Abstract
Background
The main obstacle to elucidating the role of CD4+ T cells in allergen-specific immunotherapy has been the absence of an adequately sensitive approach to directly characterize rare allergen-specific T cells without introducing substantial phenotypic modifications by in vitro amplification.
Objective
To monitor in physiological conditions, the allergen-specific CD4+ T cells generated during natural pollen exposure and during allergy vaccine.
Methods
Alder pollen allergy was used as a model for studying seasonal allergies. Allergen-specific CD4+ T cells were tracked and characterized in twelve alder pollen-allergic, six non-allergic and nine allergy vaccine-treated individuals using peptide-MHC class II tetramers.
Results
Allergen-specific CD4+ T cells were detected in all of the alder pollen-allergic and non-allergic subjects tested. Pathogenic responses (CRTH2 expression and TH2-cytokine production) are specifically associated with terminally differentiated (CD27−) allergen-specific CD4+ T cells, which dominate in allergic individuals but are absent in non-allergic individuals. In contrast, CD27 expressing allergen-specific CD4+ T cells are present at low frequencies in both allergic and non-allergic individuals and reflect classical features of the protective immune response with high expression of IL-10 and IFN-γ. Restoration of a protective response during allergen-specific immunotherapy appears to be due to the preferential deletion of pathogenic (CD27−) allergen-specific CD4+ T cells accompanied by IL-10 induction in surviving CD27+ allergen-specific CD4+ T cells.
Conclusions
Differentiation stage divides allergen-specific CD4+ T cells into two distinct subpopulations with unique functional properties and different fates during allergen-specific immunotherapy.
Keywords: Immunotherapy, allergy, pollen, T cells, CD4, peptide-MHC class II tetramer, peripheral tolerance, differentiation stage, ex vivo
INTRODUCTION
It is now firmly established that allergen-specific CD4+ memory T lymphocytes play a central role in both the induction and control of allergic inflammation1–4. One century after its first introduction, allergen-specific immunotherapy (SIT), also called allergy vaccine therapy, remains the only curative treatment for type I allergy. Despite the proven efficacy of both sublingual and subcutaneous routes of allergen-SIT5;6, little is known about T cell involvement in the generation of a protective immune response during allergen-SIT. The main obstacle to elucidating the role of CD4+ T cells in allergen-SIT has been the absence of an adequately sensitive approach to directly characterize rare allergen-specific CD4+ T cells without introducing substantial phenotypic modifications by in vitro amplification. However, recent progress in peptide-MHC class II (pMHCII) tetramer staining has allowed reliable direct ex vivo visualization of antigen-specific CD4+ T cells7;8, enabling quantification and characterization of these cells in a setting closer to their natural physiological state.
In this study, we used alder pollen allergy as a model for studying seasonal allergies. Alder is a cross reactive pollen and sufferers may also experience problems with birch, hazelnut and oak pollen9. We used an ex vivo pMHCII-tetramer approach to assess the allergen-specific CD4+ T cell response in allergic and non-allergic individuals. We also utilized longitudinal analysis to elucidate underlying T cell mechanisms that accompany either allergic inflammation or tolerance induction to the major alder pollen allergen Aln g 1 in subjects treated with allergen-SIT. This is a highly relevant approach for investigating regulation of the response to environmental allergens in healthy individuals and the development of hypersensitivity in allergic individuals. By establishing a clear link between the differentiation stages of allergen-specific CD4+ memory T cells and both their functional capacity and susceptibility to apoptosis, our data suggest a novel mechanism for allergy vaccine therapy in which the duration and dose of antigen exposure may be the driving force behind immune modulation of the allergen-specific CD4+ T cell response.
METHODS
Subjects
Subjects were recruited at the allergy clinic at Virginia Mason Medical Center (Seattle, WA). All subjects were HLA-typed by sequence-specific oligonucleotide primers using Unitray SSP Kits (Invitrogen, Carlsbad, CA). Alder pollen-allergic subjects (n=12) and patients before receiving allergen-SIT (n=9) were selected based on their clinical symptoms, a positive prick test and positive IgE reactivity using the ImmunoCap test (Phadia AB, Uppsala, Sweden) with alder pollen extracts (test score ≥ 3). For subjects with no history of atopy (n=6), the non-allergic clinical status was confirmed by a lack of IgE reactivity and a negative in vitro basophil activation assay with alder pollen extracts (Table E1). Patients after successful allergen-SIT (n=7) had undergone subcutaneous SIT for a minimum of 3 years. Treatment was considered efficacious when patients had a significant reduction in clinical symptoms and when their drug usage needs during pollen season decreased significantly. The study was approved by the Institutional Review Board of Benaroya Research Institute (Seattle, WA).
Tetramer reagents and Tetramer Guided Epitope Mapping
Biotinylated HLA-DR molecules were purified as described10. A total of 19 overlapping Aln g 1 peptides (20 aa long with a 12 residue overlap) spanning the entire Aln g 1 sequence were synthesized (Mimotopes, Clayton, Australia). For epitope mapping, peptides were divided into 3 pools of 5 peptides each plus a 4th pool of 4 peptides (Table E2). These peptide mixtures were loaded into the biotinylated HLA-DR proteins to generate pooled tetramers as described11. Cells were cultured with peptide pools for 14 days and then stained with pooled peptide tetramers. Cells from wells which gave positive staining were stained again using individual peptide MHC class-II (pMHCII) tetramers from the positive pool. pMHCII-tetramers loaded with irrelevant peptides were used as negative controls.
