Abstract
Treating high fat fed/low dose streptozotocin-diabetic rats; model of type 2 diabetes, with ilepatril (vasopeptidase inhibitor, blocks neutral endopeptidase (NEP) and angiotensin converting enzyme (ACE)) improved vascular and neural function. Next, studies were performed to determine the individual effect of inhibition of NEP and ACE on diabetes-induced vascular and neural dysfunction. High fat fed rats (8 weeks) were treated with 30 mg/kg streptozotocin (i.p.) and after 4 additional weeks, were treated for 12 weeks with ilepatril, enalapril (ACE inhibitor) or candoxatril (NEP inhibitor) followed by analysis of vascular and neural function. Glucose clearance was impaired in diabetic rats and was not improved with treatment although treatment with ilepatril or candoxatril partially improved insulin stimulated glucose uptake by isolated soleus muscle. Diabetes caused slowing of motor and sensory nerve conduction, thermal hypoalgesia, reduction in intraepidermal nerve fiber (IENF) profiles and impairment in vascular relaxation to acetylcholine and calcitonin gene-related peptide (CGRP) in epineurial arterioles of the sciatic nerve. Inhibition of NEP improved nerve conduction velocity and inhibition of NEP or ACE improved thermal sensitivity and protected IENF density. Ilepatril and candoxatril treatment of diabetic rats was efficacious in improving vascular responsiveness to acetylcholine in epineurial arterioles; whereas all three treatments improved vascular response to CGRP. These studies suggest that inhibition of NEP and ACE activity is an effective approach for treatment of type 2 diabetes neural and vascular complications.
Keywords: diabetes, diabetic neuropathy, vascular relaxation, neutral endopeptidase, vasopeptidase inhibitor, angiotensin converting enzyme
1. Introduction
Evidence suggests that there are at least five major pathways involved in the development of diabetic neuropathy: metabolic, vascular, immunologic, neurohormonal growth factor deficiency, and extracellular matrix remodeling (Brownlee, 2005). In light of the complicated etiologies, an effective treatment for diabetic neuropathy has not yet been identified. This may be due in part to the monotherapy approach that has been applied.
My laboratory has focused on the role of microvascular dysfunction in the development and progression of diabetic neuropathy. Studies in both types 1 and 2 diabetic rats have demonstrated that impaired vascular reactivity precedes the development of nerve dysfunction (Coppey, et al. 2000; 2002). Moreover, our studies have indicated that increased oxidative stress is a factor leading to impaired vascular function in diabetes (Coppey, et al. 2001a; 2001b). Based on these data we have focused our studies on therapies that reduce oxidative stress and improve vascular function. This has led to the study of the effect of vasopeptidase inhibitors (Davidson, et al. 2007; 2009; Oltman, et al. 2009). Vasopeptidase inhibitors simultaneously inhibit neutral endopeptidase and angiotensin converting enzyme activity (Weber, 1999). We and others have demonstrated that angiotensin converting enzyme inhibition is an effective treatment for diabetic vascular and neural complications (Reja, et al. 1995; Malik, et al. 1998; Coppey, et al. 2006). Neutral endopeptidase degrades vasoactive peptides including natriuretic peptides, adrenomedullin, bradykinin and calcitonin gene-related peptide and is found in many tissues (Pu and Schiffrin, 2001; Gonzalez, et al. 1998; Edwards, et al. 1999, Ebihara, et al. 2003; Broccolini, et al. 2006). Neutral endopeptidase activity has been shown to be activated by protein kinase C, which is increased in vascular tissues by diabetes (Suzki, et al. 2002; Kikkawa, et al. 2004). Therefore, vasopeptidase inhibitors are a likely treatment for vascular dysfunction.
We have previously reported that treating type 1 and 2 diabetic rats with ilepatril, a vasopeptidase inhibitor, improved vascular and neural complications (Davidson, et al. 2007; 2009; 2011). The objective of this study was to determine the individual role of neutral endopeptidase and angiotensin converting enzyme inhibition on vascular and neural complications in high fat fed/low dose streptozotocin diabetic rats.
The high fat fed/low dose streptozotocin diabetic rats are an animal model for type 2 diabetes (Reed, et al. 2000; Srinivasan, et al. 2005). Rats fed a high fat diet do not become hyperglycemic presumably due to compensatory hyperinsulinemia (Reed, et al. 2000). However, treating high fat fed rats with a low dose of streptozotocin damages insulin producing β-cells so that hyperglycemia develops even though insulin levels are similar or even higher than in chow fed normoglycemia rats (Reed, et al. 2000). The diabetes in these rats is analogous to the development of human type 2 diabetes when the decline in hyperinsulinemia is not able to compensate for insulin resistance and hyperglycemia occurs (Reed, et al. 2000). In our hands this rat models late stage type 2 diabetes (Davidson, et al. 2011).
2. Materials and methods
Unless stated otherwise all chemicals used in these studies were obtained from Sigma Chemical Co. (St. Louis, MO).
2.1. Animals
Male Sprague-Dawley (Harlan Sprague Dawley, Indianapolis, IN) rats 10–11 weeks of age were housed in a certified animal care facility and food (Harlan Teklad, #7001, Madison, WI) and water were provided ad libitum. All institutional (approval ACURF #0210257) and NIH guidelines for use of animals were followed. At 12 weeks of age the rats were separated into five groups. Four of these groups were placed on a high fat diet (D12451; Research Diets, New Brunswick, NJ). The high fat diet contained 24 gm% fat, 24 gm% protein and 41 gm% carbohydrate. The primary source of the increased fat content in the diet was soybean oil and lard. The remaining group was maintained on the control diet (Harlan Teklad, #7001, Madison, WI), which contained 4.25 gm% fat. Rats were maintained on the high fat diet for 8 weeks. Afterwards, these rats were treated with streptozotocin (30 mg/kg in 0.9% NaCl. i.p.). Diabetes was verified 96 h later by evaluating blood glucose levels with the use of glucose-oxidase reagent strips (Lifescan Inc., Milpitas, CA). Rats having blood glucose level of 11.1 mM or greater were considered to be diabetic. These four groups of rats were maintained on the high fat diet for an additional 4 weeks. Afterwards, three of the diabetic groups were placed on the high fat diet containing enalapril, ((ACE inhibitor) MERCK 500 mg/kg in the diet), candoxatril ((neutral endopeptidase inhibitor) Pfizer 300 mg/kg in the diet) or ilepatril, (vasopeptidase inhibitor), Sanofi Aventis 500 mg/kg in the diet). The dose of the drugs used in this study was based on previous studies and pharmacokinetic data (Azizi, et al. 2006; Coppey, et al. 2001b; 2006; Davidson, et al. 2007; Newaz, et al. 2010). The other group of diabetic rats remained on the high fat diet. These diets were maintained for 12 weeks.
