Abstract
Background
Although it is known that periodontal MMP-8 expression is associated with periodontal disease, the information concerning the periodontal MMP-8 expression in diabetic patients with periodontal disease is insufficient.
Materials and Methods
Periodontal tissue specimens were collected from 7 patients without periodontal disease and diabetes (Group 1), 15 patients with periodontal disease alone (Group 2) and 10 patients with both periodontal disease and diabetes (Group 3). The frozen sections were prepared and MMP-8 protein expression was detected using immunohistochemistry and quantified. For in vitro study, human U937 mononuclear cells were pre-exposed to normal or high glucose and then treated with LPS.
Results
The nonparametric Kruskal-Wallis test showed that the difference in MMP-8 protein levels among the three groups were statistically significant (p = 0.003). Nonparametric analysis using Jonckheere-Terpstra test showed a tendency of increase in periodontal MMP-8 levels across Group 1 to Group 2 to Group 3 (p = 0.0002). In vitro studies showed that high glucose and LPS had a synergistic effect on MMP-8 expression.
Conclusion
Our current study showed an increasing trend in MMP-8 protein expression levels across patients without both periodontal disease and diabetes, patients with periodontal disease alone and patients with both diseases.
Keywords: Periodontal diseases, Diabetes mellitus, MMP-8, Gene expression
Patients with either type 1 or type 2 diabetes have increased prevalence and severity of periodontal disease when compared with nondiabetic subjects (Mealey, 1999). Thus, periodontal disease has been considered as another diabetic complication besides cardiovascular disease, nephropathy, retinopathy, neuropathy and peripheral vascular diseases (Grossi and Genco, 1998). Although earlier studies proposed that altered immune response, impaired host defense and increased susceptibility to infection in diabetic patients were the underlying mechanisms for greater risk for periodontal disease in diabetic patients (Mealey, 1999), increasing evidence in recent years has indicated a crucial role of pathogen-triggered inflammatory response from host in the pathogenesis of diabetes-associated periodontal disease (Salvi et al., 2005, Salvi et al., 1998, Nishimura et al., 2007, Genco et al., 2005). It has been shown that diabetic patients with periodontal disease have significantly higher levels of inflammatory mediators such as interleukin-1 (IL-1)β, tumor necrosis factor (TNF)α, and prostaglandin E2 (PGE2) in the gingival crevicular fluid (GCF) as compared to nondiabetic patients, and mononuclear phagocytes isolated from diabetic patients have exaggerated inflammatory responses to lipopolysaccharide (LPS) (Salvi et al., 1998). The studies from our group also showed that periodontal IL-6 mRNA and protein levels increased across patients with neither periodontal disease nor diabetes, patients with periodontal disease alone, and patients with both diseases (Cole et al., 2008, Ross et al., 2010). In supporting these findings from patient studies, our in vitro studies have shown that high glucose in culture medium increased proinflammatory cytokine production by mononuclear cells in response to LPS (Maldonado et al., 2004).
Both LPS and proinflammatory cytokines such as IL-6, IL-1β and TNFα are potent stimulators for matrix metalloproteinase (MMP) expression by mononuclear cells (Li et al., 2010). MMPs belong to a family of zinc-dependent endopeptidases and are capable of degrading collagen and other matrix proteins in connective tissue (Davidson et al., 2002). Numerous studies have shown that elevated levels of MMPs, especially MMP-8 (neutrophil collagenase), in GCF, saliva and mouth rinse samples are associated with the progression of periodontal disease (Mancini et al., 1999, Uitto et al., 2003, Sorsa et al., 2004, Leppilahti et al., Lee et al., 1995, Mantyla et al., 2006, Mantyla et al., 2003). Although the pathological role of MMP-8 in periodontal disease is well known, the information regarding the relationship between periodontal MMP-8 expression and diabetes remains controversial.
By analysis of MMP-9 expression in gingival tissue using Western blot and gelatin zymography, Kumar et al. reported that MMP-8 level was increased by 2 folds in chronic periodontitis patients with diabetes compared to chronic periodontitis patients without diabetes (Kumar et al., 2006). Collin et al. also reported that advanced periodontitis in type 2 diabetes was related to elevated salivary MMP-8 levels (Collin et al., 2000). However, a recent study by Buduneli and coworkers showed that diabetes did not significantly affect GCF levels of MMP-8 (Kardesler et al., 2010). Costa et al. also reported that salivary MMP-9 concentrations were elevated regardless of periodontal inflammation in patients with diabetes (Costa et al., 2010). Thus, more investigations are necessary to determine if the state of diabetes in patients with periodontal disease is associated with increased periodontal MMP-8 expression.
In this study, we recruited 32 patients with or without periodontal disease and diabetes and collected periodontal tissue specimens at the time of necessary surgical intervention and analyzed periodontal MMP-8 protein levels using immunohistochemistry. Results showed that periodontal MMP-8 expression is significantly increased across patients with neither periodontal disease nor diabetes, patients with periodontal disease alone, and patients with both diseases.
