Abstract
Objective
To test the hypotheses that obesity due to a very high fat diet induces knee osteoarthritis, and that short-term wheel running exercise protects against obesity-induced knee osteoarthritis by reducing systemic inflammation and metabolic dysregulation.
Methods
Male C57BL/6J mice were fed either a control (13.5% kcal fat) or very high fat diet (60% kcal fat) from 12–24 wks of age. From 20–24 wks, half of the animals were housed with running wheels. Knee osteoarthritis severity was determined via histopathology, and serum cytokines were measured using a multiplex bead immunoassay and ELISAs. Body composition was quantified by dual-energy X-ray absorptiometry, and insulin resistance was assessed by glucose tolerance testing.
Results
A very high fat diet increased osteoarthritis scores and serum leptin, adiponectin, KC (mouse analog of IL-8), MIG (monokine induced by interferon-gamma, or CXCL9), and interleukin 1 receptor antagonist (IL-1Ra) levels in proportion to percent body fat, which increased 3-fold compared to controls. Wheel running reduced osteoarthritis progression in the medial femur of obese mice. Exercise disrupted the clustering of cytokine expression and improved glucose tolerance without reducing body fat or cytokine levels.
Conclusion
Obesity induced by a very high-fat diet causes osteoarthritis and systemic inflammation in proportion to body fat. Increased joint loading is not sufficient to explain the increased incidence of knee osteoarthritis with obesity as wheel running is protective rather than damaging. Exercise improves glucose tolerance and disrupts the co-expression of pro-inflammatory cytokines, suggesting that increased aerobic exercise may act independent of weight loss in promoting joint health.
Keywords: obesity, exercise, knee joint, inflammation, body fat, cartilage, proteoglycan
Introduction
Osteoarthritis (OA) is a progressive degenerative joint disease characterized by joint pain, impaired mobility, and degenerative changes in joint tissues such as the articular cartilage. It is the most common form of arthritis (1), and one of the manifestations of this disease is a reduction in physical activity levels. People with self-reported arthritis are less likely to achieve the recommended levels of moderate and vigorous activity, presumably due to joint pain associated with more intense, weight-bearing activities. People with arthritis are also more likely to be active for shorter durations and to have increased periods of inactivity compared to non-arthritic peers (2, 3).
Obesity is one of the most clinically important contexts for studying the interaction between OA and physical activity. Obesity is a significant risk factor for OA at sites throughout the body, especially the knee (4–6). However, the increased incidence of OA in non-weightbearing joints in the hand (7) suggest that systemic factors associated with obesity, such as pro-inflammatory mediators or adipokines, contribute to the onset of OA. Obesity also increases the risk of chronic diseases such as diabetes and heart disease, and recent studies indicate that arthritis-associated reductions in physical activity may underlie these co-morbidities. Adults with diabetes or heart disease who were also diagnosed with arthritis were 30% more likely to be physically inactive when adjusted for age, sex, and body mass index (BMI) (8, 9). Thus, arthritis may hinder the self-management of diabetes and heart disease by impairing physical activity levels.
Despite evidence indicating that arthritis reduces physical activity levels, staying physically active, including participating in strength training and aerobic exercise, is one of several self-management recommendations for reducing arthritis pain and improving function (10, 11). A potential concern of this recommendation, however, is that increased joint loading may exacerbate joint damage. This concern is heightened for obese individuals whose risk of knee OA is mediated, in part, by changes in knee joint alignment that contribute to changes in the magnitude and distribution of mechanical loads throughout the joint (4, 12). Thus, in obese individuals, further increases in joint loading with increased physical activity may accelerate joint degeneration.
Current evidence on how increased physical activity affects the risk of developing OA in people that are obese is inconclusive (13). Two studies indicate that increased physical activity in obese individuals accelerates the development of knee OA (14, 15). However, recent larger-scale longitudinal studies are not consistent with these findings. In a prospective community-based study, Felson and colleagues reported that increased recreational physical activity did not alter the risk for radiographic or symptomatic knee OA in overweight or obese individuals (16). Hootman et al. (2003) also reported that BMI did not alter the relationship between moderate physical activity and risk for self-reported physician diagnosed knee OA in men or women (2). Thus, moderate levels of recreational physical activity do not appear to increase or decrease the risk of developing incident knee OA in overweight or obese individuals. Furthermore, Messier and colleagues showed that combining diet and exercise treatment for 18 months in older overweight and obese individuals with knee OA significantly improved physical function, pain, and mobility without altering joint space width (17).
Exercise may impart protective effects on joint pain and function via effects on systemic inflammatory mediators. Pro-inflammatory cytokines are elevated with obesity and physical inactivity and may provide a non-biomechanical mechanism by which obesity increases the risk of developing OA (5, 18). A number of adipose tissue derived cytokines, termed adipokines, are associated with knee OA in humans and have been shown in animal models and in vitro studies to stimulate pro-inflammatory signaling cascades, matrix metalloprotease expression, and neurogenic inflammation [reviewed by (6, 19, 20)]. In humans, increased levels of physical activity are generally associated with reduced concentrations of circulating pro-inflammatory mediators, such as C-reactive protein and tumor necrosis factor-alpha (TNF-α) (21). Interleukin 6 (IL-6), which is pro-inflammatory under certain conditions, is released at high levels from exercising muscle and has been shown to exert anti-inflammatory effects on TNF-α and endotoxin-stimulated inflammation (22). Although it is unclear if exercise training reduces adipokine gene expression in obese humans, both forced (i.e., treadmill) and voluntary (i.e., wheel) exercise training in diet-induced obese mice reduced visceral fat adipokine gene expression (23, 24).
The purpose of the present study was to examine the effect of a very high-fat diet on the development of knee OA in exercised or sedentary mice. In previous studies, we showed that diet-induced obesity increased knee OA in proportion to body fat gain (25), whereas chow fed leptin-impaired mice showed significant increases in adiposity without increased OA (26). Our previous study, as well as others (27), induced knee OA by feeding mice diets containing up to 45% of kcals from fat [reviewed in (28)]. In the current study, we attempted to stimulate higher levels of systemic inflammation and knee OA by evaluating the effect of a very high-fat diet (i.e., 60% kcal from fat). Although this level of fat content is not common in human diets, it is typical of some low-carbohydrate (e.g., “Atkins”) diets (29). Our primary outcome was site-specific changes in knee joint OA. Our secondary analyses evaluated the effect of diet and activity on body composition, glucose tolerance, and serum pro- and anti-inflammatory cytokine levels. Finally, we conducted multivariate correlation analyses to evaluate the effects of body fat, running activity, and knee OA on systemic inflammatory markers.
