Abstract
The hypothesis that life span extension by caloric restriction (CR) is contingent upon the attenuation of macromolecular oxidative damage was tested in two different strains of mice: the C57BL/6, whose life span is extended by CR, and the DBA/2, in which CR has relatively minor or no impact on longevity. Mice were fed ad libitum (AL) or restricted to 40% lesser food, starting at 4 months of age. Protein damage was measured as protein-linked adducts of 4-hydroxy-2-nonenal (HNE) and malondialdehyde (MDA) in skeletal muscle mitochondria at 6- and 23-months of age. Protein-HNE and -MDA content increased with age in C57BL/6 mice and CR significantly attenuated these augmentations. Metalloprotease 1, NADP-dependent mitochondrial malic enzyme (isoform 2) and citrate synthase were identified by mass spectroscopy to contain HNE/MDA adducts. DBA/2 mice exhibited little effect of age or CR on protein HNE/MDA content in skeletal muscle mitochondria. In contrast, protein-HNE levels in liver mitochondria showed a significant increase with age in AL-fed mice of both strains, and CR caused significant attenuation of this damage. Overall, results indicated that the age-related increase in protein oxidative damage and its abatement by CR are genotype- and tissue- specific, and not a universal phenomenon.
Keywords: HNE-protein conjugates, oxidative stress, protein oxidative damage, mitochondrial proteins, food restriction
1. Introduction
Although numerous hypotheses have been proposed, the nature of the mechanisms causing age-associated losses in physiological fitness remains unclear. Decrease in the amount of energy (food) intake, or caloric restriction (CR), has been widely employed to explore the causality of senescence, partly because of the assumption that the mechanisms by which CR prolongs life span are essentially similar to those implicated in the normal aging process. Accordingly, numerous comparisons of the age-related changes have been made between ad libitum (AL) fed animals and their cohorts fed a lesser amount of food, conventionally 30 – 40% for mice and rats. In general, results of such comparisons have indicated that the magnitude of a vast majority of age-related changes, observed in the AL animals, is attenuated by CR (Ramsey et al., 2000; Masoro, 2005; Sohal and Weindruch, 1996). Consequently, it has not been possible to unambiguously identify a subset of age-related alterations that are specifically linked to the longevity-extending effect of CR.
Contrary to the historical assumption that longevity extension by CR is a virtually universal phenomenon, studies in this and other laboratories have suggested that the CR effect on life span is genotype-specific. For instance, under the AL feeding regimen, male C57BL/6 and DBA/2 mice were found to achieve similar life spans; however, gradual imposition of CR by 40%, starting at 4–5 months of age, caused an approximately 25% extension of the life span in C57BL/6 mice, but the longevity of DBA/2 was unaffected (Forster et al., 2003). Results of previous studies suggest that CR has either no effect (Fernandes et al., 1976) or a relatively minor longevity prolongation effect in male DBA/2 mice (Turturro et al., 1999). More recently, Liao et al. (2009) have reported that among 41 recombinant inbred strains of mice, 40% CR lengthened the life spans of only 10 strains, whereas, it shortened it in a majority of the strains. We have previouly advocated that the genotypes, in which life span is relatively insensensitive to CR, may provide ‘negative controls” for discerninig the effects of CR that are associated with life span extension from those that relate to CR, but not life span.
In this context, the main objective of this study was to determine whether the normal aging process and the extension of life span by CR are, respectively, associated with the accrual and the attenuation of macromolecular oxidative damage. Indeed, numerous studies in the literature have indicated that the steady-state concentrations of the products of free radical attacks on various macromolecules are elevated during aging, and the rate of accumulation of such damage is lowered by CR (Masoro, 2005; Sohal and Weindruch, 1996). Nonetheless, studies reporting a CR-induced decrease in oxidative damage were overwhelmingly conducted in strains of mice or rats that exhibited a robust extension of life span under CR. Thus it remains unknown whether or not CR lowers the level of oxidative damage without affecting life span. The resolution of this, as well as the over-arching issue, namely whether or not oxidative stress/damage is a determinant of life span, is deemed important in understanding the causality of the aging process, especially in the context of the recent expressions of skepticism about the validity of the oxidative stress hypothesis of aging (Buffenstein et al., 2008; Perez et al., 2009; Speakman and Selman, 2011; Van Raamsdonk and Hekimi, 2010).
Accordingly, a comparison was made between the levels of mitochondrial protein oxidative damage in C57BL/6 and DBA/2 mice, whose life spans are, respectively, robustly prolonged or relatively little affected by CR (Fernandes et al., 1976; Forster et al., 2003; Turturro et al., 1999). Protein oxidative damage was measured as the amounts of lipid peroxidation products, 4-hydroxy-2-nonenal (HNE) and malondialdehyde (MDA), conjugated with mitochondrial proteins. Initially, the hind leg skeletal muscles were the main focus of our studies, but later the scope of the investigation was broadened for the purpose of inter-organ comparison to include the liver mitochondria. Skeletal muscle mitochondria were selected because our previous studies on the C57BL/6 mice had indicated that protein and lipid oxidative damage in such mitochondria increased during aging and that CR attenuated such damage (Lass et al., 1998), thereby providing a foundation for the feasibility of the present study. Furthermore, mitochondria are widely regarded as a particularly appropriate model for studying the association between oxidative damage and aging because they are the predominant intra-cellular sites of superoxide anion radical and hydrogen peroxide, whose rates of generation increase during aging (Ferguson et al., 2005; Gruber et al., 2008; Kwong and Sohal, 2000; Sastre et al., 2003). The latter is hypothesized to emanate from the progressive accrual of oxidative damage to mitochondrial proteins (Sohal and Dubey, 1994). In the current studies, it was hypothesized that if longevity is causally associated with mitochondrial protein structural damage, the amount of damage should increase with age and CR should attenuate such a rise to a greater extent in the C57BL/6 than in the DBA/2 mice.
2. Experimental procedures
2.1. Materials
Electrophoretic supplies were procured from BIO-RAD; rabbit anti-HNE (HNE11-S) and anti-MDA (MDA 11-S) antisera were obtained from Alpha Diagnostic; and ECL Plus Western Blotting Detection System was from Amersham Biosciences.
2.2. Animals
Studies were conducted in adherance to the Guidelines for the Care and Use of Laboratory Animals, promlugated by the National Institutes of Health and approved by the University of Southern California. Thirty nine DBA/2J and 27 C57BL/6J male mice were obtained from The Jackson Laboratory (Bar Harbor, ME) at 8–9 weeks of age and housed singly under a 12- h light/dark cycle. Mice were fed NIH-31 diet ad libitum until 14 weeks of age, after which cohorts were placed gradually under a CR regimen, with a 10% reduction in the amount of food consumed by the AL fed mice during the first week, 20% in the second week and 40% in the third week and thereafter. The CR mice were fed a vitamin-fortified NIH-31 diet to equalize the intake of essential nutrients. The amount of food, consumed by the AL mice, was monitored throughout the duration of the study in order to maintain the level of intake by the CR mice at 60% of the AL group. Mice were euthanized at 6 or 23 months of age, i.e., following 2 or 19 months on the 40% CR regimen.
