Abstract
3,4-methylenedioxymethamphetamine (MDMA; ecstasy) binds with high affinity to the norepinephrine transporter (NET), making the noradrenergic system a potential target during fetal exposure. Recent data indicates that adult rats that had been prenatally exposed to MDMA display persistent deficits in working memory and attention; behaviors consistent with abnormal noradrenergic signaling in the forebrain. The present study was designed to investigate whether prenatal exposure to MDMA from embryonic days 14–20 affects the structure and/or function of the noradrenergic system of the rat on postnatal day 21. Offspring that were prenatally exposed to MDMA exhibited an increase in noradrenergic fiber density in the prelimbic region of the prefrontal cortex and the CA1 region of the hippocampus that was not accompanied by an increase in the number of noradrenergic neurons in the locus coeruleus. Direct tissue autoradiography using tritiated nisoxetine demonstrated that while NET binding was not altered in the prelimbic cortex, the dentate gyrus, or the locus coeruleus, it was increased in the CA1, CA2, and CA3 regions of the hippocampus. Basal levels of norepinephrine were increased in the prefrontal cortex and the nucleus accumbens of MDMA-exposed rats, as compared to saline-treated controls. These findings indicate that prenatal exposure to MDMA results in structural changes in the noradrenergic system as well as functional alterations in NE neurotransmission in structures that are critical in attentional processing.
Keywords: norepinephrine, prefrontal cortex, hippocampus, locus coeruleus
1. Introduction
The availability and perceived safety of MDMA is on the rise in the United States, and is accompanied by a resurgence in self-reported use [20]. In a study conducted by the Substance Abuse and Mental Health Services Association (SAMHS) it was estimated that approximately 1.1 million Americans used MDMA for the first time in 2009, nearly double the number of first time users reported in 2005 (615,000) [41]. Because many of these individuals are of child-bearing age [61], the risk for accidental prenatal exposure to MDMA has increased as well.
Prenatal exposure to MDMA increases dopaminergic neurite density in target structures, including the prefrontal cortex (PFC), the striatum (STR), and the nucleus accumbens (NAC) in the rat [24]. Increased dopaminergic neurite density was detectable as early as postnatal day 3 (P3), and was more pronounced by P21, suggesting that prenatal MDMA exposure may alter the postnatal developmental trajectory of these systems.
In addition to targeting the DA system, MDMA also binds with high affinity to the norepinephrine transporter (NET)[3]. Despite this fact, only one study has sought to investigate the effects of prenatal MDMA exposure on the noradrenergic system. In this report, Kelly and colleagues demonstrate that adult rats that had been exposed prenatally to MDMA had a significantly greater level of local cerebral glucose utilization in the locus coeruleus (LC) as compared to control animals, indicating heightened basal activity [21]. The fact that these differences were found in adult animals suggests that prenatal MDMA exposure may affect the initial “wiring” of the noradrenergic system, resulting in lasting changes in norepinephrine (NE) signaling.
We have demonstrated that MDMA-exposed rats display an exaggerated behavioral response to novelty [24,51], which is consistent with functional changes in NE signaling. This suggests that prenatal MDMA may in fact have significant and lasting changes on the NE system. We hypothesized that similar to the DA system, prenatal exposure to MDMA would induce collateral sprouting of noradrenergic fibers. Specifically, the behavioral abnormalities observed in MDMA-exposed rats suggested there may be functional hyperinnervation of the prelimbic (Cg3) area of the PFC and the hippocampus (HIPP) [12,23,40]. To examine this, dopamine beta hydroxylase immunoreactive (DBHir) neurons in the LC, and neurites in the Cg3 and HIPP, were quantified using stereologic methods. In order to validate the hypothesis that changes in fiber density reflect functional changes in connectivity, NET binding was assessed in these regions using quantitative autoradiography. Finally, HPLC was used to quantify basal levels of NE and its metabolite 3-methoxy, 4-hydroxyphenylglycol (MHPG) in brain regions associated with working memory and attentional processing, including the PFC, STR, NAC, HIPP, and the brainstem (BS). We hypothesized that hyperinnervation of these structures would result in elevated basal levels of NE and MHPG.
2. Methods
All assays were conducted in MDMA- and saline-exposed offspring on postnatal day (P21). P21 is the age when rats are typically weaned. In addition, it has been demonstrated that the distribution pattern and density of noradrenergic innervation of structures in the forebrain is similar to that observed in the adult by P21 [2,26,28]. Examining offspring at P21 also reduces the likelihood that any observed differences are transient changes induced by acute effects of MDMA. Finally, behavioral indications of impaired attention are observed in MDMA-exposed rats as early as P21, suggesting that there should be evidence of an underlying change in the brain to account for the observed behavioral changes [24].
2.1 Drug Administration
Timed-pregnant (embryonic day 10; E10) Sprague-Dawley rats (Zivic Miller) were housed individually in an AALAC-approved temperature-(21°C) and humidity-(45%) controlled room. Dams were randomly assigned to one of two groups, receiving either MDMA (15mg/kg) or saline (SAL; 1 ml/kg) subcutaneous injections twice per day, 8 hours apart, from E14–E20. The dose chosen for this study was extrapolated from the approximate dose during a typical human consumption [31]. Litters were delivered on embryonic day 21 (E21) and were culled on the following full postnatal day (P1) to eight (4 male, 4 female) offspring in the MDMA-exposed litters and ten (5 male, 5 female) offspring in the SAL-exposed litters. This technique has been shown to equalize the litter weights, as MDMA-treated litters are often born at lower birth weights as a result of the drug’s anorectic effects on the dam [57]. The offspring remained housed with the dam prior to experimentation. Only one male/female pair per litter was assigned to a dependent measure in order to prevent litter bias [16]. With the exception of total enumeration of the locus coeruleus and NET binding quantitation which used males only, each dependent measure began with 6 male and female offspring.
The animal facility was accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care and complied with all Federal animal care and use guidelines. Protocols were all approved by the Institutional Animal Care and Use Committee of Rush University Medical Center and the University of Cincinnati.
