Abstract
Objective
To evaluate whether oral naproxen or transdermal estradiol decreases bleeding and spotting in women initiating the levonorgestrel-releasing intrauterine system (LNG-IUS).
Study Design
We conducted a randomized controlled trial of naproxen, estradiol, or placebo administered over the first 12 weeks of LNG-IUS use. Participants completed a written bleeding diary. We imputed missing values and performed an intention-to-treat analysis.
Results
There were 129 women randomized to naproxen (n=42), estradiol (n=44), or placebo (n=43). The naproxen group was more likely to be in the lowest quartile of bleeding and spotting days compared to placebo, 42.9% versus 16.3% (p=0.03). In the multivariable analysis, the naproxen group had a 10% reduction in bleeding and spotting days (RRadj 0.90, 95%CI 0.84–0.97) compared to placebo. More frequent bleeding and spotting was observed in the estradiol group (RRadj 1.25, 95%CI 1.17–1.34).
Conclusions
Administration of naproxen resulted in a reduction in bleeding and spotting days compared to placebo. (150 words)
Keywords: Contraception, Treatment for irregular bleeding with hormonal contraception, Levonorgestrel intrauterine system, Progestin-only contraception
Introduction
The levonorgestrel intrauterine system (LNG-IUS) is one of the most effective methods of reversible contraception available in the United States. The most commonly reported side effect of the LNG-IUS is irregular bleeding; 20–33% of women report spotting or bleeding as a side effect.1,2 Although the incidence of heavy bleeding, irregular bleeding, and spotting decreases over the first six months of use2, irregular bleeding is the most frequently cited reason for discontinuation of the LNG-IUS.3,4 Up to 66% of women who request removal of the LNG-IUS will do so within the first six months of use.2 If women are appropriately counseled about the expected bleeding side effects, they may be less likely to discontinue use due to unscheduled bleeding.5
Effective treatments for irregular bleeding caused by progestin-only contraceptives have not been well-identified.6 Previous studies have shown that treatment with mefenamic acid, a non-steroidal anti-inflammatory drug (NSAID), decreases the number of bleeding days in both users of the levonorgestrel subdermal implant and depot medroxyprogesterone acetate (DMPA).7,8 Several studies have shown a reduction in irregular bleeding in levonorgestrel implant users with ethinyl estradiol administration9,10,, while another study failed to see an effect with transdermal estradiol.11 Likewise, estrogen treatment has not been shown to consistently reduce the frequency of irregular bleeding in DMPA users.12–14
Decreasing the irregular bleeding associated with progestin-only contraceptives has the potential to improve satisfaction and increase continuation rates. Our primary objective was to compare bleeding and spotting in new users of the LNG-IUS randomized to oral naproxen, transdermal estradiol, or oral placebo over a 12-week treatment period. Secondary objectives included comparison of bleeding patterns in the 4-week post-treatment period and participant satisfaction over the study period.
Materials and Methods
We conducted a randomized trial of women initiating the LNG-IUS (Mirena®, Bayer Healthcare Pharmaceuticals, Montville, NJ) for contraception among women enrolling in a contraceptive cohort study conducted at a university research clinic. This study, the Contraceptive CHOICE Project, has been previously described.15 Participants were recruited from November 2008 to January 2010 and follow-up was conducted through May 2010. Women were eligible if they were English-speaking, willing to avoid additional use of exogenous hormones (e.g. oral contraceptives) and non-steroidal anti-inflammatory drugs (NSAIDs) for the duration of the study, and willing to comply with the study protocol by adhering to the medication regimen, keeping the bleeding diary, attending follow-up visits, and completing the telephone surveys as scheduled. Exclusion criteria included known or suspected pregnancy; contraindications to estrogen or NSAID use; current use of medications that alter estrogen metabolism; current regular use of a NSAID; current diagnosis of menorrhagia, metrorrhagia, symptomatic uterine fibroids, or endometrial polyps; use of DMPA within the previous 6 months; postpartum in the past 4 weeks; induced or spontaneous abortion in the past 4 weeks; currently breastfeeding; or previous use of the LNG-IUS. We obtained approval from the Human Research Protection Office at Washington University in Saint Louis prior to recruitment and all women provided written consent before participation in the study. The trial was registered with clinicaltrials.gov (NCT00789802).