Materials and Methods
Patients
Thirty-two patients including 7 patients without periodontal disease and diabetes (Group 1), 15 patients with periodontal disease alone (Group 2), and 10 patients with both diseases (Group 3) were recruited for this study. Seven patients who had surgeries for dental disorders such as crown lengthening, extractions, and periodontal plastic surgery served as controls in Group 1. Periodontal evaluation was conducted in these patients to exclude periodontal disease and periodontal tissues were collected. Twenty-five patients in Groups 2 and 3 who presented clinical attachment loss (CAL) > 5 mm in 2 or more teeth at the 6-week non-surgical reevaluation appointment, which met the diagnostic criteria for chronic periodontitis according to the classification of 1999 (Flemmig, 1999), were recruited for the study. The oral examination, including full mouth periodontal examination such as periodontal probing depth (PPD) and CAL measurements using the cement-enamel junction (CEJ) as a reference point, was conducted as previously described (Salvi et al., 1997). The exclusion criteria were: serum creatinine ≥ 1.6 mg/dl, abnormal hepatic function, hemoglobinopathy, unwillingness to sign the informed consent form or enter the study, aggressive periodontitis, platelet and coagulation disorders. The patients in Groups 2 and 3 received periodontal surgery and the diseased periodontal tissue was collected from the site with greatest PPD or CAL or both. Each patient provided one tissue sample that was removed from one periodontal site. Patients in Group 3 had hemoglobin A1c (HbA1c) test before their surgeries to document glycemic control status. HbA1c was not evaluated for patients professing to be nondiabetics. All patients provided informed consent for the specimen collection. The study protocol and consent form were approved by the University Institutional Review Board.
Immunohistochemical Analysis of MMP-8 Expression
Periodontal tissue samples were frozen in TBS freezing media immediately after surgery and stored at −80°C. Using a cryostat, 5 µm sections were cut and mounted on slides before being placed in 95% of ethanol for 10 minutes and then washed with PBS. Sections were blocked with filtered PBS containing 2% normal goat serum and 0.5% non-fat dry milk for 20 minutes. The sections were incubated with monoclonal antibody against MMP-8 (1:150 dilution) (Santa Cruz Biotechnology, Santa Cruz, CA) for 45 minutes. After washing with PBS twice for 5 minutes, sections were then incubated with biotinylated-goat anti-mouse antibody (1:250 dilution) (Vector Laboratories, Burlingame, CA) for 30 minutes. After washing, the sections were incubated with avidin DH and biotinylated horseradish peroxidase H complex (ABC kit, Vector Laboratories) for 30 minutes and with diaminobenzidine solution (Sigma, St. Louis, MO) for 10 minutes. Counterstaining was preformed with hematoxylin (Fisher Scientific, Pittsburgh, PA). Sections stained with normal mouse IgG as primary antibody were used as negative control. Five slides from each sample were used for analysis.
Image Analysis
Images were taken using a computer-operated Zeiss Axiovert 200M inverted microscope with a Photometrix Cascade 512 CCD digital camera. Images were analyzed with the Photoshop software (version 10; Adobe Systems, San Jose, CA). The method to use “Similar” feature to select a particular color staining on a digitized immunohistochemical image has previously been described in detail (Lehr et al., 1997). Briefly, a standard was created by selecting an area of 0.5 cm × 1.0 cm from a tissue section that had desired brown color from MMP-8 immunostaining. The cursor of the Magic Wand tool was clicked on the standard to make a selection, and the area of the standard was highlighted. To specify how broad a range of color the Magic Wand tool should include in the selection, the Tolerance value in the Magic Wand Options palette was set to 100. Using the “Similar” command, all the areas with the brown color that is similar to the standard on an image being determined were highlighted. The quantification was done using the “Histogram” command in the “Image” menu, which showed the pixels of the highlighted area. The pixels of the highlighted area were normalized to the total tissue area.
Cell Culture
U937 mononuclear phagocytes (Sundstrom and Nilsson, 1976) were purchased from American Type Culture Collection (Manassas, VA). The cells were cultured in a 5% CO2 atmosphere in RPMI 1640 medium (GIBCO, Invitrogen Cop. Carlsbad, CA) containing normal glucose (5 mM) or high glucose (25 mM), 10% fetal calf serum, 1% MEM non-essential amino acid solution, and 0.6 g/100 ml of HEPES. Five and 25 mM of glucose have been used commonly as the concentrations of normal and high glucose, respectively (Nareika et al., 2008, Nareika et al., 2007, Sundararaj et al., 2009).
Real-Time Polymerase Chain Reaction (PCR)
Total RNA was isolated from cells using the RNeasy minikit (Qiagen). First-strand complementary DNA (cDNA) was synthesized with the iScript™ cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). Real-time PCR was performed in duplicate using 25 µl of reaction mixture containing 1.0 µl of RT mixture, 0.2 µM of both primers (5’ sequence: AACGCACTAACTTGACCTACAG; 3’ sequence: CTCCAGAGTTCAAAGGCATCC) and 12.5 µl of iQ™ SYBR Green Supermix (Bio-Rad Laboratories). Real-time PCR was run in the iCycler™ real-time detection system (Bio-Rad Laboratories). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control (5’ primer sequence: GAATTTGGCTACAGCAACAGGGTG; 3’ primer sequence: TCTCTTCCTCTTGTGCTCTTGCTG). The average starting quantity (SQ) of fluorescence units was used for analysis.
Statistical Analysis
Nonparametric analysis of variance (ANOVA) using the Kruskal-Wallis procedure was used to text for any differences in the continuous MMP-8 levels among groups. To test for monotonicity across the groups nonparametrically, the Jonckheere-Terpstra trend test was performed. A rank-based ANOVA was performed to explore the effects of age and gender. Differences in gender and race among the groups were evaluated using chisquare tests with p-value computing using Monte Carlo sampling as appropriate for the small size of this study. Analyses were performed using R version 2.9.1. (Broberg, 2010, Hothorn et al., 2008, R Development Core Team, 2009). A p-value less than 0.05 was considered statistically significant. For cell culture studies, data were presented as mean ± SD. Student t tests were performed to determine the statistical significance of gene expression among different experimental groups. A p-value less than 0.05 was considered statistically significant.