2.2 Immunohistochemistry
Pups were anesthetized with an i.p. injection of ketamine/xylazine (285 mg/kg; 9.5 mg/kg). Each subject was then perfused with ice cold saline (10 ml/min; 5 min), followed by 4% paraformaldehyde solution (10 ml/min; 10 min). After perfusion, the brains were placed in fresh 4% paraformaldehyde solution overnight, switched to a 30% sucrose solution and refrigerated until processing. Brains were cut in 40 μm coronal sections on a sliding microtome, saving every 6th section for DBH immunohistochemistry. Following several washes in Tris-buffered saline (TBS) containing 0.05% Triton X-100 (TBS-Tx; pH 7.2), sections were incubated for 20 minutes in a TBS solution containing 0.1M sodium periodate to eliminate endogenous peroxidase activity. The tissue was then incubated in a phosphate buffered saline solution (PBS) containing 5% normal horse serum and 1% bovine serum albumin for 1 hour to reduce background staining. Primary antibody solution (0.1 M PBS, pH 7.4 containing 0.4% TritonX-100 and 3% normal horse serum and mouse anti-DBH (1:4000; ImmunoStar, Hudson, WI)) was added to each well and incubated at room temperature overnight. On the following day, sections were washed six times in TBS-Tx and incubated for 1 hour in biotinylated horse anti-mouse (1:200; Vector, Burlingame, CA). Following 3 washes in TBS-Tx, the tissue was incubated for 75 minutes in an avidin-biotin complex solution (1:500 ABC kits; Vector). The cells were visualized with 0.05% 3,3-diaminobenzidine and 0.03% hydrogen peroxide. To intensify the staining, 2.5% nickel II sulfate was added to the chromogen solution.
2.3 Fiber and Cell Estimates
Stereology
Stereologic principles were used to estimate the density of DBH+ fibers in the Cg3 and the HIPP [55]. Analysis was conducted using the software program Stereo Investigator (MicroBrightfield, Williston, VT), in combination with an Olympus BX-51 microscope (Melville, NY) and an Optronics Microfire camera. The number of counting site and radius of the probe necessary to achieve a coefficient of error <0.1 were determined prior to this application. DBHir fibers intersecting the boundary of the “space balls” probe were marked as the operator focused through the Z-axis at each sampling site. The estimated fiber density for each structure was derived by dividing the total estimated fiber length (mm) by the estimated volume (mm3) of all sections. All sections were analyzed by a single investigator, blind to the conditions.
Prelimbic Cortex
Four sections, matched for level, were analyzed per subject (Figure 1A). The anterior medial Cg3 (plates 6–9 Paxinos and Watson [37]) was outlined under low magnification (1.25x) and counts were performed at 100x magnification, using a plan-apo oil immersion objective with a 1.4 numerical aperture. The probe was set at a Merz radius of 15 μm, with a 2 μm guard zone between the surface of the section and the outer edge of the probe. To estimate volume, the section thickness at every fifth site was determined empirically.
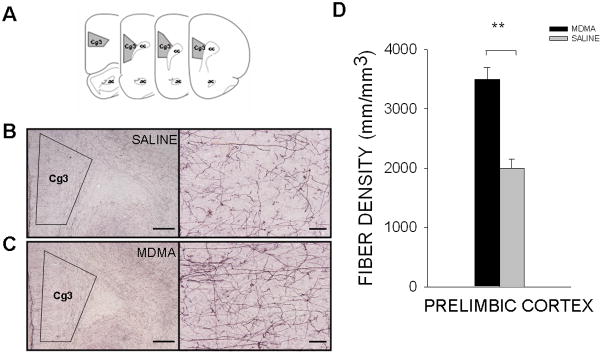
Figure 1. Noradrenergic innervation of the prelimbic cortex.
(A) Schematic diagram of the area in which DBHir fiber density was quantified. Representative images of DBHir fiber density in the prelimbic cortex of (B) saline- and (C) MDMA-exposed offspring (2x magnification, scale bar = 500 μm; 20x magnification, scale bar = 50 μm). (D) Prenatal MDMA treatment significantly increased the density of DBHir fibers in the prelimbic cortex on P21. (Cg3, prelimbic cortex; DBHir, dopamine beta hydroxylase immunoreactive)
Hippocampus
Fiber density estimates were made in rostral sections of the HIPP, as this region is known to receive a dense noradrenergic innervation, which arises exclusively from the LC [1,35,36,38,52]. In addition, the rostral HIPP has been specifically implicated in the response to novelty and spatial memory [18,25,50], which we have previously demonstrated were impaired in MDMA-exposed offspring. Three sections of the rostral HIPP, matched for level, were analyzed per subject. The CA1, CA2, CA3 and dentate gyrus were outlined under low magnification (1.25x), and counts were performed at 100x. The probe was set at a Merz radius of 8 μm, with a 1 μm guard zone. Section thickness was determined empirically at each sampling site.
Total Enumeration
Because stereologic analysis requires a minimum of 3 sampling sites per subject, small structures, such as the LC, must be quantified using total enumeration. Using the Stereo Investigator software, the optical fractionator probe was employed (grid size 54.81 × 89.38 μm) to quantify all cells within the LC. The volume fraction of the entire nucleus was determined (Cavalleri Method) based upon the entire series of sections for each animal. This value was used to normalize the actual number of counted cells to an estimate of the total number of cells in the nucleus.
2.4 Autoradiography
Animals were sacrificed by decapitation and the brains were rapidly dissected and frozen on dry ice. They were stored at −80° C until being processed, at which time they were equilibrated to −20°C in a cryostat (Leica) and sectioned at 20 μm. Serial sagittal sections were taken at each of 3 levels to allow for comparison of specific and nonspecific binding. The sections were taken from the Cg3 (Bregma +3.24 mm), the rostral HIPP (Bregma -4.44 mm), and LC (-9.48 mm). They were thaw-mounted onto Super Frost Plus slides (Fisher Scientific, Pittsburgh, PA) and stored at −80° C.
Sections were incubated in 10 mM Na2HPO4, 300 mM NaCl, and 5 mM KCl (filtered; pH = 7.4) along with 2nM [3H] nisoxetine (PerkinElmer, Boston MA) for 4 hours at 4°C. Slides used to determine nonspecific binding were incubated in the presence of 1 μM mazindol (Sigma-Aldrich, Saint Louis, MO) for the same duration. Following this incubation, slides were rinsed in 3 five minute dips in fresh buffer, and 1 five second dip in water. Incubations were carried out at 4°C and slides were stored at −20°C. Slides were exposed to [3H]-sensitive Hyperfilm (GE Healthcare, Piscataway, NJ) alongside calibrated [3H] microscale standards in tungsten cassettes for 7 weeks at 4°C. The film was developed under safelight darkroom conditions using Kodak GBX developer (5 minutes), followed by a 5% acetic acid stop solution (5 minutes), GBX fixer (5 minutes), and a 15 minute rinse in continuously running cool water. Optical density of the autoradiograms was quantified using Scion Image software (Scion Corporation, Frederick, MD). The optical density was then converted to nCi/mg tissue using the tritiated microscales (GE Healthcare, Piscataway, NJ) to predict molar quantities of bound ligand. Using the known specific activity of the ligand, this value was converted into fmol/mg tissue. Representative autoradiograms are presented as pseudocolored images using ImageJ software (version 1.41; National Institutes of Health, Bethesda, MD) [32, 36].
