Abstract
Calcitriol, the active metabolite of vitamin D, has been shown to have significant effects on the brain. These actions include reducing the severity of some central nervous system lesions, possibly by upregulating trophic factors such as glial cell line-derived neurotrophic factor (GDNF). GDNF has substantial effects on the nigrostriatal dopamine (DA) system of young adult, aged and lesioned animals. Thus, the administration of calcitriol may lead to significant effects on nigrostriatal DA neuron functioning. The present experiments were designed to examine the ability of calcitriol to alter striatal DA release, and striatal and nigral tissue levels of DA. Male Fischer-344 rats were administered vehicle or calcitriol (0.3, 1.0, or 3.0 μg/kg, s.c.) once daily for 8 consecutive days. Three weeks later in vivo microdialysis experiments were conducted to measure basal and stimulus evoked overflow of DA from the striatum. Basal levels of extracellular DA were not significantly affected by the calcitriol treatments. However, the 1.0 and 3.0 μg/kg doses of calcitriol led to increases in both potassium and amphetamine evoked overflow of striatal DA. Although post-mortem tissue levels of striatal DA were not altered by the calcitriol injections, nigral tissue levels of DA and its main metabolites were increased by both the 1.0 and 3.0 μg/kg doses of calcitriol. In a separate group of animals GDNF levels were augmented in the striatum and substantia nigra after eight consecutive daily injections of calcitriol. These results suggest that systemically administered calcitriol can upregulate dopaminergic release processes in the striatum and DA levels in the substantia nigra. Increases in levels of endogenous GDNF following calcitriol treatment may in part be responsible for these changes. The ability of calcitriol to lead to augmented DA release in the striatum suggests that calcitriol may be beneficial in disease processes involving dopaminergic dysfunction.
Keywords: Calcitriol, Dopamine, Striatum, Substantia Nigra, GDNF, Microdialysis
1. Introduction
Calcitriol (1,25-dihydroxyvitamin D3), the active metabolite of vitamin D3, is currently used clinically to treat several conditions such as hypocalcemia and hypoparathyroidism. While its classical role in bone metabolism and calcium homeostasis are well known, calcitriol has also been shown to modulate several other physiological processes including cell growth, apoptosis and immune responses (Dusso et al., 2005). Calcitriol has also been shown to have numerous effects in the nervous system (Fernandes de Abreu et al., 2009; Garcion et al., 2002). Although its ability to cross the blood brain barrier is limited, calcitriol is taken up by the brain in proportion to free circulating levels perfusing the blood brain barrier (Gascon-Barre and Huet, 1983); and vitamin D receptors are extensively distributed throughout the brain (Eyles et al., 2005; Prufer et al., 1999; Stumpf and O’Brien, 1987). Furthermore, the enzyme responsible for the final step in calcitriol synthesis has been localized to neuronal and glial cells (Eyles et al., 2005), and it has been demonstrated that activated microglial cells can produce calcitriol (Neveu et al., 1994b). Taken together, these results suggest that calcitriol may have important physiological functions in the central nervous system.
One of the apparent functions of calcitriol in the nervous system is modulation of trophic factors. Several studies have indicated that calcitriol regulates the expression of trophic factors in glial and neuroblastoma cell lines (Neveu et al., 1994a; Veenstra et al., 1997a and 1997b). One of the trophic factors that is regulated by calcitriol is glial cell line-derived neurotrophic factor (GDNF). Calcitriol has been shown to increase expression of GDNF in vitro in C6 glioma cells (Naveilhan et al., 1996), and increase release of GDNF from human glioblastoma cells (Verity et al., 1999). In vivo, calcitriol administration has been shown to increase expression and protein levels of GDNF in the brain (Sanchez et al., 2002 and 2009; Wang et al., 2000).
In young adult animals a single intracerebral administration of GDNF has significant and long-lasting effects on brain dopamine (DA) systems. These effects include changes in tissue levels of DA in the nigrostriatal system (Cass and Peters, 2010; Hudson et al., 1995; Martin et al., 1996;), augmentation of stimulus-evoked overflow of DA from the striatum (Hebert et al., 1996; Xu and Dluzen, 2000), and behavioral changes that suggest activation of dopaminergic systems (Hudson et al., 1995; Kobayashi et al., 1998; Martin et al., 1996). Similar changes have been reported in aged animals following acute or chronic administration of GDNF (Emerich et al., 1996; Grondin et al., 2003; Hebert and Gerhardt, 1997; Lapchak et al., 1997). Because of its striking effects on nigrostriatal DA neurons in normal and aged animals, GDNF has also been extensively evaluated as a potential therapeutic agent in several animal models of Parkinson’s disease (Bjorklund et al., 2000; Cass et al., 1998; Gash et al., 1998; Grondin et al., 2002; Kordower et al., 2000).
Previous studies have documented that calcitriol can increase brain GDNF levels, and that GDNF leads to increases in dopaminergic activity in the brain. Thus, it is possible that calcitriol could lead to alterations in dopaminergic processes in the brains of normal animals. Understanding how calcitriol affects DA dynamics in intact animals may provide insight into its potential as a possible therapeutic agent for conditions associated with dopaminergic dysfunction. The purpose of the present study was to determine if calcitriol treatment can alter striatal DA release, and striatal and nigral tissue levels of DA, in normal animals. In vivo microdialysis was used to evaluate potassium- and amphetamine-evoked overflow of DA, and to monitor basal extracellular levels of DA and its primary metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) from the striatum of rats treated with various doses of calcitriol. Postmortem tissue levels of DA and metabolites in the striatum and substantia nigra were determined at the conclusion of each experiment, and, in a separate group of animals the ability of calcitriol to upregulate GDNF levels was evaluated.