2.3. Preparation of mitochondrial protein
Mice were killed by cervical dislocation and the hind leg skeletal muscles and liver were removed, weighed and placed in ice-cold antioxidant buffer containing 50 mM potassium phosphate buffer (pH 7.4), 2 mM EDTA and 0.1 mM butylated hydroxytoluene. Muscle was minced with razor blades, homogenized in 10 vol of isolation buffer containing 120 mM KCl, 20 mM HEPES (pH 7.4), 2 mM MgCl2, 1mM EGTA and 0.5 mg/ml BSA, and filtered through 4 layers of cheesecloth to remove the fat and fibrous tissue (Birch-Machin et al. 1993). The filtrate was centrifuged at 17,000 g for 10 min and the resulting pellet was resuspended in 10 vol of isolation buffer and centrifuged at 7,000 g for 10 min. The pellet was re-suspended in 10 vol of a buffer containing 300 mM sucrose, 2 mM HEPES (pH 7.4) and 0.1 mM EGTA, and centrifuged at 1200 g for 10 min. The pellet was discarded and the supernatant was centrifuged at 12,000g for 10 min. The resulting mitochondrial pellet was resuspended in the latter buffer and frozen at −80 °C. Liver was homogenized in a buffer containing 0.25 M sucrose, 3 mM EDTA, 10 mM Tris buffer, ph 7.4 and centrifuged according to the procedure described by Lash and Sall (1993). Mitochondrial protein content was measured using a bicinchoninic acid protein assay kit and BSA as a standard (Pierce, Rockford, IL, USA).
2.4. Western blot analysis
SDS-PAGE was carried out in 10% separating gels by loading equal amounts of mitochondrial protein (8 ug) in each well. Proteins were transferred onto membranes at 90 V for 1 h at 4 °C. The membranes were blocked in 5% non-fat milk in Tris-buffered saline [10 mM Tris-HCl (pH 7.5), 150 mM NaCl] and Tween 20 at 37 °C for 30 min, washed thrice, 5 min each, with TBST and incubated with primary antibodies overnight at 4 °C. Antibodies against 4-hydroxy-2-nonenal (HNE) and malondialdehyde (MDA) were diluted 1:5000 in 3% non-fat milk in TBST buffer. After washing, the membranes were incubated with a goat anti-rabbit IgG antibody, conjugated to horseradish peroxidase (secondary antibody; dilution 1:100000), for 1.5 h at room temperature. Proteins were visualized using an enhanced chemiluminescence kit. The intensities of immunoreactive bands were quantified by scanning densitometry and analyzed with Quantity One (Bio-Rad).
2.5 Identification of HNE- and MDA-modified proteins
HNE- and MDA-modified proteins were identified by the procedure described by Yarian et al. (2005). Briefly, coomassie blue stained protein bands from SDS–PAGE, corresponding to purified HNE-modified proteins, were excised, destained, reduced with dithiothreitol, alkylated with iodoacetamide and digested with sequencing-grade trypsin (Promega) at 37 °C overnight. Tryptic digestion products were extracted from the gel with a 5% formic acid/50% acetonitrile solution and evaporated with SpeedVac (ThermoSavant). The dried tryptic digest samples were resuspended in 10 µL of 60% formic acid and separated on a BioBasic-18 100 mm × 0.18 mm reverse phase capillary column (ThermoFinnigan). Mass analysis was conducted using a ThermoFinnigan LCQ Deca XP Plus ion trap mass spectrometer, equipped with a nanospray ion source.
2.6 Statistical analysis
The total normalized immunodensities of HNE- and MDA-adducts were considered in separate 3-way analyses of variance, with Age, Strain and Diet as between groups factors. Data for individual bands were considered together in a 4-way ANOVA involving the same factors, with Band as a within-groups factor. In the case of a significant 3-way interaction, hypothesis-based individual comparisons of AL and CR groups of matching age and strain were performed, as well as planned comparisons assessing the effect of age and strain for mice under the AL feeding condition. The 8 comparisons were performed using single degree-of-freedom F tests involving the error term from the overall analysis. The alpha level was set at 0.05 for all analyses.
3. Results
3.1. Age- and CR- related changes in amounts of HNE- and MDA- adducts in skeletal muscle mitochondrial proteins of C57BL/6 and DBA/2 mice
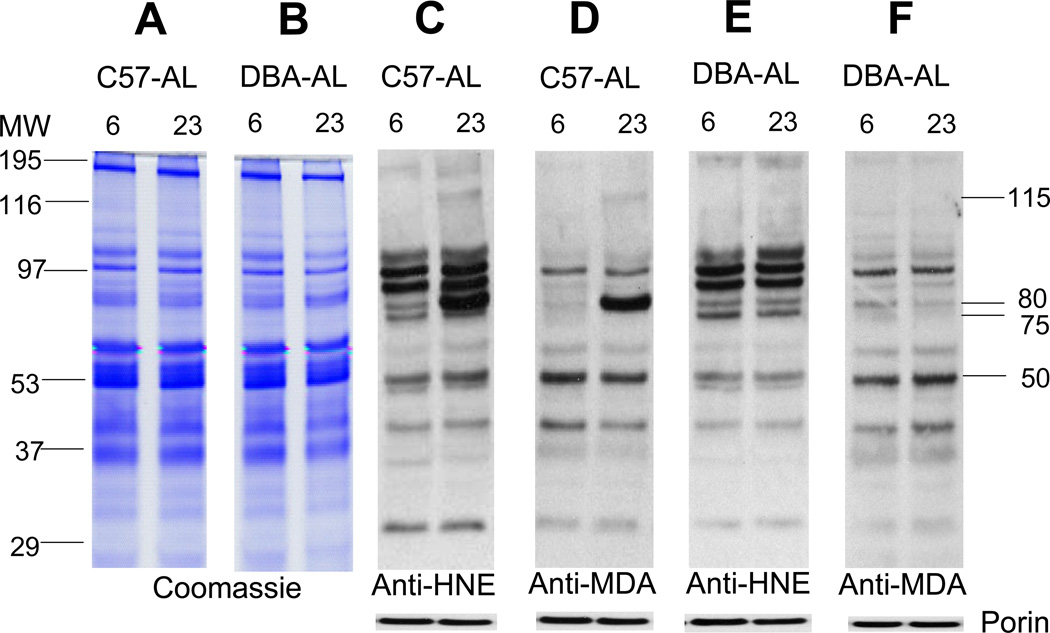
To determine the effects of age, diet, and strain on the pattern of protein expression, aliquots of skeletal muscle mitochondrial proteins from 6- and 23-month-old, AL- and CR- fed, C57BL/6 and DBA/2 mice, were separated by SDS–PAGE and stained with Coomassie blue. Several distinct protein bands of varying densities were clearly discernable in all groups, however, there were no obvious age-, strain- or diet--associated differences in the staining densities of specific bands among the different groups, suggesting that the relative amounts of the major proteins were not notably affected by age, CR, or strain (Figs. 1, 2). Corresponding sets of aliquots of mitochondrial protein were resolved by SDS-PAGE and processed for Western analysis, using specific anti-HNE or anti-MDA antibodies. Up to 10 anti-HNE- and 6 anti-MDA- positive protein bands of varying densities could be discerned in both the 6- and 23-month-old, C57BL/6 and DBA/2 mice (Fig. 1). Comparisons of the Western blots with the Coomassie-stained gels suggested that the densities of the immunopositive bands, putatively reflecting the amounts of the HNE- or MDA-adducts, were independent of the level/abundance of the protein in these bands. For instance, the relatively high abundance ~ 195 kDa protein band in the Coomassie-stained gel showed little reactivity with anti-HNE or anti-MDA antibodies in the Western blots, whereas the low abundance ~30 kDa band exhibited comparatively strong reactivity with anti-HNE antibody, but not with anti-MDA antibody (Fig. 1).
