Abstract
In rats neonatal (+)-methamphetamine (MA) exposure and maternal separation stress increase corticosterone during treatment and result in learning and memory impairments later in life. Early-life stress also changes later responses to acute stress. We tested the hypothesis that neonatal MA exposure would alter adult corticosterone after acute stress or MA challenge. Rats were treated with MA (10 mg/kg × 4/day), saline, or handling on postnatal (P) days 11–15 or 11–20 (days that lead to learning and memory impairments at this dose). As adults, corticosterone was measured before and after 15 min forced swim (FS) or 15 min forced confinement (FC), counterbalanced, and after an acute MA challenge (10 mg/kg) given last. FS increased corticosterone more than FC; order and stress type interacted but did not interact with treatment; treatment interacted with FS but not with FC. In the P11–15 regimen, MA-treated rats showed more rapid increases in corticosterone after FS than controls. In the P11–20 regimen, MA-treated rats showed a trend toward more rapid decrease in corticosterone after FS. No differences were found after MA challenge. The data do not support the hypothesis that neonatal MA causes changes in adult stress responsiveness to FS, FC, or an acute MA challenge.
Keywords: Methamphetamine, neonatal methamphetamine, stress, forced swim, corticosterone, forced confinement
1. Introduction
Methamphetamine (MA) is a widely abused psychostimulant. If women users become pregnant, passive transplacental exposure of the fetus occurs since MA readily crosses the placenta. In an ovine model, MA reaches higher concentrations in the fetus than in the ewe (Burchfield et al., 1991). The problem is not insignificant for human exposure. Recent data show that 24% of pregnant women seeking drug treatment from federally-supported treatment centers (83% of all treatment centers are federally supported) report MA as their primary drug of abuse, up from 8% in 1994 (Terplan et al., 2009). Despite this, relatively little is known about the effects of prenatal MA exposure on the neurodevelopmental outcomes of exposed children.
Human case-control studies indicate that prenatally MA-exposed infants exhibit reduced birth weight, length, and head circumference, higher rates of intraventricular hemorrhage, and anemia (Chomchai et al., 2004;Dixon, 1989;Dixon and Bejar, 1989;Little et al., 1988;Oro and Dixon, 1987;Smith et al., 2008), poor sleep, vomiting, tremors, poor feeding, and withdrawal (Chomchai et al., 2004;Dixon, 1989;Oro and Dixon, 1987). Exposed children show deficits in visual motor integration, attention, psychomotor speed, spatial, and verbal memory (Chang et al., 2004;Chang et al., 2009), reduced novel object recognition (Struthers and Hansen, 1992), and decreased volume of the hippocampus, putamen, and globus pallidus (Chang et al., 2004).
In order to better understand the role of early exposure to MA, we developed an animal model for the equivalent of third trimester pregnancy with respect to brain development in rats. The model is based on a number of regional brain growth comparisons and include the fact that cells in the dentate gyrus continue to proliferate to postnatal day (P) 19 which is approximately equivalent to post-conception day 240 in humans (Bayer et al., 1993). In addition, a large database on cross-species comparisons of brain development shows P11 in the rat corresponds with human in-utero brain development at 26 weeks for cortical and 19 weeks for limbic structures (Clancy et al., 2007b;Clancy et al., 2007a). We previously demonstrated that P11–15 and P11–20 MA exposure results in learning deficits in the Cincinnati (CWM) and Morris (MWM) water mazes (Williams et al., 2002;Williams et al., 2003c;Vorhees et al., 2009) and that the shorter interval (P11–15) induces effects smaller than those induced by the longer interval (P11–20) (Vorhees et al., 2008;Williams et al., 2003b). These exposure periods also produce morphological changes in the dentate gyrus and nucleus accumbens (Williams et al., 2004).
The exposure period used in these experiments overlaps with the stress hyporesponsive period (SHRP; ~P4–14), a period when the adrenal gland exhibits dampened responses to environmental perturbations (Sapolsky and Meaney, 1986). Depending upon the stressor, corticosterone (CORT) responses during the SHRP may be present but are smaller than those observed in adults. Previous studies using stressors or endotoxin exposure during the SHRP show long-term alterations in hypothalamic-pituitary-adrenal (HPA) axis reactivity during adulthood (Aisa et al., 2007;Biagini et al., 1998;Felszeghy et al., 2000;Hodgson et al., 2001;Kalinichev et al., 2002;Kamphuis et al., 2002;Shanks et al., 1995;Wigger and Neumann, 1999). Maternal separation during the SHRP leads to hypersecretion of CORT following a later stressor exposure and increased anxiety-related behavior (Aisa et al., 2007;Biagini et al., 1998;Kalinichev et al., 2002;Wigger and Neumann, 1999). In addition, alterations in HPA axis reactivity are associated with cognitive deficits in the MWM and novel object recognition (Aisa et al., 2007). We previously demonstrated reduced CORT levels following the last trial of MWM testing in rats neonatally exposed to MA (Skelton et al., 2007). We also showed that a single dose of MA increased CORT 30 and 105 min following treatment on any day between ages P1–19. The pattern across days showed a U-shaped function in good agreement with that of the SHRP. MA also induced increases in ACTH during this interval (Williams et al., 2000). Given that MA increases CORT during the SHRP and does so more than a physical or psychological stressor (Grace et al., 2008), we tested the hypothesis that neonatal MA exposure would alter HPA axis reactivity when the animals were exposed to an acute stressor as adults. An initial experiment showed that rats treated with 5 mg/kg MA from P11–20 had reduced CORT levels following 15 min of forced swim (FS) as adults compared to controls, however in that experiment FS was conducted after Barnes maze testing (Williams et al., 2003a). Based on these findings, we hypothesized that neonatal MA exposure would reduce adult responses to acute stress in behaviorally naïve rats and that the effect would be greatest in those exposed to MA longer (P11–20) than in those exposed for a shorter time (P11–15). In order to determine whether stress intensity affected outcome, we tested three stressors: FS, forced confinement (FC), and acute MA challenge. FS and FC were counterbalanced and MA challenge was always last. FC used the FS apparatus but without water so that the environment was identical to that used for FS.