2. Materials and methods
2.1. Animals
Male Fischer-344 rats 14-15 weeks old were obtained from Harlan Laboratories (Indianapolis, IN). The animals weighed 277-303 g at the start of the experiments and 8 animals were included in each treatment group. The animals were housed in pairs under a 12-hr light-dark cycle with food and water freely available. All animal use procedures were approved by the Animal Care and Use Committee at the University of Kentucky and were in strict accordance with National Institutes of Health guidelines. All efforts were made to minimize the number of animals used and to minimize their pain and discomfort.
2.2. Calcitriol injections
Animals were injected once daily for eight consecutive days with calcitriol (0.3, 1.0 or 3.0 μg/kg/day) or vehicle. All injections were administered subcutaneously. The calcitriol (Sigma Chemical Co., St. Louis, MO) was first dissolved in propylene glycol at a concentration of 100 μg/ml. For injections the calcitriol in propylene glycol was diluted into 0.9% saline so that the final volume given was 1 ml/kg of body weight. Vehicle treated animals were injected with propylene glycol diluted in 0.9% saline.
2.3. In Vivo Microdialysis
Microdialysis studies were conducted three weeks after the last vehicle or calcitriol injection. The animals were anesthetized with urethane (1.25–1.50 g/kg, i.p.) and placed into a Kopf stereotaxic frame. Body temperature was maintained at 37°C with a heating pad coupled to a rectal thermometer. Microdialysis probes (CMA/11 probes, 3.0 mm length of dialysis membrane; CMA/Microdialysis, Acton, MA) were slowly lowered into both the left and right striata (0.0 mm anterior to bregma, 3.0 mm lateral from midline, tip of probe 6.3 mm below the surface of the brain). The probes were perfused at a rate of 1.2 μl/min with artificial cerebrospinal fluid containing 145 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, 1.0 mM MgCl2, 0.2 mM ascorbic acid, and 2.0 mM NaH2PO4 (pH adjusted to 7.4 using NaOH). Dialysate fractions were collected at 20-min intervals. Following a two-hour equilibration period and the collection of 3 baseline fractions, DA overflow was stimulated by increasing the KCl concentration in the perfusate to 100 mM (NaCl reduced to 47.7 mM) for a single 20-min fraction, and then two hours later by adding 100 μM d-amphetamine to the perfusate for a single 20-min fraction. Five final fractions with normal artificial cerebrospinal fluid were collected following the d-amphetamine stimulation. Dialysate samples were immediately frozen on dry ice and stored at −80°C until assayed for DA, DOPAC and HVA.
2.4. Tissue collection and HPLC analysis
After collecting the dialysate fractions the urethane-anesthetized animals were killed by decapitation and their brains rapidly harvested and chilled in ice-cold saline. A coronal slice of brain 2 mm thick at the level of the dialysis probes was made with the aid of an ice-chilled brain mold (Rodent Brain Matrix, ASI Instruments, Warren, MI). The location of all dialysis probes was confirmed to be centered in the dorsal striatum at the level of the crossing of the anterior commissure. The striatum was then dissected from each half of the slice. The substantia nigra was dissected from both sides of a 2 mm thick coronal slice through the midbrain. The tissue pieces were placed in preweighed vials, weighed, frozen on dry ice, and then stored at −80°C until assayed for DA and metabolites by high performance liquid chromatography (HPLC). For tissue analysis the samples were prepared as previously described (Cass et al., 2003). For dialysis samples, 20 μl of the dialysate was injected directly onto the HPLC column.
The HPLC system consisted of a Beckman model 118 pump and model 507 autoinjector, and an ESA model 5200A Coulochem II electrochemical detector with a model 5011 dual-detector analytical cell (detector 1 set at +350 mV and detector 2 set at −300 mV). Separations were carried out using an ESA Hypersil ODS 3 μm particle C18 column (4.6 mm × 80 mm). Flow rate was 1.4 ml/min and the mobile phase was a 0.17 M citrate-acetate buffer containing 5 mg/l EDTA, 90 mg/l octanesulfonic acid, and 7–8 % methanol (pH 4.1). Chromatograms were recorded from both detectors using dual-channel strip chart recorders. Retention times of standards were used to identify peaks, and peak heights were used to calculate recovery of internal standard and amount of DA and metabolites.
2.5. Striatal and nigral GDNF levels
Rats were treated with calcitriol or vehicle once daily for eight consecutive days and striatal and nigral tissue was harvested 2–3 hours after the last injection as described above. GDNF levels were measured using Promega EmaxTM ImmunoAssay Kits as described previously (Smith et al., 2006; Yurek and Fletcher-Turner, 2001).
2.6. Data analysis
All dialysis probes were calibrated in vitro prior to use to determine acceptable probes (recovery of DA at least 12%). However, values were not corrected for in vitro recoveries as uncorrected values may be better correlated to true values (Glick et al., 1994). Basal levels of DA and metabolites were defined as the average value in the three fractions preceding stimulation by excess potassium. Dialysis data were expressed as nM concentration of DA or metabolite in the dialysate and, for evoked overflow, as the total amount of DA in the dialysate above baseline following stimulation with potassium or amphetamine. Tissue levels of DA and GDNF were expressed as ng/g wet weight of tissue. For animals used in dialysis experiments data from the left and right sides of the brain were averaged to get a single value for each time point and neurochemical per animal. Results were analyzed statistically using analysis of variance (ANOVA) as indicated in the results section. When the ANOVA results indicated significant effects, Newman-Keuls tests were used for post hoc comparisons.