2.2. Glucose tolerance and insulin stimulated glucose uptake by isolated soleus muscle
Glucose tolerance was determined by injecting rats with a saline solution containing 2 g/kg glucose, i.p., after an overnight fast. Rats were briefly anesthetized with isoflurane and the glucose solution was injected. Immediately prior to the glucose injection and at 15, 30, 45, 60, 120, 180 and 240 min blood samples from the tip of the tail that was lanced were taken to measure circulating glucose levels using glucose oxidase reagent strips (Lifescan Inc., Milpitas, CA). Fasting basal levels of insulin and leptin was also determined using Luminex technology (Davidson, et al. 2010). Briefly, 10 μL of standards or sample was mixed with reagent buffer and placed in the appropriate wells of the 96 well plate. Afterwards, 25 μL of the mixed beads containing antibody to the protein of interest and the plate was agitated on a plate shaker for 16 h at 4° C. After washing, the detection antibody was added and incubated for 1 h with agitation. After washing, Sheath Fluid was added to each well to resuspend the beads and the plate was run on the Luminex system. Glucose uptake by isolated soleus muscle was determined as described by Bouskila et al (2008) and Rogers et al. (2009). Briefly, isolated soleus muscle were suspended in 10 ml Kreb-Ringer bicarbonate buffer containing (in mM) 117 NaCl, 4.7 KCl, 2.5 CaCl2 2H2O, 1.2 KH2PO4, 1.2 MgSO4 7H2O, 24.6 NaHCO3, pH 7.5, plus 5.6 mM glucose at 37°C. The buffer was continually gassed by bubbling with a mixture of 95% O2 and 5% CO2. After a 30 min equilibration period the muscle was incubated for an additional 30 min with or without 100 nM insulin. Afterwards, [3H]-2-deoxyglucose (0.5 μCi/ml) and [14C]-mannitol (0.5 μCi/ml) was added and incubation continued for 45 min. Following this incubation muscle was snap frozen in liquid N2 to terminate glucose uptake and the frozen muscle solubilized in 1M NaOH at 80°C and neutralized with 1 M HCl. Radioactivity in muscle samples was determined by dual label liquid scintillation counting and corrected for extracellular and intracellular space and tissue weight. Data was presented as insulin stimulated glucose uptake over basal. Determination of diacylglycerol and ceramide in isolated soleus muscle was performed according to Priess et al. (1986) as modified by Bielawska et al. (2001) using the diglyceride kinase assay.
2.3. Thermal nociceptive response
Thermal nociceptive response in the hindpaw was measured using the Hargreaves method as previously described (Oltman, et al. 2008). Briefly, the rat was placed in the observation chamber on top of the thermal testing apparatus and allowed to acclimate to the warmed glass surface (30°C) and surroundings for a period of 15 min. The mobile heat source was maneuvered so that it was under the heal of the hindpaw and then activated, a process that activates a timer and locally warms the glass surface, when the rat withdrew its paw, the timer, and the heat source was turned off and the time was recorded. The timer was defaulted to go off after 25 sec to avoid injury to the rat. Following an initial recording, which was discarded, two measurements were made for each hindpaw, with a rest period of 5 min between each measurement. The mean of the measurements reported in sec were used as the thermal nociceptive response.
2.4. Motor and sensory nerve conduction velocity and biological and oxidative stress markers
On the day of terminal studies rats were weighed and anesthetized with Nembutal i.p. (50 mg/kg, i.p., Abbott Laboratories, North Chicago, IL). Non-fasting blood glucose was determined. Hemoglobin A1C levels were determined using a Glyco-tek affinity column kit (Helena Laboratories, Beaumont, TX). Serum samples were collected for determination of free fatty acid, triglyceride and free cholesterol, using commercial kits from Roche Diagnostics, Mannheim, Germany; Sigma Chemical Co., St. Louis, MO; and Bio Vision, Mountain View, CA, respectively. Serum thiobarbituric acid reactive substances levels were determined as a marker of oxidative stress as previously described (Oltman, et al. 2005). Briefly, 200 μl of serum was boiled in 0.75 ml of phosphoric acid (0.19 M), 0.25 ml thiobarbituric acid (0.42 mM) and 0.3 ml water for 60 min. Afterwards, the samples were precipitated with methanol/NaOH and centrifuged for 5 min. The supernatant was measured fluorometrically at excitation wavelength of 532 nm and emission wavelength of 553 nm. Standards were prepared by the acid hydrolysis of 1,1,3,3-tetraethoxypropane. The data was reported as μg/ml serum. ACE activity in the serum was quantitated using a colorimetric assay kit from ALPCO diagnostics (Windham, NH) and the data presented as mU/ml serum (Coppey, et al. 2006). One unit of ACE activity is defined as the amount of enzyme required to release one μmol of hippuric acid per minute and per liter of serum at 37°C.
Motor nerve conduction velocity (MNCV) was determined as previously described using a noninvasive procedure in the sciatic-posterior tibial conducting system (Coppey, et al. 2000). The left sciatic nerve was stimulated first at the sciatic notch and then at the Achilles tendon. Stimulation consisted of single 0.2-ms supramaximal (8 V) pulses through a bipolar electrode (Grass S44 Stimulator, Grass Medical Instruments, Quincy, MA). The evoked potentials were recorded from the interosseous muscle with a unipolar platinum electrode and displayed on a digital storage oscilloscope (model 54600A, Hewlett Packard, Rolling Meadows, IL). MNCV was calculated by subtracting the distal from the proximal latency measured in milliseconds from the stimulus artifact of the take-off of the evoked potential and the difference was divided into the distance between the 2 stimulating electrodes measured in millimeters using a Vernier caliper Sensory nerve conduction velocity (SNCV) was determined using the digital nerve as described by Obrosova et al (Obrosova, et al. 2004). Briefly, hindlimb SNCV was recorded in the digital nerve to the second toe by stimulating with a square-wave pulse of 0.05-ms duration using the smallest intensity current that resulted in a maximal amplitude response. The sensory nerve action potential was recorded behind the medial malleolus. Sixteen responses were averaged to obtain the position of the negative peak. The maximal SNCV was calculated by measuring the latency to the onset/peak of the initial negative deflection and the distance between stimulating and recording electrodes. The MNCV and SNCV were reported in meters per second.
2.5. Intraepidermal nerve fiber density in the hindpaw
Immunoreactive intraepidermal nerve fiber profiles, which are primarily sensory nerves, were visualized using confocal microscopy. Samples of skin of the right hindpaw were fixed, dehydrated and embedded in paraffin. Sections (7 μm) were collected and immuno stained with anti-PGP9.5 antibody (rabbit anti human, AbD Serotic, Morpho Sys US Inc., Raleigh, NC) over night followed by treatment with secondary antibody Alexa Fluor 546 goat anti rabbit (Invitrogen, Eugene, OR). Profiles were counted by two individual investigators that were blinded to the sample identity. All immunoreactive profiles within the epidermis were counted and normalized to epidermal length (Beiswenger, et al. 2008; Davidson, et al. 2010).