2.5 HPLC
Unperfused brains were extracted and immediately frozen on dry ice. Frozen slices containing structures of interest (PFC, STR, NAC, HIPP, BS) were removed in 1 mm slabs using a stainless steel brain block. Each structure was dissected on a solid state cold plate (Thermoelectric Cooling, Chicago, IL) set at −10°C. A perchlorate/sodium metabisulfite solution was added to each frozen sample, and samples were homogenized using an ultrasonic tissue homogenizer (Biologics, Gainesville, VA). A portion of the homogenate was reserved for protein analysis via the BCA protein assay (Pierce, Rockford, IL). The remaining suspension was spun at 10,500 g for 12 minutes in a refrigerated centrifuge (4°C). The supernatant was separated on a Microsorb MV C-18 column (5 μm, 4.6 × 250 mm; Varian Corp Walnut Creek, CA) and examined for NE and MHPG. Compounds were detected using a 12 channel coulometric array detector (CoulArray 5200, ESA, Chelmsford, MA) attached to a Waters 2695 Solvent Delivery System (Waters Corp, Milford, MA) under the following conditions: Flow rate of 1 ml/min, detection potentials: 50,175,350,400, 525 mV; scrubbing potential: 650 mV. The mobile phase consisted of a 10% methanol solution containing 0.1 M citric acid, 0.075 M Na2HPO4, 0.8 mM heptanesulfonic acid (pH 4.1). Unknown samples were quantified against a 6 point standard curve with a minimum r2 of 0.97. Quality control samples of known concentrations were interspersed within each run to ensure calibration.
2.6 Statistical Analyses
The mean ± SEM was calculated for each animal for each parameter. Significance was determined by the Student’s t-test, a one or a two-way ANOVA (when gender was a factor). Significant main effects or interactions were analyzed by post-hoc pairwise comparison using the Holm-Sidak correction. P values less than 0.05 were considered statistically significant.
3. Results
3.1 Fiber Density
MDMA-exposed offspring displayed a 69.2% [F(1,23)=12.778, p<0.01] increase in DBHir neurites in the Cg3 region of the PFC, as compared to SAL-treated offspring on P21 (Figure 1). In the HIPP, DBH+ fiber density was increased 32.1% in the CA1 region in MDMA-exposed animals, as compared to control animals [F(1,15)=6.307, p<0.05] (Figure 2). There were no observable differences in fiber density in the CA2 region (MDMA 3428 ± 332 mm/mm3; SAL 2682 ± 318 mm/mm3), the CA3 region (MDMA 6493 ± 586 mm/mm3; SAL 5877 ± 560 mm/mm3), or the dentate gyrus (MDMA 6696 ± 513 mm/mm3; SAL 5561 ± 491 mm/mm3) (Figure 2). There were no significant differences in males versus females, nor was there an interaction between the treatment condition and gender.
Figure 2. Noradrenergic innervation of the rostral hippocampus.
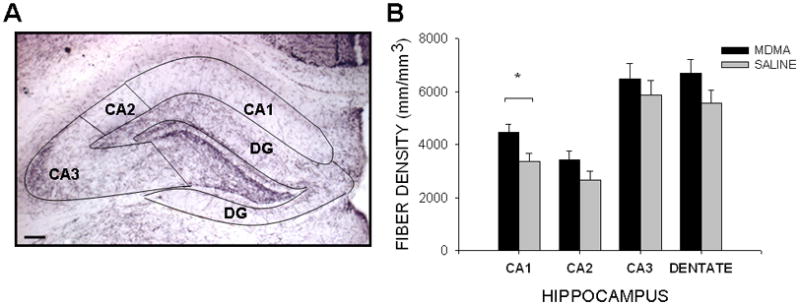
(A) DBH staining of the hippocampus allowed for clear delineation of the regions of interest (4x magnification; scale bar = 100 μm). (B) Prenatal MDMA increased DBHir fiber density in the CA1 region of hippocampus. (DBHir, dopamine beta hydroxylase immunoreactive; DG, dentate gyrus)
3.2 LC Counts
Although noradrenergic innervation of the Cg3 region of the PFC and the CA1 region of the HIPP was increased, there was no difference in the total number of DBH+ neurons in the LC following MDMA treatment (MDMA 4490 ± 145; SAL 4091 ± 408).
3.3 NET Binding
Despite the increase in noradrenergic fiber density, there was no difference in NET binding in the Cg3 region of the PFC (MDMA 32.91 ± 0.88; SAL 28.07 ± 2.84 fmol/mg). MDMA-exposed rats did show an increase in NET binding in the CA1 (39.3%) [F(1,9)=7.01, p<0.05], CA2 (83.2%) [F(1,9)=33.93, (p<0.001)], and CA3 region (32.1%) [F(1,9)=12.81, p<0.01] of the HIPP (Figure 3). No differences were seen in the dentate gyrus (MDMA 41.86 ± 2.83; SAL 35.14 ± 1.94 fmol/mg). NET binding was not altered in the LC following MDMA exposure (MDMA 461.79 ± 29.40; SAL 385.45 ± 48.84 fmol/mg).
Figure 3. NET binding in the rostral hippocampus.