3. Results
3.1. Dialysate levels of DA and metabolites
Basal extracellular dialysate levels of DA and metabolites in urethane anesthetized rats are shown in Table 1. The results were analyzed using one-way ANOVA, and, while there was a trend for basal DA levels to increase with increasing doses of calcitriol, the increase did not reach statistical significance. However, with both DOPAC and HVA there was a significant effect. In the animals treated with the highest dose of calcitriol basal DOPAC levels were increased by 31%, and basal HVA levels were increased by 23%, compared to the vehicle treated control animals.
Table 1.
Basal dialysate levels of DA, DOPAC and HVA from the striatum of animals treated for eight days with vehicle or calcitriol. Dialysis experiments were performed three weeks after the final day of treatment.
| Striatal dialysate level (nM)
|
|||
|---|---|---|---|
| Calcitriol Dose | DA | DOPAC | HVA |
| Vehicle | 4.03 ± 0.44 | 1575 ± 82 | 911 ± 39 |
| 0.3 μg/kg/day | 4.96 ± 0.41 | 1699 ± 84 | 982 ± 38 |
| 1.0 μg/kg/day | 5.32 ± 0.44 | 1779 ± 86 | 1012 ± 47 |
| 3.0 μg/kg/day | 5.77 ± 0.60 | 2056 ± 43a | 1116 ± 26a |
Values are mean ± SEM from 8 animals per group.
p < 0.05 vs. vehicle group (one-way ANOVA followed by Newman-Keuls post hoc comparisons).
The complete time course for the dialysis experiments for DA levels is shown in Fig. 1. Overall extracellular DA levels from the animals treated with either 1.0 or 3.0 μg/kg/day of calcitriol were greater than those from the vehicle group (p < 0.05 for both; main effect of treatment group). In order to facilitate comparisons of the potassium and amphetamine evoked overflow of DA between the groups the data were expressed as total amount of DA in the dialysate fractions above basal levels following stimulation by excess potassium or d-amphetamine (Fig. 2). Compared to the vehicle treated control animals, potassium-evoked overflow of DA was increased by 57% in the animals treated with 1.0 μg/kg/day of calcitriol, and by 42% in the animals treated with 3.0 μg/kg/day of calcitriol (p < 0.05 for both). Similarly, amphetamine-evoked DA overflow was increased by 36% and 32%, respectively, in the animals treated with 1.0 or 3.0 μg/kg/day of calcitriol, compared to the vehicle treated group (p < 0.05 for both).
Figure 1.
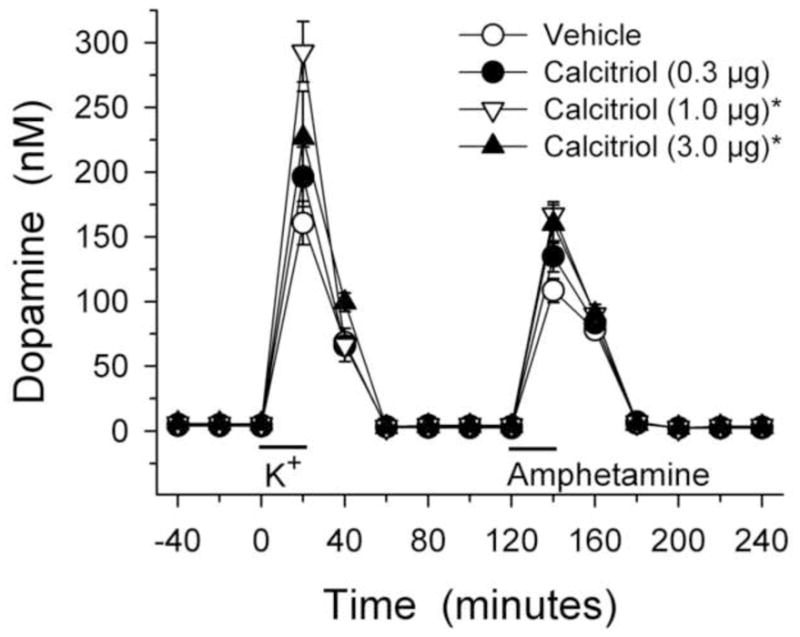
Dialysate levels of DA from the striata of animals treated with vehicle or calcitriol for eight consecutive days. Microdialysis experiments were performed three weeks after the last day of treatment. Excess potassium (100 mM) was included in the perfusate for 20-min starting at 0 min (horizontal bar above K+), and 100 μM amphetamine was included in the perfusate for 20-min starting at 120 min (horizontal bar above Amphetamine). Values shown are mean ± SEM from 8 animals per group. * p < 0.05 vs. vehicle group (mixed ANOVA with time of dialysis sample collection as a within factor, and treatment group as a between factor; significant main effect of treatment group (p < 0.01) followed by Newman-Keuls post-hoc comparisons).
Figure 2.