2.6. Vascular reactivity in epineurial arterioles
Videomicroscopy was used to investigate in vitro vasodilatory responsiveness of epineurial arterioles vascularizing the region of the sciatic nerve as previously described (Coppey, et al. 2000; 2001b; 2006). The vessels used for these studies were generally oriented longitudinally in relation to the sciatic nerve; however, radially oriented vessels were also used on occasion. The arterioles used in this study should be regarded as epineurial rather than perineurial vessels. To isolate these vessels, the common iliac was exposed, and the branch points of the internal pudendal and superior gluteal arteries were identified. The vessels were then clamped, and tissue containing these vessels and the branches at the internal pudendal and superior gluteal arteries were dissected en bloc. The block of tissue was immediately submerged in a cooled (4°C), oxygenated (20% O2, 5% CO2, and 75% N2) Krebs-Henseleit physiological saline solution (PSS) of the following composition (in millimoles per liter): NaCl 118, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 20, Na2EDTA 0.026, and glucose 5.5. Branches of the superior gluteal and internal pudendal arteries (60- to 100-μm internal diameter and 1–2 mm in length) were carefully dissected and trimmed of fat and connective tissue. Both ends of the isolated vessel segment were cannulated with glass micropipettes filled with PSS (4°C) and secured with 10–0 nylon Ethilon monofilament sutures (Ethicon, Cornelia, GA). The pipettes were attached to a single pressure reservoir (initially set at 0 mmHg) under condition of no flow. The organ chamber containing the cannulated vessels was then transferred to the stage of an inverted microscope (CK2; Olympus, Lake Success, NY). Attached to the microscope were a closed-circuit television camera (WV-BL200; Panasonic, Secaucus, NJ), a video monitor (Panasonic), and a video caliper (VIA-100K; Boeckeler Instruments, Tucson, AZ). The organ chamber was connected to a rotary pump (Masterflex; Cole Parmer Instrument, Vernon Hills, IL), which continuously circulated 37°C oxygenated PSS at 30 ml/min. The pressure within the vessel was then slowly increased to 40 mmHg. At this pressure, we found that KCl gave the maximal constrictor response. Therefore, all of the studies were conducted at 40 mmHg. Internal vessel diameter (resolution of 2 μm) was measured by manually adjusting the video micrometer. After a 30-min equilibration, KCl was added to the bath to test vessel viability. Vessels failing to constrict by at least 30% were discarded. After they were washed with PSS, vessels were incubated for 30 min in PSS and then constricted with U46619 (10−8 to 10−7 mol/l) (Cayman Chemical, Ann Arbor, MI) to 30–50% of passive diameter. Afterwards, cumulative concentration-response relationships were evaluated for acetylcholine (10−8 – 10−4 M) and calcitonin gene-related peptide (10−11 – 10−8 M) using vessels from each group of rats. At the end of each dose response curve for acetylcholine or calcitonin gene-related peptide papaverine (10−5 M) was added to determine maximal vasodilation.
2.7. Data Analysis
Results are presented as mean ± S.E.M. Comparisons between the treatment groups and control and non-treated diabetic rats were conducted using one-way ANOVA and Bonferroni post test comparison (Prism software; GraphPad, San Diego, CA). Concentration response curves for acetylcholine and calcitonin gene-related peptide were compared using a two-way repeated measures analysis of variance with autoregressive covariance structure using proc mixed program of SAS (Coppey, 2001b; 2006). A P value of less than 0.05 was considered significant.
3. Results
3.1. Effect of treatment of high fat/streptozotocin diabetic rats with enalapril, candoxatril or ilepatril on weight and blood glucose
Data in Table 1 demonstrate that untreated or treated diabetic rats failed to gain weight as compared to non-diabetic control rats (Table 1). However, only the diabetic rats treated with enalapril weighed significantly less than control rats at the end of the study period. All diabetic rats were hyperglycemic at the end of the study period as indicated by significantly elevated blood glucose and hemoglobin A1C levels (Table 1). Treatment of diabetic rats did not significantly change blood glucose levels compared to untreated diabetic rats.
Table 1.
Effect of Treatment of High Fat/Streptozotocin Diabetic Rats with enalapril, candoxatril or ilepatril on Change in Body Weight, Blood Glucose and Hemoglobin A1C
| Determination | Control (9) | Diabetic (9) | Diabetic + enalapril (8) | Diabetic + candoxatril (9) | Diabetic + ilepatril (8) |
|---|---|---|---|---|---|
| Start weight (g) | 343 ± 4 | 350 ± 5 | 335 ± 2 | 336 ± 3 | 340 ± 2 |
| End weight (g) | 505 ± 13 | 446 ± 14 | 396 ± 6a | 474 ± 28 | 432 ± 17 |
| Blood glucose (mg/dl) | 108 ± 6 | 457 ± 23a | 583 ± 11a | 330 ± 61a | 481 ± 59a |
| Hb A1C (%) | 6.6 ± 0.5 | 16.6 ± 0.5a | 20.5 ± 1.1a | 12.4 ± 1.2a | 17.8 ± 1.8a |
Data are presented as the mean ± S.E.M.
P < 0.05 compared to control. Parentheses indicate the number of experimental animals.
3.2. Effect of treatment of high fat/streptozotocin diabetic rats with enalapril, candoxatril or ilepatril on serum lipid and thiobarbituric acid reactive substances levels and ACE activity
Data in Table 2 demonstrate that serum thiobarbituric acid reactive substances, a marker for oxidative stress, were significantly increased in diabetic rats. Treating diabetic rats with enalapril, candoxatril or ilepatril reduced serum thiobarbituric acid reactive substances but only in ilepatril treated rats was this difference significant compared to untreated diabetic rats. Diabetes caused a significant increase in serum triglycerides, free fatty acids and cholesterol levels. Treating diabetic rats with candoxatril or ilepatril but not enalapril reduced serum triglyceride levels but the decrease was not significant compared to untreated diabetic rats. Treatment of diabetic rats with enalapril, candoxatril or ilepatril did not lower serum free fatty acid levels. Treating diabetic rats with ilepatril significantly decreased serum cholesterol levels compared to untreated diabetic rats. Serum cholesterol levels were also lowered in diabetic rats treated with enalapril but this difference was not significant compared to untreated diabetic rats. Treating diabetic rats with enalapril or ilepatril lowered serum ACE activity compared to control and untreated diabetic rats. This indicates that the dose used for enalapril and ilepatril in these studies was an effective dose based on the inhibition of angiotensin converting enzyme. There is no assay available to confirm whether the dose of candoxatril used in these studies was sufficient to inhibit neutral endopeptidase in vivo although candoxatril treatment resulted in effects that were similar to that of ilepatril suggesting that the dose of candoxatril was within the effective range. As previously shown serum insulin and leptin levels were not significantly changed between control and untreated and treated diabetic rats (Davidson et al. 2011)
Table 2.
Effect of Treatment of High Fat/Streptozotocin Diabetic Rats with enalapril, candoxatril or ilepatril on Serum Thio Barbituric Acid Reactive Substances, Triglycerides, Free Fatty Acids, Cholesterol and ACE activity
| Determination | Control (9) | Diabetic (9) | Diabetic + enalapril (8) | Diabetic + candoxatril (9) | Diabetic + ilepatril (8) |
|---|---|---|---|---|---|
| Thiobarbituric acid reactive substances (μg/ml) | 0.82 ± 0.04 | 1.07 ± 0.07a | 0.97 ± 0.10 | 0.87 ± 0.07 | 0.83 ± 0.07b |
| Triglycerides (mg/dl) | 29 ± 3 | 107 ± 19a | 86 ± 17a | 65 ± 16 | 61 ± 15 |
| Free fatty acids (mmol/l) | 0.08 ± 0.02 | 0.41 ± 0.05a | 0.46 ± 0.10a | 0.35 ± 0.06a | 0.38 ± 0.04a |
| Cholesterol (mg/ml) | 1.8 ± 0.3 | 4.5 ± 0.8a | 2.2 ± 0.3 | 3.7 ± 0.5 | 1.8 ± 0.3b |
| ACE (mU/ml/min) | 54 ± 3 | 89 ± 4a | 2 ± 1a,b | 78 ± 5 | 16 ± 2a,b |
Data are presented as the mean ± S.E.M.