Results
Clinical Data
Table 1 shows the clinical data for patient age, gender, race, smoking status, PPD and CAL.
Table 1.
Patients and Periodontal Disease in Three Groups
| Group 1 (patients without diabetes and periodontal disease) |
Group 2 (patients with periodontal disease alone) |
Group 3 (patients with diabetes and periodontal disease) |
||
|---|---|---|---|---|
| Patient number (periodontal sites from which samples were collected) | 7 (7) | 15 (15) | 10 (10) | |
| Age (mean±SD) | 53 ± 19 | 48 ± 13 | 55 ± 13 | |
| Gender | Female | 6 | 6 | 4 |
| Male | 1 | 9 | 6 | |
| Female/Male | 6.0 | 0.67 | 0.67 | |
| Race & Ethnicity | White (non-Hispanic) | 7 | 9 | 6 |
| Black (non-Hispanic) | 0 | 5 | 4 | |
| Hispanic | 0 | 1 | 0 | |
| Periodontal Probing Depth (PPD) | NE | 6.8 ± 3.1 | 5.9 ± 2.5 | |
| Clinical Attachment Loss (CAL) | NE | 6.2 ± 3.3 | 7.3 ± 2.4 | |
NE: not examined. The data presented are mean ± SD.
Age
The mean ± SD in Groups 1, 2 and 3 was 59 ± 15, 50 ± 13 and 57 ± 13, respectively. No significant difference in mean age was found among the 3 groups.
Gender
The ratios of female/male in Groups 1, 2 and 3 were 6/1 (6.0), 5/10 (0.50), and 4/6 (0.67), respectively. The proportion of females did not differ significantly among the groups (p=0.15).
Race
The white vs. black ratios in Groups 1, 2 and 3 were 7/0 (7.0), 9/5 (1.8), and 6/4 (1.5), respectively. The proportion of blacks to whites did not differ significantly among the three groups (p=0.16).
Periodontal disease
The means of PPD from the sampled sites in Group 2 and Group 3 were 6.8 ± 3.1 mm and 5.9 ± 2.5 mm, respectively. The means of CAL from the sampled sites in Group 2 and Group 3 were 6.2 ± 3.3 mm and 7.3 ± 2.4 mm, respectively. No significant difference of the means of PPD and CAL from the sampled sites was found between Group 2 and Group 3.
Diabetes
All patients in Group 3 had type 2 diabetes. HbA1c tests indicate that among the participants with diabetes, 9 patients had good glycemic control (HbA1c <7%) while 1 patient had poor glycemic control (HbA1c>8%). The average duration of diabetes for diabetic patients in Group 3 is 10 ± 6 years, ranging from 1 to 20 years.
Analysis of Periodontal Expression of MMP-8 Protein in Three Groups
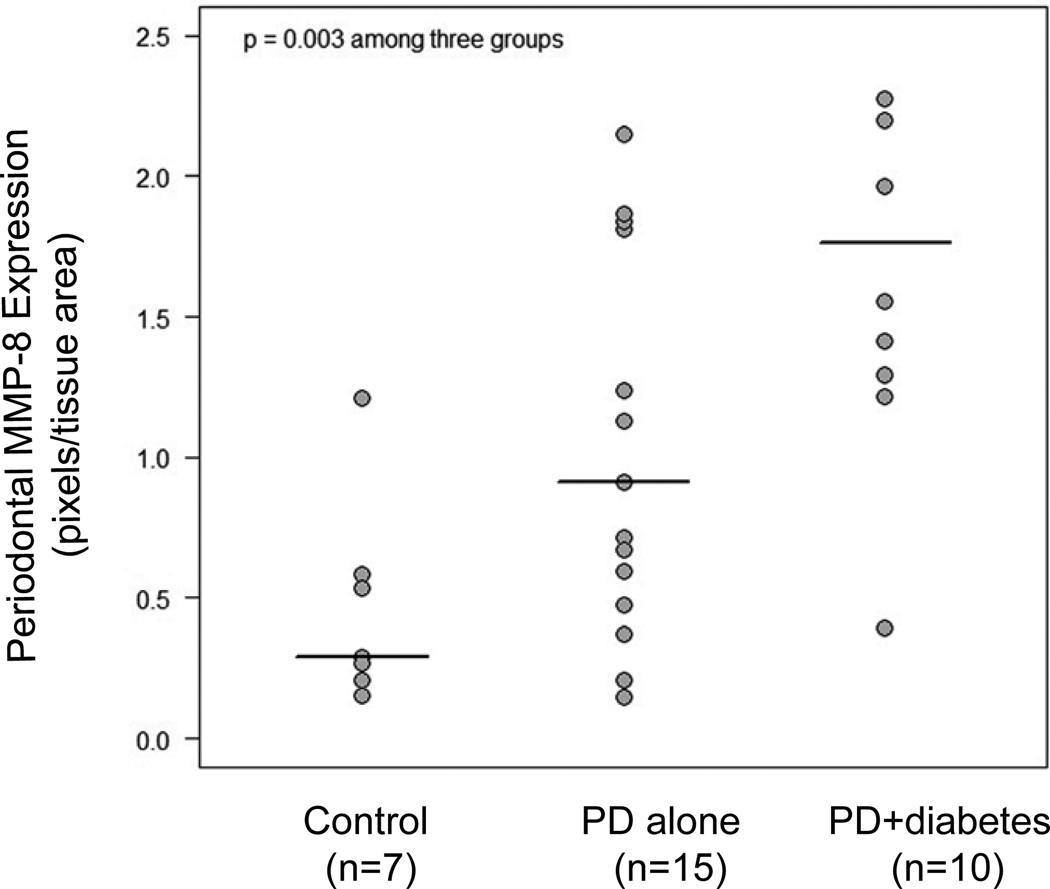
Nonparametric ANOVA (Kruskal-Wallis) yielded a p-value of 0.003, indicating significant differences among the three groups in median MMP-8 protein levels (Fig. 1). Nonparametric analysis using the Jonckheere-Terpstra test showed an increasing trend in MMP-8 protein expression levels across Group 1, Group 2 and Group 3 (p=0.0002). Neither age (p=0.37) nor gender (p=0.24) was significantly associated with MMP-8 levels.