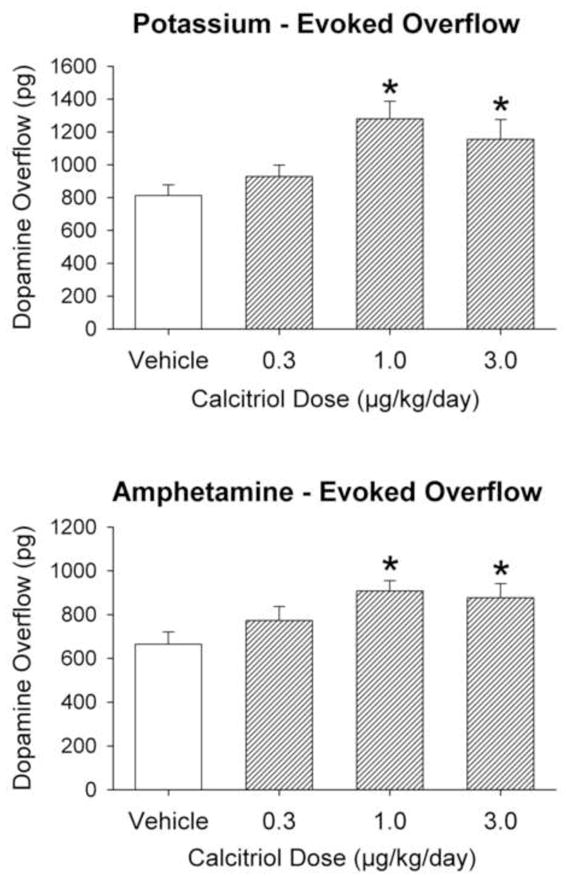
Potassium-evoked (top panel) and amphetamine-evoked (bottom panel) overflow of DA from the striatum of animals treated with vehicle or calcitriol for eight consecutive days. Microdialysis experiments were performed three weeks after the last day of treatment. Values shown are mean ± SEM from 8 animals per group. * p < 0.05 vs. vehicle group (one way ANOVA followed by Newman-Keuls post-hoc comparisons).
3.2. Tissue levels of DA
Striatal and nigral tissue levels of DA, DOPAC and HVA are shown in Table 2. The calcitriol treatments had no significant effect on striatal levels of DA or DOPAC. However, the highest dose of calcitriol, 3.0 μg/kg/day, did increase striatal levels of HVA by 26% compared to vehicle treated control animals (p < 0.05). The effects of the calcitriol treatments were more substantial in the substantia nigra, where both the 1.0 and 3.0 μg/kg/day doses of calcitriol led to significant increases in DA and metabolites. For the 1.0 and 3.0 μg/kg/day doses of calcitriol, the respective increases in nigral DA levels were 17% and 20%, the increases in DOPAC levels were 21% and 23%, and the increases in HVA levels were 46% and 55% (p < 0.05 for all). Metabolite to DA ratios were also calculated as an indicator of potential changes in DA turnover. However, there were no significant differences between any of the doses of calcitriol in either the striatum or substantia nigra for DOPAC/DA ratios, HVA/DA ratios, or (DOPAC+HVA)/DA ratios (data not shown), suggesting that the calcitriol treatments did not have a significant effect on DA turnover.
Table 2.
Tissue levels of DA, DOPAC and HVA from the striatum and substantia nigra of animals treated for eight days with vehicle or calcitriol. Tissue was harvested three weeks after the final day of treatment.
| Region and calcitriol dose | Tissue content (ng/g wet weight of tissue)
|
||
|---|---|---|---|
| DA | DOPAC | HVA | |
| Striatum | |||
| Vehicle | 15,474 ± 552 | 2,428 ± 108 | 1,373 ± 41 |
| 0.3 μg/kg/day | 16,183 ± 460 | 2,496 ± 73 | 1,409 ± 98 |
| 1.0 μg/kg/day | 15,900 ± 980 | 2,657 ± 164 | 1,591 ± 92 |
| 3.0 μg/kg/day | 16,477 ± 654 | 2,664 ± 125 | 1,743 ± 60a |
| Substantia Nigra | |||
| Vehicle | 764 ± 16 | 143 ± 4.0 | 65 ± 2.0 |
| 0.3 μg/kg/day | 788 ± 31 | 152 ± 9.3 | 70 ± 5.1 |
| 1.0 μg/kg/day | 891 ± 41 a | 173 ± 9.1 a | 95 ± 7.8 a |
| 3.0 μg/kg/day | 914 ± 38 a | 176 ± 8.7 a | 101 ± 5.2 a |
Values are mean ± SEM from 8 animals per group.
p < 0.05 vs. vehicle group (one-way ANOVA followed by Newman-Keuls post hoc comparisons).
3.3 Tissue levels of GDNF
In a separate group of animals, tissue levels of GDNF were measured on the eighth day of vehicle or calcitriol treatment. In the striatum, eight days of calcitriol treatment led to 80% and 82% increases in GDNF levels in the animals treated with the 1.0 and 3.0 μg/kg/day doses of calcitriol, respectively (p < 0.05), compared to vehicle injected control animals (Fig. 3). In the substantia nigra, eight days of calcitriol treatment led to increases in GDNF levels of 131% and 107% in the animals treated with the 1.0 and 3.0 μg/kg/day doses of calcitriol, respectively (p < 0.05), compared to the control animals (Fig. 3).
Figure 3.