We randomized participants to one of 3 arms; oral naproxen 500 mg, transdermal estradiol 0.1 mg, or oral placebo. Participants randomized to either naproxen or placebo took the study medication twice daily for the first five days of each 4 week period, starting on the day following LNG-IUS insertion (day 1). We chose naproxen because it is inexpensive, widely available in the United States, and can be taken twice daily. We administered the naproxen for the first 5 days of a 4 week period as this regimen has been previously investigated in several other studies of NSAIDS for the treatment of progestin-induced irregular bleeding.7,8,10 Women assigned to estradiol also started the patch the day following insertion (day 1) and used it continuously, changing the patch weekly. We chose transdermal estradiol as a non-oral route and changing the patch weekly would likely be preferable to women choosing a long-acting method of contraception. Each group continued with the treatment regimen for the first 12 weeks of LNG-IUS use. A 90-day reference period is typically recommended for studying bleeding patterns associated with hormonal contraception16; however, we used a 12-week or 84-day treatment period as the transdermal estradiol patch was changed weekly and we administered the treatment for three consecutive 4-week blocks. Allocation of participants was equal between arms. A biostatistician not involved in enrollment created the randomization scheme using computer generated number tables. Participants were recruited, enrolled, and assigned to study group by a research nurse. The treatment arm assignments were contained in sequentially numbered, opaque envelopes opened by the research nurse after the LNG-IUS insertion. The naproxen and placebo arms were blinded to the participant and the research team; the estradiol arm was open label as no transdermal placebo was available. A research pharmacist who was not involved in the study packaged and dispensed the study medications.
Individuals were provided with a 12-week supply of the study medication and study diaries to record bleeding and spotting and medication compliance. Bleeding was defined as any bleeding which required more than 1 panty liner, tampon, or pad in a day. Spotting was defined as any bleeding which required 1 or less panty liner, tampon, or pad in a day. Participants returned diaries by mail every 4 weeks for the 16 weeks of the study period. We conducted telephone surveys at 4, 8, and 16 weeks and an in-person follow-up visit at 12 weeks. Surveys asked about bleeding patterns, compliance with study medication, satisfaction with bleeding patterns and the LNG-IUS, and continuation of the LNG-IUS. Participants were reminded to return the bleeding diary at each telephone contact and were compensated for participation.
Statistical Analysis
We compared baseline demographic and reproductive characteristics between groups using the chi-square test. Our primary outcome was the total number of bleeding and spotting days during the 12 weeks of treatment period. Exploratory analysis of the data showed right-skewed distribution of bleeding and spotting days. Right or positive skew means that the majority of the values lie to the left of the mean and the distribution is asymmetric rather then normally distributed. When data is skewed, comparison of means is not appropriate. We performed non-parametric testing with the Mann-Whitney test to investigate differences in the distribution of bleeding and spotting days between naproxen and placebo and estradiol and placebo. As a greater percentage of bleeding and spotting days were concentrated in the first half of the study period, we divided the total bleeding and spotting days into quartiles and compared the quartile distribution of each study arm using Fisher’s exact test. The naproxen and estradiol arms were each compared to the placebo arm and not to each other; therefore, correction for multiple pair-wise comparisons was not indicated. We performed univariate and multivariable Poisson regression to measure the relative risk of bleeding and spotting days in the treatment arms and to control for baseline characteristics that were not equally distributed in the study arms. We planned a priori to control for any covariates not equally distributed between the study groups. Poisson regression is appropriate in this setting as bleeding and spotting days are count data.17 Satisfaction and continuation were compared using the chi-square test. All analyses were performed using STATA 10 (StataCorp LP, College Station, TX.)
In order to perform an intention-to-treat analysis of bleeding and spotting days, we imputed missing bleeding and spotting data for the 12-week treatment period using STATA 10.0. The “impute” command estimates missing values using selected covariates. We found the following baseline characteristics were associated with the number of reported bleeding and spotting days: average amount of bleeding during menses, duration of menses in days, age of menarche, parity, and amenorrhea in previous 12-months. These covariates were included in the model for the impute command. Similar distributions of data were found between the dataset of actual data reported by participants and the combined imputed and reported dataset (data not shown).