Materials and Methods
Animals
Twenty 8 wk old male C57BL/6J mice were housed in the Duke University Vivarium with temperature-controlled quarters (20–22°C), a 12:12-h light-dark cycle, and ad libitum access to water and control chow (Purina Rodent Chow 5001; 13.5% kcal fat). At 12 wks of age, half of the mice (N=10) were placed on a high-fat diet with 60% of kcals from fat (D12492; Research Diets, New Brunswick, NJ). Animals were housed in individual-cage housing (13×13×30 cm) with a locked running wheel (11 cm diameter). From 20–24 wks of age, running wheels were unlocked for half of the control and high-fat fed animals, thereby allowing for voluntary wheel-running activity (N=5 per diet). Total daily running distance was calculated as previously described (30). Intraperitoneal glucose tolerance tests were performed 48 h after the last bout of voluntary running in mice that were fasted overnight (31). Just prior to euthanasia, lean body mass and body fat content were measured under anesthesia using a dual-energy X-ray absorptiometry system (PIXImus2 DEXA, Faxitron X-ray Corp.). Percent body fat was measured as body fat content, excluding the head, divided by total body mass. Hearts were harvested upon death and weighed to determine the effect of exercise on changes in heart mass per unit lean body mass. All experiments were approved by the Duke University Institutional Animal Care and Use Committee.
Evaluation of osteoarthritis
Degenerative joint changes were evaluated by histological analysis as described previously (25). Representative samples from a given joint were graded for each animal from the cartilage load bearing regions of both femur and tibia in both the medial and lateral compartments. The sections were stained with hematoxylin, fast green, and Safranin-O, and then evaluated for degenerative changes by three blinded graders according to the modified Mankin scoring system for mouse articular cartilage (32). The scoring system included the categories of cartilage structure [0–11], tidemark duplication [0–3], loss of Safranin-O staining [0–8], fibrocartilage formation [0–2], chondrocyte cloning above the tidemark [0–3], presence of hypertrophic chondrocytes below the tidemark [0–3], and relative subchondral bone thickness [0–2]. Scores for each section were averaged between graders resulting in total scores between 0 and 30 for each location (medial femur, medial tibia, lateral femur, lateral tibia).
Cartilage glycosaminoglycan (GAG) content was further assessed in the stained histological sections using NIS-Elements Basic Research software (Nikon) to quantify the Safranin-O staining intensity of the manually selected uncalcified cartilage region. Green pixel intensity was selected instead of red because unstained background regions contain high pixel intensities for both red and green channels and the inverse relationship between Safranin-O staining intensity and green pixel intensity minimizes background error. Pixel intensity was calculated as relative intensity units (RIU) using the growth plate and the subchondral bone as maximal and minimal GAG content calibration sites, respectively, within each section as follows: Cartilage GAG Loss (%RIU) = 100×[1− {(Isb − Ic)/(Isb − Igp)}], where I is the pixel intensity of the subchondral bone (Isb), uncalcified cartilage (Ic), and growth plate (Igp). This quantitative method was significantly correlated with the semi-quantitative scores for Safranin-O staining loss from the modified Mankin OA scoring (R2=0.64, p<0.0001).
Cytokine and adipokine measurements
Blood was collected in anesthetized mice just prior to euthanasia and allowed to clot for 30–60 minutes at room temperature. Blood was then centrifuged for 15 minutes at 3500 r.p.m., and the serum was aliquoted for immediate storage at −80°C until analysis. Serum concentrations of adiponectin (ACRP30), leptin, IL-1α, and IL-1Ra were quantified by a sandwich ELISA specific for mouse (Linco #EZMADP-60K, Linco #EZML-82K, Quantikine #MLA00, and MRA00, R&D Systems, respectively). Intra- and inter-assay coefficients of variation for ACRP30, leptin, IL-1α, and IL-1Ra were 5.7% and 5.6%, 3% and 2.7%, 4.2% and 4.5%, and 2.4% and 5.7%, respectively. The following cytokines and chemokines were measured using a 20-plex multiplex bead immunoassay (Biosource, #LMC0006), specific to mouse, with the Luminex 100 instrument: IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12, IL-17, KC (mouse analog of IL-8), interferon-gamma-induced protein (IP-10, or CXCL10), monokine induced by interferon gamma (MIG, or CXCL9), macrophage inflammatory protein-1 (MIP-1α, or CCL3), and TNF-α. All samples were analyzed as recommended by the manufacturer.
Statistical Analysis
Statistical differences between diet (Control vs. HF) and activity type (Sedentary vs. Exercised) were determined using a two-factor analysis of variance (ANOVA) (JMP® 8; SAS Institute, Cary, NC, USA). When cytokine concentrations were below the lowest levels of quantification, a value of one half the lowest level of quantification was used for statistical purposes. Statistical analyses were performed on log-transformed values for serum cytokine data to correct for non-normal distributions. Post-hoc analyses were performed using Tukey’s HSD test for significance. In cases where data were not normally distributed (e.g., subchondral thickness score), Wilcoxon Mann-Whitney tests were performed. We also constructed bivariate and multivariate models to evaluate the independent and dependent effects of percent body fat, activity, and knee OA on systemic inflammatory markers. Statistical significance was reported at the 95% confidence level (p < 0.05), and repeated-testing error was controlled for using a 5% false discovery rate correction (33). To evaluate cytokine expression clustering and associations with metabolic function, pair-wise cluster analyses were performed using Pearson product-moment correlation analysis within sedentary and exercised animals. All data were reported as mean ± SEM.