P < 0.05 compared to control;
p < 0.05 compared to diabetic. Parentheses indicate the number of experimental animals.
3.3. Effect of treatment of high fat/streptozotocin diabetic rats with enalapril, candoxatril or ilepatril on glucose tolerance
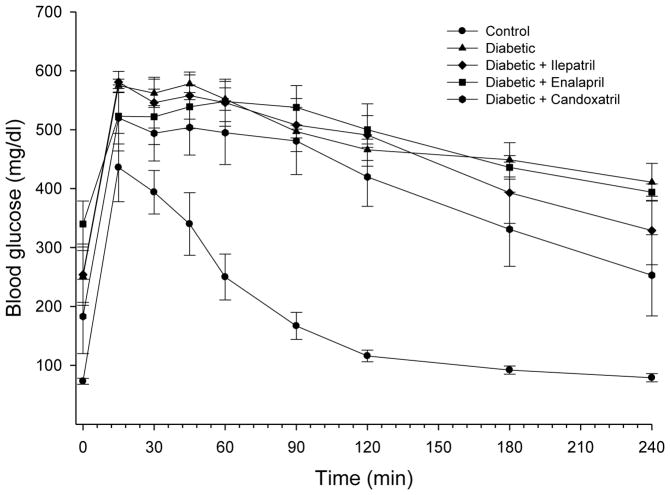
Data in Fig. 1 demonstrate that glucose utilization is significantly impaired in diabetic rats. Treating diabetic rats with enalapril, candoxatril or ilepatril did not improve glucose utilization. To examine the effect of treatment of high fat/streptozotocin diabetic rats on insulin stimulated glucose utilization we examined insulin-stimulated glucose uptake by isolated soleus muscle from control and high fat/streptozotocin diabetic rats treated without or with enalapril, candoxatril or ilepatril. Insulin (100 nM) stimulated glucose uptake by soleus muscle by 1.99 ± 0.02, 1.47 ± 0.02* 1.51 ± 0.04*, 1.65 ± 0.02 and 1.72 ± 0.05 fold for control and diabetic rats treated without or with enalapril, candoxatril or ilepatril, respectively (mean ± S.E.M. from 8–9 individual rats for each group, * P < 0.05, compared to control).
Fig. 1. Effect of treating high fat/streptozotocin diabetic rats with enalapril, candoxatril ilepatril on glucose tolerance.
Glucose tolerance was determined as described in the Methods section. Data are presented as the mean ± S.E.M. in mg/dl. The area under the curve was significantly different for high fat/streptozotocin diabetic rats (p < 0.01), high fat/streptozotocin diabetic rats treated with enalapril (p < 0.01), candoxatril (p < 0.05) or ilepatril (p < 0.05) vs. control. The number of rats in each group was the same as shown in Table 1.
3.4. Effect of treatment of high fat/streptozotocin diabetic rats with enalapril, candoxatril or ilepatril on diacylglycerol and ceramide levels in soleus muscle
Data in Table 4 demonstrate that ceramide levels are significantly increased in soleus muscle from diabetic rats. Likewise diacylglycerol levels in soleus muscle from diabetic rats are increased but the difference was not significant compared to control rats. Treating diabetic rats with candoxatril or ilepatril significantly decreased ceramide levels in soleus muscle. Ceramide levels in soleus muscle were also decreased in diabetic rats treated with enalapril but this difference was not significant compared to untreated diabetic rats. Soleus muscle diacylglycerol levels were corrected toward control levels in treated diabetic rats.
3.5. Effect of treatment of high fat/streptozotocin diabetic rats with enalapril, candoxatril or ilepatril on nerve conduction velocity, thermal nociception and intraepidermal nerve fiber density
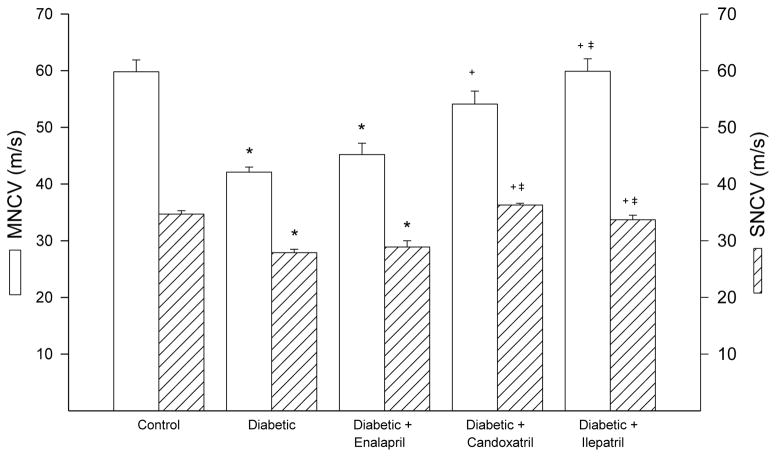
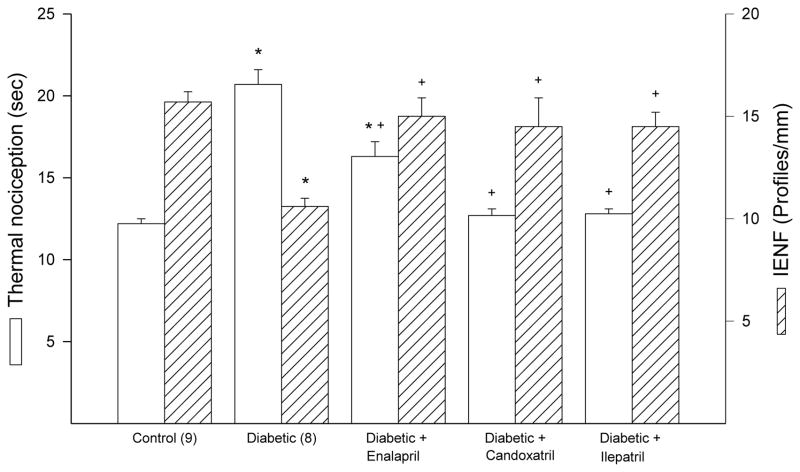
Motor and sensory nerve conduction velocity was significantly decreased diabetic rats and this was significantly improved by treatment with candoxatril or ilepatril (Fig. 2). In contrast, treating diabetic rats with enalapril did not improve motor or sensory nerve conduction velocity (Fig. 2). Data in Fig. 3 demonstrate that diabetic rats are hypoalgesic to thermal stimuli and this was significantly improved when diabetic rats were treated with enalapril, candoxatril or ilepatril. However, the difference in thermal nociception between control and diabetic rats treated with enalapril remained significant. Intraepidermal nerve fiber profiles in the hindpaw of diabetic rats are significantly decreased and this was prevented by treatment with enalapril, candoxatril or ilepatril (Fig. 3).
Fig. 2. Effect of treating high fat/streptozotocin diabetic rats with enalapril, candoxatril ilepatril on motor and sensory nerve conduction velocity.