Fig. 1.
Coomassie-blue-stained gels and Western blots of HNE- and MDA-modified proteins in mitochondria from hind leg skeletal muscles of ad libitum (AL) fed mice at 6- and 23 months of age. From left: Panels A an B, mitochondrial proteins (8 µg) from C57BL/6 and DBA/2 mice, respectively, were resolved on an SDS/polyacrylamide gel and stained with Coomassie blue. MW indicates the molecular weight markers. Panels C, D, E and F are representative immunoblots of the mitochondrial proteins from the two strains of mice, reacting with anti-HNE or anti-MDA polyclonal antibodies, at 6 and 23 months of age. To confirm equal protein loading and provide for normalization, the membranes were re-probed with anti-porin antibody, as shown at the bottom of the panels.
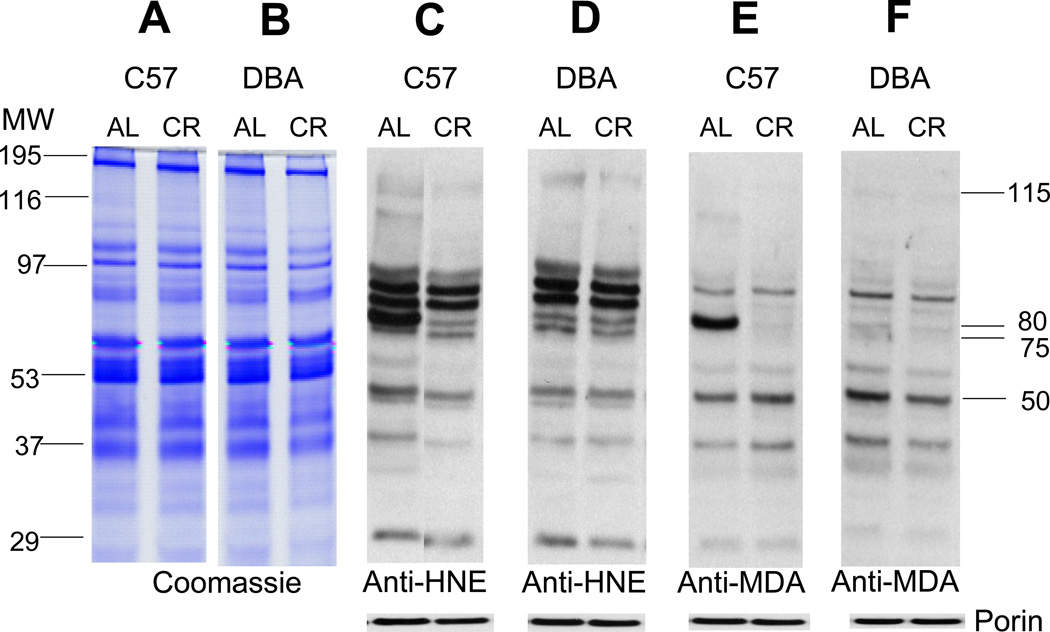
Fig. 2.
Coomassie-blue-stained gels and Western blots of HNE- and MDA-modified proteins in mitochondria from skeletal muscles of 23-month-old C57BL/6 and DBA/2 mice, fed ad libitum (AL) or maintained on the calorically restricted (CR) diet since 4-months of age. Panels A and B are Coomassie blue-stained gels. Panels C, D, E and F are representative immunoblots of mitochondrial proteins reacting with anti-HNE or anti-MDA polyclonal antibodies. To confirm equal protein loading and provide for normalization, the membranes were re-probed using anti-porin antibody (shown at the bottom of the panels).
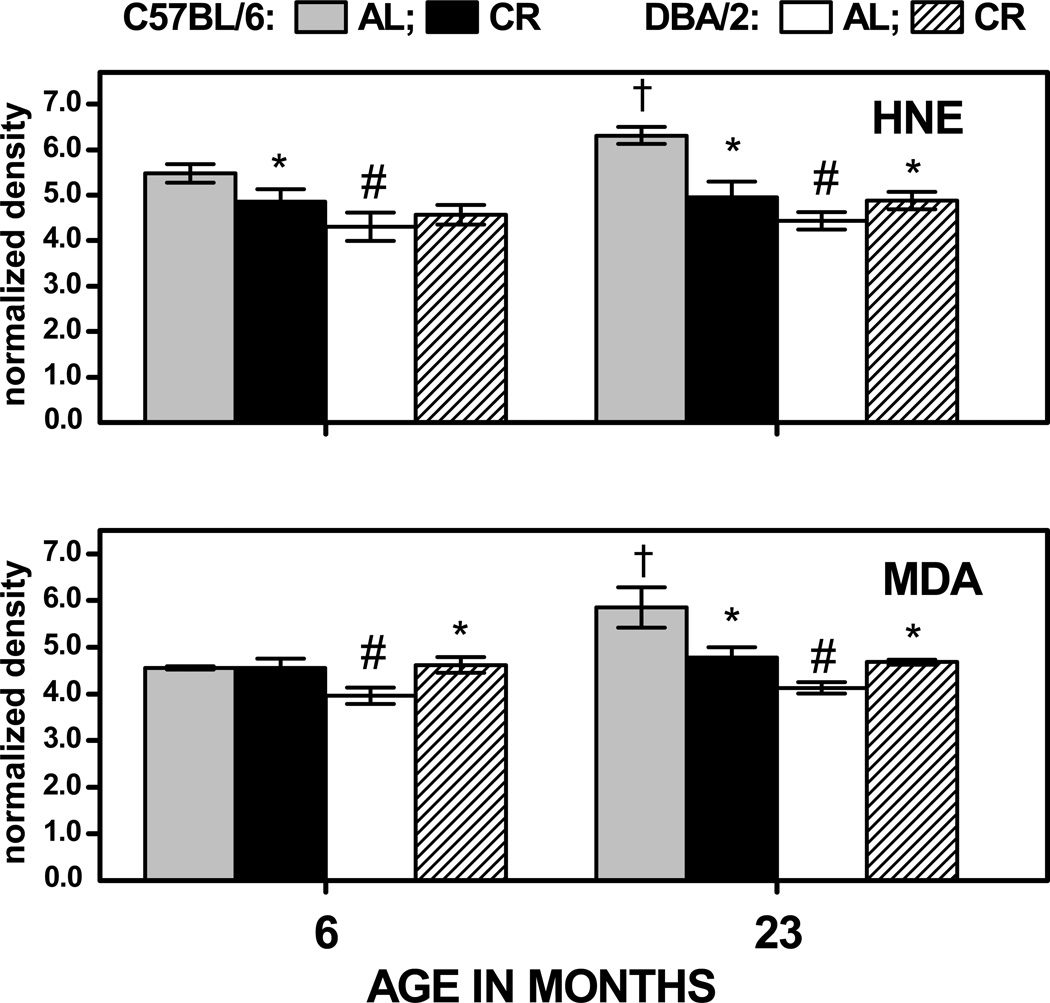
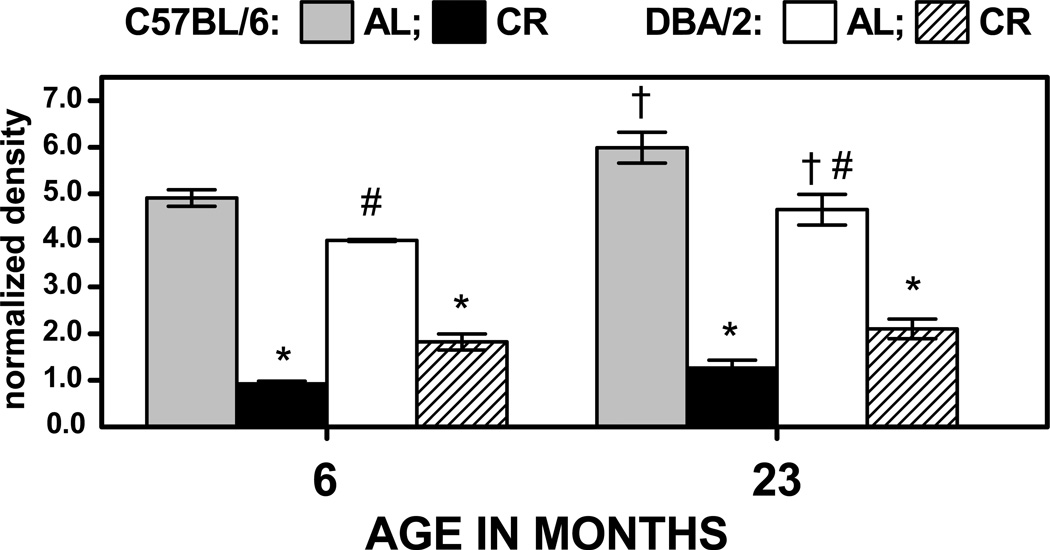
The total HNE- or MDA- protein content was quantified as the density of the entire immunoreactive material, using porin protein as a standard (Fig. 3). In the AL- fed, C57BL/6 mice, HNE and MDA content was significantly increased (by 15% and 29%, respectively), during 6 to 23 months of age; however, there was no significant age-related accretion in the total amount of HNE- or MDA-positive material in the DBA/2 mice. The total amounts of HNE and MDA adducts at 6 months of age were, respectively, 25% and 14% higher in the AL fed C57BL/6 than in DBA/2 mice, and 42% higher for both adducts at 23 months of age. In C57BL/6 mice, CR decreased the total HNE content by 11% at 6 months and 22% at 23 months of age; whereas in the DBA/2 mice, CR had no effect on the total HNE content at 6 months and increased it by 10% at 23 months of age. Caloric restriction had no impact on total MDA content at 6 months in C57BL/6 mice, but decreased it by 18% at 23 months of age. In contrast, CR enhanced the total MDA content in DBA/2 mice by 15% at 6 