Results
Effect of diet and activity on body composition, running distance, and glucose tolerance
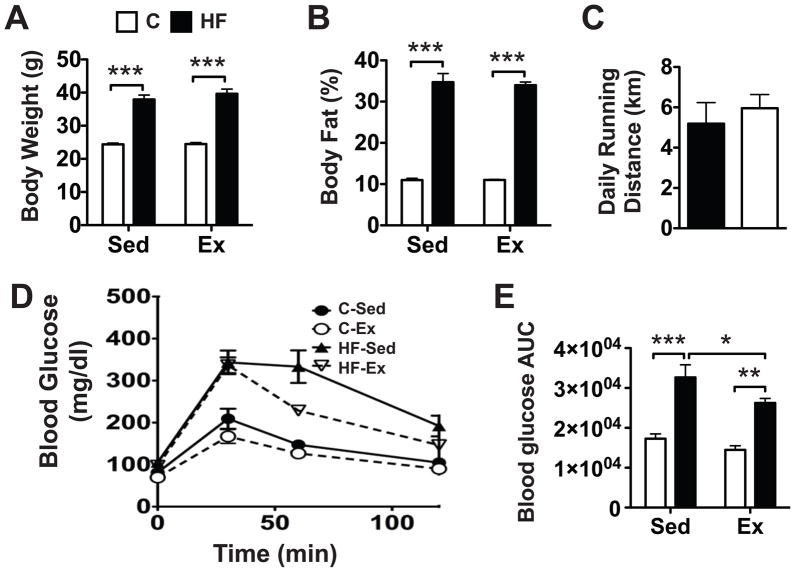
12 wks of feeding animals a very high-fat diet resulted in a 55% increase in body mass (p<0.001; Fig. 1A). This dietary effect was unaltered in animals undergoing voluntary wheel running; there was no independent effect of activity (p=0.37) or an interaction with activity and diet (p=0.43) on body weight. Body weight was nearly the same among both sedentary and exercised animals fed either the control chow diet (24.4 g vs 24.5 g, respectively) or the high-fat diet (37.9 g vs 39.7 g, respectively). As with body weight, percent body fat was dramatically increased with high-fat feeding (3.2-fold, p<0.001) and was unaffected by activity (p=0.76, Fig. 1B). Percent body fat was the same between sedentary and exercised control animals (11.0%) and similar for those fed a high-fat diet (34.7% vs. 34.0%, respectively).
Figure 1.
Effect of a high-fat (HF) diet and activity on body composition and glucose tolerance. A HF diet significantly increased body weight (A) and percent body fat (B) in both sedentary and exercised animals, despite similar nightly running distances (C). Blood glucose levels following a glucose tolerance test (D) showed that a HF diet significantly decreased glucose clearance from the blood in both sedentary and exercised mice, as indicated by increased blood glucose area under the curve (AUC) levels. (E) Exercise reduced the blood glucose AUC in HF fed mice. AUC units are mg/dl•min. Values are mean ± SEM. *p<0.05, **p<0.01, ***p<0.001.
Despite the significantly increased body weight and percent body fat in the high-fat fed animals, diet did not significantly alter the average daily running distance (Fig. 1C). Running exercise increased heart mass per unit lean body mass of both control diet and high-fat fed animals (p<0.001), consistent with an adaptive response to increased levels of activity. Relative heart mass, which was not affected by diet (p=0.26), increased by 16% in control animals and 9% in high-fat fed animals with exercise (data not shown). As shown in previous reports (31), animals fed a high-fat diet display impaired glucose tolerance (Fig. 1D). Despite no effect of exercise on body weight or percent fat, exercise partially rescued glucose tolerance in high-fat fed animals as shown by a 20% reduction in the area under the curve (p<0.05) (Fig. 1E).
Histological Assessment of Knee OA
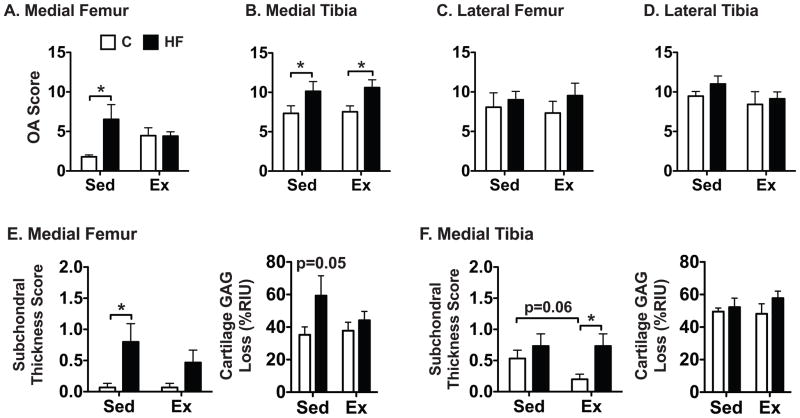
The effect of diet and activity on knee OA was assessed at four locations in the joint—medial femur, medial tibia, lateral femur, and lateral tibia. The greatest changes due to diet and activity were observed in the medial compartment (Fig. 2) where high-fat feeding led to an overall increase in knee OA scores in the medial femur and tibia with high-fat feeding (Fig. 3A,B). Medial femur OA scores increased the most in sedentary animals, where high fat feeding led to a 3.6-fold increase relative to controls (p<0.05). Total OA scores, however, were greatest in the medial tibia (Fig. 3B). Diet and activity treatments did not alter OA scores in the lateral compartment (Fig. 3C,D).
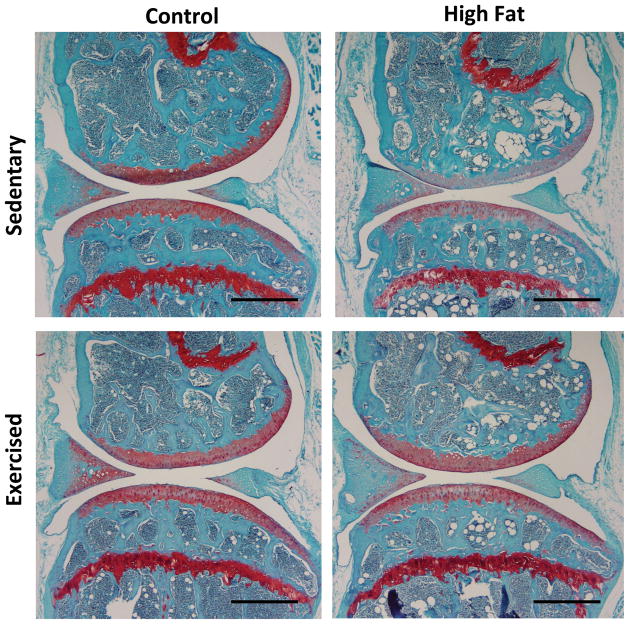
Figure 2.
Representative sagittal knee sections from the medial compartment in sedentary or exercised mice fed a control or high-fat diet. Sections were stained with hematoxylin, fast green, and Safranin-O. A high-fat diet increased the modified Mankin OA score in the medial femoral condyle and tibial plateau in sedentary animals. The medial femoral condyle in high-fat sedentary animals was characterized by a loss of Safranin-O staining, indicating a loss of glycosaminoglycan content. Scale bars = 500 μm.
Figure 3.
Site-specific modified Mankin OA scores (A–D) as a function of diet and activity. A very high-fat (HF) diet increased OA in the medial femoral condyle and tibial plateau in sedentary animals. 4 wks of wheel running prevented this diet effect in the medial femoral condyle but not in the medial tibial plateau. Changes in subchondral bone thickness and the loss of Safranin-O staining intensity are shown as a function of diet and activity for the medial femur (E) and tibia (F). A high-fat diet increased subchondral bone thickness in a site and activity specific manner, and it also increased cartilage GAG loss in the medial femur, which was recovered with exercise. Cartilage GAG loss was calculated relative to the pixel intensities of Safranin-O staining in the growth plate (0% loss) and the subchondral bone (100% loss). RIU=relative intensity units. Values = mean ± SEM. *p<0.05.