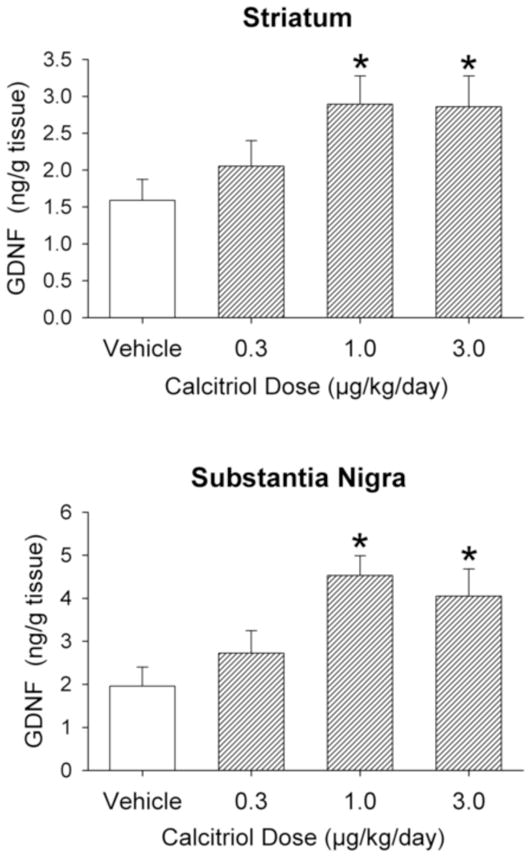
Striatal (top panel) and substantia nigra (bottom panel) GDNF levels from animals treated with vehicle or calcitriol for eight consecutive days. Tissue was harvested on the eighth day of treatment. Values shown are mean ± SEM from 8-10 animals per group. * p < 0.05 vs. vehicle group (one way ANOVA followed by Newman-Keuls post-hoc comparisons).
4. Discussion
The present study was undertaken to determine if administration of calcitriol would lead to changes in DA release in the striatum or in DA content in the striatum or substantia nigra of normal young adult rats. The results demonstrated that calcitriol, at doses of 1.0 or 3.0 μg/kg/day for eight days, led to significant changes in DA release and content in the nigrostriatal system three weeks after the end of the calcitriol injections.
Previous in vivo studies that examined the protective effects of calcitriol for DA neurons (Sanchez et al., 2009; Smith et al., 2006; Wang et al., 2001), or the ability of calcitriol to alter GDNF levels (Sanchez et al., 2002 and 2009; Wang et al., 2000) examined a single dose of calcitriol (1.0 μg/kg/day). In the current study we examined the effects of three doses of calcitriol on DA neurons and GDNF levels. The lowest dose used, 0.3 μg/kg/day, did not have statistically significant effects on any of the parameters examined. The middle and higher doses (1.0 and 3.0 μg/kg/day) both had significant, and similar, effects. On a few of the parameters examined only the 3.0 μg/kg/day dose, and not the 1.0 μg/kg/day dose, was significantly different from controls (basal dialysate levels of DOPAC and HVA, and striatal tissue levels of HVA). However, there were no differences between the 1.0 and 3.0 μg/kg/day groups when compared to each other. Thus, the differences between the effects of the middle and high doses were relatively minor. In addition, during the 8 days of calcitriol injections, the animals treated with the 1.0 μg/kg/day dose did not lose body weight, while the animals treated with the 3.0 μg/kg/day dose did lose weight during the treatment (% change in body weight from day 1 to day 8 of treatment: 1.0 μg/kg/day group, +0.24 ± 0.49%; 3.0 μg/kg/day group, −13.43 ± 1.56%; p < 0.001, t-test). Thus, the highest dose, while effective, may have been high enough to produce adverse side effects, possibly due to calcitriol-induced hypercalcemia (Chavhan et al., 2011; Wang et al., 2000). Therefore, for the parameters examined in the present study, the 1.0 μg/kg/day was the optimal dose for producing changes in DA neuron functioning and GDNF levels.
Other in vivo studies, all using the 1.0 μg/kg/day dose, have also examined the ability of calcitriol to alter GDNF levels. Wang et al. (2000) found that eight consecutive daily injections of calcitriol led to a 92% increase in cortical levels of GDNF. Sanchez et al. (2002) report a 40% increase in striatal GDNF protein levels after seven consecutive days of treatment, and Smith et al. (2006) report a 37% increase in nigral GDNF levels after eight days of calcitriol. In the current study we found an 80% increase in striatal GDNF levels, and a 131% increase in nigral GDNF levels, with the 1.0 μg/kg/day dose after eight days of treatment. Thus, the present results support previous studies and provide further evidence that calcitriol can act as a modulator of endogenous GDNF levels.
The dose response of calcitriol on GDNF levels was similar to the dose response for changes in DA overflow and nigral tissue DA levels. In addition, the effects observed on evoked DA overflow and tissue levels of DA are similar to what has been reported in rats treated with GDNF. For example, following the injection of a single dose of GDNF into the brain of unlesioned rats, previous studies have reported increases, or a trend for an increase, in potassium-evoked overflow of striatal DA (Cass and Manning, 1999; Cass and Peters, 2010; Hebert et al., 1996; Hebert and Gerhardt, 1997; Xu and Dluzen, 2000), and increases in amphetamine-evoked overflow of striatal DA (Cass and Peters, 2010; Hebert et al., 1996; Hebert and Gerhardt, 1997). In addition, tissue levels of DA in the substantia nigra are also significantly increased following a single intracerebral administration of GDNF (Cass and Manning 1999; Cass and Peters, 2010; Hebert et al., 1996; Hudson et al., 1995; Martin et al., 1996). Thus, the parallel changes in dopaminergic parameters and levels of GDNF, along with the previously documented effects of GDNF, suggest that the calcitriol-induced changes in DA release and tissue DA levels may be due in part to upregulation of endogenous GDNF levels. However, we cannot rule out the possibility that other effects of calcitriol may be responsible for the present results. For instance, calcitriol has been shown to regulate expression of other trophic factors, such as transforming growth factor- ß, nerve growth factor and neurotrophin-3 (Neveu et al., 1994a; Saporito et al., 1994; Veenstra et al., 1997a and 1997b), which could affect neuronal function; and calcitriol has been shown to increase levels of tyrosine hydroxylase expression (Puchacz et al., 1996; Sanchez et al., 2009), which could modulate dopaminergic processes. Thus, further studies will be necessary to more completely define the mechanism by which calcitriol affects DA systems.