Ex vivo analysis of Aln g 1-reactive CD4+ T cells
Magnetic bead enrichment of pMHCII-tetramer-positive CD4+ T cells was performed as previously described7. Briefly, 30 to 60 million PBMCs in culture medium at a concentration of 150 million/ml were stained with 20 μg/ml PE-labeled tetramers at room temperature for 100 min. Cells were then washed twice and incubated with anti-PE magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) at 4 °C for another 20 min. The cells were washed again and were enriched using a Miltenyi magnetic column. Frequency was calculated as previously described8. For phenotyping studies, cells in the bound fractions were stained with antibodies against markers of interest or corresponding isotype-matched mAbs. A combination of a violet fluorescent reactive dye (ViViD; Invitrogen, Carlsbad, CA) as a viability marker, CD19 (Dako) and CD14 (BD Pharmingen) was used to exclude dead cells, B cells and monocytes from the analysis, respectively. For Foxp3 (eBioscience) or Ki-67 (BD Bioscience) staining, tetramer and surface staining was performed first, followed by fixation/permeabilization and staining as previously described12. Data acquisition was performed on an LSRII flow cytometer (Becton Dickinson) and analyzed using FlowJo software (Treestar, Ashland, Ore).
Intracellular cytokine staining
CD4+ T cells cultured for 14 days with specific immunodominant peptide were stained with corresponding PE-conjugated pMHCII-tetramers for 60 min at 37 °C. Cells were then stimulated with 50 ng/mL PMA and 1 μg/mL ionomycin in the presence of 10 μg/ml monensin in 1ml of complete medium for 6 h at 37 °C, 5% CO2. For cytokine staining, surface staining was performed first, followed by fixation/permeabilization as per the manufacturer’s protocol (eBioscience). Cells were then stained with various combinations of antibodies for IFN-γ, IL-17, IL-10 and IL-13 (all Biolegend), IL-4 (eBioscience) and IL-5 (Miltenyi) or corresponding isotype-matched mAbs. After 30 min at 4 °C, cells were washed and immediately analyzed by flow cytometry.
Statistical analysis
The nonparametric Mann-Whitney U test was used for unpaired comparisons between groups, whereas the nonparametric Wilcoxon matched pairs test was used for paired comparison. All statistical analysis was performed with the GraphPad Prism software version 5.0a (GraphPad Software, La Jolla, CA).
RESULTS
Allergen-specific CD4+ T cells are present in allergen-tolerant individuals
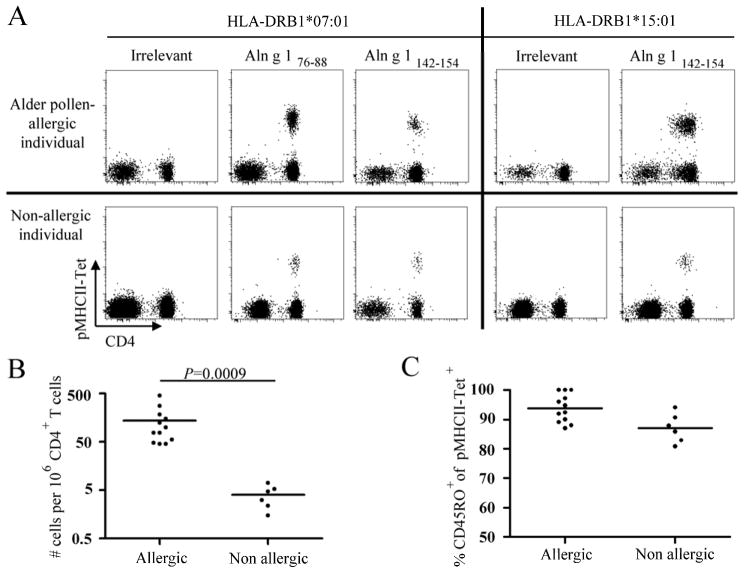
A prerequisite for utilizing pMHCII-tetramers to interrogate CD4+ T cells is the identification of MHC class II-restricted epitopes within the antigen of interest. We used the tetramer-guided epitope mapping (TGEM) approach11 to identify CD4+ T cell epitopes within Aln g 1, the major allergen from Alnus glutinosa (data not shown). We selected DRB1*07:01 and DRB1*15:01 for our study as these alleles were prevalent in our cohort of alder pollen-allergic subjects. We identified two peptides, Aln g 176–88 and Aln g 1142–154, as minimal epitopes for DRB1*07:01, and Aln g1142–154 as a minimal DRB1*15:01-restricted epitope in allergic subjects (Table E3). We next produced and used PE-labeled DRB1*07:01 and DRB1*15:01 pMHCII-tetramers corresponding to these epitopes to track Aln g 1-specific CD4+ T cells ex vivo using an anti-PE magnetic bead enrichment technique7. This allowed direct detection and quantification of allergen-specific CD4+ T cells with minimal manipulation, thereby circumventing the potential bias introduced by in vitro amplification. We consistently detected Aln g 1-specific CD4+ T cells in both alder pollen-allergic and non-allergic individuals (Fig. 1A). The average frequency of Aln g 1-specific CD4+ T cells was significantly higher in alder pollen-allergic subjects than in non-allergic subjects (Fig. 1B) and these cells displayed a predominantly CD45RO+ memory phenotype in all individuals examined (Fig. 1C), implying natural antigen presentation which is critical for clinically relevant epitopes. These data demonstrate the presence of Aln g 1-specific memory CD4+ T cells in alder pollen-allergic and non-allergic subjects that react to the same immunodominant epitopes. However, the absence of a pathologic response in non-allergic individuals is accompanied by a significantly lower number of Aln g 1-specific CD4+ T cells.