2 Methods
2.1 Animals and conditions
Male (251–275 g) and nulliparous female (151–175 g) Sprague-Dawley CD IGS rats were obtained from Charles River Laboratories (Raleigh, NC) and male offspring were the subjects used. Rats were acclimated to the vivarium (14 h dark: 10 h light, lights on at 600 h with temperature and humidity controlled) for at least one week prior to breeding and were housed in polycarbonate cages. Stainless steel enrichment enclosures were placed in cages starting on embryonic day 1 and throughout the experiment (Vorhees et al., 2008). P0 was designated as the day of birth. On P1, pups were removed from their mothers, weighed, sexed, and culled to 10 with at least 4 males. On P7, pups were marked for identification by ear punch and separated from the dam and housed in pairs on P28. Animals had ad libitum access to food and water. Protocols were approved by the Institutional Animal Care and Use Committee. The vivarium is accredited by AAALAC.
2.2 Treatments
Males from each litter were placed in one of three treatment groups: (1) weighed-only (WEIGH), (2) saline (SAL), or (3) (+)-methamphetamine HCl (MA: 10 mg/kg expressed as the freebase, NIDA, > 95% pure) in a volume of 3 ml/kg. Extra males in a litter received MA in case of loss of the first MA-treated pup but was otherwise not used in order to maintain the design such that no more than 1 pup/treatment was tested from any given litter. Treatment was given 4 times per day at 2 h intervals from P11–15 or P11–20, intervals previously shown to cause learning impairments (Vorhees et al., 2008;Williams et al., 2003c). SAL or MA was given s.c. in the dorsum. Twenty litters were initially assigned to each regimen; however catheter clogging reduced this number; therefore, in order to compensate additional litters were added. The final number of litters was 19–28 per treatment regimen. Offspring were weighed on P1, P7, during treatment, and weekly thereafter.
2.3 Surgical procedures
Jugular catheters were surgically implanted once the animals were older than P90. Rats were moved to a surgical suite and anesthetized using isoflurane. The neck was shaved and swabbed with betadine and alcohol. Rats were anesthetized by placing them in an airtight chamber and isoflurane (4–5%) vapor was infused into the chamber until the animal was unresponsive. The rat was then removed and anesthesia maintained using a nose-cone and continuous flow isoflurane (2–2.5%). Using sterile methods, an incision was made lateral to the midline and the jugular vein exposed and occluded with silk suture superior to the catheter insertion. An incision was made in the jugular and an inserter (Becton-Dickinson and Co., Franklin Lakes, NJ) introduced to guide the catheter (Braintree Scientific Inc., Braintree, MA) to a point superior to the aorta. The inner diameter of the catheter port was 58.4 μm, the catheter body was 27.9 μm, and the intravascular tippet 30.5 μm (Braintree Scientific Inc.). The catheter was pre-filled with 10 IU/ml heparinized saline and connected to a 3 ml syringe using a 23 gauge blunt needle (Braintree Scientific Inc.) which remained connected to the catheter until it was firmly secured. The position of the catheter was kept consistent and secured using another silk suture fastened around a node on the catheter and at the opening of the jugular incision. The suture posterior to the incision was used to anchor and further secure the catheter. After securing the catheter, blood was drawn to check for patency. Once flow was established, the 3 ml syringe was disconnected and a plug inserted (Braintree Scientific Inc.). The catheter was tunneled from the incision in the neck and exited through an incision in the dorsum between the shoulders. It was secured using sutures and the incision was closed around the catheter. Sensorcaine® was applied to incision sites. Both exterior suture sites were sealed and the animal placed in an incubator at 28°C until anesthetic recovery. Post-surgery animals were housed singly to prevent damage to the catheter. Approximately 0.2 ml of a heparinized glycerol (50 IU/ml) catheter lock solution was injected in the catheter to maintain patency.