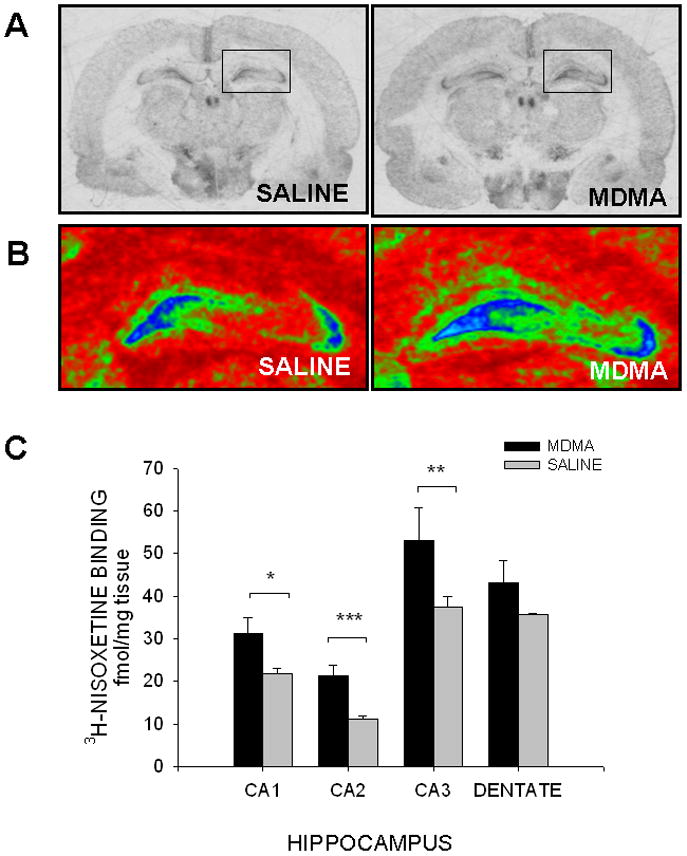
(A) Representative autoradiograms of coronal sections containing the rostral hippocampus of saline- and MDMA-exposed offspring. (B) Pseudocolored autoradiograms illustrating 3H-nisoxetine binding in the rostral hippocampus. (Blue and green indicate high levels of NET binding.) (C) Prenatal exposure to MDMA increased NET binding in the CA1, CA2, and CA3 region of the hippocampus. NET binding was not significantly different in the dentate gyrus of MDMA-exposed pups. (NET, norepinephrine transporter)
3.4 Neurochemistry
Neurochemistry results are summarized in Table 2.
MDMA-exposed offspring showed a 15% increase in NE in the PFC, as compared to SAL-treated controls [F(1,18)=6.96, p<0.05]. There were no differences in the level of MHPG, or in NE turnover in this region. No differences were seen in NE, MHPG or NE turnover in the striatum in MDMA vs. SAL treated animals. A 79.6% increase in NE was observed in the NAC of MDMA-treated animals, as compared to control animals [F(1,22)=11.90, p<0.01]. MHPG levels were below detectable limits in this structure. Despite the increase in NET binding in the HIPP, there were no differences in NE, MHPG, or NE turnover in this region. There were also no discernable differences in NE, MHPG, or NE turnover in the brainstem across groups.
4. Discussion
The current study demonstrated that prenatal exposure to MDMA affects the morphology and function of the noradrenergic system in the rat at P21. In addition to an increase in noradrenergic innervation of the Cg3 region of the PFC and the CA1 region of the HIPP, MDMA treatment increased NET binding in the CA1, CA2 and CA3 regions of the HIPP, and increased basal NE levels in the PFC and the NAC, as compared to SAL-treated controls.
While the postnatal culling procedure used in this study was effective in normalizing the weight disparity in MDMA- versus SAL-treated litters, this measure did not control for the potential effects of MDMA-induced anorexia in the dam during gestation. This is of particular concern, as gestational malnutrition has been shown to produce both acute and long-term changes in the central nervous system [5,13]. Importantly, however, the effects of early undernutrition on the brain catecholamine systems have been shown to be transient, with normal levels restored by nutritional rehabilitation [56]. The process of reducing the number of pups in MDMA-exposed litters is intended to have a similar effect with regard to nutritional recovery, and should therefore ameliorate any effects of maternal undernutrition on the brain NE system. It is also important to note that the neurochemical and morphological changes observed in this study are not consistent with those reported in neonatal malnutrition paradigms [8,29,43], or in comparable studies which utilized pair-fed pregnant dams [7,9,48,49]. Together, this suggests that the neurochemical sequelae observed in MDMA-treated offspring are directly related to the pharmacologic action of the drug.
Variance associated with differences in maternal care as a result of drug administration was not investigated in the current study and is another potential confounding factor in this study. It has been shown, for example, that neonatal exposure to MDMA (P1–4) results in increased activity and ultrasonic vocalizations, behaviors that can specifically affect maternal responsivity [60]. While maternal behavior has not been specifically examined following prenatal MDMA exposure, comparable studies in cocaine-exposed rats have failed to demonstrate differences in pup-induced maternal behavior [19], nesting, or pup retrieval [15]. In addition, several studies have sought to determine whether litter-specific effects are observed following neonatal MDMA exposure. These studies demonstrated that the behavioral deficits observed in MDMA-exposed rat pups in which a “split-litter” design was employed (one offspring per litter, per dependent measure), could be replicated using a “within-litter” design, in which offspring from a single litter were used for each dependent measure [6,58]. While this study sought to determine the direct effects of MDMA on the brain, in reality, the fetus is inextricably linked to the mother, and is thus susceptible to the potential influence of a number of maternal factors. The potential consequences of issues such as maternal malnutrition and impoverished neonatal care may, in fact, have significant consequences on brain growth and development in human infants. Further investigation of the combined effects of MDMA’s pharmacologic action and its effects on maternal factors is therefore warranted, and would be of considerable value to the field.
Transient increases in catecholamines have been shown to occur in both the PFC and the NAC during initial exposure to a novel enclosure [39]. If basal levels of NE are already augmented in MDMA-exposed offspring as suggested by this study, novelty-induced increases in NE release could alter attentional processing. In the rat, an increased attentional response to novelty can manifest as increased exploratory activity. This is consistent with our previous report which found that rats exposed prenatally to MDMA exhibited heightened responsivity to a novel cage environment as juveniles (P21) [24], which persists into adulthood (P61–P62) [51]. When these behavioral alterations are considered in the context of the neuroanatomical and neurochemical changes observed in this study, they suggest that prenatal MDMA-induced changes in the NE system may affect attentional processing, contributing to a reduced habituation to novelty in these animals.
We have shown that prenatal exposure to MDMA results in an increase in NET binding in the CA1, CA2 and CA3 regions of the HIPP. This increase could be indicative of enhanced NE sprouting in this region, or may represent a form of compensatory upregulation following the sustained elevation of synaptic NE during the MDMA treatment regimen. Noradrenergic fiber density was increased in the CA1 region of the HIPP, consistent with the former explanation, however fiber density was comparable to controls in the CA2 and CA3 region, suggesting that the relative innervation of these regions is comparable in both groups, but NET is upregulated in MDMA-treated subjects. We have previously reported findings consistent with the latter hypothesis in the DA system [27]. In this study, we demonstrated that MDMA increases Slc6a3 (DAT) gene expression in primary mesencephalic DA neurons in vitro, and that this was not a result of an increase in neurite density. Because this phenomenon is observed in both the NE and the DA system, it is possible that transporter upregulation could be important in mediating the various downstream neurochemical and morphological abnormalities observed in these systems following prenatal MDMA exposure. Thus, a key goal of future studies will be to investigate the relationship between upregulation of catecholaminergic transporters and the pathological development of these systems.