Figure 1.
Frequencies of Aln g 1-specific T cells. A, Ex vivo DRB1*07:01 and DRB1*15:01/Aln g 1-pMHCII-tetramer staining of total live CD3+ T cells. Data are representative of at least 3 individuals per group. B, Ex vivo frequencies of Aln g 1-specific CD4+ T cells during the alder pollen season. C, Percentage memory (CD45RO+) cells within Aln g 1-specific CD4+ T cells. B and C, each data point represents a single epitope in a single donor. Differences between groups were analyzed by Mann-Whitney U-test.
Allergen-specific CD4+ T cells are functionally active during pollen season
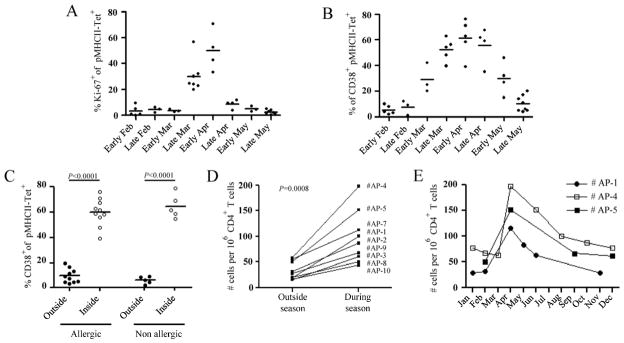
To determine whether natural exposure to alder pollen induces the activation and proliferation of Aln g 1-specific CD4+ T cells in both groups of subjects, we examined expression of the nuclear protein Ki-67, an indicator of T cell proliferation in vivo13, and CD38, a cell-surface activation marker14, outside and during the alder pollen season. In each of the alder pollen-allergic individuals tested, Ki-67 (Fig. 2A) and CD38 (Fig. 2B) expression within Aln g 1-specific CD4+ T cells dramatically increased between March and April, a window of time that correlates with elevated levels of alder pollen (Fig. E1) and increased allergy symptoms. After the alder pollen season, the percentage of Aln g 1-specific cells expressing Ki-67 and CD38 declined rapidly, returning to baseline levels by May. The low frequency of allergen-specific CD4+ T cells in non-allergic individuals precluded determination of Ki-67 expression in those subjects; however, the level of CD38 expression could be examined. CD38 expression was significantly up-regulated in non-allergic individuals during alder pollen season, suggesting a recently generated population of activated CD4+ T cells (Fig. 2C).
Figure 2.
Dynamics of the Aln g 1-specific T cell response. Ex vivo longitudinal analysis of Ki-67 (A) and CD38 expression (B) within Aln g 1-specific CD4+ T cells in allergic individuals. C, Percentage of Aln g 1-specific CD4+ T cells expressing CD38. D, Ex vivo frequencies of Aln g 1-specific CD4+ T cells in ten allergic individuals, outside and during the alder pollen season. E, Ex vivo Aln g 1-specific CD4+ T cell frequencies plotted over time for three representative allergic individuals. A-C, Each data point in each time period represents a single epitope in a single donor. C-D, Differences between groups were analyzed by the Wilcoxon matched pairs test.
Increased Ki-67 expression among Aln g 1-specific CD4+ T cells indicates that these cells are actively dividing during the symptomatic period; accordingly, we observed in all alder pollen-allergic individuals a 2 to 5-fold higher ex vivo frequency in alder pollen season than out of season (Fig. 2D). The frequency of Aln g 1-specific CD4+ T cells reached a peak in April, decreased rapidly for the first 2 months after alder pollen season, and then slowly returned to baseline (Fig. 2E). Together, these data demonstrate allergen recognition by T cells in both allergic and non-allergic subjects during periodic natural allergen exposure.