2.4 Blood collection and plasma isolation
On the day following surgery, a blood sample was collected. This continued for 3 days to ensure proper catheter function and to familiarize animals with the procedure. Blood drawn on the third day following surgery was used to determine pre-experimental baseline CORT levels. On days 4, 5, and 6 post-surgery, one of 3 stressors was given. On these days, blood was taken at 6 time points: 0 (immediately upon removal from the home cage) and 15, 30, 60, 90, and 120 min following the start of stressor exposure. Following each blood collection, catheters were flushed with 10 IU/ml heparinized saline. Blood was collected on ice and plasma separated from cells by centrifugation at 1300 RCF at 4°C for 10 min and stored at −80ºC until CORT was measured. The red blood cells were resuspended in physiologic saline and reintroduced in the animals through the catheter after the next blood sample was taken. Approximately 0.2 ml of blood was obtained in each draw and 0.1 ml of plasma was collected with an identical volume (0.1 ml) of saline used to resuspend the red blood cells. After the final red blood cell replacement each day, the catheter was again flushed with 0.2 ml injection of 50 IU/ml heparinized glycerol.
2.5 Stressors
Stressors were applied between 1000 and 1145 h to account for ultradian and circadian rhythms. On days 4 and 5 post-surgery, animals received either FS on day-4 and FC on day-5 or the reverse. FS was conducted in a 15 cm diameter by 46 cm tall PVC cylinder filled with water to a depth of 35 cm (22 ± 1°C). FC was performed in the same apparatus but without water, i.e., rats were placed in the cylinder and were free to move around within it but were confined to the 16 cm diameter amount of space. Both FS and FC were given for 15 min. 24 h after FS or FC, animals were given an injection of 10 mg/kg MA.
2.6 Corticosterone assessment
Plasma CORT was assayed using Octeia ELISA kits (IDS, Fountain Hills, AZ) and each sample was diluted 1:3 and assayed according to the manufacturer’s protocol. The ELISAs were measured on a SpectraMax Plus microtiter plate reader (Molecular Devices, Sunnyvale, CA). The intra- and inter-assay variability is under 5% and 10%, respectively, for this assay.
2.7 Statistics
Corticosterone data were analyzed using general linear model analysis of variance (ANOVA) with two repeated measure factors. The model for the CORT data for each regimen (exposure age P11–15 vs. P11–20) was a 3 treatment × 2 stress type × 2 order × 6 time point ANOVA (2-between, 2-within) with regimen as an additional factor in an omnibus ANOVA performed first. The model for the day-3 MA challenge data was a 3 treatment × 2 order × 6 time point ANOVA (2-between, 1-within). For these analyses, variance-covariance matrices found to be significantly non-spherical were corrected using Greenhouse-Geisser adjusted F-ratios. For body weight, mixed models were used as there was only one repeated measure factor. In this case, the variance-covariance matrix was tested for best fit to structural models and the autoregressive-1 (AR1) model chosen in conjunction with Kenward-Roger degrees of freedom. Significant interactions were analyzed using simple-effect slice ANOVAs except when comparing treatment groups for each regimen; in these cases t-tests for independent samples were used (2-tailed). Significance was set at p ≤ 0.05. Data were analyzed using SAS version 9.2 (SAS Institute, Cary, NC).
2. Results
2.8 Body weight
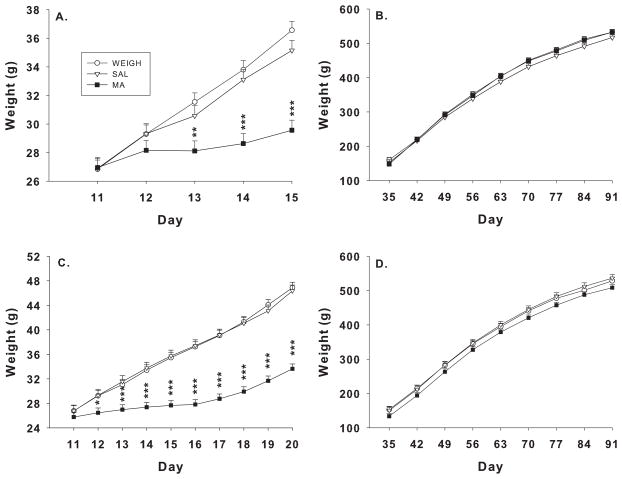
For the P11–15 regimen body weights, there were main effects of treatment, F(2, 38) = 7.67, p< 0.002, day, F(4, 144) = 126.48, p< 0.0001, and the interaction of treatment × day, F(8, 144) = 13.21, p< 0.0001 during dosing. The MA group had reduced weight gain compared to SAL or WEIGH groups. The treatment × day interaction revealed that the MA group had significantly reduced weight gain compared with SAL or WEIGH groups on P13–15 (Figure 1A). Adult weights were not significantly different among groups (Figure 1B) including no differences between the WEIGH and SAL groups.
Figure 1.
Body weights during dosing and after weaning. Neonatal rats were weighed-only (WEIGH) or treated with 10 mg/kg MA or SAL 4 times daily from P11–15 (A–B) or P11–20 (C–D). (A) MA-treated rats had reduced weight gain from P13–15 compared to either control. Following the dosing period, MA animals rebounded and gained weight at rates comparable to controls (B). Similar observations were made for the P11–20 group except the decreased weight was observed beginning on P12 (C), but no differences were detected in adulthood (D). ***p< 0.001, **p< 0.01, *p< 0.05 compared to WEIGH or SAL.