Motor and sensory nerve conduction velocity was examined as described in the Methods section. Data are presented as the mean ± S.E.M. in m/sec. The number of rats in each group was the same as shown in Table 1. * p < 0.05 compared to control rats; + p < 0.05 compared to diabetic rats; ‡ p < 0.05 compared to diabetic + enalapril rats.
Fig. 3. Effect of treating high fat/streptozotocin diabetic rats with enalapril, candoxatril ilepatril on thermal nociception and intraepidermal nerve fiber density.
Thermal nociception and intraepidermal nerve fiber density was examined as described in the Methods section. Data are presented as the mean ± S.E.M. for thermal nociception in sec and intraepidermal nerve fiber profiles per mm. The number of rats in each group was the same as shown in Table 1. * P < 0.05 compared to control rats; + P < 0.05 compared to diabetic rats.
3.6. Effect of treatment of high fat/streptozotocin diabetic rats with enalapril, candoxatril or ilepatril on vascular relaxation by epineurial arterioles
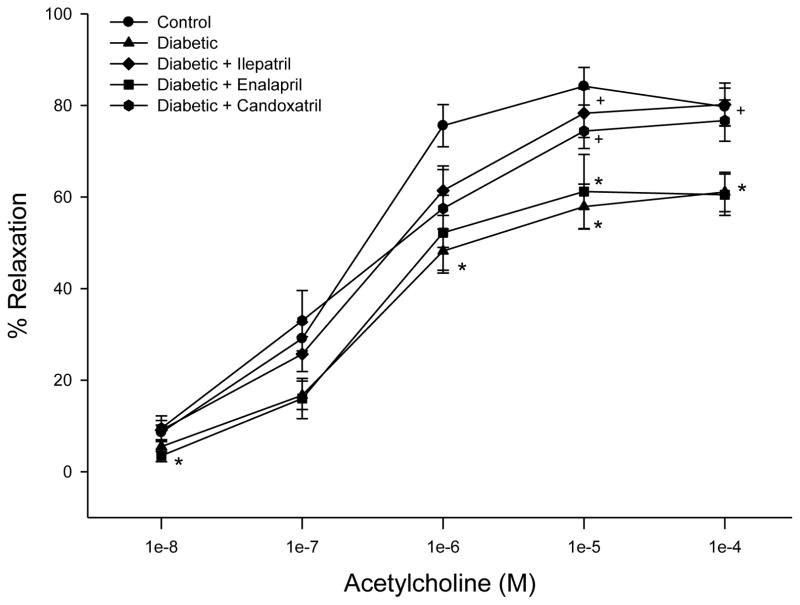
We have previously demonstrated that streptozotocin-induced diabetes caused a decrease in acetylcholine- and calcitonin gene-related peptide-mediated vascular relaxation that was improved with treatment using ilepatril or enalapril (Coppey, et al. 2006; Davidson, et al. 2007). We have also previously shown that treating high fat/streptozotocin diabetic rats with ilepatril improved vascular relaxation to acetylcholine and calcitonin gene-related peptide (Davidson, et al. 2011). In this study we compared the effect of treating high fat/streptozotocin diabetic rats with enalapril or candoxatril on acetylcholine- and calcitonin gene-related peptide-mediated vascular relaxation (Fig. 4 and Fig. 5). Treatment of diabetic rats with ilepatril or candoxatril significantly improved acetylcholine-mediated vascular relaxation compared to untreated diabetic rats (Fig. 4). In contrast, treatment of diabetic rats with enalapril did not improve acetylcholine-mediated vascular relaxation in epineurial arterioles (Fig. 4).
Fig. 4. Effect of treating high fat/streptozotocin diabetic rats with enalapril, candoxatril ilepatril on vascular relaxation by acetylcholine in epineurial arterioles.
Pressurized arterioles (40 mm Hg and ranging from 60–100 μm luminal diameter) were constricted with U46619 (30–50%) and incremental doses of acetylcholine were added to the bathing solution while recording steady state vessel diameter. Data are presented as the mean of % relaxation ± S.E.M. For these studies two vessels were collected from each rat, studied and the data combined. The number of rats in each group was the same as shown in Table 1. * P < 0.05, compared to control; + P < 0.05, compared to diabetic rats.
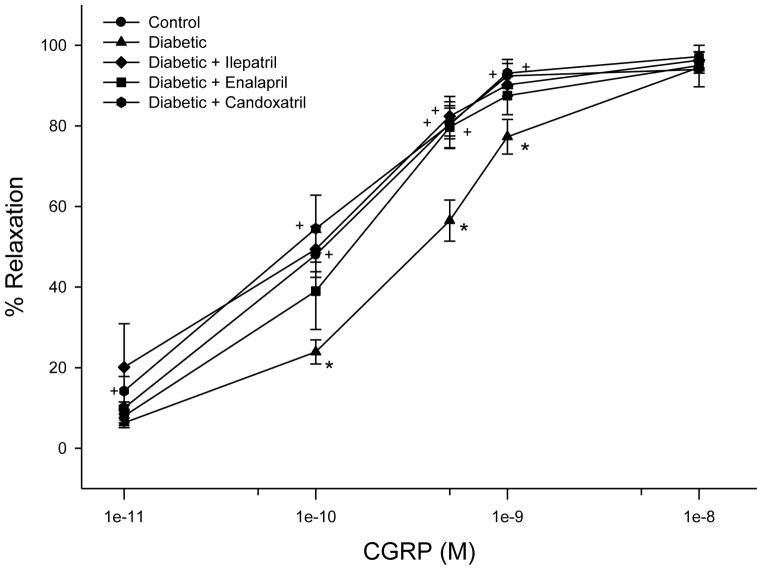
Fig. 5. Effect of treating high fat/streptozotocin diabetic rats with enalapril, candoxatril ilepatril on vascular relaxation by calcitonin gene-related peptide in epineurial arterioles.
Arterioles were treated as described in Fig. 4. Incremental doses of calcitonin gene-related peptide (CGRP) were added to the bathing solution while recording steady state vessel diameter. Data are presented as the mean of % relaxation ± S.E.M. The number of rats in each group was the same as shown in Table 1. * P < 0.05, compared to control rats; + P < 0.05, compared to diabetic rats.
Fig. 5 provides data on the effect of diabetes and treatments on vascular relaxation mediated by calcitonin gene-related peptide in epineurial arterioles. Calcitonin gene-related peptide is the most potent vasodilator of epineurial arterioles that we have identified (Yorek, et al. 2004). Diabetes causes a significant decrease in vascular relaxation to calcitonin gene-related peptide and this was significantly improved by treating diabetic rats with enalapril, candoxatril or ilepatril.
Diabetes caused a significant decrease in blood flow in the sciatic nerve and all treatments corrected this deficit with blood flow in ilepatril treated rats exceeding the blood flow in control rats by about 2 fold (data not shown).