Allergen-specific CD4+ T cells are distinct in allergic and non-allergic subjects
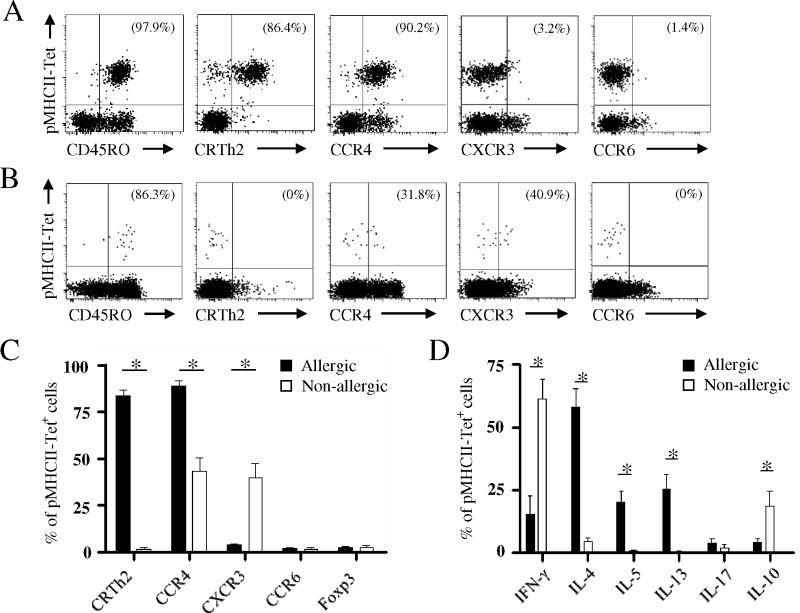
To characterize Aln g 1-specific CD4+ T cell responses in each group, we next directly examined the expression of various markers by Aln g 1-specific CD4+ T cells (Fig. 3A and B). We observed that most allergen-specific CD4+ T cells from alder pollen-allergic individuals expressed the prostaglandin D2 receptor, CRTH2, a marker associated with T helper (TH) type-2 cytokine-producing cells15, and CCR4, a TH-2-associated chemokine receptor that allows trafficking to non-lymphoid sites such as the skin and airway mucosa16. These cells did not express the TH-17 associated chemokine receptor CCR617 (Fig. 3A and C). In contrast to cell surface marker expression in allergic individuals, Aln g 1-specific CD4+ memory T cells from non-allergic individuals did not express CRTH2 and only a small percent expressed CCR4 (Fig. 3B and C). Moreover, a higher proportion of Aln g 1-specific CD4+ T cells from non-allergic individuals expressed CXCR3, a TH1-associated chemokine receptor that has been reported to correlate with allergen-specific IFN-γ production18. However, although we cannot rule out the possibility that our ex vivo analysis may not reflect the local events in specific tissues, we observed no significant difference in the proportion of peripheral CD127−CD25HIFoxp3+ Aln g 1-specific CD4+ T cells between allergic and non-allergic individuals (Fig. 3C) or between in season and out of season time points (data not shown).
Figure 3.
Phenotype of Aln g 1-specific T cells. Representative flow cytometry plots showing ex vivo multicolor phenotyping of Aln g 1-specific CD4+ T cells in allergic (A) and non-allergic individuals (B). C, Ex vivo phenotype of Aln g 1-specific CD4+ T cells. D, Cytokine production by Aln g 1-specific CD4+ T cells. A and B, Percentages of tetramer-positive cells expressing the given marker are indicated in the upper right quadrant. C and D, Data are mean ± SEM from at least 6 individuals per group. Differences between groups were analyzed by Mann-Whitney U-test. * P < 0.001.
In support of these phenotyping experiments, we next functionally characterized the allergen-specific CD4+ T cell responses. In alder pollen-allergic individuals, Aln g 1-specific CD4+ T cells produced TH2-associated cytokines, including abundant IL-4, IL-5 and IL-13, but produced no IL-17 and only small amounts of IFN-γ (Fig. 3D). In non-allergic individuals, allergen-specific CD4+ T cells produced IFN-γ and IL-10 but TH2-cytokines were absent. Interestingly, allergen-specific CD4+ T cells co-expressed IL-10 and IFN-γ in those individuals (Fig. E2). This result, together with the observation that peripheral allergen-specific CD4+ T cells are rarely CD127−CD25HIFoxp3+ demonstrate that peripheral tolerance to allergen is associated with TH1 and TR1 cells.
CD27 expression defines functionally distinct allergen-specific T cell subsets
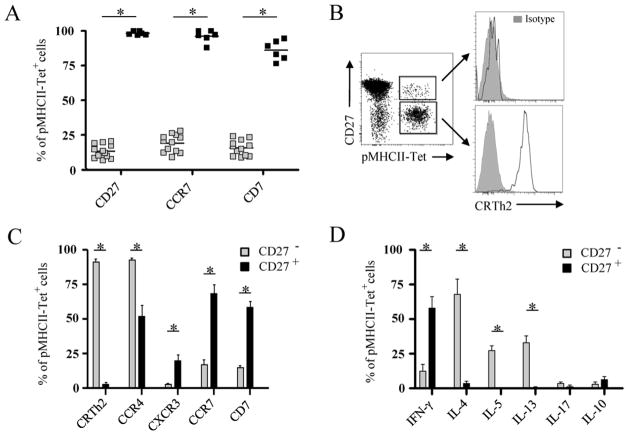
Human CD4+ memory T cells progress through stages of post-thymic differentiation that are each characterized by a distinct expression of CD27 and CCR7 on CD4+ memory T cells19–21. In the context of allergy, a recent study demonstrated that birch pollen-specific CD4+ memory T cells expressed CCR7 in non-allergic individuals but not in allergic individuals12. In light of these findings, we postulated that the functional differences of allergen-specific CD4+ memory T cells between allergic and healthy subjects may depend upon their stage of differentiation. Here we found that Aln g 1-specific CD4+ memory T cells from non-allergic individuals expressed CD27 and CCR7, while those from alder pollen-allergic individuals mainly lacked CD27 and CCR7 expression (Fig. 4A), characterizing them as terminally differentiated effector cells. In support of this finding, Aln g 1-specific CD4+ T cells from alder pollen-allergic individuals expressed lower levels of CD7 than those from non-allergic subjects. Loss of this marker is also consistent with the phenotype of highly mature memory T cells22;23.