Wheel running exercise prevented the increase in OA in the medial femur caused by feeding sedentary mice a very high fat diet as indicated by a significant diet-activity interaction (p=0.04). Exercise mitigated the dietary effect primarily via changes in subchondral bone thickness and cartilage GAG loss (Fig. 3E). As a result, there was no difference in the medial femur OA score between control and high-fat fed exercised animals (Fig. 3A). Exercise did not affect the overall OA score for the medial tibia, although exercise tended to decrease the subchondral bone thickness in control animals, which resulted in a significantly reduced subchondral bone thickness compared to high-fat fed exercised animals (Fig. 3F). This pattern was also observed for the lateral femur and tibia (data not shown). Unlike the medial femur, cartilage GAG loss in the medial tibia was not altered by diet or exercise (Fig. 3F). Overall, the effect of four weeks of wheel exercise on OA severity was modest. Exercise did not increase OA scores at any location in diet-induced obese mice, and in the medial femur, exercise protected against OA induced by a very high fat diet.
Effect of Diet and Activity on Systemic Cytokines
Out of a total of 23 cytokines, chemokines, and growth factors analyzed, 12 were detectable in the majority of samples from at least one of the experimental diet and activity groups (Table 1). High-fat feeding led to a significant increase in the serum concentrations of two CXC-family chemokines, KC and MIG, as well as the adipokines leptin and adiponectin (Table 1). High fat feeding also increased the levels of the anti-inflammatory cytokine IL-1Ra but decreased the anti-inflammatory cytokine IL-4. Compared to sedentary controls, high-fat feeding had the greatest effect on leptin levels, with more than a 30-fold increase, followed by KC and IL-1Ra concentrations, which increased about 6-fold compared to controls. None of these dietary effects were substantially altered by wheel running exercise. However, wheel running significantly increased the serum concentrations of the CXC chemokine IP-10 (3-and 1.8-fold greater in control and high-fat diet fed animals, respectively) (Table 1). Exercise also altered IFN-γ concentrations, although the direction of the effect depended on diet as indicated by a significant diet-activity interaction (p<0.05; Table 1). IFN-γ concentrations were reduced with exercise in control diet animals but increased with exercise in high-fat fed animals.
Table 1.
Effect of diet and activity on serum cytokines, chemokines, and growth factors
| Control | High Fat | ANOVA Factors (p-value) | |||||
|---|---|---|---|---|---|---|---|
| Parameter | Sed | Ex | Sed | Ex | Diet | Activity | Diet*Activity |
| IL-1α | 33 ± 15 | 33 ± 6 | 37 ± 10 | 45 ± 4 | 0.19 | 0.39 | ns |
| IL-1β | 195 ± 91 | 26 ± 14a | 38 ± 19a | 44 ± 36a | 0.24 | 0.08 | ns |
| IL-1Ra | 11 ± 3 | 23 ± 13 | 67 ± 17 | 77 ± 24 | <0.001* | 0.27 | ns |
| IL-4 | 73 ± 26 | 17 ± 7a | 9 ± 0a | 9 ± 0a | <0.01* | 0.03 | ns |
| IL-10 | 69 ± 48 | 647 ± 552 | 21 ± 0 | 322 ± 191 | 0.94 | 0.09 | ns |
| IFN-γ | 9.3 ± 4.0 | 1.7 ± 0.9a | 0.4 ± 0.0a | 7.3 ± 6.8a | 0.10 | 0.55 | <0.05 |
| IP-10 | 87 ± 48a | 263 ± 38 | 91 ± 49a | 161 ± 97a | 0.25 | <0.02 | ns |
| KC | 67 ± 60a | 38 ± 27a | 446 ± 82 | 442 ± 59 | <0.001* | 0.75 | ns |
| MIG | 31 ± 27a | 4 ± 0a | 70 ± 34 | 109 ± 40 | <0.001* | 0.98 | ns |
| FGF basic | 504 ± 158 | 291 ± 114 | 849 ± 844a | 420 ± 193 | 0.13 | 0.94 | ns |
| Leptin | 8 ± 2 | 6 ± 2 | 279 ± 86 | 372 ± 96 | <0.001* | 0.63 | ns |
| ACRP30 | 8.8 ± 0.5 | 9.7 ± 0.2 | 12.4 ± 0.4 | 12.1 ± 1.3 | <0.001* | 0.64 | ns |
Values are reported as pg/ml, except for ACRP30 (adiponectin) (μg/ml). Data were log-transformed for statistical analysis.
Statistical significance (p<0.05) controlling for 5% false discovery rate correction for multiple comparisons.
Greater than 50% of animals in this group had cytokine concentrations below the lowest level of quantification, which for statistical purposes are given a value of one half the lowest level of quantification. Results are not reported for some cytokines and growth factors because few, if any, values were above the lowest level of quantification for any group. One half the lowest levels of quantification in pg/ml for these cytokines are: GM-CSF (7.8), IL-2 (6.0), IL-5 (6.8), IL-6 (6.1), IL-12 (5.5), IL-13 (10.3), IL-17 (9.3), MCP-1 (4.9), MIP-1α (6.9), TNF-α (14.8), and VEGF (6.5).
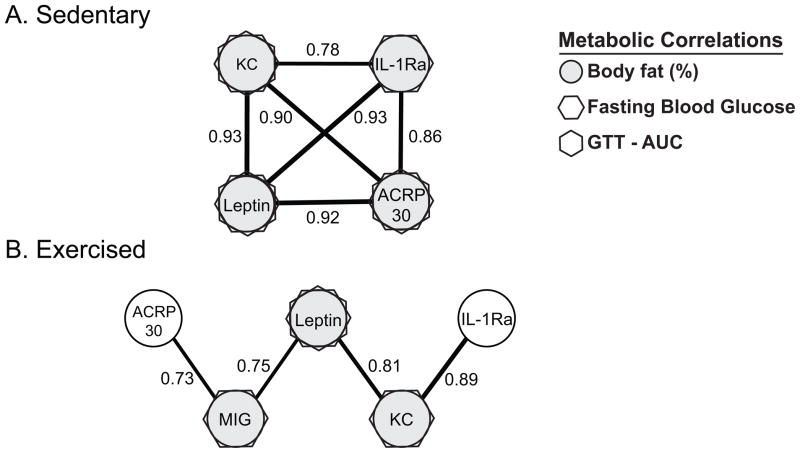
Exercise had a substantial effect on the clustering of cytokines associated with obesity, which was determined by performing activity-specific pair-wise correlation analyses. Within sedentary animals, including both control and high-fat groups, adipokines associated with increased adiposity, fasting blood glucose levels, and glucose intolerance were strongly co-expressed (Fig. 4). Within the exercised animals, however, the adipokine-centered cluster was disconnected and associations with glucose intolerance were weakened. Exercise also stimulated MIG to be expressed in association with adiposity, leptin, and adiponectin.