months of age and 13% at 23 months. The different age-dependent effects of the diet in the two different strains resulted in a significant Age × Strain × Diet interaction when the normalized density data for HNE and MDA were considered in 3-way analyses of variance (ps < 0.025). Thus, the effect of age and CR on HNE- and MDA- adducts in skeletal muscle differed in the two strains of mice: in the AL-fed C57BL/6 mice, the total levels of the HNE- and MDA-adducts increased with age and CR virtually prevented these age-related elevations, whereas, in the DBA/2 mice, age had no effect on HNE or MDA content and CR tended to increase the HNE and MDA level. Altogether, data suggested that the age-associated increase in mitochondrial protein oxidative damage, indicated by the levels of HNE/MDA adducts, is dependent upon the genotype and is not a universally occurring phenomenon.
Fig. 3.
Amounts of total HNE- and MDA-immunopositive protein in skeletal muscle mitochondria of C57BL/6 and DBA/2 mice at 6 and 23 months of age. Mice were fed AL or gradually put on 40% CR at 4 months of age. Data represent the aggregate amounts of HNE-(top) and MDA- (bottom) immunopositive material. Values are mean ± SD of 3 independent mitochondrial preparations, each consisting of tissue pooled from two mice. † p < 0.05 for planned comparison between 6- and 23-month-old AL-fed mice of the matching strain; * p < 0.05 for comparison between CR- and AL- fed mice of matching age and strain; # p < 0.05 for comparison between AL-fed C57BL/6 vs DBA/2 mice of matching age.
3.2. Effect of age and CR on amounts of HNE- and MDA-adducts in specific mitochondrial protein bands in C57BL/6 and DBA/2 mice
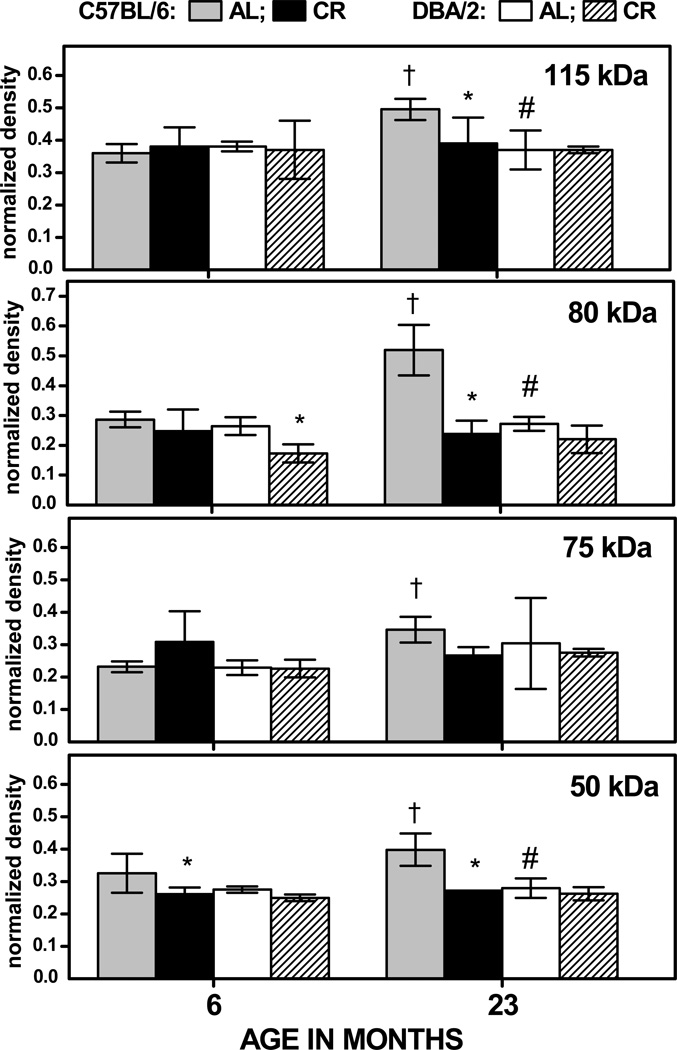
Densities of the individual HNE/MDA immunopositive bands were also monitored separately in order to determine whether the effects of age, strain or diet occurred uniformly or differentially. Among the ten HNE-positive protein bands, with MW of ~ 115-, 100-, 95-, 90-, 80-, 75-, 50-, 43-, 35- and 30-kDa, that were discernable at 6 months of age in C57BL/6 mice, only four, with MW of 115-, 80-, 75- and 50-kDa, exhibited an age- and/or CR-related alteration in immunodensity (Figs. 1, 2). Out of the six MDA-positive bands, with MW of ~ 115-, 90-, 80-, 50-, 43-, 35- and 30-kDa, only three, with MW of 115, 80- and 50-kDa, showed apparent age- or CR-associated variations in signal intensity. It should be noted that the latter three bands exhibited the presence of both HNE- and MDA-adducts (Figs.1, 2).