Figure 4.
CD27 expression defines two distinct subsets of allergen-specific T cells. A, Percentage of CD27+, CCR7+, and CD7+ cells among Aln g 1-specific CD4+ T cells within the allergic (grey) and non-allergic (black) group. Each square represents a single donor. B, CRTH2 expression (open histogram) by CD27+ and CD27− Aln g 1-specific CD4+ T cells in allergic individuals. C, Ex vivo phenotype of CD27− and CD27+ Aln g 1-specific CD4+ T cells in allergic individuals. D, Cytokine production by CD27− and CD27+ Aln g 1-specific CD4+ T cells in allergic individuals. Data are mean ± SEM from at least 7 individuals. Differences between groups were analyzed by Mann-Whitney U-test. * P < 0.001.
Only a small proportion of allergen-specific CD4+ memory T cells expressed CD27 in allergic subjects. This motivated us to explore whether this fraction represents a subset of allergen-specific CD4+ memory T cells that is functionally distinct from the CD27− Aln g 1-specific CD4+ memory T cell subset. Multicolor staining of cell surface markers revealed that CD27+ allergen-specific CD4+ T cells from alder pollen-allergic subjects expressed little to no CRTH2 while the CD27− Aln g 1-specific CD4+ T cell fraction expressed high levels of CRTH2 (Fig. 4B). Both of these subsets up regulated CD38 expression during the pollen season (data not shown) indicating that they actively respond to natural allergen exposure. Further characterization revealed that CXCR3 was coordinately expressed with CD27 and a lower proportion of those cells expressed CCR4 (Fig. 4C and E3 A). Cytokine analysis of CD27+ and CD27− Aln g 1-specific CD4+ T cells in alder pollen-allergic individuals revealed that CD27− allergen-specific CD4+ T cells produced TH2 cytokines while CD27+ allergen-specific CD4+ T cells secreted IFN-γ (Fig. 4D and E3 B). In total, these results indicate that CD27+ allergen-specific CD4+ T cells in allergic individuals are phenotypically and functionally similar to allergen-specific CD4+ T cells in non-allergic subjects, while CD27− allergen-specific CD4+ T cells are phenotypically and functionally distinct. Thus, allergen-specific CD4+ memory T cells can be divided into two distinct subpopulations with unique functional properties: a CD27− subpopulation which represents allergen-specific TH2 cells and a CD27+ subpopulation which represents allergen-specific TH1 and TR1 cells.
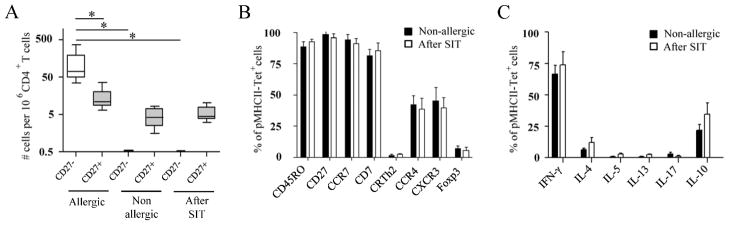
SIT induces preferential deletion of TH-2 cells
Clinical improvement in allergic individuals receiving allergen immunotherapy is associated with decreased in the ratio of allergen-specific TH2/TH1 and TH2/TR1 cells24–25. To directly assess the immunological changes induced during allergen-SIT, we examined the frequency and surface phenotype of Aln g 1-specific CD4+ T cells in alder pollen-allergic patients during and after allergy vaccine therapy. In patients after successful allergen-SIT (n=7), we consistently observed low frequencies of Aln g 1-specific CD4+ T cells that were almost exclusively CD27+ (Fig. 5A). Based on reports that loss of CD27 expression is an irreversible phenomenon associated with cells with a shorter life-span19;26–27, we postulated that the reduced frequency of Aln g 1-reactive T cells observed in patients after successful allergen-SIT was due to the depletion of the CD27− Aln g 1-specific CD4+ T cells. Interestingly, all different groups of subjects had equivalent frequencies of CD27+ Aln g 1-specific CD4+ T cells (Fig. 5A). In addition, surface marker expression and cytokine profiles of CD27+ allergen-specific CD4+ memory T cells were remarkably similar regardless of whether they were isolated from non-allergic or patients after successful allergen-SIT (Fig. 5B and C). Together, our observations suggest that allergen-SIT may induce the selective deletion of allergen-specific CD27− CD4+ T cells, likely due to their increased susceptibility to apoptosis22;26. To further corroborate these findings, we followed the Aln g 1-specific CD4+ T cell response in two alder pollen-allergic subjects at multiple time points during the course of allergen-SIT. This analysis revealed that the CD27− (TH2) allergen-specific CD4+ T cell subset progressively declined after allergen-SIT treatment was initiated. In contrast, the CD27+ (TH1/TR1) subset remained relatively stable but became progressively dominant, apparently due to the decrease of the CD27− (TH2) population (Fig. E4 A and B). Together, these data suggest that the overall reduction in the frequency of allergen-specific CD4+ memory T cells during allergen-SIT may occur mainly through depletion of the TH-2 subset, leading to an increase in the allergen-specific TH1/TH2 and TR1/TH2 cell ratio.