For the P11–20 regimen, there were main effects of treatment, F(2, 52.3) = 35.95, p< 0.0001, day, F(9, 438) = 138.69, p< 0.0001, and the treatment × day interaction, F(18, 438) = 10.57, p< 0.0001 during dosing. The MA group had reduced weight gain compared with SAL or WEIGH. The treatment × day interaction showed that the MA group had reduced weight gain from P12–20 (Figure 1C) compared with the SAL or WEIGH groups. There were no significant differences on adult body weight (Figure 1D), including no differences between the SAL and WEIGH groups.
2.9 Corticosterone after forced swim or confinement
CORT assays for each of the regimens (P11–15 and P11–20) were temporally separated. This resulted in absolute CORT values that were not identical for the two data sets. In order to compare responses to the stressors across data sets, the magnitude of change relative to the baseline for each data set were expressed in terms of fold-change. Baseline CORT concentrations for each regimen are provided in the figure captions (Figure 2 caption for the P11–15 regimen and Figure 3 caption for the P11–20 regimen). ANOVA comparing the four baseline CORT levels regimen × order showed no significant differences. For both treatment regimens, plasma CORT showed an inverted U-shaped function for the FS groups whereas only slight changes were seen in the FC groups.
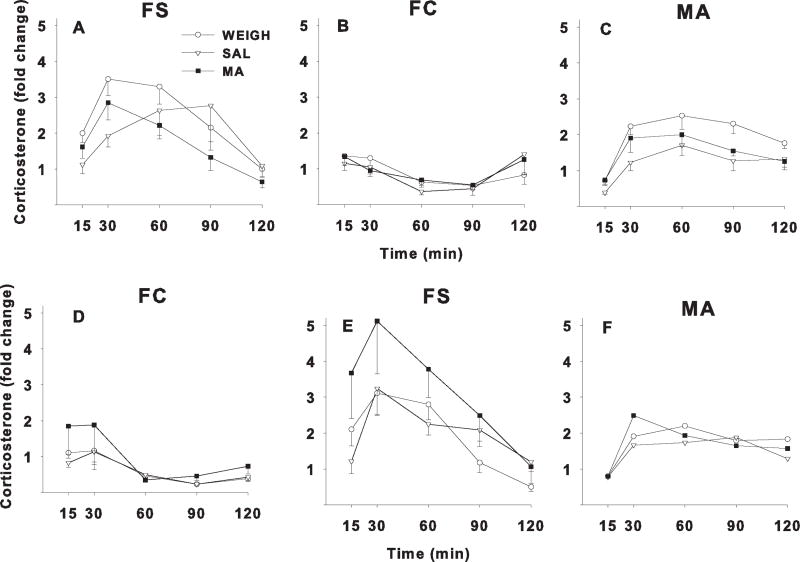
Figure 2.
Relative change in plasma CORT levels in adult rats following one of three stressors. Rats were treatment on P11–15 with MA, SAL, or were only weighed (WEIGH). FS = forced swim, FC = forced confinement, MA = methamphetamine. A–C: groups given FS on day-1, FC on day-2, and MA on day-3. D–F: groups given FC on day-1, FS on day-2, and MA on day-3. Arrows indicate when each stressor was administered after the time 0 blood sample. Fold change was calculated relative to average baseline determined at time −24 h and time 0, where time 0 = sample taken immediately prior to the stressor (FS or FC) and time −24 h was taken 24 h prior to time 0. Mean ± SEM (N) baseline CORT values for the FS-FC condition were FS group = 15.2 ± 1.1 (19) and FC group = 19.9 ± 1.3 (19) mg/dl. CORT values for the FC-FS condition were FS group = 18.4 ± 1.5 (20) and FC group = 18.2 ± 1.4 (20) ng/ml.
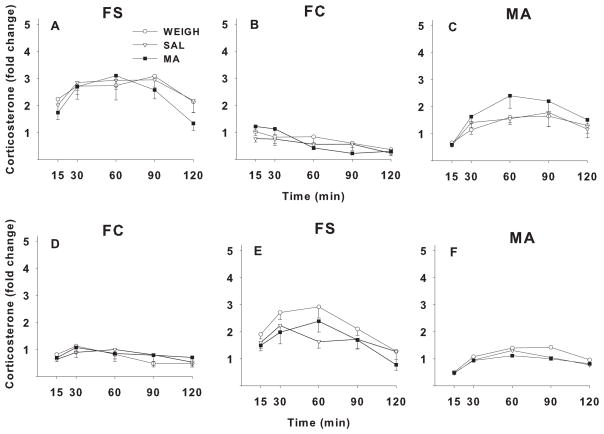
Figure 3.
Relative change in plasma CORT levels in adult rats following one of three stressors. Rats were treatment on P11–20 with MA, SAL, or were only weighed (WEIGH). FS = forced swim, FC = forced confinement, MA = methamphetamine. A–C: groups given FS on day-1, FC on day-2, and MA on day-3. D–F: groups given FC on day-1, FS on day-2, and MA on day-3. Arrows indicate when each stressor was administered after the time 0 blood sample. Fold change was calculated relative to average baseline determined at time −24 h and time 0, where time 0 = sample taken immediately prior to the stressor (FS or FC) and time −24 h was taken 24 h prior to time 0. Mean ± SEM (N) baseline CORT values for the FS-FC condition were FS group = 13.1 ± 0.9 (28) and FC group = 16.2 ± 1.6 (28) mg/dl. CORT values for the FC-FS condition were FS group = 13.6 ± 0.9 (24) and FC group = 12.9 ± 0.8 (24) ng/ml.