4. Discussion
The purpose of these studies was to determine the individual role of angiotensin converting enzyme and neutral endopeptidase activity on vascular and nerve complications that occur in high fat fed/low dose streptozotocin-diabetic rats. We had previously demonstrated that dual inhibition of angiotensin converting enzyme and neutral endopeptidase activity by the vasopeptidase inhibitor ilepatril prevented vascular and neural dysfunction in diabetic rats (Davidson, et al. 2007; 2011). In this study we found that inhibition of neutral endopeptidase activity was more effective than inhibition of angiotensin converting enzyme activity in preventing slowing of motor and sensory nerve conduction velocity in this type 2 diabetic rat model. Inhibition of angiotensin converting enzyme or neutral endopeptidase activity was similarly effective in protecting intraepidermal nerve fiber density. However, inhibition of neutral endopeptidase activity was more effective than inhibition of angiotensin converting enzyme activity in preserving thermal sensitivity of the hindpaw. Vascular relaxation to acetylcholine and calcitonin gene-related peptide was impaired significantly in untreated diabetic rats. Treating diabetic rats with an angiotensin converting enzyme inhibitor did not improve vascular relaxation to acetylcholine. In contrast, treatment of diabetic rats with neutral endopeptidase inhibitor significantly improved vascular relaxation in response to acetylcholine. Treatment of diabetic rats with either angiotensin converting enzyme inhibitor or neutral endopeptidase inhibitor significantly improved vascular relaxation to calcitonin gene-related peptide.
Inhibiting angiotensin converting enzyme and/or neutral endopeptidase provided positive benefits toward improving diabetes-induced vascular and neural complications. In contrast, these treatments were not effective in improving insulin resistance as measured by glucose clearance. However, treating diabetic rats with candoxatril or ilepatril but not enalapril did improve insulin-stimulated glucose uptake by isolated soleus muscle. This latter result is in agreement with previous studies performed with obese Zucker rats. Arbin, et al. (2001) found that dual inhibition of angiotensin converting enzyme and neutral endopeptidase improved insulin mediated glucose disposal more effectively than monotherapy and this effect was linked to increased activation of the kinin-nitric oxide pathway. In a similar independent study it was found that Omapatrilat, a vasopeptidase inhibitor, induced insulin sensitization and increased myocardial glucose uptake in obese Zucker rats and that the effect of Omapatrilat was greater than Ramipril in part due to stimulation of the B2 receptor (Wang, et al. 2003). Later this group reported that treatment of obese Zucker rats with a vasopeptidase inhibitor increased muscle glucose uptake independent of insulin signaling (Wong, et al. 2006). In two of these studies protection of bradykinin from degradation by neutral endopeptidase was found to improve insulin action (Arbin, et al. 2001; Wang, et al. 2003). We attribute the lack of effect of treating diabetic rats with candoxatril or ilepatril on glucose clearance to the inability of these rats to produce/release a sufficient amount of insulin in order to improve glucose utilization in vivo. The high fat fed/low dose streptozotocin diabetic rat models late stage type 2 diabetes and pancreatic production of insulin is probably insufficient to reduce blood glucose levels following an administration of a glucose load (Reed, et al. 2000; Davidson, et al. 2011). Interestingly, it has been shown that natriuretic peptides promote muscle mitochondrial biogenesis and fat oxidation as to prevent obesity and glucose intolerance (Miyashita, et al. 2009). The natriuretic peptides are degraded by neutral endopeptidase (Gonzalez, et al. 1998; Potter, 2011). Because neutral endopeptidase is expressed in skeletal muscle in relatively large amounts and being located on the cell surface, neutral endopeptidase is able to hydrolyze peptides in the vicinity of their receptors thereby neutralizing their bioactivity (Yorek, 2008; Broccolini, et al. 2006). Improved bioactivity of bradykinin and natriuretic peptides may also explain why levels of ceramide and diacylglycerol in soleus muscle are reduced in candoxatril or ilepatril treated rats compared to untreated diabetic rats. Increased levels of ceramide and diacylglycerol in muscle are implicated in insulin resistance and perturbing insulin signaling pathways (DeFronzo, 2010; Zhang, et al. 2011; Eckardt, et al. 2011). Since bradykinin and natriuretic peptides may have a role in regulating glucose and fatty acid metabolism by muscle protecting their bioactive function by preventing degradation may reduce levels of ceramide and diacylglycerol in muscle and improve insulin signaling and could provide a therapeutic approach for treatment of obesity and type 2 diabetes (Yorek, 2008; Broccolini, et al. 2006).
We have obtained conflicting results regarding the efficacy of treatment of neuropathy with enalapril compared to candoxatril or ilepatril in type 1 compared to type 2 diabetic rats. In type 1 streptozotocin diabetic rats enalapril treatment improved nerve conduction velocity, thermal sensitivity and intraepidermal nerve fiber density (Oltman, et al. 2011). However, treatment with ilepatril was found to be more efficacious then enalapril in improving sensory nerve function (Oltman, et al. 2011). In these studies enalapril treatment failed to improve motor nerve conduction and was partially successful improving sensory nerve function. Like in type 1 diabetic rats treatment with ilepatril of high fat/low dose streptozotocin diabetic rats improved both motor and sensory nerve function. There could be multiple reasons for this outcome. In these studies treatment of diabetic rats with ilepatril tended to reduce serum triglyceride levels and thio barbituric acid reactive substances, a serum marker for oxidative stress, to a greater degree than treatment with enalapril. In addition, treatment with ilepatril significantly improved vascular relaxation to acetylcholine in epineurial arterioles whereas; enalapril treatment did not improve acetylcholine-mediated vasodilation. These results could indicate that the enalapril treated diabetic rats remain under metabolic and oxidative stress and this may result in minimal to less than optimal improvement in nerve function.
The lack of improvement in vascular relaxation of epineurial arterioles to acetylcholine in diabetic rats treated with enalapril compared to diabetic rats treated with candoxatril or ilepatril is likely due to protection of C-type natriuretic peptide. We have previously demonstrated that epineurial arterioles express C-type natriuretic peptide in the endothelium and that C-type natriuretic peptide likely is responsible for the endothelium-derived hyperpolarizing factor component of acetylcholine-mediated vascular relaxation (Davidson, et al. 2007). Neutral endopeptidase degrades natriuretic peptides thereby reducing their biological activity (Gonzalez, et al. 1998; Potter, 2011). We have shown that diabetes causes an increased expression of neutral endopeptidase in epineurial arterioles (Davidson, et al. 2007). Therefore, increased expression of neutral endopeptidase would likely cause an increased degradation of C-type natriuretic peptide limiting the biological activity of C-type natriuretic peptide and reducing acetylcholine-mediated vascular reactivity. In contrast, inhibition of angiotensin converting enzyme or neutral endopeptidase was effective in improving the diabetes-induced impairment in vascular relaxation to calcitonin gene-related peptide. One possible explanation for this is that relaxation to calcitonin gene-related peptide is independent of the endothelium and that inhibition of angiotensin converting enzyme or neutral endopeptidase protected the bioactivity of the smooth muscle layer to calcitonin gene-related peptide.
5. Conclusions
Studies were performed on the effect of treating high fat/streptozotocin diabetic rats, an animal model for late stage type 2 diabetes, with enalapril, candoxatril or ilepatril on vascular reactivity and nerve function. We found that inhibition of neutral endopeptidase improved nerve conduction velocity and acetylcholine-mediated vascular relaxation of epineurial arterioles; whereas inhibition of angiotensin converting enzyme provided little improvement of these endpoints. In contrast, inhibition of neutral endopeptidase or angiotensin converting enzyme improved thermal hypoalgesia, innervation of the skin of the hindpaw and cacitonin gene-related peptide-mediated vascular relaxation of epineurial arterioles. Overall, inhibition of both angiotensin converting enzyme and neutral endopeptidase may be an effective approach for treatment of diabetic vascular and neural complications.