The primary purpose of the NET is to regulate signaling by rapidly clearing synaptic NE. It is therefore tempting to conclude that the increase in NET expression observed in the HIPP would result in greater reuptake and attenuated postsynaptic NE signaling. However, without additional information, it is difficult to speculate on the actual physiological effects of increased NET expression. Receptor binding autoradiography does not, for example, distinguish between internalization versus cell surface expression. This limitation may be particularly important as amphetamine has been shown to reduce cell surface expression of human DAT [42]. (The sequence motif targeted in DAT internalization is conserved across SLC6 carriers, and is functional in the homologous NET [17,47]. If MDMA acts via a similar mechanism, it is possible that surface NET expression is actually reduced in this region. Additional experiments focused on post-synaptic receptor expression are planned, and will provide significant insight into the relationship between prenatal MDMA and its long-term effects on NE signaling. Regardless, we hypothesized that the deficits in attention and working memory we observed in MDMA-exposed animals [24,51] were the result of abnormal NE signaling in the HIPP. The current data provide support for this hypothesis, demonstrating that prenatal MDMA affects NE innervation and NET expression in this region. Future studies will examine this phenomenon in greater detail, and will seek to determine the mechanism underlying MDMA-mediated alterations in NE signaling.
Norepinephrine is critical in mediating communication between the CA1 region of the HIPP and the entorhinal cortex [34]. Aberrant NE signaling in the CA1 region in MDMA-exposed animals, as suggested by the changes in innervation and NET binding observed in this study, may compromise communication with this region, resulting in deficits in spatial learning and working memory. This is consistent with our recently reported behavioral data, which found that adult rats that had been exposed prenatally to MDMA had difficulty developing effective strategies to locate a hidden platform which had previously been associated with a hanging cue in the Morris Water Maze (MWM) [51]. In future studies, it will be necessary to determine whether NE signaling is in fact altered in MDMA-exposed animals during this task, and to investigate whether performance can be improved with clinically relevant drugs, such as methylphenidate (Ritalin®), or the NET specific inhibitor atomoxetine (Strattera®).
Although we hypothesized that NE innervation and signaling would be enhanced in the dentate gyrus following prenatal MDMA exposure, there were no discernable differences in DBHir fiber density or NET binding. The reason that changes in NE signaling are apparent in the hippocampus proper, but not the dentate may lie in the difference in timing of the development and NE innervation of these structures. The pyramidal cells of CA1, CA2 and CA3 develop prenatally, while the majority of granule cells of the dentate gyrus are formed postnatally [4]. Similarly, NE innervation of the hippocampus proper begins on E18, while innervation of the dentate becomes apparent on P4 [28]. It is therefore possible that only the HIPP structures that are actively being innervated during MDMA exposure are affected.
While research in the field of drug addiction has primarily focused on the brain DA system, it is known that the NE system is important in mediating some aspects of drug abuse, including reward saliency and stress-induced relapse [46,53]. We have found that prenatal MDMA increases basal NE levels in both the PFC and the NAC, areas which are critical in the brain’s reward circuitry [54]. The fact that these differences are observed at P21, a time equivalent to early adolescence in humans, is especially relevant, as many behaviors that precede experimentation and compulsive substance abuse, including novelty-seeking and impulsivity, are most prevalent during this period of development [10,33,44,59]. Future studies examining this and other reward-motivated behaviors during adolescence are warranted.
We have previously demonstrated that prenatal MDMA dramatically increases DA innervation of the PFC, and that this is not accompanied by an increase in DA neurons in the ventral tegmental area (VTA) [24]. The current study found a comparable effect in the NE system, demonstrating that prenatal exposure to MDMA increases NE innervation of the PFC without increasing the number of NE neurons in the LC. Interestingly, although both DA and NE fiber density was increased in this region, DA levels were comparable to controls [24], while NE levels in MDMA-treated pups were significantly greater than controls. Studies using pharmacological and/or genetic manipulation of catecholaminergic transporters have consistently demonstrated that extracellular DA clearance in the PFC is mediated, almost exclusively, by the NET [11,32,62]. In addition, a recent study in the Rictor Null mouse, a mutant characterized by increased NET expression in the cortex, found that NE levels were increased, while DA levels were decreased in the cortex [45]. Presumably, enhanced NET function in these mice increases presynaptic DA uptake, which is subsequently converted to NE before being re-released. It is disappointing that we did not observe any significant differences in NET binding in the PFC, however due to the previously discussed limitations of the techniques used in this study, it is still possible that a similar mechanism may underlie the changes observed in DA and NE in this region. The NE system has also been shown to be particularly important in maintaining and protecting DA circuitry. Thus, it is possible that MDMA-mediated changes in NE innervation may actually facilitate the previously documented DA hyperinnervation of the PFC. A similar phenomenon has been demonstrated in the tottering (tg/tg) mouse mutant, which is characterized by NE hyperinnervation of the forebrain. In these mice, DA terminals in the STR were preserved following treatment with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a DA neurotoxin, to a greater degree than in WT mice [22]. While there is no evidence to suggest that MDMA is neurotoxic to DA neurons, the same protective factors derived from NE nerve terminals in the tottering/MPTP paradigm may promote growth and hyperinnervation of the DA system following prenatal MDMA. Regardless as to whether the NE system is involved in this particular phenomenon, the DA and NE systems co-innervate a number of brain nuclei, where they interact to coordinate both motor and cognitive tasks [14,30,32,62]. Future studies are therefore needed to investigate the nature of the interaction between these two systems following prenatal exposure to MDMA.
5. Conclusions
Prenatal exposure to MDMA results in changes in the structure and function of the NE system in rats on P21, as characterized by hyperinnervation of the Cg3 region of the PFC and the CA1 region of the HIPP, increased NET binding in the CA1, CA2, and CA3 region of the HIPP, and elevated basal levels of NE in the PFC and the NAC. This is the first study to specifically address the consequences of prenatal MDMA on the developing NE system. Together, these data provide insight into the neuroanatomical and neurochemical profile that may underlie behavioral deficits in individuals exposed prenatally to MDMA.
Table 1.