Omnibus significant effects were stressor (p < 0.0001), regimen × stressor × order (p < 0.01), time (p < 0.0001), treatment × time (p < 0.01), order × time (p < 0.01), regimen × time (p < 0.0001), regimen × order × time (p = 0.05), stressor × time (p < 0.0001), stressor × order × time (p < 0.02), regimen × stressor × time (p < 0.0001), and treatment × regimen × stressor × time (p = 0.05). Since two of the interactions involved treatment, follow-up ANOVAs were conducted for each regimen.
For the P11–15 regimen, there were main effects of stressor, F(1,33) = 65.9, p< 0.0001, and time, F(4,132) = 23.8, p< 0.0001, but not of treatment. The stressor main effect was caused by FS producing greater increases in CORT than FC regardless of order. There were also interactions of treatment × time, F(8,132) = 2.5, p < 0.05, order × time, F(4,132) = 3.6, p < 0.03, and stressor × time, F(4,132) = 23.8, p < 0.0001. Follow-up analyses of the treatment × time interaction showed there were significant treatment effects only at 15 min, F(2,36) = 3.5, p < 0.05. Pairwise comparisons showed that MA increased CORT at 15 min compared with SAL (p < 0.05) but not compared with WEIGH: mean (fold change) ± SEM (N): WEIGH = 273.0 ± 33.8 (15); SAL = 222.0 ± 32.3 (12); MA = 305.3 ± 59.4 (12).
For the P11–20 regimen, there were main effects of stressor, F(1,46) = 183.1, p< 0.0001, and time, F(4,184) = 27.7, p< 0.0001, but no main effect of treatment. As before, the main effect of stressor was caused by FS producing larger increases in CORT than FC. There were interactions of stressor × order, F(1,46) = 12.7, p<0.001, stressor × time, F(4,184) = 14.8, p< 0.0001, treatment × stressor × time, F(8,184) = 2.2, p < 0.05, and stressor × order × time, F(4,184) = 5.00, p < 0.002. Follow-up analyses of the treatment × stressor × time interaction showed there were no treatment effects at any time after FC. For FS, there was no significant treatment effect at any time, however a trend was seen at 120 min, F(2,46) = 2.7, p < 0.08. The group means (fold change) ± SEMs (N) were as follows: WEIGH = 193.0 ± 28.6 (17); SAL = 220.2 ± 41.7 (14); MA = 172.8 ± 33.2 (21); pairwise comparisons based on the p < 0.08 trend, showed that the MA group had lower responses to FS at 120 min compared with the SAL and WEIGH groups (p < 0.05), suggesting a faster return to basal levels in MA-exposed offspring.
2.10 Corticosterone after acute methamphetamine
MA challenge was given last and there were no treatment main effects and no treatment-related interactions. The analysis revealed main effects of regimen, F(1,79) = 8.9, p < 0.01, and time, F(4,316) = 61.1, p < 0.0001. There were significant interactions of regimen × order, F(1,79) = 4.5, p < 0.05, and regimen × time, F(4,316) = 3.5, p < 0.02. ANOVAs on each regimen showed that for the P11–15 regimen, the only significant effect was time, F(4,132) = 38.6, p < 0.0001. For the P11–20 regimen, the only significant effects were order, F(1,46) 6.4, p < 0.02, and time, F(4,184) = 26.7, p < 0.0001).
3 Discussion
3.1 Developmental MA exposure and adult corticosterone
It has been shown that early life stress has lasting effects on HPA axis reactivity (Aisa et al., 2007;Biagini et al., 1998;Felszeghy et al., 2000;Hodgson et al., 2001;Kalinichev et al., 2002;Kamphuis et al., 2002;Shanks et al., 1995;Wigger and Neumann, 1999). The present results suggest that developmental MA treatment has little or no effect on the adult CORT response despite the fact that it has been shown previously to impair learning and memory. The absence of larger effects was surprising given that separation stress at these ages results in altered adult stress reactivity (Aisa et al., 2007). We conclude that developmental MA treatment and maternal separation operate through different mechanisms with little overlap. It may be that the increase in CORT caused by neonatal MA exposure is not linked to a psychological determinate, whereas maternal separation has a clear link between the removal of the dam and the increase in CORT response. No differences in baseline CORT were seen as a function of neonatal MA treatment either.
We previously showed that neonatal MA exposure (P11–20) caused a reduced adult CORT response (Williams et al., 2003a), but in that experiment the animals were tested for CORT after a series of behavioral tests, suggesting that exposure to other procedures may modify the MA-induced response. We also showed that FS or FC exposure to rats during the same period as used here, i.e., P11–15 or P11–20, caused increased CORT but not to the degree caused by MA, suggesting that developmental MA and short-term stressor exposure are very different effects and one does not recapitulate the other.