The microdialysis experiments were carried out three weeks after ending the calcitriol injections. This interval was chosen as it is a time point when significant changes in DA release and content have been demonstrated in rats following GDNF injection (Cass and Peters, 2010; Hebert et al., 1996; Hebert and Gerhardt, 1997). In addition, several studies have demonstrated significant behavioral or neurochemical effects 3 to 4 weeks after GDNF treatment (for example: Aoi et al., 2000; Cass et al., 2000; Date et al., 1998; Hoffer et al., 1994; Salvatore et al., 2004). Thus, if GDNF is involved with the effects of calcitriol, the three week interval should, and did, represent a time point likely to demonstrate potential effects of calcitriol on DA neurons.
The timing for the duration of the calcitriol treatment and for the measurement of GDNF levels (8 days) was based on previous calcitriol studies. Wang et al. (2001) found that eight days of calcitriol partially protected against the neurotoxic effects of 6-hydroxydopamine (6-OHDA), and Sanchez et al. (2009) found that seven days of calcitriol, given before or after a 6-OHDA lesion, led to a reduction in 6-OHDA-induced loss of tyrosine hydroxylase positive neurons in the substantia nigra. In addition, seven or eight consecutive days of calcitriol administration (1.0 μg/kg/day) has been shown to upregulate GDNF expression and protein levels in the brain (Sanchez et al., 2002 and 2009; Smith et al., 2006; Wang et al., 2000). Thus, we choose eight days of treatment for examining the potential ability of calcitriol to augment dopaminergic release processes and tissue DA and GDNF levels.
In conclusion, the systemic administration of calcitriol led to increases in evoked overflow of DA from the striatum, to increases in DA levels in the substantia nigra, and to increases in endogenous GDNF levels in the striatum and substantia nigra. Taken together, the results of this study indicate that calcitriol can augment dopaminergic processes in the nigrostriatal system of normal animals and provide further evidence that calcitriol may be beneficial in treating disease processes involving dopaminergic dysfunction.
Highlights.
Effects of calcitriol (active metabolite of vitamin D) were studied in normal rats.
Calcitriol increases stimulus- evoked overflow of dopamine from the striatum.
Calcitriol increases tissue levels of dopamine in the substantia nigra.
Calcitriol upregulates GDNF levels in the striatum and substantia nigra.
Calcitriol may have therapeutic potential for disorders such as Parkinson’s disease.
Acknowledgments
This study was supported in part by United States Public Health Service Grants DA22314, NS60924 and NS50311. None of the authors have a conflict of interest of any type in association with this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoi M, Date I, Tomita S, Ohmoto T. The effect of intrastriatal single injection of GDNF on the nigrostriatal dopaminergic system in hemiparkinsonian rats: behavioral and histological studies using two different dosages. Neurosci Res. 2000;36:319–325. doi: 10.1016/s0168-0102(00)00097-3. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Kirik D, Rosenblad C, Georgievska B, Lundberg C, Mandel RJ. Towards a neuroprotective gene therapy for Parkinson’s disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model. Brain Res. 2000;886:82–98. doi: 10.1016/s0006-8993(00)02915-2. [DOI] [PubMed] [Google Scholar]
- Cass WA, Manning MW. GDNF protection against 6-OHDA-induced reductions in potassium-evoked overflow of striatal dopamine. J Neurosci. 1999;19:1416–1423. doi: 10.1523/JNEUROSCI.19-04-01416.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass WA, Peters LE. Neurturin effects on nigrostriatal dopamine release and content: comparison with GDNF. Neurochem Res. 2010;35:727–734. doi: 10.1007/s11064-010-0128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass WA, Kearns CM, Gash DM. Protective and regenerative properties of GDNF in the central nervous system. In: Mattson MP, editor. Neuroprotective Signal Transduction. Humana Press; Totowa, NJ: 1998. pp. 145–161. [Google Scholar]
- Cass WA, Manning MW, Bailey SL. Restorative effects of GDNF on striatal dopamine release in rats treated with neurotoxic doses of methamphetamine. Ann NY Acad Sci. 2000;914:127–136. doi: 10.1111/j.1749-6632.2000.tb05190.x. [DOI] [PubMed] [Google Scholar]
- Cass WA, Harned ME, Peters LE, Nath A, Maragos WF. HIV-1 Protein Tat Potentiation of Methamphetamine-Induced Decreases in Evoked Overflow of Dopamine in the Striatum of the Rat. Brain Res. 2003;984:133–142. doi: 10.1016/s0006-8993(03)03122-6. [DOI] [PubMed] [Google Scholar]