Figure 5.
Effect of allergen-SIT on Aln g 1-specific CD4+ T cells. A, Average frequencies of CD27− and CD27+ Aln g 1-specific CD4+ T cell in twelve allergic and six non-allergic individuals and seven patients after successful SIT. Ex vivo phenotypes (B) and cytokine production (C) in Aln g 1-specific CD4+ T cells between non-allergic individuals and patients after successful SIT. Data are mean ± SEM from at least 6 individuals per group. Differences between groups were analyzed by Mann-Whitney U-test. * P < 0.001.
DISCUSSION
In the present study we utilized an ex vivo pMHCII-tetramer approach to demonstrate that the degree of differentiation of pollen allergen-specific CD4+ memory T cells is correlated with their functional activities. These data have several important implications for understanding the basic immunologic mechanisms involved in the amelioration of allergic symptoms during allergen-SIT. First, we demonstrate that allergic and non-allergic individuals have functionally and phenotypically distinct circulating allergen-specific CD4+ T cells which can be clearly differentiated based on their differentiation stage. Both of these subsets actively respond to natural allergen exposure, but they appear to play markedly different roles in allergic disease. While we detected CD27+ allergen-specific memory CD4+ T cells in both allergic and non-allergic subjects, we exclusively detected CD27− allergen-specific memory CD4+ T cells in allergic subjects. CD27− allergen-specific CD4+ T cells were associated with TH2 cytokine production, providing a clear functional connection with allergic disease. In contrast, CD27+ allergen-specific CD4+ T cells were associated with IFN-γ and IL-10 production and represent the only subset observed in non-allergic individuals, implying that these cells are protective and play a role in tolerance to allergens. Finally, the chronic high-dose allergen stimulation during allergen-SIT may induce a total depletion of allergen-specific CD27− T cells with no significant changes in the frequency of CD27+ allergen-specific memory CD4+ T cells. These data suggest a novel mechanism in which the depletion of allergen-specific TH2 cells, rather than an increase in allergen-specific regulatory T cells, could be a key event in restoring tolerance during allergen-SIT.
One of the noteworthy findings in this study is that allergen-specific CD4+ T cells may have different levels of sensitivity to allergen-SIT based on their differentiation state. The observation that only the CD27+ allergen-specific CD4+ T cell subset persists in allergic patients after successful allergen-SIT suggests that these protective allergen-specific CD4+ T cells possess a survival benefit during allergen-SIT that correlates with CD27 expression26. While the frequency of CD27+ allergen-specific CD4+ memory T cells remained relatively constant during allergen-SIT, our data demonstrate a dramatic reduction in the overall frequency of allergen-reactive T cells and an accompanying change in the ratio of allergen-specific TH2/TH1 and TH2/Tr1 cells.
A course of allergen-SIT involves repeated administration of escalating doses of the sensitizing allergen over months or years. In a recent study, a novel role for apoptosis in allergen-SIT has been suggested28. Given that functional deletion of highly mature antigen-specific CD4+ T cells can occur when the antigen dose is both extremely high and persistent29;30, it is possible that allergen-SIT acts by this mechanism. The absence of CD27− allergen-specific CD4+ T cells in the SIT-treated group, even a long period of time after treatment, is notable and suggests a mechanism of deletion rather than T cell homing to tissue sites. In addition, we found that allergen-specific TH2 cells also display an absence of CD7 expression, which has been associated with cells highly sensitive to activation-induced cell death22–23. Thus, we propose that allergen-specific TH2 cells, which are typically in the final stages of differentiation, are more susceptible to apoptosis during allergen-SIT than CD27+ (protective) allergen-specific CD4+ T cells.
IL-10 plays an important role in both induction and maintenance of specific T cell tolerance and may be implicated in the mechanism of desensitization31;32. Here, we show that allergen-specific TH1-like TR1 cells are the IL-10-producing cell type that becomes increasingly dominant during the course of allergen-SIT. It has been demonstrated that during repetitive activation with high dose antigen, the principal source of IL-10 is TH1 cells33 and that such conditions result in the differentiation of IL-10-producing TH1 cells and maximal IL-10 expression34–36. Differentiation and induction of IL-10-secreting TR1 cells has been recently demonstrated to be negatively regulated by the TH2 cytokine IL-433;37;38. In addition, it has been recently suggested that production of IL-4 causes TH2 cells to become refractory to regulatory T cell suppression39;40, suggesting that allergen-specific TH2 cell depletion is a critical first step in the induction of a protective response during allergen-SIT. Interestingly, it has been shown that during such persistent stimulation, IL-10-secreting TR1 cells are generated from naïve cells in the presence of IL-1041;42, suggesting sequential induction and maintenance of a tolerogenic response. Hence, in addition to depletion of allergen-specific CD4+ TH2 cells, the mechanism of action of allergen-SIT may also include the enhancement of IL-10 production by the remaining CD27+ allergen-reactive T cells and generation of new protective allergen-specific Tr1 cells by repeated high-dose allergen stimulation.