We calculated that 99 women (33 in each group) would be required to detect a 25% reduction in the total number of bleeding and spotting days with an alpha of 0.05 and 90% power. We estimated a mean number of 39 bleeding and spotting days during the 12-week treatment period with a standard deviation of 12 days based on a prior study of bleeding and spotting in new LNG-IUS users.18 Assuming a 10% loss to follow-up, we planned to enroll 38 women in each group for a total of 114 participants. Approximately halfway through enrollment, we observed a greater than expected loss to follow-up and we added an additional 15 participants for a final sample size of 129 subjects.
Results
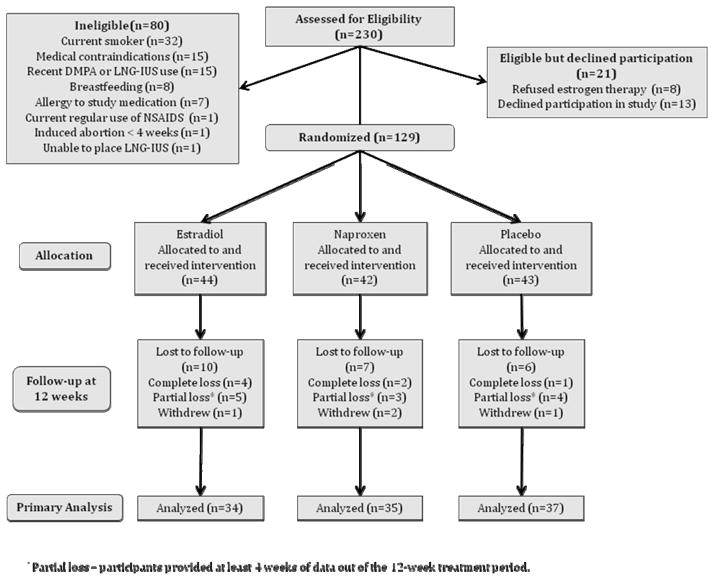
There were 129 women randomized to one of the 3 study arms. Figure 1 shows study participants by treatment arm and follow-up. One hundred and six women (82.2%) returned complete bleeding diaries for the 12-week treatment period. Twelve women (9.3%) provided partial data and 7 women (5.4%) returned no bleeding diaries at all. There were 4 additional participants who provided no data or partial data because they withdrew from the study. One withdrew from the estradiol group with complaints of headaches; two withdrew from the naproxen group, one because of pregnancy, and one due to complaints of chest pain; and one woman withdrew from the placebo group citing lack of time for participation. There was one additional withdrawal after the 12-week treatment period in the placebo group due to LNG-IUS expulsion. There were a total of 23 participants missing 47 bleeding diaries from the 12-week period for 12.1% missing data. Women who were lost to follow-up were more likely to be black (p<0.01), otherwise there were no significant differences in demographic and reproductive characteristics between responders and non-responders.
Figure 1.
Consort Study Flowchart
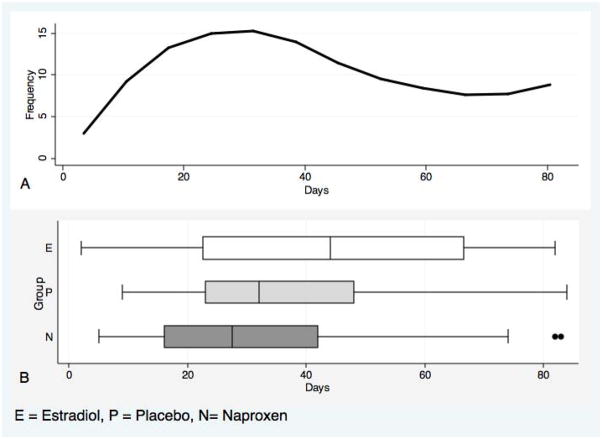
Table 1 shows the demographic and reproductive characteristics of the 3 groups. Baseline characteristics were similar among the 3 groups except body mass index (BMI) was higher in the estradiol group (p=0.05) and women in the naproxen group were more likely to report irregular menses prior to enrollment (p<0.01). Figure 2A shows the distribution of the total bleeding and spotting days over the study period for all participants combined. Figure 2B shows box plots of the distribution of bleeding and spotting days for each study arm. The median number of bleeding and spotting days was 27.5 (range 5–83) in the naproxen group, 44 (2–82) in the estradiol group, and 32 (9–84) in the placebo group. Using non-parametric testing, the distribution of bleeding and spotting days was not significantly different in the naproxen group compared to placebo (p=0.15) or the in the estradiol group compared to placebo (p=0.10).