Figure 4.
Correlation cluster diagrams of serum chemokine and cytokine concentrations within (A) sedentary (N=10: 5 control and 5 HF) and (B) exercised mice (N=10, 5 control and 5 HF). Filled circles and symbols identify cytokines that are positively correlated with the metabolic parameters indicated in the legend. Sedentary mice express a highly correlated cytokine cluster associated with increased adiposity and glucose dysregulation. In exercised mice, this cytokine cluster is significantly disrupted; monocyte-induced interferon-γ (MIG) is co-expressed, and adiponectin (ACRP30) and IL-1Ra are no longer associated with adiposity or glucose dysregulation. Numerical values represent the pairwise Pearson product-moment correlations. Only correlations with p<0.05 are shown. Line thickness is proportional to correlation strength.
Associations Among Adiposity, Inflammation, and OA
To better understand the independent and dependent effects of percent body fat, activity (with and without exercise), and knee OA on systemic inflammatory markers, we constructed both bivariate least-squares and multivariate generalized linear models to identify which variables (i.e., diet, body fat, or knee OA) remained independently associated with inflammatory markers in the multivariate model (Table 2). The bivariate correlation analyses revealed significant positive correlations between four different categorizations of knee OA and percent body fat. The adipokines KC, MIG, adiponectin, IL-1Ra, and leptin were also positively associated with percent body fat. Furthermore, KC, leptin, and IL-1Ra were positively correlated with the total knee OA score as well as some additional sub-compartmental OA scores (Table 2). Two multivariate generalized linear models were tested to determine if any of the cytokines significantly associated with body fat or knee OA in the bivariate models remained independently associated with knee OA after statistically controlling for body fat and activity. Model I was based on the total knee OA score and Model II was based on the tibial OA score. Both Models were significant predictors of KC, MIG, adiponectin, IL-1Ra, and leptin serum concentrations; however, only percent body fat remained significantly associated with the cytokine levels (Table 2). Interestingly, adiponectin was negatively associated with the tibial knee OA score, although this association did not remain significant when controlling for a 5% false discovery rate.
Table 2.
Independent and dependent associations between systemic cytokines and body fat, knee OA, and activity level
| Bivariate (r) | % Body Fat | Pro-inflammatory | Anti-inflammatory | |||
|---|---|---|---|---|---|---|
| ln(KC) | ln(MIG) | ln(Leptin) | ln(ACRP30) | ln(IL-1Ra) | ||
| % Body Fat | - | 0.88*** | 0.68** | 0.93*** | 0.72*** | 0.71** |
| Knee OATotal | 0.56* | 0.56* | 0.24 | 0.51* | 0.27 | 0.57* |
| Knee OATibia | 0.58** | 0.45 | 0.31 | 0.54* | 0.15 | 0.37 |
| Knee OAMedial | 0.60** | 0.53* | 0.35 | 0.41 | 0.39 | 0.35 |
| Knee OAMedial, Tibia | 0.61** | 0.46* | 0.43 | 0.44 | 0.18 | 0.34 |
| Knee OAMedial, Femur | 0.43 | 0.44 | 0.19 | 0.27 | 0.46 | 0.26 |
|
| ||||||
| Multivariate (β) | ||||||
| Model I | ||||||
| Whole Model (r2) # | - | 0.91***1 | 0.51**1 | 0.87***1 | 0.59**1 | 0.80***1 |
| Knee OATotal | - | 0.01 | −0.04 | < −0.01 | < −0.01 | 0.02 |
| % Body Fat | - | 0.15***1 | 0.11**1 | 0.18***1 | 0.02***1 | 0.08***1 |
| Activity | - | −0.20 | −0.34 | 0.06 | −0.01 | −0.21 |
| Model II | ||||||
| Whole Model (r2) # | - | 0.81***1 | 0.51**1 | 0.88***1 | 0.64***1 | 0.58***1 |
| Knee OATibia | - | −0.08 | −0.10 | 0.02 | −0.04* | −0.04 |
| % Body Fat | - | 0.15***1 | 0.10**1 | 0.18***1 | 0.02***1 | 0.08**1 |
| Activity | - | −0.33 | −0.29 | 0.07 | < −0.01 | −0.31 |
Log-transformed pro- and anti-inflammatory cytokines from all experimental animals (N=20) were used as independent variables to determine their associations with percent body fat and various knee OA scores using bivariate pairwise correlations and multivariate Generalized Linear Modeling (GLM) analyses.
r2 values are not possible in GLM and therefore are performed in a multivariable model without GLM to provide an estimate of how much variation the model explains.
p<0.05,
p<0.01,
p<0.001;
p<0.05 controlling for 5% false discovery rate correction for the multivariate analyses.
Discussion
Obesity is one of the most clinically important contexts for studying the pathogenesis of OA because, while it is one of strongest risk factors for OA, it is also potentially modifiable by diet and exercise. Furthermore, the limitations in physical activity caused by OA may restrict the treatment of other obesity-associated diseases, such as diabetes and heart disease (8, 9). Strength training and aerobic exercise reduce arthritis pain and improve function (10, 11); however, the mechanism(s) responsible for these physical therapeutic approaches are not well understood. Furthermore, it is not clear if increased joint loading will increase or decrease pre-radiographic OA markers in an obese population. In the present article, we show that a very high-fat diet induces knee OA in association with increased adiposity, systemic pro-inflammatory mediators, and glucose intolerance. Furthermore, we show that moderate running wheel exercise improves glucose tolerance and disrupts pro-inflammatory cytokine networks in the absence of weight loss or decreased adiposity. Exercise also mitigates the severity of knee OA in the medial femur of obese mice, consistent with the improvements observed in metabolic regulation and inflammation.