Collectively, our results suggest that depletion of allergen-specific TH2 cells may be a key event during clinical tolerance induction, which proposes a new paradigm for understanding why duration and allergen dose could be key factors for successful allergen-SIT. In this context, the use of synthetic peptide fragments of allergen molecules which could allow the administration of higher doses of allergen without increasing the risk of anaphylaxis is an attractive approach for improving the efficacy and safety of current allergy treatment.
Supplementary Material
Key Messages.
Peptide-MHC class II tetramer staining allows reliable direct ex vivo visualization of allergen-specific CD4+ T cells in a setting closer to their natural physiological state.
CD27 expression distinguishes protective (TH1/TR1) from pathogenic (TH2) allergen-specific CD4+ T cells.
Allergen-SIT restores the balance between pathogenic and protective T cell responses by preferential deletion of terminally differentiated (TH2) cells and IL-10 induction in surviving CD27+ allergen-specific CD4+ T cells.
Acknowledgments
We thank Jennifer Heaton for help with subject recruitment. We also thank G.T. Nepom and S.F. Ziegler for comments on the manuscript as well as members of our laboratory for discussions and suggestions.
Declaration of all sources of funding: NIH contract HHSN272200700046C
Abbreviations
- FITC
Fluorescein isothiocyanate
- Foxp3
Forkhead box P3
- Aln g
Alnus glutinosa
- HLA
Human histocompatibility leukocyte antigen
- MHC
Major histocompatibility complex
- PBMC
Peripheral blood mononuclear cell
- PE
Phycoerythrin
- PHA
Phytohemagglutinin
- pMHCII
Peptide-MHC class II
- SIT
Allergen-specific immunotherapy
- TH
T helper
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004;199:1567–75. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maggi E. T-cell responses induced by allergen-specific immunotherapy. Clin Exp Immunol. 2010;161:10–18. doi: 10.1111/j.1365-2249.2010.04148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akkoc TA, Akdis M, Akdis CA. Update in the Mechanisms of Allergen-Specific Immunotheraphy. Allergy Asthma Immunol Res. 2011;3:11–20. doi: 10.4168/aair.2011.3.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–71. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 5.Durham SR, Walker SM, Varga EM, Jacobson MR, O’Brien F, Noble W, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–75. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 6.Wilson DR, Lima MT, Durham SR. Sublingual immunotherapy for allergic rhinitis: systematic review and meta-analysis. Allergy. 2005;60:4–12. doi: 10.1111/j.1398-9995.2005.00699.x. [DOI] [PubMed] [Google Scholar]
- 7.Scriba TJ, Purbhoo M, Day CL, Robinson N, Fidler S, Fox J, et al. Ultrasensitive detection and phenotyping of CD4+ T cells with optimized HLA class II tetramer staining. J Immunol. 2005;175:6334–43. doi: 10.4049/jimmunol.175.10.6334. [DOI] [PubMed] [Google Scholar]
- 8.Kwok WW, Roti M, Delong JH, Tan V, Wambre E, James EA, et al. Direct ex vivo analysis of allergen-specific CD4+ T cells. J Allergy Clin Immunol. 2010;125:1407–09. doi: 10.1016/j.jaci.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebner C, Ferreira F, Hoffmann K, Hirschwehr R, Schenk S, Szépfalusi Z, et al. T cell clones specific for Bet v I, the major birch pollen allergen, cross-react with the major allergens of hazel, Cor a I, and alder, Aln g I. Mol Immunol. 1993;30:1323–9. doi: 10.1016/0161-5890(93)90093-q. [DOI] [PubMed] [Google Scholar]
- 10.Novak EJ, Liu AW, Nepom GT, Kwok WW. MHC class II tetramers identify peptide-specific human CD4(+) T cells proliferating in response to influenza A antigen. J Clin Invest. 1999;104:63–7. doi: 10.1172/JCI8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwok WW, Gebe JA, Liu A, Agar S, Ptacek N, Hammer J, et al. Rapid epitope identification from complex class-II-restricted T-cell antigens. Trends Immunol. 2001;22:583–8. doi: 10.1016/s1471-4906(01)02038-5. [DOI] [PubMed] [Google Scholar]
- 12.Van Overtvelt L, Wambre E, Maillere B, von Hofe E, Louise A, Balazuc AM, et al. Assessment of Bet v 1-specific CD4+ T cell responses in allergic and non allergic individuals using MHC class II peptide tetramers. J Immunol. 2008;180:4514–22. doi: 10.4049/jimmunol.180.7.4514. [DOI] [PubMed] [Google Scholar]
- 13.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–5. [PubMed] [Google Scholar]
- 14.Malavasi F, Funaro A, Alessio M, DeMonte LB, Ausiello CM, Dianzani U, et al. CD38: a multi-lineage cell activation molecule with a split personality. Int J Clin Lab Res. 1992;22:73–80. doi: 10.1007/BF02591400. [DOI] [PubMed] [Google Scholar]