Table 1.
Baseline Characteristics of Participants by Treatment Group
| Estradiol (n=44) n (%) |
Naproxen (n=42) n (%) |
Placebo (n=43) n (%) |
P* | |
|---|---|---|---|---|
| Age (years) | ||||
| <21 | 5 (11.4) | 5 (11.9) | 4 (9.3) | 1.00 |
| 21–29 | 30 (68.2) | 28 (66.7) | 30 (69.8) | |
| 30+ | 9 (20.5) | 9 (21.4) | 9 (20.9) | |
|
| ||||
| Race | 0.72 | |||
| White | 14 (31.8) | 18 (42.9) | 19 (44.2) | |
| Black | 26 (59.1) | 22 (52.4) | 22 (51.2) | |
| Other | 4 (9.1) | 2 (4.7) | 2 (4.6) | |
|
| ||||
| Hispanic | 1 (2.3) | 0 (0) | 3 (7.0) | 0.22 |
|
| ||||
| Marital Status | 0.10 | |||
| Single | 28 (65.1) | 27 (65.9) | 18 (43.9) | |
| Cohabiting/Married | 11 (25.6) | 11 (26.8) | 21 (51.2) | |
| Separated/Divorced | 4 (9.3) | 3 (7.3) | 2 (4.9) | |
|
| ||||
| Education | 0.32† | |||
| Some HS | 11 (25.6) | 6 (14.6) | 6 (14.6) | |
| HS/Some college | 20 (46.5) | 23 (56.1) | 17 (41.5) | |
| College/graduate degree | 12 (27.9) | 12 (29.3) | 18 (43.9) | |
|
| ||||
| Low SES | 0.36† | |||
| Yes | 18 (40.9) | 23 (54.8) | 23 (53.5) | |
| No | 26 (59.1) | 19 (45.2) | 20 (46.5) | |
|
| ||||
| BMI (kg/m2) | ||||
| <25 | 12 (27.3) | 15 (35.7) | 22 (51.2) | 0.05† |
| 25–29.9 | 10 (22.7) | 15 (35.7) | 9 (20.9) | |
| 30+ | 22 (50.0) | 12 (28.6) | 12 (27.9) | |
|
| ||||
| Parity | 0.21 | |||
| 0 | 20 (45.4) | 22 (53.4) | 22 (51.1) | |
| 1–2 | 23 (52.3) | 14 (33.3) | 18 (41.9) | |
| 3+ | 1 (2.3) | 6 (14.3) | 3 (7.0) | |
|
| ||||
| Regular menses prior to enrollment | <0.01† | |||
| Yes | 39 (88.6) | 26 (61.9) | 35 (81.4) | |
| No | 5 (11.4) | 16 (38.1) | 8 (18.6) | |
HS–high school; SES–socioeconomic status measured by receipt of government assistance or reporting difficulty paying for basic necessities; BMI–body mass index
Percentages may not add to 100 due to rounding.
All p values calculated using Fisher exact test unless otherwise specified
P value calculated using chi-square test
Figure 2.
Distribution of the Total Number of Bleeding and Spotting Days for the Entire Study Population (2A) Showing Right-Skewed Distribution and Distribution of Bleeding and Spotting by Study Arm Presented as Box Plots (2B).