Diet-induced obesity significantly increased OA in the medial femur and tibia compared to control animals. The two OA score factors that were most sensitive to the diet-induced obesity in the medial femur were a greater loss of Safranin-O staining and an increase in the subchondral bone thickness. These histologic changes are consistent with a previous study of diet-induced obesity and OA in which female C57BL/6J mice were fed a 45% kcal fat diet over the course of 45 weeks (25). However, in this previous study, mice showed substantial variability in adiposity, which was correlated with OA severity in the high-fat diet group. Male C57BL/6J mice also show variability in weight gain when fed a 45% kcal fat diet (34); therefore, the 60% kcal fat diet used in the present study appears to produce more consistent gains in body weight, body fat, and associated joint degeneration. The observed changes in OA pathology in the medial but not the lateral compartment are consistent with other mouse OA models, such as the STR/ort mouse (35), and suggest that local structural or biomechanical factors interact with obesity-associated changes to increase OA severity.
Four weeks of running wheel exercise produced modest changes in knee histopathology. For most locations, the effect was neutral; however, within the medial femur of high-fat fed animals, exercise tended to be protective against OA. This effect was mediated in part by improved Safranin-O staining in the cartilage, an indicator of increased sulfated GAG content. Previous mouse studies have reported both protective and damaging effects of wheel running exercise on the spontaneous development of knee OA (36, 37). These studies, along with additional rodent exercise studies involving spontaneous or induced knee OA, support the following general conclusions. First, the effect of running exercise on knee OA is age-dependent, with protective or neutral effects observed in younger but not older animals (38, 39). Second, moderate running exercise is generally protective when it is initiated prior to substantial disruption of the collagen network but may be damaging if initiated after cartilage degeneration has begun (40). In the current study, the protective effect of running exercise in the medial femoral condyle and the neutral effects elsewhere within high-fat fed mice are consistent with these general conclusions since running exercise was initiated at a relatively young age (20 wks) and prior to substantial cartilage structural changes.
We hypothesized that wheel-running exercise protects against the development of obesity-induced knee OA by reducing systemic inflammation. Our results show that 4 wks of running, in the absence of changes in body weight or percent body fat, does not result in a significant lowering of serum cytokine levels. This was observed by comparing cytokine levels between diet and activity groups (Table 1) and by testing for a significant effect of activity as a dependent predictor of cytokine variation in two multivariate models (Table 2). Wheel running did, however, disrupt the clustering of cytokine expression levels, such as those associated with increased adiposity and glucose dysregulation (Fig. 4). These disruptions, such as the loss of associations between body fat and fasting blood glucose with adiponectin and IL-1Ra levels, indicate that exercise is sufficient to moderate metabolic-associated inflammation even when overall cytokine and adiposity levels do not change. MIG, a T-cell chemoattractant, was significantly associated with adiponectin and leptin in exercised animals only, suggesting that T-cell activation may play a role in exercise-mediated disruption of adiposity and inflammation as observed in humans (41, 42). Exercise also improves glucose tolerance and disrupts immunometabolic associations, further supporting the important role of aerobic physical activity in metabolic regulation independent of weight loss. Thus, similar to findings in humans (43), these results suggest that ‘fitness’, independent of ‘fatness’, is sufficient to improve metabolic regulation and disrupt cytokine co-expression, changes that may protect against obesity-associated knee OA.
Although the current study shows significant correlations between knee OA and the cytokines KC, leptin, and IL-1Ra, these correlations can be attributed to the strong association between adiposity and knee OA. Previously, we showed that serum leptin was independently associated with knee OA in diet-induced obese female C57BL/6J mice (25). In the current study, adiponectin but not leptin showed a marginally significant negative correlation with the tibial OA score in a multivariate model. The negative correlation indicates that when statistically controlling for the positive relationship between percent body fat and adiponectin levels, increased levels of adiponectin are associated with decreased tibial OA scores. Although the mechanistic basis for this relationship is not known, adiponectin may provide an important link between metabolic signaling pathways and cartilage homeostasis (44) as it is an important positive mediator of glucose metabolism (45) and can stimulate both pro- and anti-catabolic effects in chondrocytes (46, 47).
Our findings share a number of important similarities to recent clinical studies investigating the effect of BMI and physical activity on early-stage OA. In patients with unicompartmental radiographic knee OA, BMI was negatively associated with cartilage GAG content in the non-radiographic OA compartment, as measured using dGEMRIC (48). The authors suggest that the non-radiographic OA compartment is the compartment that is at risk for early-stage disease progression. Similarly, in patients who had undergone a recent meniscectomy, BMI was negatively associated with cartilage GAG content in the affected compartment (49). Thus, as we found in the current study with high-fat feeding, obesity is a significant risk factor for proteoglycan loss in early-stage OA. Moreover, moderate exercise and an overall increase in self-reported physical activity levels have been shown to improve knee cartilage GAG content in patients who underwent a partial medial meniscus resection 3–5 years previously (50).
A potential limitation of the study is that it does not contain pre-exercise data for serum cytokines or joint OA status. This information may be critical for properly testing the hypothesis that exercise protects against the development of obesity-induced knee OA by reducing systemic inflammation. Moreover, sampling synovial fluid cytokine levels may provide additional insights between local changes in inflammation and the effects of exercise on OA. Future studies incorporating these measurements may reveal new insights into the mechanisms responsible for obesity-induced OA and the protective effects of exercise in this model.
In conclusion, a very high-fat diet induces early-stage knee OA in association with increased adiposity, systemic inflammation, and glucose intolerance. The independent contribution of systemic inflammatory mediators to knee OA in this model are not clear because inflammation is primarily associated with increased adiposity, not knee OA. Nevertheless, increased joint loading per se is not sufficient to explain the increased incidence of knee OA in diet-induced obese mice as voluntary wheel running is protective or neutral rather than damaging. Exercise improves glucose tolerance and disrupts the co-expression of pro-inflammatory cytokines, suggesting that increased aerobic exercise may act independent of weight loss in promoting joint health.
Acknowledgments
This study was supported by grants from the NIH (AR50245, EB01630, AR48182, AR48852, AG15768 to F. Guilak, AR51672 to T. Griffin, and AR050429 to Z. Yan) and an Arthritis Investigator Award to T. Griffin from the Arthritis Foundation.
This work was supported by grants from the NIH (AR50245, EB01630, AR48182, AR48852, AG15768 to F. Guilak, AR51672 to T. Griffin, and AR050429 to Z. Yan) and an Arthritis Investigator Award to T. Griffin from the Arthritis Foundation. We thank Bridgette Furman and Holly Leddy for their help with histological grading. We also thank Daniel Schmitt, Frank Keefe, and William Kraus for many insightful discussions.
Footnotes
The authors declare no competing financial interests or other conflicts of interest with regard to this work.