- Chavhan SG, Brar RS, Banga HS, Sandhu HS, Sodhi S, Gadhave PD, Kothule VR, Kammon AM. Clinicopathological studies on vitamin D(3) toxicity and therapeutic evaluation of aloe vera in rats. Toxicol Int. 2011;18:35–43. doi: 10.4103/0971-6580.75851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date I, Aoi M, Tomita S, Collins F, Ohmoto T. GDNF administration induces recovery of the nigrostriatal dopaminergic system both in young and aged parkinsonian mice. NeuroReport. 1998;9:2365–2369. doi: 10.1097/00001756-199807130-00039. [DOI] [PubMed] [Google Scholar]
- Dusso AS, Brown AJ, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol. 2005;289:F8–F28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Plone M, Francis J, Frydel BR, Winn SR, Lindner MD. Alleviation of behavioral deficits in aged rodents following implantation of encapsulated GDNF-producing fibroblasts. Brain Res. 1996;736:99–110. [PubMed] [Google Scholar]
- Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1α-hydroxylase in human brain. J Clin Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Fernandes de Abreu DA, Eyles D, Feron F. Vitamin D, a neuro-immunomodulator: implications for neurodegenerative and autoimmune diseases. Psychoneuroendocrinology. 2009;34S:S265–S277. doi: 10.1016/j.psyneuen.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13:100–105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- Gascon-Barre M, Huet PM. Apparent [3H]1,25-dihydroxyvitamin D3 uptake by canine and rodent brain. Am J Physiol. 1983;244:E266–E271. doi: 10.1152/ajpendo.1983.244.3.E266. [DOI] [PubMed] [Google Scholar]
- Gash DM, Zhang Z, Gerhardt GA. Neuroprotective and neurorestorative properties of GDNF. Ann Neurol. 1998;44(suppl 1):S121–S125. doi: 10.1002/ana.410440718. [DOI] [PubMed] [Google Scholar]
- Glick SD, Dong N, Keller RW, Jr, Carlson JN. Estimating extracellular concentrations of dopamine and 3,4-dihydroxyphenylacetic acid in nucleus accumbens and striatum using microdialysis: relationships between in vitro and in vivo recoveries. J Neurochem. 1994;62:2017–2021. doi: 10.1046/j.1471-4159.1994.62052017.x. [DOI] [PubMed] [Google Scholar]
- Grondin R, Zhang Z, Yi A, Cass WA, Maswood N, Andersen AH, Elsberry DD, Klein MC, Gerhardt GA, Gash DM. Chronic, controlled GDNF infusion promotes structural and functional recovery in advanced parkinsonian monkeys. Brain. 2002;125:2191–2201. doi: 10.1093/brain/awf234. [DOI] [PubMed] [Google Scholar]
- Grondin R, Cass WA, Zhang Z, Stanford JA, Gash DM, Gerhardt GA. Glial cell line-derived neurotrophic factor increases stimulus-evoked dopamine release and motor speed in aged rhesus monkeys. J Neurosci. 2003;23:1974–1980. doi: 10.1523/JNEUROSCI.23-05-01974.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert MA, Gerhardt GA. Behavioral and neurochemical effects of intranigral administration of glial cell line-derived neurotrophic factor on aged Fischer 344 rats. J Pharmacol Exp Ther. 1997;282:760–768. [PubMed] [Google Scholar]
- Hebert MA, Van Horne CG, Hoffer BJ, Gerhardt GA. Functional effects of GDNF in normal rat striatum: presynaptic studies using in vivo electrochemistry and microdialysis. J Pharmacol Exp Ther. 1996;279:1181–1190. [PubMed] [Google Scholar]
- Hoffer BJ, Hoffman A, Bowenkamp K, Huettl P, Hudson J, Martin D, Lin LFH, Gerhardt GA. Glial cell line-derived neurotrophic factor reverses toxin-induced injury to midbrain dopaminergic neurons in vivo. Neurosci Lett. 1994;182:107–111. doi: 10.1016/0304-3940(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Hudson J, Granholm AC, Gerhardt GA, Henry MA, Hoffman A, Biddle P, Leela NS, Mackerlova L, Lile JD, Collins F, Hoffer BJ. Glial cell line-derived neurotrophic factor augments midbrain dopaminergic circuits in vivo. Brain Res Bull. 1995;36:425–432. doi: 10.1016/0361-9230(94)00224-o. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Ogren SO, Hoffer BJ, Olson L. Dopamine D1 and D2 receptor-mediated acute and long-lasting behavioral effects of glial cell line-derived neurotrophic factor administered into the striatum. Exp Neurol. 1998;154:302–314. doi: 10.1006/exnr.1998.6952. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Deglon N, Aebisher P. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Miller PJ, Jiao S. Glial cell line-derived neurotrophic factor induces the dopaminergic and cholinergic phenotype and increases locomotor activity in aged Fischer 344 rats. Neuroscience. 1997;77:745–752. doi: 10.1016/s0306-4522(96)00492-7. [DOI] [PubMed] [Google Scholar]
- Martin D, Miller G, Cullen T, Fischer N, Dix D, Russell D. Intranigral or intrastriatal injections of GDNF: effects on monoamine levels and behavior in rats. Eur J Pharmacol. 1996;317:247–256. doi: 10.1016/s0014-2999(96)00756-x. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Neveu I, Wion D, Brachet P. 1,25-Dihydroxyvitamin D3, an inducer of glial cell line-derived neurotrophic factor. NeuroReport. 1996;7:2171–2175. doi: 10.1097/00001756-199609020-00023. [DOI] [PubMed] [Google Scholar]