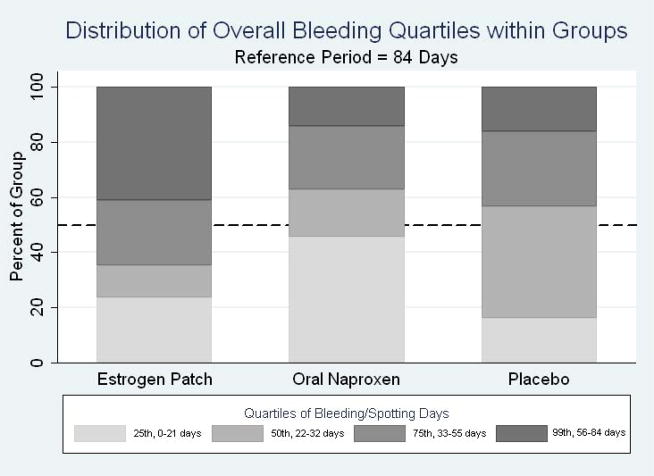
Figure 3 shows the quartile distribution for each study arm. The difference in quartile distribution of bleeding and spotting days was statistically significant for the naproxen group compared to placebo (p=0.03), and the estradiol group compared to placebo (p<0.01). Women in the naproxen group were more likely to be in the lowest quartile of bleeding and spotting (2 to 21 days) than women in the placebo group (42.9% compared to 16.3%). Women in the estradiol arm were more likely to be in the highest quartile of bleeding and spotting (54–84 days) compared to women in the placebo group (40.9% versus 18.6%, p=0.02).
Figure 3.
Distribution of Bleeding and Spotting Days by Quartile for Each Group Over the 12-week Treatment Period*
*25th percentile for bleeding and spotting ranged from 0–21 days over the 12 week treatment period. Quartiles for 50%, 75%, and 99% were in the ranges of 22–33, 34–54, and 55–84 days respectively.
Table 2 shows the results of the univariate and multivariable Poisson regression. After adjusting for BMI and irregular menses at baseline, treatment with naproxen showed a small, statistically significant reduction in the number of bleeding and spotting days (RRadj 0.90, 95%CI 0.84–0.97). Treatment with estradiol was associated with an increased number of bleeding and spotting days (RRadj 1.25, 95%CI 1.17–1.34). A BMI in the overweight and obese range was associated with a small reduction in the number of bleeding and spotting days (RRadj 0.86, 95%CI 0.82–0.95 and RRadj 0.91, 95%CI 0.85–0.97, respectively). There was no difference in the number of bleeding and spotting days between the three groups during the 4 week post-treatment period. Women in the estradiol group were less likely to report consistent use of the study medication at 4 weeks (74.4% versus 88.1% and 90.7% in the naproxen and placebo groups respectively, p=0.05). There was no difference in reported use at 8 weeks (p=0.40). “Always” or “sometimes” use was reported by more than 90% of participants at 4, 8, and 12-week time-points.
Table 2.
Univariate and Multivariable Poisson Regression of Participant Baseline Characteristics Associated with Total Bleeding and Spotting Days
| Unvariate | Mutivariable | |
|---|---|---|
| Characteristic | RR (95%CI) | RR (95%CI)* |
| Study Group | ||
| Estradiol | 1.22 (1.15–1.31) | 1.25 (1.17–1.34) |
| Placebo | Ref | Ref |
| Naproxen | 0.88 (0.82–0.94) | 0.90 (0.84–0.97) |
| BMI | ||
| 17.1–24.9 | Ref | Ref |
| 25–29.9 | 0.89 (0.83–0.96) | 0.86 (0.82–0.95) |
| 30+ | 0.97 (0.91–1.03) | 0.91 (0.85–0.97) |
| Irregular Menses Past 3 Months | 0.90 (0.84–0.97) | 0.96 (0.89–1.03) |
BMI–body mass index
Model adjusted for study group, BMI, and irregular menses at baseline
Satisfaction with bleeding patterns is shown in Table 3. Women in the estradiol group were more likely to be dissatisfied with their bleeding pattern at 4 weeks; 39.5% of women in the estradiol group were dissatisfied with their bleeding compared to 9.5% and 11.6% of women in the naproxen and placebo groups respectively (p=0.01). There was no difference in satisfaction between the placebo and naproxen group at 4 weeks. Satisfaction with bleeding patterns improved over time in all groups. At 12 weeks, more than 85% of women reported being “somewhat” or “very” satisfied with their bleeding pattern and satisfaction did not differ by treatment arm (p=0.12). Satisfaction with the LNG-IUS was greater than 94% at 12 weeks in all 3 study groups.