Figure 1.
Statistical analysis of periodontal MMP-8 protein expression among Group 1 (patients with neither periodontal disease nor diabetes), Group 2 (patients with periodontal disease alone), and Group 3 (patients with both diseases). MMP-8 protein expression was detected in periodontal tissue and quantified as described in Materials and Methods. The Kruskal-Wallis test was performed to analyze the difference of MMP-8 expression levels among the three groups. Horizontal lines indicate the group median levels. PD, periodontal disease.
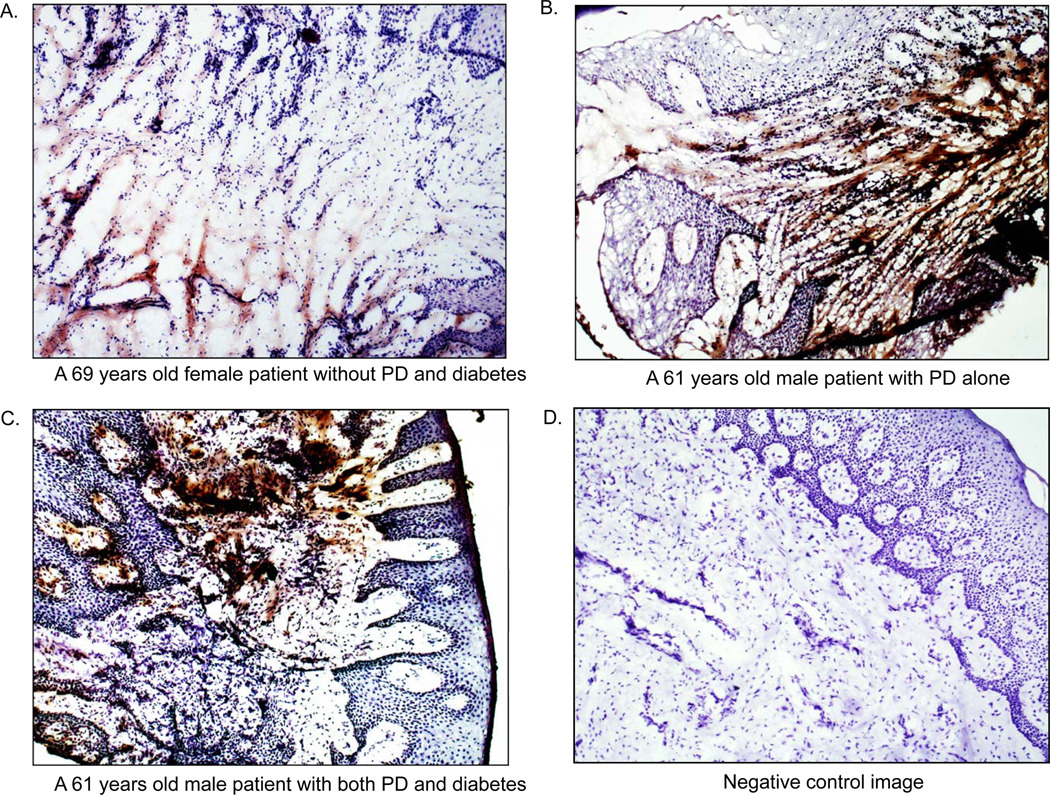
Representative images of periodontal tissue sections with MMP-8 immunostaining are presented in Fig. 2. These images showed that the intensity and area of MMP-8 immunostaining was increased in patients with periodontal disease (Group 2) as compared to control patients (Group 1) and further increased in patients with both periodontal disease and diabetes (Group 3) as compared to patients with periodontal disease alone (Group 2). The most immunostaining of MMP-8 appeared to be associated with cells (Fig. 2).
Figure 2.
Representative images of periodontal MMP-8 protein immunostaining. A: Immunostaining of MMP-8 in periodontal tissue collected from a 69 years old female without periodontal disease (PD) and diabetes. B: Immunostaining of MMP-8 in periodontal tissue collected from a 61 years old male with PD alone. C: Immunostaining of MMP-8 in periodontal tissue collected from a 61 years old male with both PD and diabetes. D: Negative control for MMP-8 immunostaining using normal mouse IgG as control primary antibody.