Table 3.
Effect of Treatment of High Fat/Streptozotocin Diabetic Rats with enalapril, candoxatril or ilepatril on Soleus Muscle Diacylglycerol and Ceramide levels
| Determination | Control (9) | Diabetic (9) | Diabetic + enalapril (8) | Diabetic + candoxatril (9) | Diabetic + ilepatril (8) |
|---|---|---|---|---|---|
| Diacylglycerol (nmol/mg wet wt) | 4.1 ± 0.6 | 7.6 ± 0.5 | 4.3 ± 1.1 | 4.6 ± 1.6 | 4.3 ± 0.9 |
| Ceramide (pmol/mg wet wt) | 295 ± 16 | 715 ± 65a | 409 ± 37 | 314 ± 26b | 272 ± 17b |
Data are presented as the mean ± S.E.M.
P < 0.05 compared to control;
p < 0.05 compared to diabetic. Parentheses indicate the number of experimental animals.
Acknowledgments
This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development, by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK073990 from NIH and by a research grant from the Juvenile Diabetes Research Foundation.
Footnotes
The content of this manuscript are new and solely the responsibility of the authors and do not necessarily represent the official views of the granting agencies. The authors have no conflicts of interest to report. The authors would like to extend their appreciation to Sanofi Aventis, MERCK, and Pfizer for supplying ilepatril, enalapril and candoxatril, respectively for these studies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arbin V, Claperon N, Fournie-Zaluski MC, Roques BP, Peyroux J. Effects of combined neutral endopeptidase 24–11 and angiotensin-converting enzyme inhibition on femoral vascular conductance in streptozotocin-induced diabetic rats. Br J Pharmacol. 2000;130:1297–1304. doi: 10.1038/sj.bjp.0703442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizi M, Bissery A, Peyrard S, Guyene TT, Ozoux ML, Floch A, Menard J. Pharmacokinetics and pharmacodynamics of the vasopeptidase inhibitor AVE7688 in humans. Clin Pharmacol Ther. 2006;79:49–61. doi: 10.1016/j.clpt.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Beiswenger KK, Calcutt NA, Mizisin AP. Epidermal nerve fiber quantification in the assessment of diabetic neuropathy. Acta Histochem. 2008;110:351–362. doi: 10.1016/j.acthis.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielawska A, Perry DK, Hannun YA. Determination of ceramides and diglycerides by the diglyceride kinase assay. Analytical Biochem. 2001;298:141–150. doi: 10.1006/abio.2001.5342. [DOI] [PubMed] [Google Scholar]
- Bouskila M, Hirshman MF, Jensen J, Goodyear LJ, Sakamoto K. Insulin promotes glycogen synthesis in the absence of GSK3 phosphorylation in skeletal muscle. Am J Physiol Endocrinol Met. 2008;294:E28–35. doi: 10.1152/ajpendo.00481.2007. [DOI] [PubMed] [Google Scholar]
- Broccolini A, Gidaro T, Morosetti R, Gliubizzi C, Servidei T, Pescatori M, Tonali PA, Ricci E, Mirabella M. Neprilysin participates in skeletal muscle regeneration and is accumulated in abnormal muscle fibres of inclusion body myositis. J Neurochem. 2006;96:777–789. doi: 10.1111/j.1471-4159.2005.03584.x. [DOI] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications, a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Coppey LJ, Davidson EP, Dunlap J, Lund DD, Yorek MA. Slowing of motor nerve conduction velocity in streptozotocin-induced diabetic rats is preceded by impaired vasodilation in arterioles that provide circulation to the sciatic nerve. Int J Exp Diabetes Res. 2000;1:131–143. doi: 10.1155/EDR.2000.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Yorek MA. Effect of treating streptozotocin-induced diabetic rats with sorbinil, myo-inositol or aminoguanidine on endoneurial blood flow, motor nerve conduction velocity and vascular function of epineurial arterioles of the sciatic nerve. Int J Experimental Diab Res. 2002;3:21–36. doi: 10.1080/15604280212525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppey LJ, Gellett JS, Davidson EP, Dunlap J, Lund DD, Salvemini D, Yorek MA. Effect of M40403 Treatment of diabetic rats on endoneurial blood flow, motor nerve conduction velocity, and vascular function of epineurial arterioles of the sciatic nerve. Brit J Pharmacol. 2001a;134:121–129. doi: 10.1038/sj.bjp.0704216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppey LJ, Davidson EP, Rinehart TW, Gellett JS, Oltman CL, Lund DD, Yorek MA. ACE inhibitor or angiotensin II receptor antagonist attenuates diabetic neuropathy in streptozotocin-induced diabetic rats. Diabetes. 2006;55:341–348. doi: 10.2337/diabetes.55.02.06.db05-0885. [DOI] [PubMed] [Google Scholar]
- Coppey LJ, Gellett JS, Davidson EP, Dunlap J, Lund DD, Yorek MA. Effect of antioxidant treatment of streptozotocin-induced diabetic rats on endoneurial blood flow, motor nerve conduction velocity, and vascular reactivity of epineurial arterioles of the sciatic nerve. Diabetes. 2001b;50:1927–1937. doi: 10.2337/diabetes.50.8.1927. [DOI] [PubMed] [Google Scholar]
- Davidson EP, Coppey LJ, Kleinschmidt TL, Oltman CL, Yorek MA. Vascular and Neural Dysfunction in obese Zucker rats: Effect of AVE7688. Experimental Diabetes Res. 2009;2009(2009):912327. doi: 10.1155/2009/912327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson EP, Kleinschmidt TL, Oltman CL, Lund DD, Yorek MA. Treatment of Streptozotocin-induced Diabetic Rats with AVE7688, a Vasopeptidase Inhibitor, on Vascular and Neural Disease. Diabetes. 2007;56:355–362. doi: 10.2337/db06-1180. [DOI] [PubMed] [Google Scholar]
- Davidson EP, Coppey LJ, Calcutt NA, Oltman CL, Yorek MA. Diet Induced Obesity in Sprague Dawley Rats: Effect on Microvascular and Neural Function. Diabetes Metabolism Res Rev. 2010;26:306–18. doi: 10.1002/dmrr.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson EP, Coppey LJ, Holmes A, Dake B, Yorek MA. Effect of treatment of high fat fed/low dose streptozotocin-diabetic rats with Ilepatril on vascular and neural complications. European J Pharmacol. 2011;688:497–506. doi: 10.1016/j.ejphar.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes and atherosclerosis; the missing links. Diabetologia. 2010;53:1270–1287. doi: 10.1007/s00125-010-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara F, Di Marco GS, Juliano MA, Casarini DE. Neutral endopeptidase expression in mesangial cells. J Renin-Angiotensin-Aldosterone System. 2003;4:228–233. doi: 10.3317/jraas.2003.037. [DOI] [PubMed] [Google Scholar]
- Eckardt K, Taube A, Eckel J. Obesity-associated insulin resistance in skeletal muscle: role of lipid accumulation and physical inactivity. Rev Endocrin Metab Disorders. 2011;12:163–172. doi: 10.1007/s11154-011-9168-2. [DOI] [PubMed] [Google Scholar]