Table 3.
Reported participant satisfaction with bleeding pattern at 4, 8, and 12 week time points for the estradiol, naproxen, and placebo study arms.
| Time Point | Satisfaction withB leeding | Estradiol | Naproxen | Placebo | Pvalue |
|---|---|---|---|---|---|
| 4 weeks | Satisfied | 24 (57.2) | 24 (57.2) | 26 (60.5) | 0.01* |
| Somewhat Satisfied | 10 (23.3) | 14 (33.3) | 12 (27.9) | ||
| Not Satisfied | 17 (39.5) | 4 (9.5) | 5 (11.6) | ||
|
| |||||
| 8 week | Satisfied | 21 (40.0) | 27 (69.2) | 23 (59.0) | 0.06* |
| Somewhat Satisfied | 13 (30.9) | 8 (20.5) | 10 (25.6) | ||
| Not Satisfied | 8 (19.1) | 4 (10.3) | 6 (15.4) | ||
|
| |||||
| 12 week | Satisfied | 26 (70.3) | 26 (72.2) | 34 (87.2) | 0.12§ |
| Somewhat Satisfied | 6 (16.2) | 6 (16.7) | 5 (12.8) | ||
| Not Satisfied | 5 (13.5) | 4 (11.1) | 0 | ||
All values are in n(%)
P value calculated using chi-square test
P value calculated using Fisher’s exact test
There were few serious adverse events reported during the treatment period. One participant in the naproxen arm had her LNG-IUS removed due to complaints of chest pain. She was subsequently evaluated in the emergency department and diagnosed with gastroesophageal reflux disease. There was also one expulsion in the placebo group, which occurred after the 12-week treatment period. There were no other reported adverse events and no other women discontinued their LNG-IUS during the study period.
Comment
We found that administration of naproxen was associated with a reduction in the number of bleeding and spotting days and administration of estradiol was associated with an increased number of bleeding and spotting days during the first 12 weeks of LNG-IUS use compared to placebo. Although the number of bleeding and spotting days decreased over time in all 3 groups, there were overall fewer bleeding and spotting days in the naproxen group. Our findings are consistent with the results of a Cochrane analysis of 2 randomized trials, which found a reduction in irregular bleeding among DMPA users treated with NSAIDs (RR 0.42, 95%CI 0.25–0.72).6 We observed a smaller reduction in bleeding and spotting days in the naproxen group; however, in our study participants were treated preventatively rather than therapeutically which may have attenuated the effect of the treatment. It is possible that women with specific complaints of bleeding and spotting secondary to the LNG-IUS may be more likely to benefit from treatment with naproxen rather than administering naproxen to all women initiating the LNG-IUS regardless of their bleeding and spotting
Multiple studies have investigated treatments for the management of irregular bleeding with progestin-only contraceptive methods6; however, few of these studies included the LNG-IUS. The mechanism by which NSAIDs may reduce the irregular bleeding associated with progestin-only contraception is not clear. An increase in endometrial cytokines and prostaglandins is observed in the initial months of LNG-IUS use19, which may have important effects on the vasculature of the endometrium and contribute to irregular bleeding.20 The anti-prostaglandin effect of NSAIDs may therefore reduce the irregular bleeding. As irregular bleeding does not occur in the setting of true endometrial atrophy, it is possible that the administration of the estradiol increased bleeding and spotting by providing estrogen to the endometrium, which may explain the increase in bleeding and spotting days we observed in the estradiol arm.21
Our study had several limitations. We lost 18% of our cohort to follow-up, which necessitated imputing missing values to perform an intention-to-treat analysis. Imputing missing data raises the question of the validity of the results; however, our imputation model was based on factors found to correlate with the number of total bleeding and spotting days in our study population. This is a more sophisticated method of imputation than replacing missing values with the mean or random imputation. Additionally, a comparison of the reported data and the combined imputed/reported data showed that they were similar in mean values and range. A greater number of women in the estradiol arm were lost to follow-up (22% as opposed to 14% and 14% in the naproxen and placebo groups respectively), raising the possibility of a differential bias from the imputation. However, we feel this is unlikely for the above reasons. Additionally, we had a relatively small sample size which may limit our generalizability and the ability to find statistical significance for small differences.