High Glucose and LPS Have A Synergistic Effect on MMP-8 Expression
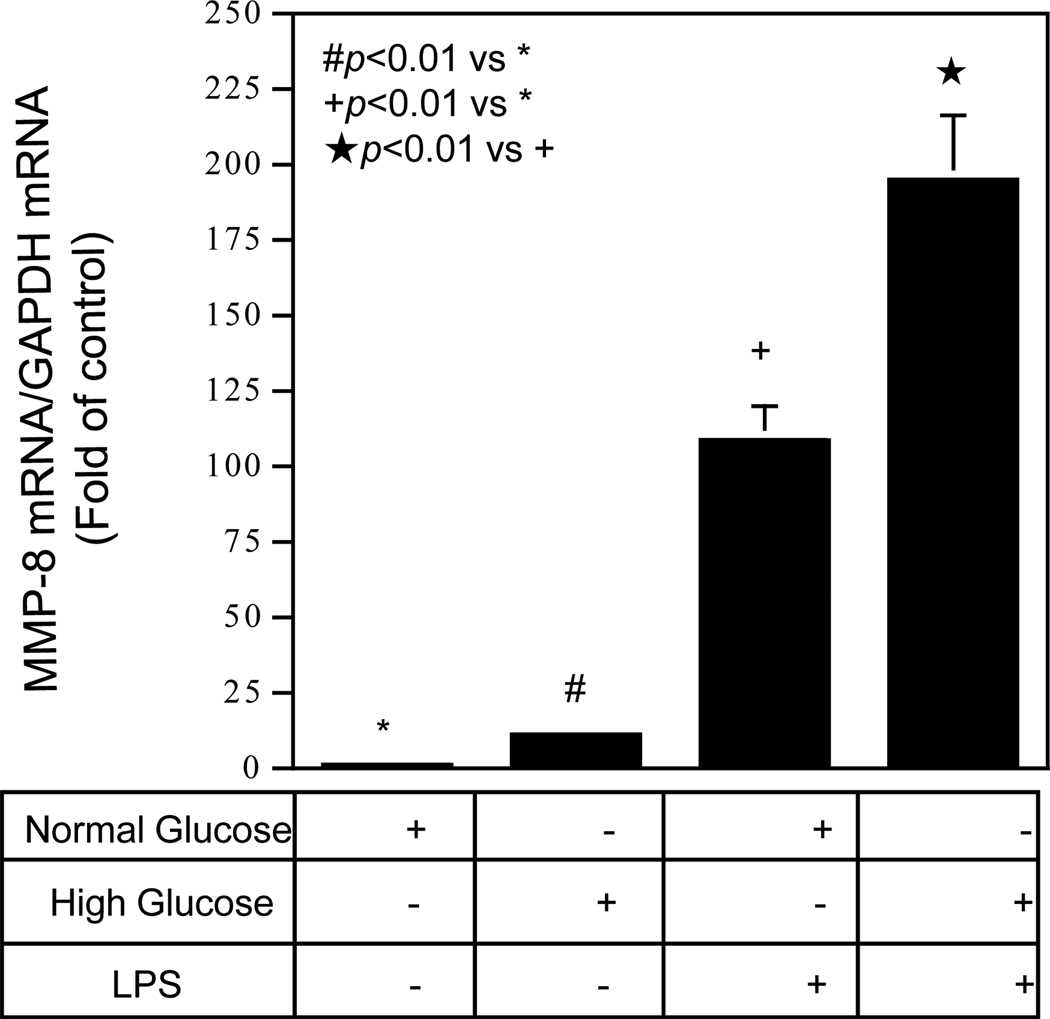
To understand the mechanism by which periodontal MMP-8 expression is increased in patients with diabetes, we hypothesized that hyperglycemia may augment MMP-8 expression in response to bacterial LPS. Since mononuclear cells play an important role in periodontal disease, we tested our hypothesis by performing in vitro studies using U937 mononuclear cells to determine if increased glucose concentration in culture medium augmented LPS-stimulated MMP-8 expression. Quantitative real-time PCR showed that high glucose and LPS increased MMP-8 expression by 2-fold as compared to normal glucose and LPS (Fig. 3), indicating that high glucose and LPS had a synergistic effect on MMP-8 expression by U937 mononuclear cells.
Figure 3.
The effect of high glucose on MMP-8 expression by U937 mononuclear cells exposed to LPS. U937 cells pre-exposed to normal (5 mM) or high (25 mM) of glucose for one week before treatment with or without 100 ng/ml of LPS for 24 h. After the treatment, RNA was isolated from cells and subjected to real-time PCR to detect MMP-8 mRNA.
Discussion
MMP-8, also called neutrophil collagenase, is expressed and released by not only neutrophils (Sorsa et al., 2004), but also a number of other types of cells including monocytes, macrophages, epithelial cells, endothelial cells and plasma cells in response to inflammatory stimuli (Prikk et al., 2001, Schubert-Unkmeir et al., 2010, Wahlgren et al., 2001). In consistence with these previous reports, our present study also showed that MMP-8 was expressed by human U937 mononuclear cells in response to LPS and high glucose (Figs. 3). Thus, it appears that MMP-8 not only plays an essential role in tissue inflammation in gingivitis that is characterized by dominant infiltration of neutrophils into the periodontal tissue, but also contributes to tissue inflammation in periodontitis that is associated with increased infiltration of monocytes.
Although MMP-8, MMP-1 and MMP-13 as collagenases target similar matrix proteins such as collagen I, II, III, VII, VIII, X, it was found that MMP-8 level was higher than MMP-1 and MMP-13 levels in GCF collected from patients with periodontal disease (Ingman et al., 1996, Lee et al., 1995). Furthermore, patient studies have shown that treatment of periodontal disease decreases MMP-8 level and its activity in GCF (Sorsa et al., 1999). Based on the findings from a large number of studies, MMP-8 was considered as a good marker for periodontal disease and thus a MMP-8 specific chair-side test has been developed to monitor periodontal health and disease (Sorsa et al., 1999). Clinical studies have provided strong evidence that MMP-8 plays an important role in periodontal disease.