Basal levels of NE, MHPG, and NE turnover on P21 following prenatal exposure to MDMA
| Prefrontal Cortex | Striatum | Nucleus Accumbens | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NE | MHPG | MHPG/NE | NE | MHPG | MHPG/NE | NE | MHPG | MHPG/NE | |
| MDMA | 2.16 ± 0.07 * | 0.59 ± 0.07 | 0.29 ± 0.04 | 1.00 ± 0.10 | 1.07 ± 0.03 | 1.22 ± 0.13 | 6.34 ± 0.56 ** | #### | #### |
| Saline | 1.88 ± 0.08 | 0.49 ± 0.07 | 0.27 ± 0.04 | 0.78 ± 0.11 | 0.98 ± 0.03 | 1.09 ± 0.13 | 3.53 ± 0.59 | #### | #### |
| Hippocampus | Brainstem | |||||
|---|---|---|---|---|---|---|
| NE | MHPG | MHPG/NE | NE | MHPG | MHPG/NE | |
| MDMA | 4.00 ± 0.31 | #### | #### | 17.36 ± 1.34 | 0.69 ± 0.25 | 0.04 ± 0.01 |
| Saline | 3.80 ± 0.33 | #### | #### | 14.46 ± 1.41 | 0.89 ± 0.26 | 0.06 ± 0.01 |
Values represent nanograms per milligram of protein (mean ± S.E.M.)
p<0.05
p<0.01
Highlights.
Prenatal exposure to MDMA increases noradrenergic innervation of the forebrain.
NET binding and NE neurochemistry are also altered by prenatal MDMA exposure.
Changes in the NE system may underlie previously reported behavioral abnormalities.
Acknowledgments
This study was funded by the National Institute on Drug Abuse: 1R21DA019261 (JWL) & 1R01DA017399 (JWL). MDMA was provided by the NIDA Research Drug Supply System.
Abbreviations
- 5-HT
serotonin
- AALAC
Association for Accreditation of Laboratory Animal Care
- BS
brainstem
- Cg3
prelimbic cortex
- DA
dopamine
- DBHir
dopamine beta hydroxylase immunoreactive
- E
embryonic day
- HPLC
high performance liquid chromatography
- LC
locus coeruleus
- MDMA
3,4-methylenedioxymethamphetamine
- MHPG
3-methoxy, 4-hydroxyphenylglycol
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NAC
nucleus accumbens
- NET
norepinephrine transporter
- P
postnatal day
- PFC
prefrontal cortex
- SAL
saline
- STR
striatum
- WCST
Wisconsin Card Sorting Task
- MWM
Morris Water Maze
Footnotes
Conflict of Interest Statement: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angel I, Schoemaker H, Arbilla S, Galzin AM, Berry C, Niddam R, Pimoule C, Sevrin M, Wick A, Langer SZ. SL-84.0418 - A NOVEL, POTENT AND SELECTIVE ALPHA-2 ADRENOCEPTOR ANTAGONIST - INVITRO PHARMACOLOGICAL PROFILE. J Pharmacol Exp Ther. 1992;263:1327–1333. [PubMed] [Google Scholar]
- 2.Antonopoulos J, Latsari M, Dori I, Chiotelli M, Parnavelas JG, Dinopoulos A. Noradrenergic innervation of the developing and mature septal area of the rat. J Comp Neurol. 2004;476:80–90. doi: 10.1002/cne.20205. [DOI] [PubMed] [Google Scholar]
- 3.Battaglia G, Brooks BP, Kulsakdinun C, De Souza EB. Pharmacologic profile of MDMA (3,4-methylenedioxymethamphetamine) at various brain recognition sites. Eur J Pharmacol. 1988;149:159–63. doi: 10.1016/0014-2999(88)90056-8. [DOI] [PubMed] [Google Scholar]
- 4.Bayer SA, Altman J. Hippocampal development in the rat: cytogenesis and morphogenesis examined with autoradiography and low-level X-irradiation. J Comp Neurol. 1974;158:55–79. doi: 10.1002/cne.901580105. [DOI] [PubMed] [Google Scholar]
- 5.Brandt I. Brain growth, fetal malnutrition, and clinical consequences. J Perinat Med. 1981;9:3–26. [PubMed] [Google Scholar]
- 6.Broening HW, Morford LL, Inman-Wood SL, Fukumura M, Vorhees CV. 3,4-methylenedioxymethamphetamine (ecstasy)-induced learning and memory impairments depend on the age of exposure during early development. J Neurosci. 2001;21:3228–35. doi: 10.1523/JNEUROSCI.21-09-03228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabrera TM, Levy AD, Li Q, van de Kar LD, Battaglia G. Prenatal methamphetamine attenuates serotonin mediated renin secretion in male and female rat progeny: evidence for selective long-term dysfunction of serotonin pathways in brain. Synapse. 1993;15:198–208. doi: 10.1002/syn.890150305. [DOI] [PubMed] [Google Scholar]
- 8.Chen JC, Turiak G, Galler J, Volicer L. Postnatal changes of brain monoamine levels in prenatally malnourished and control rats. Int J Dev Neurosci. 1997;15:257–63. doi: 10.1016/s0736-5748(96)00121-9. [DOI] [PubMed] [Google Scholar]
- 9.Church MW, Rauch HC. Prenatal cocaine exposure in the laboratory mouse: effects on maternal water consumption and offspring outcome. Neurotoxicol Teratol. 1992;14:313–9. doi: 10.1016/0892-0362(92)90037-b. [DOI] [PubMed] [Google Scholar]
- 10.Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. Novelty-seeking in rats--biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34:136–45. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- 11.Devoto P, Flore G, Pani L, Gessa GL. Evidence for co-release of noradrenaline and dopamine from noradrenergic neurons in the cerebral cortex. Mol Psychiatry. 2001;6:657–64. doi: 10.1038/sj.mp.4000904. [DOI] [PubMed] [Google Scholar]
- 12.Dias R, Honey RC. Involvement of the rat medial prefrontal cortex in novelty detection. Behav Neurosci. 2002;116:498–503. doi: 10.1037//0735-7044.116.3.498. [DOI] [PubMed] [Google Scholar]
- 13.Dobbing J. Vulnerable periods of brain development. lipids, malnutrition & the developing brain, Ciba Found Symp. 1971:9–29. [PubMed] [Google Scholar]
- 14.Guiard BP, El Mansari M, Blier P. Cross-talk between dopaminergic and noradrenergic systems in the rat ventral tegmental area, locus ceruleus, and dorsal hippocampus. Mol Pharmacol. 2008;74:1463–75. doi: 10.1124/mol.108.048033. [DOI] [PubMed] [Google Scholar]
- 15.Heyser CJ, Molina VA, Spear LP. A fostering study of the effects of prenatal cocaine exposure: I. Maternal behaviors. Neurotoxicol Teratol. 1992;14:415–21. doi: 10.1016/0892-0362(92)90052-c. [DOI] [PubMed] [Google Scholar]