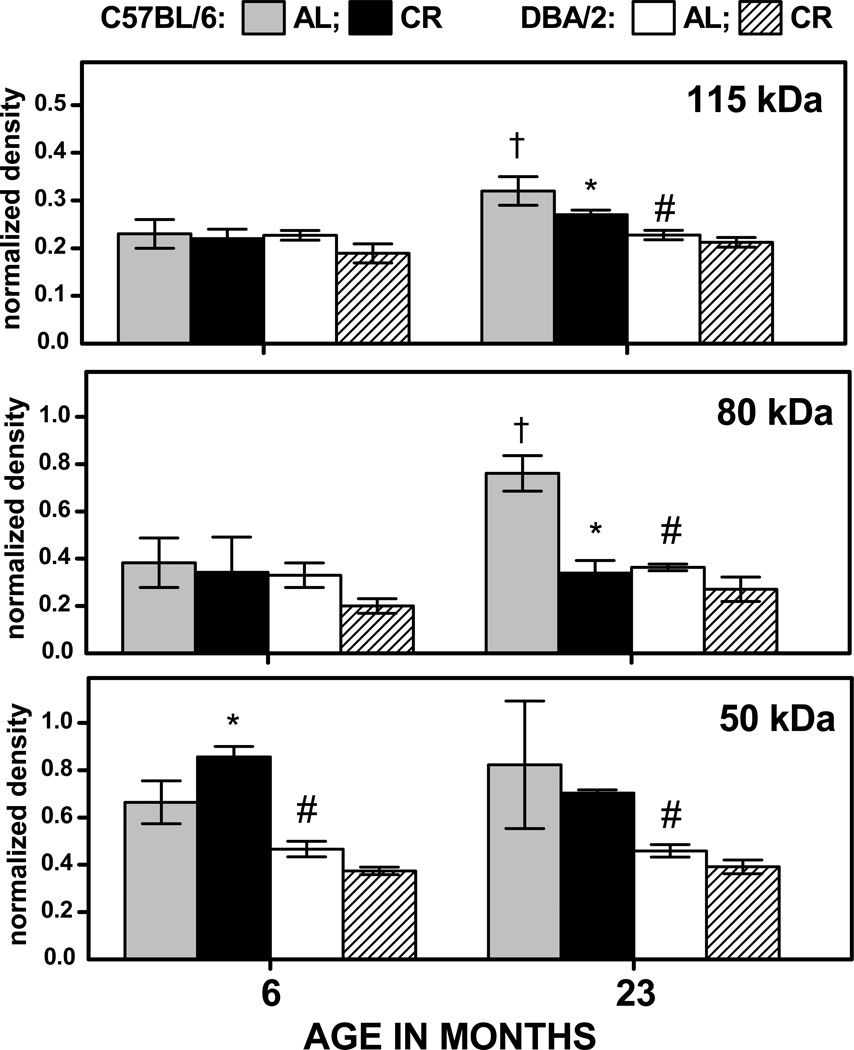
In the AL-fed, C57BL/6 mice, normalized immunodensities of HNE-adducts increased during 6 to 23 months by 38%, 87%, 51%, and 22% respectively, for the 115-, 80-, 75- and 50-kDa bands (Fig. 4). In contrast, during the same period, none of age-related changes in these bands were significant in the DBA/2 mice. Densities of the 115-, 80- and 50-kDa HNE-positive bands at 23 months of age were 33–97% higher in the AL-fed, C57BL/6 mice than in DBA/2 mice. In the C57BL/6 mice, CR resulted in a decrease of 20% in immunodensity of the 50 kDa HNE-positive band at 6 months of age and, at 23 months, the immunodensities of the 115-, 80-, and 50-kDa protein bands were, respectively, 20%, 56%, and 32% lower in the CR compared to the AL fed mice. In contrast, there were no CR-related decreases in HNE-positive bands in DBA/2 mice at 23 months of age (Fig. 4). The analysis of variance conducted on the data shown in Fig 4 suggested a significant main effect of Band (p< 0.001), as well as a significant 3-way interaction of Age, Strain and Diet (p<0.001), reflecting the strain-dependent effects of age and diet on HNE content in the different bands.
Fig. 4.
Normalized density of HNE immunostaining for 115-, 80-, 75- and 50-kDa protein bands (Figs. 1, 2) in the skeletal muscle mitochondria of C57BL/6 and DBA/2 mice as a function of age and diet. Data are expressed as the mean ± SD of 3 independent mitochondrial preparations, each consisting of tissue pooled from two mice. † p < 0.05 for planned comparison between 6- and 23-month-old AL-fed mice of the matching strain; * p < 0.05 for comparison between CR and AL fed mice of matching age and strain; # p < 0.05 for comparison between AL-fed C57BL/6 vs DBA/2 mice of matching age.
The pattern of the age- and CR-related changes in the levels of MDA adducts were similar to that of the HNE-adducts. In the AL-fed, C57BL/6 mice, the MDA content of the 115-, and 80-kDa bands increased, respectively, by 41% and 99% during 6 to 23 months of age, while the corresponding accretions in the DBA/2 mice were not significant. The densities of all three MDA-positive bands in the AL mice at 23 months of age were 41–109% higher in C57BL/6 than in the DBA/2 mice (Fig. 5). In 6-month-old, C57BL/6 mice, CR had no attenuating effect on MDA content of 80- and 115-kDa bands and, surprisingly, increased the MDA level in the 50-kDa band by 29%. In the corresponding DBA/2 mice, CR had no significant effect on the MDA content of any of the bands. At 23 months of age, the MDA content of 115- and 80-kDa bands was, respectively, 15% and 55% lower in the CR compared to the AL- fed C57BL/6 mice, whereas the corresponding changes in the DBA/2 mice were not significant (Fig. 5). The analysis of variance conducted on normalized MDA immunodensity led to the same outcome as that for HNE immunodensities. Thus, the results suggested that during aging the specific mitochondrial protein bands accrued considerably more HNE and MDA adducts in the C57BL/6 than in DBA/2 mice, and the attenuations in immunoreactivity due to long-term CR were also greater in the former than in the latter.
Fig. 5.