Other limitations that should be noted are that diaries were returned on a monthly basis, which could result in recall bias. Additionally, we defined spotting as bleeding requiring one sanitary protection (panty liner, pad, or tampon) daily which is different from the recommendations of Mishell et al 16 who define spotting as bleeding that does not require the use of sanitary protection. However, we felt that this definition was more consistent with characteristics important to women, as any bleeding, including spotting, may be perceived as bothersome. Furthermore, we analyzed bleeding and spotting together in all of our analyses, therefore the definition does not affect our primary outcome of interest.
Strengths of our study include a randomized, double-blinded, placebo-controlled study design for the oral naproxen arm. We were, unfortunately, not able to obtain placebo patches to conduct a double-blinded trial of transdermal estradiol; however, we were still able to compare it to our oral placebo arm. Lack of blinding in this arm may affect the internal validity of our findings; however, we believe the total absence of a comparison group would be a larger weakness. Additionally, we imputed missing values which allowed us to perform our analysis based on intention-to-treat principles. Our results confirm the results from prior studies which found no benefit of estradiol in the treatment of irregular bleeding associated with progestin-only contraception. 6,12,13
Naproxen is an inexpensive and widely available medication associated with relatively few side effects. Additionally, we did not observe any increase in irregular bleeding during the 4 weeks post-treatment period suggesting that naproxen administration does not alter the typical decrease in irregular bleeding seen with the LNG-IUS over time. We only administered naproxen for 5 days every 4 weeks and women were treated preventatively. It is possible that a greater reduction in bleeding and spotting days would be seen with either a longer duration of administration or when only treating women who are bothered by irregular bleeding. Further investigation of naproxen as a treatment for irregular bleeding associated with progestin-only contraceptives is warranted.
Acknowledgments
This research was supported in part by: 1) An ACOG/Bayer Healthcare Pharmaceuticals Research Award in Contraception; 2) Award number K12HD001459 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD); 3) Award number 5T35HL007815-15 from the National Institute of Health (NIH); 4) Midcareer Investigator Award in Women’s Health Research (K24 HD01298); and 5) Award Number KL2RR024994 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NICHD, NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained fromhttp://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp
Footnotes
Reprints are not available from the authors.
Disclosure: Dr. Madden is a speaker for Bayer Healthcare Pharmaceuticals. None of the other authors have a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tewari R, Kay VJ. Compliance and user satisfaction with the intra-uterine contraceptive device in Family Planning Service: the results of a survey in Fife, Scotland, August 2004. Eur J Contracept Reprod Health Care. 2006;11:28–37. doi: 10.1080/13625180500431422. [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo M, Bahamondes L, Perrotti M, Diaz J, Dantas-Monteiro C, Petta C. Bleeding patterns and clinical performance of the levonorgestrel-releasing intrauterine system (Mirena) up to two years. Contraception. 2002;65:129–32. doi: 10.1016/s0010-7824(01)00302-x. [DOI] [PubMed] [Google Scholar]
- 3.Andersson K, Odlind V, Rybo G. Levonorgestrel-releasing and copper-releasing (Nova T) IUDs during five years of use: A randomized comparative trial. Contraception. 1994;49:56–72. doi: 10.1016/0010-7824(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 4.Faundes A, Alvarez F, Diaz J. A Latin American experience with levonorgestrel IUD. Ann Med. 1993;25:149–53. doi: 10.3109/07853899309164159. [DOI] [PubMed] [Google Scholar]
