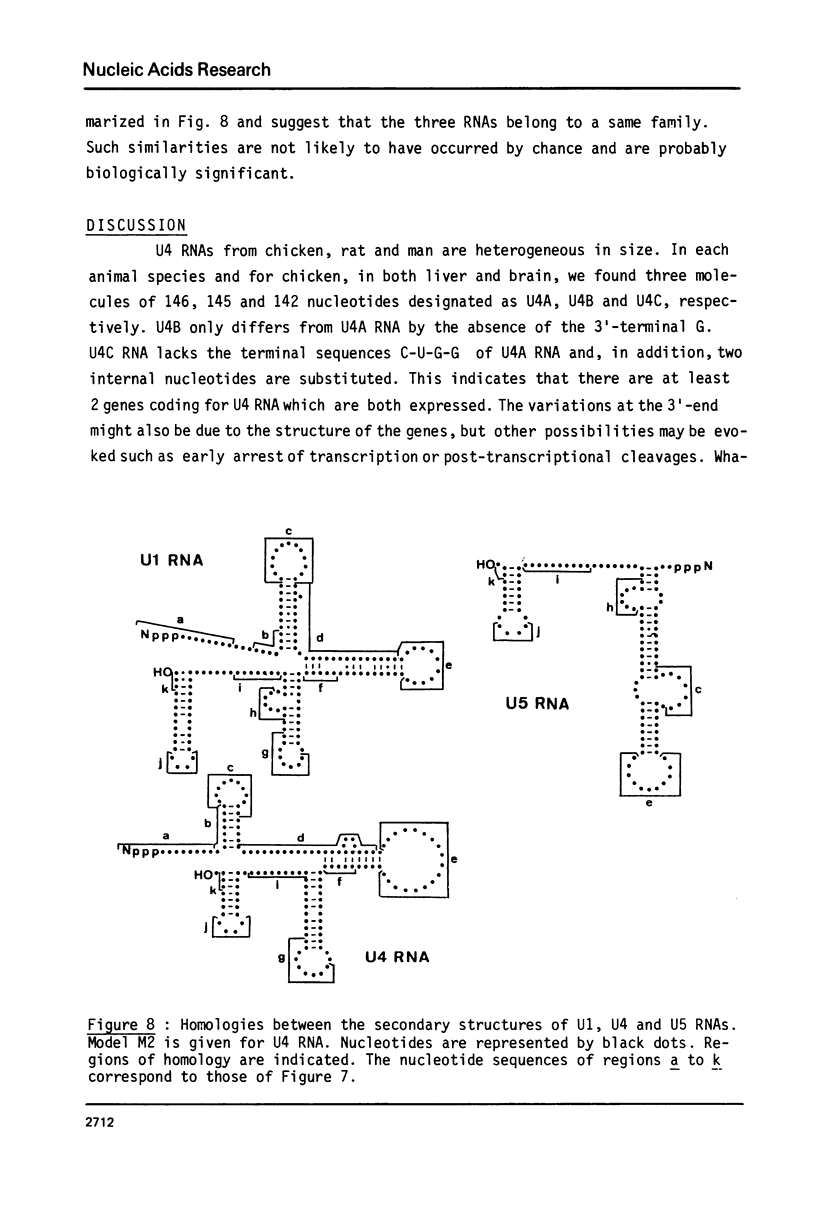
U4 RNA from chicken, rat and man was examined for nucleotide sequence and secondary structure. Three molecular species, U4A, U4B and U4C were detected in the three animal species. U4A is 146 nucleotide long and U4B RNA only lacks the 3' terminal G. four nucleotides are missing at the 3'-end of U4C RNA which, in addition, differs from U4A and U4B RNAs at two internal positions. Thus, U4C RNA is encoded by another gene as U4A and U4B RNAs. Only one nucleotide substitution occurred between chicken and man showing that U4A, U4B and U4C RNAs have been extremely conserved throughout evolution. The three molecular species are capped, they contain three psi, a 2'-P methyl A and a m6A. An additional post-transcriptional modification close to the cap structure is observed in man. On the basis on an experimental study, two models of secondary structure may be proposed for U4 RNA. The 3'domain is the same in both models and is homologous to that of U1 and U5 RNAs. It consists of a single-stranded region, containing the sequence Py-(A)2-(U)n-Gp flanked by two stable hairpins probably involved in tertiary interactions. The 5' domain is less stable than the 3' domain and its structure is different in the two models. However, a long single-stranded pyrimidine region containing modified nucleotides is found in both models as in U1 and U5 RNAs. Several other nucleotide sequence homologies related to specific features of secondary structure suggest that U1, U4 and U5 RNAs derive from a common ancestor and may have common function.