Normalized density of MDA-immunostaining for 115-, 80- and 50-kDa protein bands (Figs. 1, 2) in skeletal muscle mitochondria of C57BL/6 and DBA/2 mice as a function of age and diet. Values are mean ± SD of 3 independent mitochondrial preparations, each consisting of tissue pooled from two mice. †p < 0.05 for planned comparison between 6- and 23-month-old AL-fed mice of the matching strain; * p < 0.05 for comparison between CR- and AL-fed mice of matching age and strain; # p < 0.05 for comparison between AL-fed C57BL/6 vs DBA/2 mice of matching age.
3.3. Identification of HNE- and MDA-modified proteins in the skeletal muscle mitochondria
To identify the specific proteins with HNE- and/or MDA adducts within the 115-, 80-, 75- and 50-kDa immunoreactive bands (Fig.1), mitochondria were sonicated and separated into matrix and membrane fractions by ultracentrifugation; the soluble proteins were then separated according to their isoelectric point, in the pH range of 8 to 4, and the enriched immunopositive bands were confirmed by Western blotting, as described in the Materials and Methods section. Mass spectrometric analysis indicated the presence of pitrilysin (metalloprotease 1) in the 115 kDa band, NADP-dependent mitochondrial malic enzyme (isoform 2) in the 75 kDa band, and citrate synthase in the 50 kDa band (Table 1). We were unsuccessful in reliably identifying the immunoreactive protein in the 80-kDa band.
Table 1.
Identification of enriched proteins exhibiting HNE and MDA adducts
| Protein | NCBI accession number |
Modification (HNE or MDA reactive) |
Molecular Weight (kDa) |
Number of peptides identified |
% of sequence coverage |
|---|---|---|---|---|---|
| Pitrilysin (Metalloprotease 1) |
109506100 | HNE, MDA | 117.6 | 1 | 2 |
| NADP-dependent mitochondrial malic enzyme (isoform 2) |
239049264 | HNE | 67.2 | 14 | 17 |
| Citrate synthase | 18543177 | HNE, MDA | 50 | 9 | 14 |
3.4. Age- and CR- related changes in the amounts of HNE-adducts in liver mitochondrial proteins of C57BL/6 and DBA/2 mice
To determine whether or not tissue other than skeletal muscle is also affected by age, CR and genotype in a manner similar to that observed in skeletal muscle, the total amounts of HNE-adducts were also examined in the liver mitochondria (Fig. 6). The effect of age on HNE in the AL-fed mice differed from that observed in skeletal muscle: there were increases of 16 to 22% in both strains of mice during 6 to 23 months of age. The effect of CR on the level of liver HNE-adducts at 6 and 23 months of age differed markedly from that evident in skeletal muscle mitochondria, with both mouse strains exhibiting robust decreases in the amounts of protein-bound HNE content. In the 23-month-old C57BL/6 mice, HNE level decreased by 79% under the CR regimen, whereas in the DBA/2 mice of the same age it was attenuated by 55%; similar effects of CR were also present in the 6-month-old mice of both strains. Thus, in liver mitochondria, the ability of CR to attenuate the level of HNE-modified protein did not accord with the difference in the longevity-extending effect of CR between the C57BL/6 and DBA/2 strains.
Fig. 6.
Normalized density of total HNE-immunostaining in liver mitochondria of C57BL/6 and DBA/2 mice at 6 and 23 months of age. Mice were fed AL or gradually put on 40% CR at 4 months of age. Data represent the aggregate amounts of HNE-immunopositive material. Values are mean ± SD of 3 independent mitochondrial preparations, each consisting of tissue pooled from two mice. † p < 0.05 for planned comparison between 6- and 23-month-old AL-fed mice of the matching strain; * p < 0.05 for comparison between CR- and AL-fed mice of matching age and strain; # p < 0.05 for comparison between AL-fed C57BL/6 vs DBA/2 mice of matching age.
4. Discussion
The main objective of this study was to test the historical concept that macromolecular oxidative damage increases with age and CR retards its accretion. Results indicate that the total amounts of HNE/MDA adducts increased with age and CR attenuated the augmentations in the skeletal muscle mitochondria in the C57BL/6 mice, whose life span is extended by CR; whereas, in the DBA/2 mice, whose life span is unaffected or weakly extended by CR, there was neither an increase in the level of HNE/MDA adducts with age nor did CR cause a decrease in the amounts of these adducts. Incongruously, in the liver mitochondria of both strains of mice, the level of protein-HNE content significantly increased with age and CR caused a very substantial reduction in its amount regardless of age. The observed age-related increase in protein oxidative damage in the skeletal muscle and liver mitochondria and its attenuation by CR in C57BL/6 mice accords with the numerous reports of similar association in the literature. Nevertheless, another set of observations, namely: (i) the DBA/2 mice neither exhibit an age-related elevation in oxidative damage nor does CR have an attenuating effect on the level of such damage in skeletal muscle mitochondria, and (ii) oxidative damage in the liver of both strains of mice is attenuated by CR, are inconsistent with the historical concept of an assocition between oxidative damage, life span and CR. Instead, our findings suggest that age-related accual of protein oxidative damage, manifested as HNE/MDA adducts, and its decrease by CR, are dependent upon the tissue and the genotype, and show no consistent linkage with longevity. Indeed, the tissue-specific nature of the age-related changes in the level of mitochondrial oxidative damage has been previously reported by Davies et al. (2001) in the rat. Mitochondrial protein carbonyl content was found to decrease in liver, remain unchanged in the heart and to increase in the brain, which led these authors to conclude that there was no consistent or large-scale accumulation of mitochondrial oxidative damage during aging.
Several factors may be responsible for the lack of consistent correlations between accretion of protein oxidative damage and longevity. The steady-state levels of oxidized proteins are dependent upon the balance between two main processes, namely the rate of hydrolysis of oxidized proteins and the rate of nascent oxidation of proteins (Stadtman, 2002). Oxidized proteins are known to be more susceptible to proteolysis than the native proteins, as they are preferentially hydrolyzed by the activities of proteasomes or alkaline proteases (Agarwal and Sohal, 1994; Jung and Grune 2008; Rivett et al., 1985; Starke-Reed and Oliver, 1989; Ugarte et al., 2010). Alkaline protease activity of tissues against oxidized bovine serum albumin was reported to decline in rats by 50% in the liver and 20% in heart, and remain unaltered in the brain (Agarwal and Sohal, 1994). Keller and associates found that proteasomal activity declined with age in the rat heart, liver and brain, but not in the spleen (Dasuri et al., 2009; Li et al., 2008; Zhang et al., 2007). In contrast, houseflies showed no age-related alterations in proteolytic activity (Agarwal and Sohal, 1994). Lon-like protease activity, localized within mitochondria, decreased 5-fold in the mouse skeletal muscle and 2.5-fold in rat liver, but remained unaffected in the rat heart (Ugarte et al., 2010). Such findings suggest that age-related changes in proteasomal activity for the removal of oxidized proteins also do not follow a uniform pattern in different tissues or genotypes.