- 16.Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–8. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- 17.Holton KL, Loder MK, Melikian HE. Nonclassical, distinct endocytic signals dictate constitutive and PKC-regulated neurotransmitter transporter internalization. Nat Neurosci. 2005;8:881–8. doi: 10.1038/nn1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkins TA, Amin E, Pearce JM, Brown MW, Aggleton JP. Novel spatial arrangements of familiar visual stimuli promote activity in the rat hippocampal formation but not the parahippocampal cortices: a c-fos expression study. Neuroscience. 2004;124:43–52. doi: 10.1016/j.neuroscience.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 19.Johns JM, McMurray MS, Hofler VE, Jarrett TM, Middleton CL, Elliott DL, Mirza R, Haslup A, Elliott JC, Walker CH. Cocaine disrupts pup-induced maternal behavior in juvenile and adult rats. Neurotoxicol Teratol. 2007;29:634–41. doi: 10.1016/j.ntt.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnston L, O’Malley P, Bachman J, Shulenberg J. Overview of Key Findings. NIH Publication No 08-6418. 2008. Monitoring the Future: National Results on Adolescent Drug Abuse. [Google Scholar]
- 21.Kelly PA, Ritchie IM, Quate L, McBean DE, Olverman HJ. Functional consequences of perinatal exposure to 3,4-methylenedioxymethamphetamine in rat brain. Br J Pharmacol. 2002;137:963–70. doi: 10.1038/sj.bjp.0704961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilbourn MR, Sherman P, Abbott LC. Reduced MPTP neurotoxicity in striatum of the mutant mouse tottering. Synapse. 1998;30:205–10. doi: 10.1002/(SICI)1098-2396(199810)30:2<205::AID-SYN10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Kitchigina V, Vankov A, Harley C, Sara SJ. Novelty-elicited, noradrenaline-dependent enhancement of excitability in the dentate gyrus. Eur J Neurosci. 1997;9:41–7. doi: 10.1111/j.1460-9568.1997.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 24.Koprich JB, Chen EY, Kanaan NM, Campbell NG, Kordower JH, Lipton JW. Prenatal 3,4-methylenedioxymethamphetamine (ecstasy) alters exploratory behavior, reduces monoamine metabolism, and increases forebrain tyrosine hydroxylase fiber density of juvenile rats. Neurotoxicol Teratol. 2003;25:509–17. doi: 10.1016/s0892-0362(03)00091-6. [DOI] [PubMed] [Google Scholar]
- 25.Lee I, Kesner RP. Time-dependent relationship between the dorsal hippocampus and the prefrontal cortex in spatial memory. J Neurosci. 2003;23:1517–23. doi: 10.1523/JNEUROSCI.23-04-01517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levitt P, Moore RY. Development of the noradrenergic innervation of neocortex. Brain Res. 1979;162:243–59. doi: 10.1016/0006-8993(79)90287-7. [DOI] [PubMed] [Google Scholar]
- 27.Lipton JW, Tolod EG, Thompson VB, Pei L, Paumier KL, Terpstra BT, Lynch KA, Collier TJ, Sortwell CE. 3,4-Methylenedioxy-N-methamphetamine (ecstasy) promotes the survival of fetal dopamine neurons in culture. Neuropharmacology. 2008;55:851–9. doi: 10.1016/j.neuropharm.2008.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loy R, Moore RY. Ontogeny of the noradrenergic innervation of the rat hippocampal formation. Anat Embryol (Berl) 1979;157:243–53. doi: 10.1007/BF00304992. [DOI] [PubMed] [Google Scholar]
- 29.Marichich ES, Molina VA, Orsingher OA. Persistent changes in central catecholaminergic system after recovery of perinatally undernourished rats. J Nutr. 1979;109:1045–50. doi: 10.1093/jn/109.6.1045. [DOI] [PubMed] [Google Scholar]
- 30.Mavridis M, Degryse AD, Lategan AJ, Marien MR, Colpaert FC. Effects of locus coeruleus lesions on parkinsonian signs, striatal dopamine and substantia nigra cell loss after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in monkeys: a possible role for the locus coeruleus in the progression of Parkinson’s disease. Neuroscience. 1991;41:507–23. doi: 10.1016/0306-4522(91)90345-o. [DOI] [PubMed] [Google Scholar]
- 31.Mordenti J, Chappell W. The use of interspecies scaling in toxicokinetics. In: Yacobi A, Skelly JP, Batra VK, editors. Toxicokinetics and New Drug Development. Pergamon Press; New York: 1989. pp. 42–96. [Google Scholar]
- 32.Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci. 2002;22:389–95. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noguchi T, Yoshida Y, Chiba S. Effects of psychological stress on monoamine systems in subregions of the frontal cortex and nucleus accumbens of the rat. Brain Res. 2001;916:91–100. doi: 10.1016/s0006-8993(01)02868-2. [DOI] [PubMed] [Google Scholar]
- 34.Otmakhova NA, Lewey J, Asrican B, Lisman JE. Inhibition of perforant path input to the CA1 region by serotonin and noradrenaline. J Neurophysiol. 2005;94:1413–22. doi: 10.1152/jn.00217.2005. [DOI] [PubMed] [Google Scholar]
- 35.Palacios JM, Hoyer D, Cortes R. ALPHA-1-ADRENOCEPTORS IN THE MAMMALIAN BRAIN - SIMILAR PHARMACOLOGY BUT DIFFERENT DISTRIBUTION IN RODENTS AND PRIMATES. Brain Res. 1987;419:65–75. doi: 10.1016/0006-8993(87)90569-5. [DOI] [PubMed] [Google Scholar]
- 36.Palacios JM, Kuhar MJ. BETA-ADRENERGIC-RECEPTOR LOCALIZATION IN RAT-BRAIN BY LIGHT MICROSCOPIC AUTORADIOGRAPHY. Neurochem Int. 1982;4:473–490. doi: 10.1016/0197-0186(82)90036-5. [DOI] [PubMed] [Google Scholar]
- 37.Paxinos G, Watson C, Pennisi M, Topple A. Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J Neurosci Methods. 1985;13:139–43. doi: 10.1016/0165-0270(85)90026-3. [DOI] [PubMed] [Google Scholar]
- 38.Rainbow TC, Parsons B, Wolfe BB. QUANTITATIVE AUTORADIOGRAPHY OF BETA-1-ADRENERGIC AND BETA-2-ADRENERGIC RECEPTORS IN RAT-BRAIN. Proceedings of the National Academy of Sciences of the United States of America-Biological Sciences; 1984. pp. 1585–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rebec G, Grabner C, Johnson M, Pierce R, Bardo M. Transient increase in catecholaminergic activity in medial prefrontal cortex and nucleus accumbes shell during novelty. Neuroscience. 1996;76:707–714. doi: 10.1016/s0306-4522(96)00382-x. [DOI] [PubMed] [Google Scholar]