References
- 1.Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226–9. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 2.Hootman JM, Macera CA, Ham SA, Helmick CG, Sniezek JE. Physical activity levels among the general US adult population and in adults with and without arthritis. Arthritis Rheum. 2003;49(1):129–35. doi: 10.1002/art.10911. [DOI] [PubMed] [Google Scholar]
- 3.Shih M, Hootman JM, Kruger J, Helmick CG. Physical activity in men and women with arthritis National Health Interview Survey, 2002. Am J Prev Med. 2006;30(5):385–93. doi: 10.1016/j.amepre.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Felson DT, Goggins J, Niu J, Zhang Y, Hunter DJ. The effect of body weight on progression of knee osteoarthritis is dependent on alignment. Arthritis Rheum. 2004;50(12):3904–9. doi: 10.1002/art.20726. [DOI] [PubMed] [Google Scholar]
- 5.Griffin TM, Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev. 2005;33(4):195–200. doi: 10.1097/00003677-200510000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Sowers MR, Karvonen-Gutierrez CA. The evolving role of obesity in knee osteoarthritis. Curr Opin Rheumatol. 2010;22(5):533–7. doi: 10.1097/BOR.0b013e32833b4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliveria SA, Felson DT, Cirillo PA, Reed JI, Walker AM. Body weight, body mass index, and incident symptomatic osteoarthritis of the hand, hip, and knee. Epidemiology. 1999;10(2):161–6. [PubMed] [Google Scholar]
- 8.Bolen J, Hootman JM, Helmick CG, Murphy L, Langmaid G, Caspersen CJ. Arthritis as a potential barrier to physical activity among adults with diabetes -- United States, 2005 and 2007. MMWR. 2008;57(18):486–9. [PubMed] [Google Scholar]
- 9.Bolen J, Murphy L, Greenlund K, Helmick CG, Hootman JM, Brady TJ, et al. Arthritis as a Potential Barrier to Physical Activity Among Adults With Heart Disease ---United States, 2005 and 2007. MMWR. 2009;58(7):165–9. [PubMed] [Google Scholar]
- 10.Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, Dieppe P, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT) Ann Rheum Dis. 2003;62(12):1145–55. doi: 10.1136/ard.2003.011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roddy E, Zhang W, Doherty M, Arden NK, Barlow J, Birrell F, et al. Evidence-based recommendations for the role of exercise in the management of osteoarthritis of the hip or knee--the MOVE consensus. Rheumatology (Oxford) 2005;44(1):67–73. doi: 10.1093/rheumatology/keh399. [DOI] [PubMed] [Google Scholar]
- 12.Sharma L, Lou C, Cahue S, Dunlop DD. The mechanism of the effect of obesity in knee osteoarthritis: the mediating role of malalignment. Arthritis Rheum. 2000;43(3):568–75. doi: 10.1002/1529-0131(200003)43:3<568::AID-ANR13>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 13.Urquhart DM, Soufan C, Teichtahl AJ, Wluka AE, Hanna F, Cicuttini FM. Factors that may mediate the relationship between physical activity and the risk for developing knee osteoarthritis. Arthritis Res Ther. 2008;10(1):203. doi: 10.1186/ar2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kujala UM, Kettunen J, Paananen H, Aalto T, Battie MC, Impivaara O, et al. Knee osteoarthritis in former runners, soccer players, weight lifters, and shooters. Arthritis Rheum. 1995;38(4):539–46. doi: 10.1002/art.1780380413. [DOI] [PubMed] [Google Scholar]
- 15.McAlindon TE, Wilson PW, Aliabadi P, Weissman B, Felson DT. Level of physical activity and the risk of radiographic and symptomatic knee osteoarthritis in the elderly: the Framingham study. Am J Med. 1999;106(2):151–7. doi: 10.1016/s0002-9343(98)00413-6. [DOI] [PubMed] [Google Scholar]
- 16.Felson DT, Niu J, Clancy M, Sack B, Aliabadi P, Zhang Y. Effect of recreational physical activities on the development of knee osteoarthritis in older adults of different weights: the Framingham Study. Arthritis Rheum. 2007;57(1):6–12. doi: 10.1002/art.22464. [DOI] [PubMed] [Google Scholar]
- 17.Messier SP, Loeser RF, Miller GD, Morgan TM, Rejeski WJ, Sevick MA, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50(5):1501–10. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 18.Aspden RM. Obesity punches above its weight in osteoarthritis. Nat Rev Rheumatol. 2010 doi: 10.1038/nrrheum.2010.123. [DOI] [PubMed] [Google Scholar]
- 19.Iannone F, Lapadula G. Obesity and inflammation--targets for OA therapy. Curr Drug Targets. 2010;11(5):586–98. doi: 10.2174/138945010791011857. [DOI] [PubMed] [Google Scholar]
- 20.Lago F, Dieguez C, Gomez-Reino J, Gualillo O. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol. 2007;3(12):716–24. doi: 10.1038/ncprheum0674. [DOI] [PubMed] [Google Scholar]
- 21.You T, Nicklas BJ. Effects of exercise on adipokines and the metabolic syndrome. Curr Diab Rep. 2008;8(1):7–11. doi: 10.1007/s11892-008-0003-4. [DOI] [PubMed] [Google Scholar]
- 22.Mathur N, Pedersen BK. Exercise as a mean to control low-grade systemic inflammation. Mediators of inflammation. 2008;2008:109502. doi: 10.1155/2008/109502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradley RL, Jeon JY, Liu FF, Maratos-Flier E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. Am J Physiol Endocrinol Metab. 2008;295(3):E586–94. doi: 10.1152/ajpendo.00309.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vieira VJ, Valentine RJ, Wilund KR, Antao N, Baynard T, Woods JA. Effects of exercise and low-fat diet on adipose tissue inflammation and metabolic complications in obese mice. Am J Physiol Endocrinol Metab. 2009;296(5):E1164–71. doi: 10.1152/ajpendo.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffin TM, Fermor B, Huebner JL, Kraus VB, Rodriguiz RM, Wetsel WC, et al. Diet-induced obesity differentially regulates behavioral, biomechanical, and molecular risk factors for osteoarthritis in mice. Arthritis Res Ther. 2010;12(4):R130. doi: 10.1186/ar3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffin TM, Huebner JL, Kraus VB, Guilak F. Extreme obesity due to impaired leptin signaling in mice does not cause knee osteoarthritis. Arthritis Rheum. 2009;60(10):2935–44. doi: 10.1002/art.24854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silberberg M, Silberberg R. Age factor and high-fat diets in the evolution of osteoarthritis in mice. J Gerontol. 1957;12(1):9–13. doi: 10.1093/geronj/12.1.9. [DOI] [PubMed] [Google Scholar]