- 15.Cosmi L, Annunziato F, Galli MIG, Maggi RME, Nagata K, Romagnani S. CRTH2 is the most reliable marker for the detection of circulating human type 2 Th and type 2 T cytotoxic cells in health and disease. Eur J Immunol. 2000;30:2972–9. doi: 10.1002/1521-4141(200010)30:10<2972::AID-IMMU2972>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–83. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–46. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 18.Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–34. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP, et al. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol. 2010;11:83–9. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fritsch RD, Shen X, Sims GP, Hathcock KS, Hodes RJ, Lipsky PE. Stepwise differentiation of CD4 memory T cells defined by expression of CCR7 and CD27. J Immunol. 2005;175:6489–97. doi: 10.4049/jimmunol.175.10.6489. [DOI] [PubMed] [Google Scholar]
- 21.Mack DG, Lanham AM, Palmer BE, Maier LA, Fontenot AP. CD27 expression on CD4+ T cells differentiates effector from regulatory T cell subsets in the lung. J Immunol. 2009;182:7317–20. doi: 10.4049/jimmunol.0804305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rappl G, Schrama D, Hombach A, Meuer EK, Schmidt A, Becker JC, et al. CD7(-) T cells are late memory cells generated from CD7(+) T cells. Rejuvenation Res. 2008;11:543–56. doi: 10.1089/rej.2007.0612. [DOI] [PubMed] [Google Scholar]
- 23.Baars PA, Maurice MM, Rep M, Hooibrink B, van Lier RA. Heterogeneity of the circulating human CD4+ T cell population. Further evidence that the CD4+CD45RA-CD27− T cell subset contains specialized primed T cells. J Immunol. 1995;154:17–25. [PubMed] [Google Scholar]
- 24.Möbs C, Slotosch C, Loffler H, Jakob T, Hertl M, Pfutzner W. Birch pollen immunotherapy leads to differential induction of regulatory T cells and delayed helper T cell immune deviation. J Immunol. 2010;184:2194–03. doi: 10.4049/jimmunol.0901379. [DOI] [PubMed] [Google Scholar]
- 25.Aslam A, Chan H, Warrell DA, Misbah S, Ogg GS. Tracking antigen-specific T-cells during clinical tolerance induction in humans. PLoS One. 2010;5:e11028. doi: 10.1371/journal.pone.0011028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med. 2003;198:1369–80. doi: 10.1084/jem.20030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snyder CM, Cho KS, Bonnett EL, van Dommelen S, Shellam GR, Hill AB. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity. 2008;29:650–9. doi: 10.1016/j.immuni.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124(2):292–300. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fuller MJ, Zajac AJ. Ablation of CD8 and CD4 T cell responses by high viral loads. J Immunol. 2003;170:477–86. doi: 10.4049/jimmunol.170.1.477. [DOI] [PubMed] [Google Scholar]
- 30.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–27. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akdis CA, Blesken T, Akdis M, Wüthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nouri-Aria KT, Wachholz PA, Francis JN, Jacobson MR, Walker SM, Wilcock LK, et al. Grass pollen immunotherapy induces mucosal and peripheral IL-10 responses and blocking IgG activity. J Immunol. 2004;172:3252–9. doi: 10.4049/jimmunol.172.5.3252. [DOI] [PubMed] [Google Scholar]
- 33.Trinchieri G. Regulatory role of T cells producing both interferon gamma and interleukin 10 in persistent infection. J Exp Med. 2001;194:53–57. doi: 10.1084/jem.194.10.f53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–81. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 35.Gabrysová L, Nicolson KS, Streeter HB, Verhagen J, Sabatos-Peyton CA, Morgan DJ, et al. Negative feedback control of the autoimmune response through antigen-induced differentiation of IL-10-secreting Th1 cells. J Exp Med. 2009;206:1755–67. doi: 10.1084/jem.20082118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saraiva M, Christensen JR, Veldhoen M, Murphy TL, Murphy KM, O’Garra A. Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity. 2009;31:209–19. doi: 10.1016/j.immuni.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mantel PY, Kuipers H, Boyman O, Rhyner C, Ouaked N, Rückert B, et al. GATA3-driven Th2 responses inhibit TGF-beta1-induced FOXP3 expression and the formation of regulatory T cells. PLoS Biol. 2007;5:e329. doi: 10.1371/journal.pbio.0050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hadjur S, Bruno L, Hertweck A, Cobb BS, Taylor B, Fisher AG, et al. IL4 blockade of inducible regulatory T cell differentiation: the role of Th2 cells, Gata3 and PU.1. Immunol Lett. 2009;122:37–43. doi: 10.1016/j.imlet.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Pillemer BB, Qi Z, Melgert B, Oriss TB, Ray P, Ray A. STAT6 activation confers upon T helper cells resistance to suppression by regulatory T cells. J Immunol. 2009;183:155–63. doi: 10.4049/jimmunol.0803733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2007;104:18169–74. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 42.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.