Full text
PDF



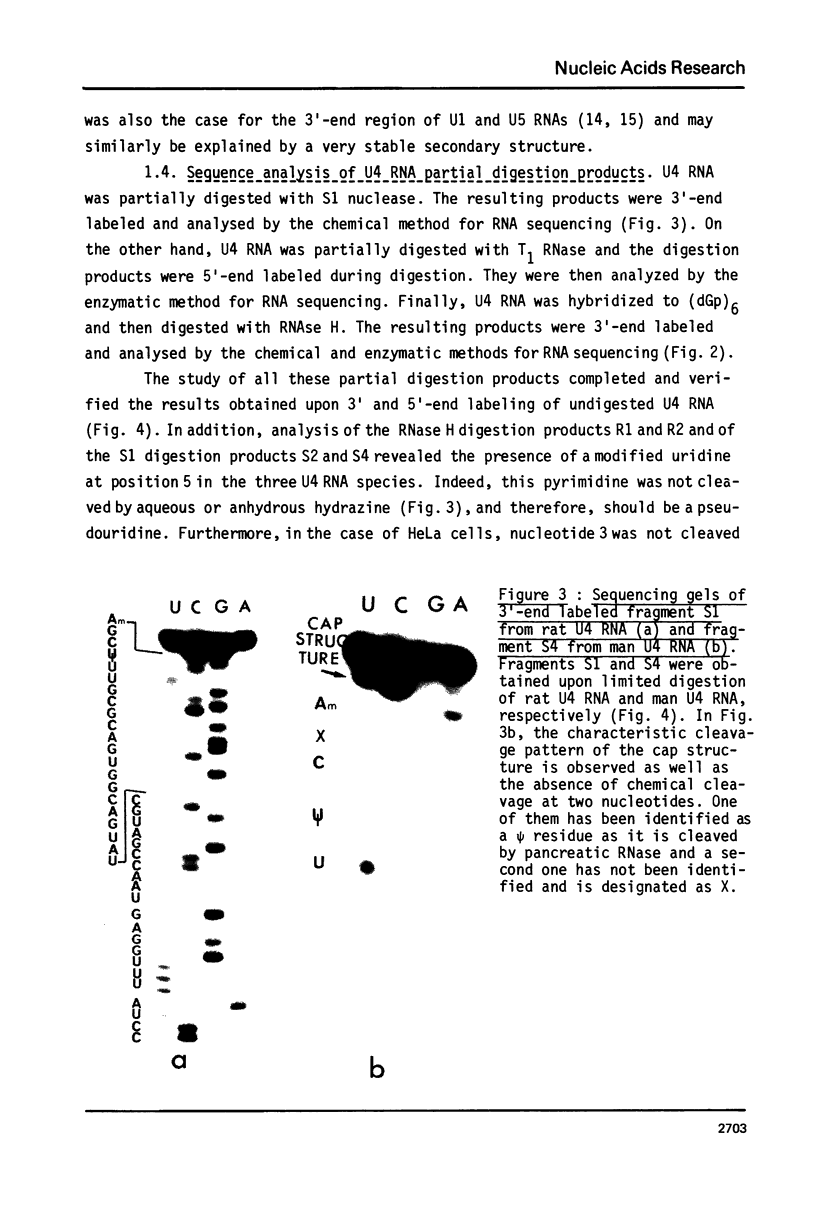







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branlant C., Krol A., Ebel J. P., Gallinaro H., Lazar E., Jacob M. The conformation of chicken, rat and human U1A RNAs in solution. Nucleic Acids Res. 1981 Feb 25;9(4):841–858. doi: 10.1093/nar/9.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol A., Ebel J. P., Lazar E., Gallinaro H., Jacob M., Sri-Widada J., Jeanteur P. Nucleotide sequences of nuclear U1A RNAs from chicken, rat and man. Nucleic Acids Res. 1980 Sep 25;8(18):4143–4154. doi: 10.1093/nar/8.18.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol A., Machatt A., Ebel J. P. The secondary structure of the protein L1 binding region of ribosomal 23S RNA. Homologies with putative secondary structures of the L11 mRNA and of a region of mitochondrial 16S rRNA. Nucleic Acids Res. 1981 Jan 24;9(2):293–307. doi: 10.1093/nar/9.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C., Krol A., Machatt M. A., Ebel J. P. Structural study of ribosomal 23 S RNA from Escherichia coli. FEBS Lett. 1979 Nov 1;107(1):177–181. doi: 10.1016/0014-5793(79)80490-1. [DOI] [PubMed] [Google Scholar]
- Brunel C., Widada J. S., Lelay M. N., Jeanteur P., Liautard J. P. Purification and characterization of a simple ribonucleoprotein particle containing small nucleoplasmic RNAs (snRNP) as a subset of RNP containing heterogenous nuclear RNA (hnRNP) from HeLa cells. Nucleic Acids Res. 1981 Feb 25;9(4):815–830. doi: 10.1093/nar/9.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deimel B., Louis C. H., Sekeris C. E. The presence of small molecular weight RNAs in nuclear ribonucleoprotein particles carrying HnRNA. FEBS Lett. 1977 Jan 15;73(1):80–84. [PubMed] [Google Scholar]
- Donis-Keller H. Site specific enzymatic cleavage of RNA. Nucleic Acids Res. 1979 Sep 11;7(1):179–192. doi: 10.1093/nar/7.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein P., Reddy R., Henning D., Busch H. The nucleotide sequence of nuclear U6 (4.7 S) RNA. J Biol Chem. 1980 Sep 25;255(18):8901–8906. [PubMed] [Google Scholar]
- Gallinaro H., Jacob M. An evaluation of small nuclear RNA in hnRNP. FEBS Lett. 1979 Aug 1;104(1):176–182. doi: 10.1016/0014-5793(79)81110-2. [DOI] [PubMed] [Google Scholar]
- Gattoni R., Stevenin J., Jacob M. Comparison of the nuclear ribonucleoproteins containing the transcripts of adenovirus-2 and HeLa cell dna. Eur J Biochem. 1980;108(1):203–211. doi: 10.1111/j.1432-1033.1980.tb04713.x. [DOI] [PubMed] [Google Scholar]