3.2 Developmental MA exposure alters stress responses given serially
The data show that serial stress exposures affect CORT levels on subsequent days (order effect) with no interaction with developmental MA exposure. This was evident for FS in the P11–15 exposure regimen in which the average response across groups given FC first then FS showed larger CORT responses than those given FS first. Order effects were less evident in the P11–20 regimen; average responses to FS were slightly larger when FS was given first vs. when given second. The reasons for this are not clear but presumably reside in the longer neonatal treatment interval in the P11–20 regimen. Why this also affects the WEIGH group is unknown unless the act of daily litter disturbance for 10 days was sufficient to change responses compared with those disturbed for only 5 days. Since dampened responses to FS were seen in P11–20 MA treated offspring after adult behavioral testing (Williams et al., 2006), it may also be that the impact of early MA-induced CORT increases are evident as interactions of multiple HPA axis activation events whereas herein the early MA exposure was not sufficient by itself to provoke an altered response to FS or FC.
Because our focus was on stages that previously showed learning and memory deficits and we previously found that P16–20 MA treatment was refractory to MA-induced learning deficits (Williams et al., 2003b), we did not include a P16–20 regimen here. It is not surprising that drug effects are age-sensitive. Cocaine has been shown to also be sensitive to the window of exposure (Stanwood and Levitt, 2004).
An examination of the WEIGH group revealed that the peak increase in CORT was different for each stressor. FS and MA produced peak CORT levels 30–60 min after stress exposure in most cases whereas FC peaked earlier, 15–30 min after stress exposure and the peaks were smaller. In addition, the effect on CORT following FS was greater than after MA challenge. This may be because the MA challenge was given third (Grace et al., 2008), however in a previous study, we showed that FS produced a greater CORT release than MA in naïve animals (Herring et al., 2008), therefore the pattern in this study in this regard are not surprising. If cross-sensitization to the previous stressors occurred it could not be determined here because we did not have a group that only received MA. Previously, (Kirby et al., 1997) showed that FS has a greater impact on the HPA axis response than other stressors such as tail pinch, cold, or immobilization. We show a similar finding and include novel environment (FC) and MA. Interestingly, others have shown a definite sensitization to subsequent stressors following an acute stressor such as immobilization (Belda et al., 2008). We, however, find an interaction between the early exposure period and the presence of sensitization.
3.3 Developmental MA exposure and the SHRP
We have previously shown that MA exposure during early development produces increases in CORT during the SHRP (Schaefer et al., 2006;Schaefer et al., 2008;Williams et al., 2000;Williams et al., 2006). We examined the effects of early MA on adult stress reactivity in order to determine if altered development of the HPA axis might persist into adulthood where it could potentially contribute to the long-term learning and memory deficits in swimming mazes observed in early MA-treated rats (Vorhees et al., 2008;Vorhees et al., 2008;Williams et al., 2002;Williams et al., 2003a;Vorhees et al., 2009). It is known that early HPA axis changes can have long-term effects on learning and memory when other stressors are used (Croiset et al., 2000;Lupien and McEwen, 1997). Corticosteroids regulate cell proliferation in brain regions important for learning and memory (Gould et al., 1991;Lupien and McEwen, 1997), including spatial learning and memory. In the present experiment, only small and short-lived increases in adult stress reactivity as indexed by CORT were observed following FS after P11–20 MA exposure. Previously we showed that a reduced response to FS was observed after the last day of MWM testing (Skelton et al., 2007). We also showed that multiple days of early MA exposure during the SHRP causes sharp initial increases in CORT that increase further with each additional day of treatment 30 min after treatment but show progressively more rapid down-regulation each day when assessed at 105 min after treatment (Williams et al., 2006). Therefore, when taken together, neonatal MA appears to have dramatic early effects on the HPA axis as measured by CORT, but little or no lasting effects on the function of the HPA axis. It may be that other stressors or some combination of behavioral testing with stressor exposure would reveal a difference that was not observed in the present experiment, but given the current data we conclude that developmental MA does not induce lasting changes in CORT reactivity coincident with the learning and memory impairments induced by the drug and therefore may not contribute to such cognitive changes.
Research highlights.
Developmental methamphetamine exposure causes learning and memory deficits and increased in corticosterone
Maternal separation and other developmental stressor also cause increased corticosterone and later learning and memory deficits
Rats were treated with methamphetamine or saline from P11–15 or P11–20 and as adults were given stressors (forced swim, forced confinement, or methamphetamine)
Forced swim increased corticosterone more than forced confinement; acute methamphetamine was intermediate
Minor interactions were found between neonatal methamphetamine exposure and force swim-induced corticosterone
No support for the hypothesis that early methamphetamine exposure alters adult corticosterone release in response to acute stress was obtained.