Our present study utilized periodontal tissue specimens removed from diabetic and nondiabetic patients to study the relationship between diabetes and periodontal expression of MMP-8. Because the periodontal specimens surgically removed from patients with periodontal disease are diseased tissues, the change in MMP-8 expression in these specimens is likely to be associated with periodontal disease. Our findings are consistent with the previous studies that utilized GCF or saliva collected from nondiabetic and diabetic patients and showed that MMP-8 level in GCF or saliva was higher in diabetic patients than that in nondiabetic patients (Kumar et al., 2006, Collin et al., 2000). We found a significant difference among 3 groups (p=0.003) and a significant trend of MMP-8 increase among 3 groups (p=0.0002). These findings, therefore, suggest that MMP-8 may be involved in diabetes-associated periodontal disease.
The data from our in vitro studies (Fig. 3) have provided clue that may partially explain why diabetic patients have increased periodontal MMP-8 expression. Since our previous studies have shown that U937 mononuclear cells and human monocytes have similar response to LPS and high glucose in their expression of genes involved in inflammation (Samuvel et al., 2010, Sundararaj et al., 2009), we used U937 cells in this study. Results from these studies showed that high glucose and LPS exert a synergistic effect on MMP-8 expression by U937 cells, indicating that hyperglycemia may be a key factor in MMP-8 upregulation in periodontal tissue. Besides hyperglycemia, patients with type 2 diabetes may also have other pathological factors such as hypercholesterolemia and hypertriglyceridemia, which are also associated with increased proinflammatory cytokines (Chan et al., 2002, Wisse, 2004). Indeed, it has been shown that the expression of proinflammatory cytokines such as IL-6 have increased expression in periodontal tissue in diabetic patients (Cole et al., 2008, Salvi et al., 1998) and proinflammatory cytokines are potent stimulators for MMP-8 expression (Wahlgren et al., 2001, Li et al., 2010). Thus, diabetic patients with a good glycemic control may also have increased periodontal MMP-8 expression if they have dyslipidemia. This may explain why the diabetic patients in this study had increased MMP-8 expression despite most of them had good glycemic control.
In summary, our study has shown an increasing trend in MMP-8 protein expression levels across patients without both periodontal disease and diabetes, patients with periodontal disease alone and patients with both diseases. To the best of our knowledge, this is the first study in which periodontal tissue removed from surgery were used to determine the periodontal MMP-8 expression in diabetic and nondiabetic patients. Although the sample size in this study was relatively small due to the limited number of diabetic patients who had surgery, our statistical analyses showed a significantly increased MMP-8 expression in diabetic patients. The findings from this study warrant further investigations in a larger population.
Clinical Relevance.
Scientific Rationale for Study
It has been well documented that periodontal MMP-8 expression is increased in patients with periodontal disease as compared to patients without periodontal disease. However, it remains unclear if periodontal MMP-8 expression is further increased in patients with both periodontal disease and diabetes as compared to patients with periodontal disease alone.
Principal Findings
The nonparametric Kruskal-Wallis test showed that the difference in MMP-8 protein levels among the three groups were statistically significant (p = 0.003). Nonparametric analysis using Jonckheere-Terpstra test showed a tendency of increase in periodontal MMP-8 levels across Group 1 to Group 2 to Group 3 (p = 0.0002). In vitro studies showed that high glucose and LPS had a synergistic effect on MMP-8 expression.
Practical Implications
MMP-8 inhibition is a potential therapeutic strategy and further studies need to prove its application and efficacy.
Acknowledgments
Sources of Funding Statement
This study was supported by NIH grant DE016353 and Merit Review Grant from Department of Veterans Affairs (to Y.H.).
Footnotes
Conflict of Interest
There is no conflict of interest to declare.
References
- Broberg P. SAGx: Statistical Analysis of the GeneChip. R package version 1.18.0. 2010 2009 URL http://www.bioconductor.org/packages/2.4/bioc/html/SAGx.html.
- Chan JC, Cheung JC, Stehouwer CD, Emeis JJ, Tong PC, Ko GT, Yudkin JS. The central roles of obesity-associated dyslipidaemia, endothelial activation and cytokines in the Metabolic Syndrome--an analysis by structural equation modelling. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2002;26:994–1008. doi: 10.1038/sj.ijo.0802017. [DOI] [PubMed] [Google Scholar]
- Cole CM, Sundararaj KP, Leite RS, Nareika A, Slate EH, Sanders JJ, Lopes-Virella MF, Huang Y. A trend of increase in periodontal interleukin-6 expression across patients with neither diabetes nor periodontal disease, patients with periodontal disease alone, and patients with both diseases. Journal of periodontal research. 2008;43:717–722. doi: 10.1111/j.1600-0765.2008.01082.x. [DOI] [PubMed] [Google Scholar]