The rate of nascent protein oxidation is determined by the balance between ROS generation and the antioxidant defenses as well as the abundance of the moleculer targets of oxidative attacks (Stadtman, 2002). In general, the rates of mitochondrial production of superoxide anion radical and hydrogen peroxide vary in different tissues and increase as a function of age (Sohal et al., 2002). It was reported in a previous study (Ferguson et al., 2008) that the rate of mitochondrial superoxide anion radical generation in the skeletal muscle and heart was similar in these two strains of mice, whereas the amounts of GSH in the heart, liver and skeletal muscle, as well as catalase activity in in skeletal muscle and heart, were significantly higher in C57BL/6 than the DBA/2 mice, suggesting that the former may be relatively more efficient in the removal of hydrogen peroxide. Nevetheless, in a separate study, skeletal muscle and liver of both strains of mice were found to display comparable levels of age-related increases in GSSG:GSH ratios and amounts of protein mixed disulfides, which are widely accepted as relatively sensitive indicators of oxidative stress (Rebrin et al., 2011). Such data do not support the role of observed inter-strain differences in pro-oxidant generation and/or antioxidant defenses as factors in the age-related variations in the accrual of mitochondrial oxidative damage reported in the current study.
However, a notable difference between the two strains of mice used here, that may arguably be partly responsible for the age- and CR-associated inter-strain differences in the amounts HNE/MDA-modified proteins, is the level of adiposity. Our previous studies indicated that the mice of these two strains weighed the same at 4 months of age and consumed similar amounts of food throughout life; however, compared to the DBA/2, the C57BL/6 had ~ 25% lower metabolic rate and during 6 to 22 months of age gained 20% more body weight and 160% greater fat mass. CR prevented fat accumulation in C57BL/6 by 56%, but had little effect in the DBA/2 mice (Sohal et al., 2009). Lipotoxic effects, emanating from the deposition of excess fat in skeletal muscle and liver, have been implicated in mitochondrial dysfunction and the exacerbation/acceleration of senescent changes (Chavez and Summers, 2010; Schrauwen et al. 2010; Zimniak, 2011). Lipolysis of triacylglycerides, stored in the intra-cellular lipid droplets, into fatty acids, such as linoleic- and arachidonic-acid, can upon peroxidation lead to the generation of lipid peroxides and subsequently products like HNE and MDA (Chavez and Summers 2010). The finding, that the amounts of HNE/MDA protein adducts increased with age and CR attenuated such augmentations to a greater extent in C57BL/6 than in DBA/2 mice, correlates with the differences in their fat mass under both, AL and CR regimens. Nonetheless, a cause-and-effect relationship between the inter-strain variation in fat mass and HNE/MDA adducts cannot be established on the basis of the current data.
In a previous studty, CR was found to have a varied effect on the redox state in these two strains of mice. Both strains showed a comparable age-related pro-oxidizing shift in the thiol redox state, indicated by levels of GSH and GSSG, GSSG:GSH ratios and the amounts of protein mixed disulfides. Relatively long-term CR (19 months) prevented these pro-oxidizing changes in the skeletal muscle and liver of C57BL/6 mice, but not in the DBA/2 mice. Even a relatively short-term CR (7 weeks) reversed the age-associated pro-oxidizing shift in the glutathione redox state in the erthrocytes of C57BL/6, but not in the DBA/2 mice. A comparison of the activities of enzymes associated with glutathione metabolism and redox state indicated that in DBA/2 mice, CR elevated the activities of glutathione peroxidase and glutathione reductase, but not glutamate-cysteine ligase, the rate-limiting enzyme in GSH synthesis. In contrast, CR enhanced the activities of glutathione peroxidase as well as GCL in the C57BL/6 mice. Thus CR failed to attenuate the pro-oxidant changes in the mouse strain (DBA/2), whose life span is not robustly prolonged by CR, suggesting that thiol redox state rather than the steady-state levels of protein oxidative damage, may be more closely associated with longevity extension by CR.
Although mitochondrial protein oxidative damage was found neither to consistenly increase with age nor show an attenuation by CR, it may not necessarily imply that such damage is unlikely to exert any deleterious functional effect. For instance, oxidative damage does increase the rate of protein turnover, which, unless matched by biosnythesis, would decrease the net content of the native proteins. Even though the total amount of oxidative damage may not increase with age, damage to some indidual proteins increases during aging, as also observed in the current study. Metalloprotease 1, NADP-dependent mitochondrial malic enzyme (isoform 2) and citrate synthase were found to undergo significant age-related increases in HNE/MDA content, however, corresponding alterations in catalytic activities were not determined. Although aconitase has been identified to be a target of structural oxidative damage and to lose activity during aging in several different tissues and genotypes, the specific proteins exhibiting oxidative damage may vary in different tissues (Sohal, 2002). Furthermore, oxidative damage to a specific protein does not, a priori. entail a loss of activity during aging. Yarian et al. (2005) identified four proteins that displayed MDA adducts in mouse heart, but only two of those showed a age-related loss of activity. It is also worth pointing out that Western blot analysis is less efficient in the detection of oxidative damage to proteins that are relatively low in abundance. Thus, it seems that no reliable inferences about the potential losses of protein function can be drawn solely on the basis of structural damage.
In conclusion, the results of this study suggest that the association between aging, oxidative damage and CR does not follow a uniform pattern in different tissues and genotypes. Although the results are partially supportive, they also contradict the historic concept that oxidative damage increases with age and the extension of life span by CR is associated with the retardation of the accrual of such damage.
Highlights.
Mice differing in life-extending response to caloric restriction (CR) were studied
Age-related increases in protein oxidation were dependent on genotype and tissue
Attenuation of protein oxidation by CR was genotype and tissue specific
The effect of CR on protein oxidation did not fully accord with life-span extension
Acknowledgements
This study was supported by the grant R01 AG 13563 from the National Institutes of Health-National Institute on Aging. We are grateful to Dr William C. Orr for his comments.