- Neveu I, Naveilhan P, Baudet C, Brachet P, Metsis M. 1,25-Dihydroxyvitamin D3 regulates NT-3, NT-4 but not BDNF mRNA in astrocytes. NeuroReport. 1994a;6:124–126. doi: 10.1097/00001756-199412300-00032. [DOI] [PubMed] [Google Scholar]
- Neveu I, Naveilhan P, Menaa C, Wion D, Brachet P, Garabedian M. Synthesis of 1,25-dihydroxyvitamin D3 by rat brain macrophages in vitro. J Neurosci Res. 1994b;38:214–220. doi: 10.1002/jnr.490380212. [DOI] [PubMed] [Google Scholar]
- Prufer K, Veenstra TD, Jirikowski GF, Kumar R. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in rat brain and spinal cord. J Chem Neuroanat. 1999;16:135–145. doi: 10.1016/s0891-0618(99)00002-2. [DOI] [PubMed] [Google Scholar]
- Puchacz E, Stumpf WE, Stachowiak EK, Stachowiak MK. Vitamin D increases expression of the tyrosine hydroxylase gene in adrenal medullary cells. Mol Brain Res. 1996;36:193–196. doi: 10.1016/0169-328x(95)00314-i. [DOI] [PubMed] [Google Scholar]
- Salvatore MF, Zhang JL, Large DM, Wilson PE, Gash CR, Thomas TC, Haycock JW, Bing G, Stanford JA, Gash DM, Gerhardt GA. Striatal GDNF administration increases tyrosine hydroxylase phosphorylation in the rat striatum and substantia nigra. J Neurochem. 2004;90:245–254. doi: 10.1111/j.1471-4159.2004.02496.x. [DOI] [PubMed] [Google Scholar]
- Sanchez B, Lopez-Martin E, Segura C, Labandeira-Garcia JL, Perez-Fernandez R. 1,25-Dihydroxyvitamin D3 increases striatal GDNF mRNA and protein expression in adult rats. Mol Brain Res. 2002;108:143–146. doi: 10.1016/s0169-328x(02)00545-4. [DOI] [PubMed] [Google Scholar]
- Sanchez B, Relova JL, Gallego R, Ben-Batalla I, Perez-Fernandez R. 1,25-Dihydroxyvitamin D3 administration to 6-hydroxydopamine-lesioned rats increases glial cell line-derived neurotrophic factor and partially restores tyrosine hydroxylase expression in substantia nigra and striatum. J Neurosci Res. 2009;87:723–732. doi: 10.1002/jnr.21878. [DOI] [PubMed] [Google Scholar]
- Saporito MS, Brown ER, Hartpence KC, Wilcox HM, Vaught JL, Carswell S. Chronic 1,25-dihydroxyvitamin D3-mediated induction of nerve growth factor mRNA and protein in L929 fibroblasts and in adult rat brain. Brain Res. 1994;633:189–196. doi: 10.1016/0006-8993(94)91539-3. [DOI] [PubMed] [Google Scholar]
- Smith MP, Fletcher-Turner A, Yurek DM, Cass WA. Calcitriol protection against dopamine loss induced by intracerebroventricular administration of 6-hydroxydopamine. Neurochem Res. 2006;31:533–539. doi: 10.1007/s11064-006-9048-4. [DOI] [PubMed] [Google Scholar]
- Stumpf WE, O’Brien LP. 1,25(OH)2 vitamin D3 sites of action in the brain. An autoradiographic study. Histochemistry. 1987;87:393–406. doi: 10.1007/BF00496810. [DOI] [PubMed] [Google Scholar]
- Veenstra TD, Londowski JM, Windebank AJ, Brimijoin S, Kumar R. Effects of 1,25-dihydroxyvitamin D3 on growth of mouse neuroblastoma cells. Dev Brain Res. 1997a;99:53–60. doi: 10.1016/s0165-3806(96)00196-4. [DOI] [PubMed] [Google Scholar]
- Veenstra TD, Windebank AJ, Kumar R. 1,25-Dihydroxyvitamin D3 regulates expression of N-myc, c-myc, protein kinase C, and transforming growth factor-ß2 in neuroblastoma cells. Biochem Biophys Res Commun. 1997b;235:15–18. doi: 10.1006/bbrc.1997.6718. [DOI] [PubMed] [Google Scholar]
- Verity AN, Wyatt TL, Lee W, Hajos B, Baecker PA, Eglen RM, Johnson RM. Differential regulation of glial cell line-derived neurotrophic factor (GDNF) expression in human neuroblastoma and glioblastoma cell lines. J Neurosci Res. 1999;55:187–197. doi: 10.1002/(SICI)1097-4547(19990115)55:2<187::AID-JNR6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chiang YH, Su TP, Hayashi T, Morales M, Hoffer BJ, Lin SZ. Vitamin D3 attenuates cortical infarction induced by middle cerebral artery ligation in rats. Neuropharmacology. 2000;39:873–880. doi: 10.1016/s0028-3908(99)00255-5. [DOI] [PubMed] [Google Scholar]
- Wang JY, Wu JN, Cherng TL, Hoffer BJ, Chen HH, Borlongan CV, Wang Y. Vitamin D3 attenuates 6-hydroxydopmaine-induced neurotoxicity in rats. Brain Res. 2001;904:67–75. doi: 10.1016/s0006-8993(01)02450-7. [DOI] [PubMed] [Google Scholar]
- Xu K, Dluzen DE. The effect of GDNF on nigrostriatal dopaminergic function in response to a two-pulse K+ stimulation. Exp Neurol. 2000;166:450–457. doi: 10.1006/exnr.2000.7515. [DOI] [PubMed] [Google Scholar]
- Yurek DM, Fletcher-Turner A. Differential expression of GDNF, BDNF, and NT-3 in the aging nigrostriatal system following a neurotoxic lesion. Brain Res. 2001;891:228–235. doi: 10.1016/s0006-8993(00)03217-0. [DOI] [PubMed] [Google Scholar]