- Collin HL, Sorsa T, Meurman JH, Niskanen L, Salo T, Ronka H, Konttinen YT, Koivisto AM, Uusitupa M. Salivary matrix metalloproteinase (MMP-8) levels and gelatinase (MMP-9) activities in patients with type 2 diabetes mellitus. Journal of periodontal research. 2000;35:259–265. doi: 10.1034/j.1600-0765.2000.035005259.x. [DOI] [PubMed] [Google Scholar]
- Costa PP, Trevisan GL, Macedo GO, Palioto DB, Souza SL, Grisi MF, Novaes AB, Jr, Taba M., Jr Salivary interleukin-6, matrix metalloproteinase-8, and osteoprotegerin in patients with periodontitis and diabetes. Journal of periodontology. 2010;81:384–391. doi: 10.1902/jop.2009.090510. [DOI] [PubMed] [Google Scholar]
- Davidson B, Reich R, Risberg B, Nesland JM. The biological role and regulation of matrix metalloproteinases (MMP) in cancer. Arkhiv patologii. 2002;64:47–53. [PubMed] [Google Scholar]
- Flemmig TF. Periodontitis. Annals of periodontology / the American Academy of Periodontology. 1999;4:32–38. doi: 10.1902/annals.1999.4.1.32. [DOI] [PubMed] [Google Scholar]
- Genco RJ, Grossi SG, Ho A, Nishimura F, Murayama Y. A proposed model linking inflammation to obesity, diabetes, and periodontal infections. Journal of periodontology. 2005;76:2075–2084. doi: 10.1902/jop.2005.76.11-S.2075. [DOI] [PubMed] [Google Scholar]
- Grossi SG, Genco RJ. Periodontal disease and diabetes mellitus: a two-way relationship. Annals of periodontology / the American Academy of Periodontology. 1998;3:51–61. doi: 10.1902/annals.1998.3.1.51. [DOI] [PubMed] [Google Scholar]
- Hothorn T, Hornik K, van de Wiel MA, Zeileis A. Implementing a class of permutation tests: the coin package. J Stat Softw. 2008;28:1–23. [Google Scholar]
- Ingman T, Tervahartiala T, Ding Y, Tschesche H, Haerian A, Kinane DF, Konttinen YT, Sorsa T. Matrix metalloproteinases and their inhibitors in gingival crevicular fluid and saliva of periodontitis patients. Journal of clinical periodontology. 1996;23:1127–1132. doi: 10.1111/j.1600-051x.1996.tb01814.x. [DOI] [PubMed] [Google Scholar]
- Kardesler L, Biyikoglu B, Cetinkalp S, Pitkala M, Sorsa T, Buduneli N. Crevicular fluid matrix metalloproteinase-8, -13, and TIMP-1 levels in type 2 diabetics. Oral diseases. 2010;16:476–481. doi: 10.1111/j.1601-0825.2010.01659.x. [DOI] [PubMed] [Google Scholar]
- Kumar MS, Vamsi G, Sripriya R, Sehgal PK. Expression of matrix metalloproteinases (MMP-8 and -9) in chronic periodontitis patients with and without diabetes mellitus. Journal of periodontology. 2006;77:1803–1808. doi: 10.1902/jop.2006.050293. [DOI] [PubMed] [Google Scholar]
- Lee W, Aitken S, Sodek J, McCulloch CA. Evidence of a direct relationship between neutrophil collagenase activity and periodontal tissue destruction in vivo: role of active enzyme in human periodontitis. Journal of periodontal research. 1995;30:23–33. doi: 10.1111/j.1600-0765.1995.tb01249.x. [DOI] [PubMed] [Google Scholar]
- Lehr HA, Mankoff DA, Corwin D, Santeusanio G, Gown AM. Application of photoshop-based image analysis to quantification of hormone receptor expression in breast cancer. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 1997;45:1559–1565. doi: 10.1177/002215549704501112. [DOI] [PubMed] [Google Scholar]
- Leppilahti J, Ahonen MM, Hernandez M, Munjal S, Netuschil L, Uitto VJ, Sorsa T, Mantyla P. Oral rinse MMP-8 point-of-care immuno test identifies patients with strong periodontal inflammatory burden. Oral diseases. 2011;17:115–122. doi: 10.1111/j.1601-0825.2010.01716.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Samuvel DJ, Sundararaj KP, Lopes-Virella MF, Huang Y. IL-6 and high glucose synergistically upregulate MMP-1 expression by U937 mononuclear phagocytes via ERK1/2 and JNK pathways and c-Jun. Journal of cellular biochemistry. 2010;110:248–259. doi: 10.1002/jcb.22532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado A, He L, Game BA, Nareika A, Sanders JJ, London SD, Lopes-Virella MF, Huang Y. Pre-exposure to high glucose augments lipopolysaccharide-stimulated matrix metalloproteinase-1 expression by human U937 histiocytes. Journal of periodontal research. 2004;39:415–423. doi: 10.1111/j.1600-0765.2004.00756.x. [DOI] [PubMed] [Google Scholar]
- Mancini S, Romanelli R, Laschinger CA, Overall CM, Sodek J, McCulloch CA. Assessment of a novel screening test for neutrophil collagenase activity in the diagnosis of periodontal diseases. Journal of periodontology. 1999;70:1292–1302. doi: 10.1902/jop.1999.70.11.1292. [DOI] [PubMed] [Google Scholar]
- Mantyla P, Stenman M, Kinane D, Salo T, Suomalainen K, Tikanoja S, Sorsa T. Monitoring periodontal disease status in smokers and nonsmokers using a gingival crevicular fluid matrix metalloproteinase-8-specific chair-side test. Journal of periodontal research. 2006;41:503–512. doi: 10.1111/j.1600-0765.2006.00897.x. [DOI] [PubMed] [Google Scholar]
- Mantyla P, Stenman M, Kinane DF, Tikanoja S, Luoto H, Salo T, Sorsa T. Gingival crevicular fluid collagenase-2 (MMP-8) test stick for chair-side monitoring of periodontitis. Journal of periodontal research. 2003;38:436–439. doi: 10.1034/j.1600-0765.2003.00677.x. [DOI] [PubMed] [Google Scholar]