- 5.Andersson K, Batar I, Rybo G. Return to fertility after removal of a levonorgestrel-releasing intrauterine device and Nova-T. Contraception. 1992;46:575–84. doi: 10.1016/0010-7824(92)90122-a. [DOI] [PubMed] [Google Scholar]
- 6.Abdel-Aleem H, d’Arcangues C, Vogelsong K, Gulmezoglu AM. Treatment of vaginal bleeding irregularities induced by progestin only contraceptives (Review) Cochrane Database of Systematic Reviews. 2007:CD003449. doi: 10.1002/14651858.CD003449.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Kaewrudee S, Taneepanichskul S, Jaisamraun U, Reinprayoon D. The effect of mefenamic acid on controlling irregular uterine bleeding secondary to Norplant use. Contraception. 1999;60:25–30. doi: 10.1016/s0010-7824(99)00059-1. [DOI] [PubMed] [Google Scholar]
- 8.Tantiwattanakul P, Taneepanichskul S. Effect of mefenamic acid on controlling irregular uterine bleeding in DMPA users. Contraception. 2004;70:277–9. doi: 10.1016/j.contraception.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Witjaksono J, Lau TM, Affandi B, Rogers PA. Oestrogen treatment for increased bleeding in Norplant users: preliminary results. Hum Reprod. 1996;11 (Suppl 2):109–14. doi: 10.1093/humrep/11.suppl_2.109. [DOI] [PubMed] [Google Scholar]
- 10.Diaz S, Croxatto HB, Pavez M, Belhadj H, Stern J, Sivin I. Clinical assessment of treatments for prolonged bleeding in users of Norplant implants. Contraception. 1990;42:97–109. doi: 10.1016/0010-7824(90)90094-c. [DOI] [PubMed] [Google Scholar]
- 11.Boonkasemsanti W, Reinprayoon D, Pruksananonda K, et al. The effect of transdermal oestradiol on bleeding pattern, hormonal profiles and sex steroid receptor distribution in the endometrium of Norplant users. Human Reproduction. 1996;11 (Suppl 2):115–23. doi: 10.1093/humrep/11.suppl_2.115. [DOI] [PubMed] [Google Scholar]
- 12.Said S, Sadek W, Rocca M, et al. Clinical evaluation of the therapeutic effectiveness of ethinyl oestradiol and oestrone sulphate on prolonged bleeding in women using depot medroxyprogesterone acetate for contraception. Human Reproduction. 1996;11:1–13. doi: 10.1093/humrep/11.suppl_2.1. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg AB, Cardenas LH, Hubbard AE, Darney PD. Post-abortion depot medroxyprogesterone acetate continuation rates: a randomized trial of cyclic estradiol. Contraception. 2002;66:215–20. doi: 10.1016/s0010-7824(02)00391-8. [DOI] [PubMed] [Google Scholar]
- 14.Dempsey A, Roca C, Westhoff C. Vaginal estrogen supplementation during Depo-Provera initiation: a randomized controlled trial. Contraception. 2010;82:250–5. doi: 10.1016/j.contraception.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Secura GM, Allsworth JE, Madden T, Mullersman JL, Peipert JF. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203(115):e1–7. doi: 10.1016/j.ajog.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishell DR, Jr, Guillebaud J, Westhoff C, et al. Recommendations for standardization of data collection and analysis of bleeding in combined hormone contraceptive trials. Contraception. 2007;75:11–5. doi: 10.1016/j.contraception.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Long JS, Freese J. Regression Models for Categorical Dependent Variables Using Stata. 2. College Station, TX: Stata Press; 2006. [Google Scholar]
- 18.Suvisaari J, Lahteenmaki P. Detailed analysis of menstrual bleeding patterns after postmenstrual and postabortal insertion of a copper IUD or a levonorgestrel-releasing intrauterine system. Contraception. 1996;54:201–8. doi: 10.1016/s0010-7824(96)00189-8. [DOI] [PubMed] [Google Scholar]
- 19.Jones RL, Critchley HO. Morphological and functional changes in human endometrium following intrauterine levonorgestrel delivery. Hum Reprod. 2000;15 (Suppl 3):162–72. doi: 10.1093/humrep/15.suppl_3.162. [DOI] [PubMed] [Google Scholar]
- 20.Guttinger A, Critchley HO. Endometrial effects of intrauterine levonorgestrel. Contraception. 2007;75:S93–8. doi: 10.1016/j.contraception.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Fraser IS. Bleeding arising from the use of exogenous steroids. Baillieres Best Pract Res Clin Obstet Gynaecol. 1999;13:203–22. doi: 10.1053/beog.1999.0018. [DOI] [PubMed] [Google Scholar]