Acknowledgments
Supported by NIH grants R01 DA006733 (CVV) and T32 ES07051 (CEG and TLS)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aisa B, Tordera R, Lasheras B, Del RJ, Ramirez MJ. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology. 2007;32:256–266. doi: 10.1016/j.psyneuen.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Belda X, Fuentes S, Nadal R, Armario A. A single exposure to immobilization causes long-lasting pituitary-adrenal and behavioral sensitization to mild stressors. Horm Behav. 2008;54:654–661. doi: 10.1016/j.yhbeh.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Biagini G, Pich EM, Carani C, Marrama P, Agnati LF. Postnatal maternal separation during the stress hyporesponsive period enhances the adrenocortical response to novelty in adult rats by affecting feedback regulation in the CA1 hippocampal field. Int J Dev Neurosci. 1998;16:187–197. doi: 10.1016/s0736-5748(98)00019-7. [DOI] [PubMed] [Google Scholar]
- Burchfield DJ, Lucas VW, Abrams RM, Miller RL, DeVane CL. Disposition and pharmacodynamics of methamphetamine in pregnant sheep. J Amer Med Assoc. 1991;265:1968–1973. [PubMed] [Google Scholar]
- Chang L, Cloak C, Jiang CS, Farnham S, Tokeshi B, Buchthal S, Hedemark B, Smith LM, Ernst T. Altered neurometabolites and motor integration in children exposed to methamphetamine in utero. Neuroimage. 2009;48:391–397. doi: 10.1016/j.neuroimage.2009.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, Ernst T. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatr Res : Neuroimaging. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Chomchai C, Na MN, Watanarungsan P, Yossuck P, Chomchai S. Methamphetamine abuse during pregnancy and its health impact on neonates born at Siriraj Hospital, Bangkok, Thailand. Southeast Asian J Trop Med Public Health. 2004;35:228–231. [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007a;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Kersh B, Hyde J, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2007b;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- Croiset G, Nijsen MJ, Kamphuis PJ. Role of corticotropin-releasing factor, vasopressin and the autonomic nervous system in learning and memory. Eur J Pharmacol. 2000;405:225–234. doi: 10.1016/s0014-2999(00)00556-2. [DOI] [PubMed] [Google Scholar]
- Dixon SD. Effects of transplacental exposure to cocaine and methamphetamine on the neonate. Western J Med. 1989;150:436–442. [PMC free article] [PubMed] [Google Scholar]
- Dixon SD, Bejar R. Echoencephalographic findings in neonates associated with maternal cocaine and methamphetamine use: Incidence and clinical correlates. J Pediatr. 1989;115:770–778. doi: 10.1016/s0022-3476(89)80661-4. [DOI] [PubMed] [Google Scholar]
- Felszeghy K, Bagdy G, Nyakas C. Blunted pituitary-adrenocortical stress response in adult rats following neonatal dexamethasone treatment. J Neuroendocrinol. 2000;12:1014–1021. doi: 10.1046/j.1365-2826.2000.00551.x. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Cameron HA, Daniels DC, McEwen BS. Adrenal steroids regulate postnatal development of the rat dentate gyrus: II. Effects of glucocorticoids and mineralocorticoids on cell birth. J Comp Neurol. 1991;313:486–492. doi: 10.1002/cne.903130309. [DOI] [PubMed] [Google Scholar]
- Grace CE, Schaefer TL, Herring NR, Skelton MR, McCrea AE, Vorhees CV, Williams MT. (+)-Methamphetamine increases corticosterone in plasma and BDNF in brain more than forced swim or isolation in neonatal rats. Synapse. 2008;62:110–121. doi: 10.1002/syn.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring NR, Schaefer TL, Tang PH, Skelton MR, Lucot JP, Gudelsky GA, Vorhees CV, Williams MT. Comparison of time-dependent effects of (+)-methamphetamine or forced swim on monoamines, corticosterone, glucose, creatine, and creatinine in rats. BMC Neurosci. 2008;9:49. doi: 10.1186/1471-2202-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson DM, Knott B, Walker FR. Neonatal endotoxin exposure influences HPA responsivity and impairs tumor immunity in Fischer 344 rats in adulthood. Pediatr Res. 2001;50:750–755. doi: 10.1203/00006450-200112000-00020. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-evans rats. Pharmacol Biochem Behav. 2002;73:131–140. doi: 10.1016/s0091-3057(02)00781-5. [DOI] [PubMed] [Google Scholar]
- Kamphuis PJ, Bakker JM, Broekhoven MH, Kunne C, Croiset G, Lentjes EG, Tilders FJ, van BF, Wiegant VM. Enhanced glucocorticoid feedback inhibition of hypothalamo-pituitary-adrenal responses to stress in adult rats neonatally treated with dexamethasone. Neuroendocrinology. 2002;76:158–169. doi: 10.1159/000064526. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Chou-Green JM, Davis K, Lucki I. The effects of different stressors on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid. Brain Res. 1997;760:218–230. doi: 10.1016/s0006-8993(97)00287-4. [DOI] [PubMed] [Google Scholar]