- 40.Rosario LA, Abercrombie ED. Individual differences in behavioral reactivity: correlation with stress-induced norepinephrine efflux in the hippocampus of Sprague-Dawley rats. Brain Res Bull. 1999;48:595–602. doi: 10.1016/s0361-9230(99)00040-4. [DOI] [PubMed] [Google Scholar]
- 41.SAMHSA; D.o.H.a.H. Services. National Survey on Drug Use and Health. 2009. [Google Scholar]
- 42.Saunders C, Ferrer JV, Shi L, Chen J, Merrill G, Lamb ME, Leeb-Lundberg LM, Carvelli L, Javitch JA, Galli A. Amphetamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism. Proc Natl Acad Sci U S A. 2000;97:6850–5. doi: 10.1073/pnas.110035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seidler FJ, Bell JM, Slotkin TA. Undernutrition and overnutrition in the neonatal rat: long-term effects on noradrenergic pathways in brain regions. Pediatr Res. 1990;27:191–7. doi: 10.1203/00006450-199002000-00021. [DOI] [PubMed] [Google Scholar]
- 44.Shiraishi H, Suzuki A, Fukasawa T, Aoshima T, Ujiie Y, Ishii G, Otani K. Monoamine oxidase A gene promoter polymorphism affects novelty seeking and reward dependence in healthy study participants. Psychiatr Genet. 2006;16:55–8. doi: 10.1097/01.ypg.0000199447.62044.ef. [DOI] [PubMed] [Google Scholar]
- 45.Siuta MA, Robertson SD, Kocalis H, Saunders C, Gresch PJ, Khatri V, Shiota C, Kennedy JP, Lindsley CW, Daws LC, et al. Dysregulation of the norepinephrine transporter sustains cortical hypodopaminergia and schizophrenia-like behaviors in neuronal rictor null mice. PLoS Biol. 2010;8:e1000393. doi: 10.1371/journal.pbio.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith RJ, Aston-Jones G. Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct. 2008;213:43–61. doi: 10.1007/s00429-008-0191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sorkina T, Hoover BR, Zahniser NR, Sorkin A. Constitutive and protein kinase C-induced internalization of the dopamine transporter is mediated by a clathrin-dependent mechanism. Traffic. 2005;6:157–70. doi: 10.1111/j.1600-0854.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 48.Spear LP, Kirstein CL, Bell J, Yoottanasumpun V, Greenbaum R, O’Shea J, Hoffmann H, Spear NE. Effects of prenatal cocaine exposure on behavior during the early postnatal period. Neurotoxicol Teratol. 1989;11:57–63. doi: 10.1016/0892-0362(89)90086-x. [DOI] [PubMed] [Google Scholar]
- 49.Spear LP, Kirstein CL, Frambes NA. Cocaine effects on the developing central nervous system: behavioral, psychopharmacological, and neurochemical studies. Ann N Y Acad Sci. 1989;562:290–307. doi: 10.1111/j.1749-6632.1989.tb21027.x. [DOI] [PubMed] [Google Scholar]
- 50.Strange BA, Fletcher PC, Henson RN, Friston KJ, Dolan RJ. Segregating the functions of human hippocampus. Proc Natl Acad Sci U S A. 1999;96:4034–9. doi: 10.1073/pnas.96.7.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thompson VB, Heiman J, Chambers JB, Benoit SC, Buesing WR, Norman MK, Norman AB, Lipton JW. Long-term behavioral consequences of prenatal MDMA exposure. Physiol Behav. 2009;96:593–601. doi: 10.1016/j.physbeh.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uprichard DC, Bechtel WD, Rouot BM, Snyder SH. MULTIPLE APPARENT ALPHA-NORADRENERGIC RECEPTOR-BINDING SITES IN RAT-BRAIN - EFFECT OF 6-HYDROXYDOPAMINE. Mol Pharmacol. 1979;16:47–60. [PubMed] [Google Scholar]
- 53.Ventura R, Latagliata EC, Morrone C, La Mela I, Puglisi-Allegra S. Prefrontal norepinephrine determines attribution of “high” motivational salience. PLoS One. 2008;3:e3044. doi: 10.1371/journal.pone.0003044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ventura R, Morrone C, Puglisi-Allegra S. Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward- and aversion-related stimuli. Proc Natl Acad Sci U S A. 2007;104:5181–6. doi: 10.1073/pnas.0610178104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- 56.Wiggins RC, Fuller GN, Seifert WE, Butler IJ, Gottesfeld Z. Catecholamines in rat brain following postnatal undernutrition and nutritional rehabilitation. J Neurosci Res. 1982;8:651–6. doi: 10.1002/jnr.490080409. [DOI] [PubMed] [Google Scholar]
- 57.Williams M, Morford L, Wood S, Rock S, AEM, Fukumura M, Wallace T, Broening H, Moran M, Vorhees C. Developmental 3,4-methylenedioxymethamphetamine (MDMA) impairs sequential and spatial but not cued learning independent of growth, litter effects or injection stress. Brain Res. 2003;968:89–101. doi: 10.1016/s0006-8993(02)04278-6. [DOI] [PubMed] [Google Scholar]
- 58.Williams MT, Morford LL, Wood SL, Rock SL, McCrea AE, Fukumura M, Wallace TL, Broening HW, Moran MS, Vorhees CV. Developmental 3,4-methylenedioxymethamphetamine (MDMA) impairs sequential and spatial but not cued learning independent of growth, litter effects or injection stress. Brain Res. 2003;968:89–101. doi: 10.1016/s0006-8993(02)04278-6. [DOI] [PubMed] [Google Scholar]
- 59.Wills TA, Vaccaro D, McNamara G. Novelty seeking, risk taking, and related constructs as predictors of adolescent substance use: an application of Cloninger’s theory. J Subst Abuse. 1994;6:1–20. doi: 10.1016/s0899-3289(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 60.Winslow JT, Insel TR. Serotonergic modulation of rat pup ultrasonic vocal development: studies with 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1990;254:212–20. [PubMed] [Google Scholar]
- 61.Winstock A, Griffiths P, Stewart D. Drugs and the dance music scene: a survey of current drug use patterns among a sample of dance music enthusiasts in the UK. Drug Alcohol Depend. 2001;64:9–17. doi: 10.1016/s0376-8716(00)00215-5. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto BK, Novotney S. Regulation of extracellular dopamine by the norepinephrine transporter. J Neurochem. 1998;71:274–80. doi: 10.1046/j.1471-4159.1998.71010274.x. [DOI] [PubMed] [Google Scholar]