- 28.Griffin TM, Guilak F. Why is obesity associated with osteoarthritis? Insights from mouse models of obesity. Biorheology. 2008;45(3–4):387–98. [PMC free article] [PubMed] [Google Scholar]
- 29.Bradley U, Spence M, Courtney CH, McKinley MC, Ennis CN, McCance DR, et al. Low-fat versus low-carbohydrate weight reduction diets: effects on weight loss, insulin resistance, and cardiovascular risk: a randomized control trial. Diabetes. 2009;58(12):2741–8. doi: 10.2337/db09-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waters RE, Rotevatn S, Li P, Annex BH, Yan Z. Voluntary running induces fiber type-specific angiogenesis in mouse skeletal muscle. Am J Physiol Cell Physiol. 2004;287(5):C1342–8. doi: 10.1152/ajpcell.00247.2004. [DOI] [PubMed] [Google Scholar]
- 31.Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, et al. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem. 2005;280(39):33588–98. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- 32.Furman BD, Strand J, Hembree WC, Ward BD, Guilak F, Olson SA. Joint degeneration following closed intraarticular fracture in the mouse knee: a model of posttraumatic arthritis. J Orthop Res. 2007;25(5):578–92. doi: 10.1002/jor.20331. [DOI] [PubMed] [Google Scholar]
- 33.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society, Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- 34.Koza RA, Nikonova L, Hogan J, Rim JS, Mendoza T, Faulk C, et al. Changes in gene expression foreshadow diet-induced obesity in genetically identical mice. PLoS Genet. 2006;2(5):e81. doi: 10.1371/journal.pgen.0020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mason RM, Chambers MG, Flannelly J, Gaffen JD, Dudhia J, Bayliss MT. The STR/ort mouse and its use as a model of osteoarthritis. Osteoarthritis Cartilage. 2001;9(2):85–91. doi: 10.1053/joca.2000.0363. [DOI] [PubMed] [Google Scholar]
- 36.Lapvetelainen T, Hyttinen M, Lindblom J, Langsjo TK, Sironen R, Li SW, et al. More knee joint osteoarthritis (OA) in mice after inactivation of one allele of type II procollagen gene but less OA after lifelong voluntary wheel running exercise. Osteoarthritis Cartilage. 2001;9(2):152–60. doi: 10.1053/joca.2000.0370. [DOI] [PubMed] [Google Scholar]
- 37.Lapvetelainen T, Hyttinen MM, Saamanen AM, Langsjo T, Sahlman J, Felszeghy S, et al. Lifelong voluntary joint loading increases osteoarthritis in mice housing a deletion mutation in type II procollagen gene, and slightly also in non-transgenic mice. Ann Rheum Dis. 2002;61(9):810–7. doi: 10.1136/ard.61.9.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hyttinen MM, Arokoski JP, Parkkinen JJ, Lammi MJ, Lapvetelainen T, Mauranen K, et al. Age matters: collagen birefringence of superficial articular cartilage is increased in young guinea-pigs but decreased in older animals after identical physiological type of joint loading. Osteoarthritis Cartilage. 2001;9(8):694–701. doi: 10.1053/joca.2001.0466. [DOI] [PubMed] [Google Scholar]
- 39.Otterness IG, Eskra JD, Bliven ML, Shay AK, Pelletier JP, Milici AJ. Exercise protects against articular cartilage degeneration in the hamster. Arthritis Rheum. 1998;41(11):2068–76. doi: 10.1002/1529-0131(199811)41:11<2068::AID-ART23>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 40.Arokoski JP, Jurvelin JS, Vaatainen U, Helminen HJ. Normal and pathological adaptations of articular cartilage to joint loading. Scand J Med Sci Sports. 2000;10(4):186–98. doi: 10.1034/j.1600-0838.2000.010004186.x. [DOI] [PubMed] [Google Scholar]
- 41.Kruger K, Mooren FC. T cell homing and exercise. Exerc Immunol Rev. 2007;13:37–54. [PubMed] [Google Scholar]
- 42.Lowder T, Padgett DA, Woods JA. Moderate exercise early after influenza virus infection reduces the Th1 inflammatory response in lungs of mice. Exerc Immunol Rev. 2006;12:97–111. [PubMed] [Google Scholar]
- 43.Church TS, Finley CE, Earnest CP, Kampert JB, Gibbons LW, Blair SN. Relative associations of fitness and fatness to fibrinogen, white blood cell count, uric acid and metabolic syndrome. Int J Obes Relat Metab Disord. 2002;26(6):805–13. doi: 10.1038/sj.ijo.0802001. [DOI] [PubMed] [Google Scholar]
- 44.Kang EH, Lee YJ, Kim TK, Chang CB, Chung JH, Shin K, et al. Adiponectin is a potential catabolic mediator in osteoarthritis cartilage. Arthritis Res Ther. 2010;12(6):R231. doi: 10.1186/ar3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bai JP, Bell R, Buckman S, Burckart GJ, Eichler HG, Fang KC, et al. Translational Biomarkers: from Preclinical to Clinical a Report of 2009 AAPS/ACCP Biomarker Workshop. AAPS J. 2011 doi: 10.1208/s12248-011-9265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen TH, Chen L, Hsieh MS, Chang CP, Chou DT, Tsai SH. Evidence for a protective role for adiponectin in osteoarthritis. Biochimica et biophysica acta. 2006;1762(8):711–8. doi: 10.1016/j.bbadis.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 47.Lago R, Gomez R, Otero M, Lago F, Gallego R, Dieguez C, et al. A new player in cartilage homeostasis: adiponectin induces nitric oxide synthase type II and pro-inflammatory cytokines in chondrocytes. Osteoarthritis Cartilage. 2008;16(9):1101–9. doi: 10.1016/j.joca.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 48.Tiderius C, Hori M, Williams A, Sharma L, Prasad PV, Finnell M, et al. dGEMRIC as a function of BMI. Osteoarthritis Cartilage. 2006;14(11):1091–7. doi: 10.1016/j.joca.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 49.Ericsson YB, Dahlberg LE, Roos EM. Effects of functional exercise training on performance and muscle strength after meniscectomy: a randomized trial. Scand J Med Sci Sports. 2009;19(2):156–65. doi: 10.1111/j.1600-0838.2008.00794.x. [DOI] [PubMed] [Google Scholar]
- 50.Roos EM, Dahlberg L. Positive effects of moderate exercise on glycosaminoglycan content in knee cartilage: a four-month, randomized, controlled trial in patients at risk of osteoarthritis. Arthritis Rheum. 2005;52(11):3507–14. doi: 10.1002/art.21415. [DOI] [PubMed] [Google Scholar]