- Edwards RM, Pullen M, Nambi P. Distribution of neutral endopeptidase activity along the rat and rabbit nephron. Pharmacology. 1999;59:45–50. doi: 10.1159/000028304. [DOI] [PubMed] [Google Scholar]
- Gonzalez W, Soleilhac JM, Fournie-Zaluski MC, Roques, Michel BP. Characterization of neutral endopeptidase in vascular cells, modulation of vasoactive peptide levels. European J Pharmacol. 1998;345:323–331. doi: 10.1016/s0014-2999(98)00038-7. [DOI] [PubMed] [Google Scholar]
- Kikkawa F, Shibata K, Suzuki T, Kajiyama H, Ino K, Nomura S, Mizutani S. Signal pathway involved in increased expression of neutral endopeptidase by gonadotropin releasing hormone in choriocarcinoma cells. Placenta. 2004;25:176–183. doi: 10.1016/j.placenta.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Malik RA, Williamson S, Abbott C, Carrington AL, Iqbal J, Schady W, Boulton AJ. Effect of angiotensin-converting-enzyme (ACE) inhibitor trandolapril on human diabetic neuropathy: randomized double-blind controlled trial. Lancet. 1998;352:1978–1981. doi: 10.1016/S0140-6736(98)02478-7. [DOI] [PubMed] [Google Scholar]
- Miyashita K, Itoh H, Tsujimoto H, Tamura N, Fukunaga Y, Sone M, Yamahara K, Taura D, Inuzuka M, Sonoyama T, Nakao K. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009;58:2880–2892. doi: 10.2337/db09-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newaz M, Yousefipour Z, Oyekan A. Natriuretic and renoprotective effect of chronic oral neutral endopeptidase inhibition in acute renal failure. Ren Fail. 2010;32:384–390. doi: 10.3109/08860221003611745. [DOI] [PubMed] [Google Scholar]
- Porter LR. Natriuretic peptide metabolism, clearance and degradation. FEBS J. 2011;278:1808–1817. doi: 10.1111/j.1742-4658.2011.08082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrosova IG, Li F, Abatan OI, Forsell MA, Komjati K, Pacher P, Szabo C, Stevens MJ. Role of poly(ADP-ribose) polymerase activation in diabetic neuropathy. Diabetes. 2004;53:711–720. doi: 10.2337/diabetes.53.3.711. [DOI] [PubMed] [Google Scholar]
- Oltman CL, Coppey LJ, Gellett JS, Davidson EP, Lund DD, Yorek MA. Progression of Vascular and Neural Dysfunction in Sciatic Nerves of Zucker Diabetic Fatty (ZDF) and Zucker Rats. Am J Physiol. 2005;289:E113–122. doi: 10.1152/ajpendo.00594.2004. [DOI] [PubMed] [Google Scholar]
- Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, Dake B, Yorek MA. Role of the effect of inhibition of neutral endopeptidase on vascular and neural complications in streptozotocin-induced diabetic rats. European J Pharmacol. 2011;650:556–562. doi: 10.1016/j.ejphar.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, Lund DD, Yorek MA. Attenuation of Vascular/Neural Dysfunction in Zucker Rats Treated with Enalapril or Rosuvastatin. Obesity. 2008;16:82–89. doi: 10.1038/oby.2007.19. [DOI] [PubMed] [Google Scholar]
- Oltman CL, Davidson EP, Coppey LJ, Kleinschmidt TL, Lund DD, Yorek MA. Treatment of Zucker Diabetic Fatty Rats with AVE7688 Improves Vascular and Neural Dysfunction. Diabetes, Obesity and Metabolism. 2009;11:223–233. doi: 10.1111/j.1463-1326.2008.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J, Loomis CR, Bishop WR, Stein R, Niedel JE, Bell RM. Quantitative measurement of sn-1,2-diacylglycerol present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986;261:8597–8600. [PubMed] [Google Scholar]
- Pu Q, Schiffrin EL. Effect of ACE/NEP inhibition on cardiac and vascular collagen in stroke-prone spontaneously hypertensive rats. Am J Hypertension. 2001;14:1067–1072. doi: 10.1016/s0895-7061(01)02157-4. [DOI] [PubMed] [Google Scholar]
- Reed MJ, Meszaros K, Entes LJ, Claypool MD, Pinkett JG, Gadbois TM, Reaven GM. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat. Metabolism. 2000;49:1390–1394. doi: 10.1053/meta.2000.17721. [DOI] [PubMed] [Google Scholar]
- Reja A, Tesfaye S, Harris ND, Ward UK. Is ACE inhibition with lisinopril helpful in diabetic neuropathy? Diabetic Med. 1995;12:307–309. doi: 10.1111/j.1464-5491.1995.tb00482.x. [DOI] [PubMed] [Google Scholar]
- Rogers NH, Witczak CA, Hirshman MF, Goodyear LJ, Greenberg AS. Estradiol stimulates Akt, AMP-activated protein kinase (AMPK) and TBC1D1/4 but not glucose uptake in rat soleus. Biochem Biophys Res Comm. 2009;382:646–650. doi: 10.1016/j.bbrc.2009.02.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52:313–320. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Suzki T, Ino K, Kikkawa F, Uehara C, Kajiyama H, Shibata K, Mizutani S. Neutral endopeptidase/CD10 expression during phorbol ester-induced differentiation of choriocarcinoma cells through the protein kinase C- and extracellular signal-regulated kinase-dependent signaling pathway. Placenta. 2002;23:475–482. doi: 10.1053/plac.2002.0820. [DOI] [PubMed] [Google Scholar]
- Wang CH, Leung N, Lapointe N, Szeto L, Uffelman KD, Giacca A, Rouleau JL, Lewis GF. Vasopeptidase inhibitor Omapatrilat induces profound insulin sensitization and increases myocardial glucose uptake in Zucker fatty rats. Circulation. 2003;107:1923–1929. doi: 10.1161/01.CIR.0000062646.09566.CC. [DOI] [PubMed] [Google Scholar]
- Weber M. Emerging treatments for hypertension: potential role for vasopeptidase inhibition. Am J Hypertension. 1999;12:139S–147S. doi: 10.1016/s0895-7061(99)00205-8. [DOI] [PubMed] [Google Scholar]
- Wong V, Szeto L, Uffelman K, Fantus IG, Lewis GF. Enhancement of muscle glucose uptake by the vasopeptidase inhibitor, Omapatrilat, is independent of insulin signaling and the AMP kinase. J Endocrinol. 2006;190:441–450. doi: 10.1677/joe.1.06396. [DOI] [PubMed] [Google Scholar]
- Yorek MA, Coppey LJ, Gellett JS, Davidson EP. Sensory nerve innervation of epineurial arterioles of the sciatic nerve containing calcitonin gene-related peptide: effect of streptozotocin-induced diabetes. Exp Diabesity Res. 2004;5:187–193. doi: 10.1080/15438600490486732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorek MA. The potential role of angiotensin converting enzyme and vasopeptidase inhibitors in the treatment of diabetic neuropathy. Curr Drug Targets. 2008;9:77–84. doi: 10.2174/138945008783431736. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ussher JR, Oka T, Cadete VJ, Wagg C, Lopaschuk GD. Cardiac diacylglycerol accumulation in high fat-fed mice is associated with impaired insulin-stimulated glucose oxidation. Cardiovasc Res. 2011;89:148–156. doi: 10.1093/cvr/cvq266. [DOI] [PubMed] [Google Scholar]