Abbreviations
- AL
ad libitum
- CR
caloric restriction
- DNP
dinitrophenylhydrazone
- HNE
4-hydroxy-2-nonenal
- MDA
malondialdehyde
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal S, Sohal RS. Aging and proteolysis of oxidized proteins. Arch. Biochem. Biophys. 1994;309:24–28. doi: 10.1006/abbi.1994.1078. [DOI] [PubMed] [Google Scholar]
- Birch-Machin M, Jackson S, Kler RS, Turnbull DM. Study of skeletal muscle mitochondrial dysfunction. In: Lash LH, Jones DP, editors. Methods in Toxicology: Mitochondrial Dysfunction. San Diego: Academic Press; 1993. pp. 51–59. [Google Scholar]
- Buffenstein R, Edrey YH, Yang T, Mele J. The oxidative stress theory of aging: embattled or invincible? Insights from non-traditional model organisms. Age. 2008;30:99–109. doi: 10.1007/s11357-008-9058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez JA, Summers SA. Lipid oversupply, selective insulin resistance, and lipotoxicity: molecular mechanisms. Biochim. Biophys. Acta. 2010;1801:252–265. doi: 10.1016/j.bbalip.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasuri K, Zhang L, Ebenezer P, Liu Y, Fernandez-Kim SO, Keller JN. Aging and dietary restriction alter proteasome biogenesis and composition in the brain and liver. Mech. Ageing Dev. 2009;130:777–783. doi: 10.1016/j.mad.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SMK, Poljak A, Duncan MW, Smythe GA, Murphy MP. Measurements of protein carbonyls, ortho- and meta- tyrosine and oxidative phosporylation complex activity in mitochondria from young and old rats. Free Radic. Biol. Med. 2001;31:181–190. doi: 10.1016/s0891-5849(01)00576-7. [DOI] [PubMed] [Google Scholar]
- Ferguson M, Mockett RJ, Shen Y, Orr WC, Sohal RS. Age-associated decline in mitochondrial respiration and electron transport in Drosophila melanogaster. Biochem. J. 2005;390:501–511. doi: 10.1042/BJ20042130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M, Rebrin I, Forster MJ, Sohal RS. Comparison of metabolic rate and oxidative stress between two different strains of mice with varying response to caloric restriction. Exp. Gerontol. 2008;43:757–763. doi: 10.1016/j.exger.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes G, Yunis EJ, Good RA. Influence of diet on survival of mice. Proc. Nat. Acad. Sci. USA. 1976;73:1279–1283. doi: 10.1073/pnas.73.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster MJ, Morris P, Sohal RS. Genotype and age influence the effect of caloric intake on mortality in mice. FASEB J. 2003;17:690–692. doi: 10.1096/fj.02-0533fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J, Schaffer S, Halliwell B. The mitochondrial free radical theory of ageing--where do we stand? Front. Biosci. 2008;13:6554–6579. doi: 10.2741/3174. [DOI] [PubMed] [Google Scholar]
- Jung T, Grune T. The proteasome and its role in the degradation of oxidized proteins. IUBMB Life. 2008;60:743–752. doi: 10.1002/iub.114. [DOI] [PubMed] [Google Scholar]
- Kwong LK, Sohal RS. Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Arch. Biochem. Biophys. 2000;373:16–22. doi: 10.1006/abbi.1999.1495. [DOI] [PubMed] [Google Scholar]
- Lash LH, Sall JM. Mitochondrial isolation from liver and kidney: strategy, techniques, and criteria for purity. In: Lash LH, Jones DP, editors. Mitochondrial dysfunction. San Diego: Academic Press; 1993. pp. 8–29. [Google Scholar]
- Lass A, Sohal BH, Weindruch R, Forster MJ, Sohal RS. Caloric restriction prevents age-associated accrual of oxidative damage to skeletal muscle mitochondria. Free Radic. Biol. Med. 1998;25:1089–1097. doi: 10.1016/s0891-5849(98)00144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C-Y, Rikke BA, Johnson TE, Diaz V, Nelson J. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2009;9:92–95. doi: 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Zhang L, Craddock J, Bruce-Keller AJ, Dasuri K, Nguyen A, Keller JN. Aging and dietary restriction effects on ubiquitination, sumoylation, and the proteasome in the heart. Mech. Ageing Dev. 2008;129:515–521. doi: 10.1016/j.mad.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech. Ageing Dev. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Perez VI, Bokov A, Van Remmen H, Mele J, Ran Q, Ikeno Y, Richardson A. Is the oxidative stress theory of aging dead? Biochim. Biophys. Acta. 2009;1790:1005–1014. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey JJ, Harper ME, Weindruch R. Restriction of energy intake, energy expenditure, and aging. Free Radic. Biol. Med. 2000;29:946–968. doi: 10.1016/s0891-5849(00)00417-2. [DOI] [PubMed] [Google Scholar]
- Rebrin I, Forster MJ, Sohal RS. Association between life-span extension by caloric restriction and thiol redox state in two different strains of mice. Free Radic. Biol. Med. 2011;51:225–233. doi: 10.1016/j.freeradbiomed.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivett AJ. Preferential degradation of the oxidatively modified form of glutamine synthetase by intracellular mammalian proteases. J. Biol. Chem. 1985;260:300–305. [PubMed] [Google Scholar]
- Sastre J, Pallardo FV, Vina J. The role of mitochondrial oxidative stress in aging. Free Radic. Biol. Med. 2003;35:1–8. doi: 10.1016/s0891-5849(03)00184-9. [DOI] [PubMed] [Google Scholar]
- Schrauwen P, Schrauwen-Hinderling V, Hoeks J, Hesselink MK. Mitochondrial dysfunction and lipotoxicity. Biochim. Biophys. Acta. 2010;1801:266–271. doi: 10.1016/j.bbalip.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Mockett RJ, Orr WC. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free Radic. Biol. Med. 2002;33:575–586. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- Sohal RS. Role of oxidative stress and protein oxidation in the aging process. Free Radic. Biol. Med. 2002;33:37–44. doi: 10.1016/s0891-5849(02)00856-0. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Dubey A. Mitochondrial oxidative damage, hydrogen peroxide release and aging. Free Radic. Biol. Med. 1994;16:621–626. doi: 10.1016/0891-5849(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Ferguson M, Sohal BH, Forster MJ. Life span extension in mice by food restriction depends on an energy imbalance. J. Nutr. 2009;139:533–539. doi: 10.3945/jn.108.100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, Selman C. The free-radical damage theory: Accumulating evidence against a simple link of oxidative stress to ageing and lifespan. Bioessays. 2011;33:255–259. doi: 10.1002/bies.201000132. [DOI] [PubMed] [Google Scholar]
- Stadtman ER, Berlett BS. Reactive oxygen-mediated protein oxidation in aging and disease. Chem. Res. Toxicol. 1997;10:485–494. doi: 10.1021/tx960133r. [DOI] [PubMed] [Google Scholar]
- Stadtman ER. Importance of individuality in oxidative stress and aging. Free Radic. Biol. Med. 2002;33:597–604. doi: 10.1016/s0891-5849(02)00904-8. [DOI] [PubMed] [Google Scholar]
- Starke-Reed PE, Oliver CN. Protein oxidation and proteolysis during aging and oxidative stress. Arch. Biochem. Biophys. 1989;275:559–567. doi: 10.1016/0003-9861(89)90402-5. [DOI] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J. Gerontol. A Biol. Sci. Med. Sci. 1999;54:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- Ugarte N, Petropoulos I, Friguet B. Oxidized mitochondrial protein degradation and repair in aging and oxidative stress. Antioxid. Redox Signal. 2010;13:539–549. doi: 10.1089/ars.2009.2998. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk JM, Hekimi S. Reactive Oxygen Species and Aging in Caenorhabditis elegans: Causal or Casual Relationship? Antioxid. Redox Signal. 2010;13:1911–1953. doi: 10.1089/ars.2010.3215. [DOI] [PubMed] [Google Scholar]
- Yarian CS, Rebrin I, Sohal RS. Aconitase and ATP synthase are targets of malondialdehyde modification and undergo an age-related decrease in activity in mouse heart mitochondria. Biochem Biophys. Res. Commun. 2005;330:151–156. doi: 10.1016/j.bbrc.2005.02.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li F, Dimayuga E, Craddock J, Keller JN. Effects of aging and dietary restriction on ubiquitination, sumoylation, and the proteasome in the spleen. FEBS Lett. 2007;581:5543–5547. doi: 10.1016/j.febslet.2007.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimniak P. Relationship of electrophilic stress to aging. Free Radic. Biol. Med. 2011;51:1087–1105. doi: 10.1016/j.freeradbiomed.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]