- Mealey B. Diabetes and periodontal diseases. Journal of periodontology. 1999;70:935–949. doi: 10.1902/jop.1999.70.8.935. [DOI] [PubMed] [Google Scholar]
- Nareika A, Im YB, Game BA, Slate EH, Sanders JJ, London SD, Lopes-Virella MF, Huang Y. High glucose enhances lipopolysaccharide-stimulated CD14 expression in U937 mononuclear cells by increasing nuclear factor kappaB and AP-1 activities. The Journal of endocrinology. 2008;196:45–55. doi: 10.1677/JOE-07-0145. [DOI] [PubMed] [Google Scholar]
- Nareika A, Maldonado A, He L, Game BA, Slate EH, Sanders JJ, London SD, Lopes-Virella MF, Huang Y. High glucose-boosted inflammatory responses to lipopolysaccharide are suppressed by statin. Journal of periodontal research. 2007;42:31–38. doi: 10.1111/j.1600-0765.2006.00911.x. [DOI] [PubMed] [Google Scholar]
- Nishimura F, Iwamoto Y, Soga Y. The periodontal host response with diabetes. Periodontology 2000. 2007;43:245–253. doi: 10.1111/j.1600-0757.2006.00171.x. [DOI] [PubMed] [Google Scholar]
- Prikk K, Maisi P, Pirila E, Sepper R, Salo T, Wahlgren J, Sorsa T. In vivo collagenase-2 (MMP-8) expression by human bronchial epithelial cells and monocytes/macrophages in bronchiectasis. The Journal of pathology. 2001;194:232–238. doi: 10.1002/path.849. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing, R Foundation for Statistical Computing. Vienna, Austria; 2009. 2009. ISBN 3-900051-07-0, URL http://www.r-project.org/. [Google Scholar]
- Ross JH, Hardy DC, Schuyler CA, Slate EH, Mize TW, Huang Y. Expression of periodontal interleukin-6 protein is increased across patients with neither periodontal disease nor diabetes, patients with periodontal disease alone and patients with both diseases. Journal of periodontal research. 2010;45:688–694. doi: 10.1111/j.1600-0765.2010.01286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi GE, Beck JD, Offenbacher S. PGE2, IL-1 beta, and TNF-alpha responses in diabetics as modifiers of periodontal disease expression. Annals of periodontology / the American Academy of Periodontology. 1998;3:40–50. doi: 10.1902/annals.1998.3.1.40. [DOI] [PubMed] [Google Scholar]
- Salvi GE, Kandylaki M, Troendle A, Persson GR, Lang NP. Experimental gingivitis in type 1 diabetics: a controlled clinical and microbiological study. Journal of clinical periodontology. 2005;32:310–316. doi: 10.1111/j.1600-051X.2005.00682.x. [DOI] [PubMed] [Google Scholar]
- Salvi GE, Yalda B, Collins JG, Jones BH, Smith FW, Arnold RR, Offenbacher S. Inflammatory mediator response as a potential risk marker for periodontal diseases in insulin-dependent diabetes mellitus patients. Journal of periodontology. 1997;68:127–135. doi: 10.1902/jop.1997.68.2.127. [DOI] [PubMed] [Google Scholar]
- Samuvel DJ, Sundararaj KP, Li Y, Lopes-Virella MF, Huang Y. Adipocyte-mononuclear cell interaction, Toll-like receptor 4 activation, and high glucose synergistically up-regulate osteopontin expression via an interleukin 6-mediated mechanism. The Journal of biological chemistry. 2010;285:3916–3927. doi: 10.1074/jbc.M109.033951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert-Unkmeir A, Konrad C, Slanina H, Czapek F, Hebling S, Frosch M. Neisseria meningitidis induces brain microvascular endothelial cell detachment from the matrix and cleavage of occludin: a role for MMP-8. PLoS pathogens. 2010;6 doi: 10.1371/journal.ppat.1000874. e1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorsa T, Mantyla P, Ronka H, Kallio P, Kallis GB, Lundqvist C, Kinane DF, Salo T, Golub LM, Teronen O, Tikanoja S. Scientific basis of a matrix metalloproteinase-8 specific chair-side test for monitoring periodontal and peri-implant health and disease. Annals of the New York Academy of Sciences. 1999;878:130–140. doi: 10.1111/j.1749-6632.1999.tb07679.x. [DOI] [PubMed] [Google Scholar]
- Sorsa T, Tjaderhane L, Salo T. Matrix metalloproteinases (MMPs) in oral diseases. Oral diseases. 2004;10:311–318. doi: 10.1111/j.1601-0825.2004.01038.x. [DOI] [PubMed] [Google Scholar]
- Sundararaj KP, Samuvel DJ, Li Y, Sanders JJ, Lopes-Virella MF, Huang Y. Interleukin-6 released from fibroblasts is essential for up-regulation of matrix metalloproteinase-1 expression by U937 macrophages in coculture: cross-talking between fibroblasts and U937 macrophages exposed to high glucose. The Journal of biological chemistry. 2009;284:13714–13724. doi: 10.1074/jbc.M806573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937) International journal of cancer. Journal international du cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Uitto VJ, Overall CM, McCulloch C. Proteolytic host cell enzymes in gingival crevice fluid. Periodontology 2000. 2003;31:77–104. doi: 10.1034/j.1600-0757.2003.03106.x. [DOI] [PubMed] [Google Scholar]
- Wahlgren J, Maisi P, Sorsa T, Sutinen M, Tervahartiala T, Pirila E, Teronen O, Hietanen J, Tjaderhane L, Salo T. Expression and induction of collagenases (MMP-8 and -13) in plasma cells associated with bone-destructive lesions. The Journal of pathology. 2001;194:217–224. doi: 10.1002/path.854. [DOI] [PubMed] [Google Scholar]
- Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. Journal of the American Society of Nephrology : JASN. 2004;15:2792–2800. doi: 10.1097/01.ASN.0000141966.69934.21. [DOI] [PubMed] [Google Scholar]