- Little BB, Snell LM, Gilstrap LC. Methamphetamine abuse during pregnancy: Outcome and fetal effects. Obstet Gynecol. 1988;72:541–544. [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Brain Res Rev. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Oro AS, Dixon SD. Perinatal cocaine and methamphetamine exposure: Maternal and neonatal correlates. J Pediatr. 1987;111:571–578. doi: 10.1016/s0022-3476(87)80125-7. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: Neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Research Reviews. 1986;11:65–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Schaefer TL, Ehrman LA, Gudelsky GA, Vorhees CV, Williams MT. Comparison of monoamine and corticosterone levels 24 h following (+)methamphetamine, (+/−)3,4-methylenedioxymethamphetamine, cocaine, (+)fenfluramine or (+/−)methylphenidate administration in the neonatal rat. J Neurochem. 2006;98:1369–1378. doi: 10.1111/j.1471-4159.2006.04034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TL, Skelton MR, Herring NR, Gudelsky GA, Vorhees CV, Williams MT. Short- and long-term effects of (+)-methamphetamine and (+/−)-3,4-methylenedioxymethamphetamine on monoamine and corticosterone levels in the neonatal rat following multiple days of treatment. J Neurochem. 2008;104:1674–1685. doi: 10.1111/j.1471-4159.2007.05112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks N, Larocque S, Meaney MJ. Neonatal endotoxin exposure alters the development of the hypothalamic-pituitary-adrenal axis: early illness and later responsivity to stress. J Neurosci. 1995;15:376–384. doi: 10.1523/JNEUROSCI.15-01-00376.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton MR, Williams MT, Schaefer TL, Vorhees CV. Neonatal (+)-methamphetamine increases brain derived neurotrophic factor, but not nerve growth factor, during treatment and results in long-term spatial learning deficits. Psychoneuroendocrinology. 2007;32:734–745. doi: 10.1016/j.psyneuen.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria A, Huestis M, Haning W, Strauss A, Grotta SD, Fallone M, Liu J, Lester BM. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicol Teratol. 2008;30:20–28. doi: 10.1016/j.ntt.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanwood GD, Levitt P. Drug exposure early in life: functional repercussions of changing neuropharmacology during sensitive periods of brain development. Curr Opin Pharmacol. 2004;4:65–71. doi: 10.1016/j.coph.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Struthers JM, Hansen RL. Visual recognition memory in drug-exposed infants. Dev Behav Pediatr. 1992;13:108–111. doi: 10.1097/00004703-199204000-00005. [DOI] [PubMed] [Google Scholar]
- Terplan M, Smith EJ, Kozloski MJ, Pollack HA. Methamphetamine use among pregnant women. Obstet Gynecol. 2009;113:1285–1291. doi: 10.1097/AOG.0b013e3181a5ec6f. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Herring NR, Schaefer TL, Grace CE, Skelton MR, Johnson HL, Williams MT. Effects of neonatal (+)-methamphetamine on path integration and spatial learning in rats: effects of dose and rearing conditions. Int J Dev Neurosci. 2008;26:599–610. doi: 10.1016/j.ijdevneu.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Skelton MR, Grace CE, Schaefer TL, Graham DL, Braun AA, Williams MT. Effects of (+)-methamphetamine on path integration and spatial learning, but not locomotor activity or acoustic startle, align with the stress hyporesponsive period in rats. Int J Dev Neurosci. 2009;27:289–298. doi: 10.1016/j.ijdevneu.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigger A, Neumann ID. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiol Behav. 1999;66:293–302. doi: 10.1016/s0031-9384(98)00300-x. [DOI] [PubMed] [Google Scholar]
- Williams MT, Blankenmeyer TL, Schaefer TL, Brown CA, Gudelsky GA, Vorhees CV. Long-term effects of neonatal methamphetamine exposure in rats on spatial learning in the Barnes maze and on cliff avoidance, corticosterone release, and neurotoxicity in adulthood. Dev Brain Res. 2003a;147:163–175. doi: 10.1016/j.devbrainres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Williams MT, Brown RW, Vorhees CV. Neonatal methamphetamine administration induces region-specific long-term neuronal morphological changes in the rat hippocampus, nucleus accumbens and parietal cortex. Eur J Neurosci. 2004;19:3165–3171. doi: 10.1111/j.0953-816X.2004.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Inman-Wood SL, Morford LL, McCrea AE, Ruttle AM, Moran MS, Rock SL, Vorhees CV. Preweaning treatment with methamphetamine induces increases in both corticosterone and ACTH in rats. Neurotoxicol Teratol. 2000;22:751–759. doi: 10.1016/s0892-0362(00)00091-x. [DOI] [PubMed] [Google Scholar]
- Williams MT, Moran MS, Vorhees CV. Refining the critical period for methamphetamine-induced spatial deficits in the Morris water maze. Psychopharmacology. 2003b;168:329–338. doi: 10.1007/s00213-003-1433-y. [DOI] [PubMed] [Google Scholar]
- Williams MT, Morford LL, Wood SL, Wallace TL, Fukumura M, Broening HW, Vorhees CV. Developmental d-methamphetamine treatment selectively induces spatial navigation impairments in reference memory in the Morris water maze while sparing working memory. Synapse. 2003c;48:138–148. doi: 10.1002/syn.10159. [DOI] [PubMed] [Google Scholar]
- Williams MT, Schaefer TL, Furay AR, Ehrman LA, Vorhees CV. Ontogeny of the adrenal response to (+)-methamphetamine in neonatal rats: The effect of prior drug exposure. Stress. 2006;9:153–163. doi: 10.1080/10253890600902842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MT, Vorhees CV, Boon F, Saber AJ, Cain DP. Methamphetamine exposure from postnatal days 11 to 20 causes impairments in both behavioral strategies and spatial learning in adult rats. Brain Res. 2002;958:312–321. doi: 10.1016/s0006-8993(02)03620-x. [DOI] [PubMed] [Google Scholar]