- Guimont-Ducamp C., Sri-Widada J., Jeanteur P. Occurrence of small molecular weight RNAs in Hela nuclear ribonucleoprotein particles containing HnRNA. Biochimie. 1977;59(8-9):755–758. doi: 10.1016/s0300-9084(77)80259-9. [DOI] [PubMed] [Google Scholar]
- Harada F., Kato N., Nishimura S. The nucleotide sequence of nuclear 4.8S RNA of mouse cells. Biochem Biophys Res Commun. 1980 Aug 14;95(3):1332–1340. doi: 10.1016/0006-291x(80)91620-4. [DOI] [PubMed] [Google Scholar]
- Harada F., Kato N. Nucleotide sequences of 4.5S RNAs associated with poly(A)-containing RNAs of mouse and hamster cells. Nucleic Acids Res. 1980 Mar 25;8(6):1273–1285. doi: 10.1093/nar/8.6.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Hardin J. A., Steitz J. A. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science. 1981 Jan 23;211(4480):400–402. doi: 10.1126/science.6164096. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Nomura M., Yates J. L., Dean D., Post L. E. Feedback regulation of ribosomal protein gene expression in Escherichia coli: structural homology of ribosomal RNA and ribosomal protein MRNA. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7084–7088. doi: 10.1073/pnas.77.12.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northemann W., Scheurlen M., Gross V., Heinrich P. C. Circular dichroism of ribonucleoprotein complexes from rat liver nuclei. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1130–1137. doi: 10.1016/0006-291x(77)90973-1. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R., Henning D., Busch H. Nucleotide sequence of nucleolar U3B RNA. J Biol Chem. 1979 Nov 10;254(21):11097–11105. [PubMed] [Google Scholar]
- Reddy R., Henning D., Busch H. Pseudouridine residues in the 5'-terminus of uridine-rich nuclear RNA I (U1 RNA). Biochem Biophys Res Commun. 1981 Feb 27;98(4):1076–1083. doi: 10.1016/0006-291x(81)91221-3. [DOI] [PubMed] [Google Scholar]
- Reddy R., Henning D., Busch H. Substitutions, insertions, and deletions in two highly conserved U3 RNA species. J Biol Chem. 1980 Jul 25;255(14):7029–7033. [PubMed] [Google Scholar]
- Rogers J., Wall R. A mechanism for RNA splicing. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1877–1879. doi: 10.1073/pnas.77.4.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Stevenin J., Gallinaro-Matringe H., Gattoni R., Jacob M. Complexity of the structure of particles containing heterogeneous nuclear RNA as demonstrated by ribonuclease treatment. Eur J Biochem. 1977 Apr 15;74(3):589–602. doi: 10.1111/j.1432-1033.1977.tb11428.x. [DOI] [PubMed] [Google Scholar]