Abstract
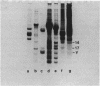
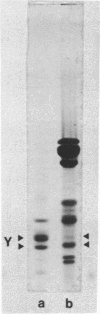
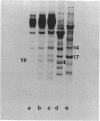
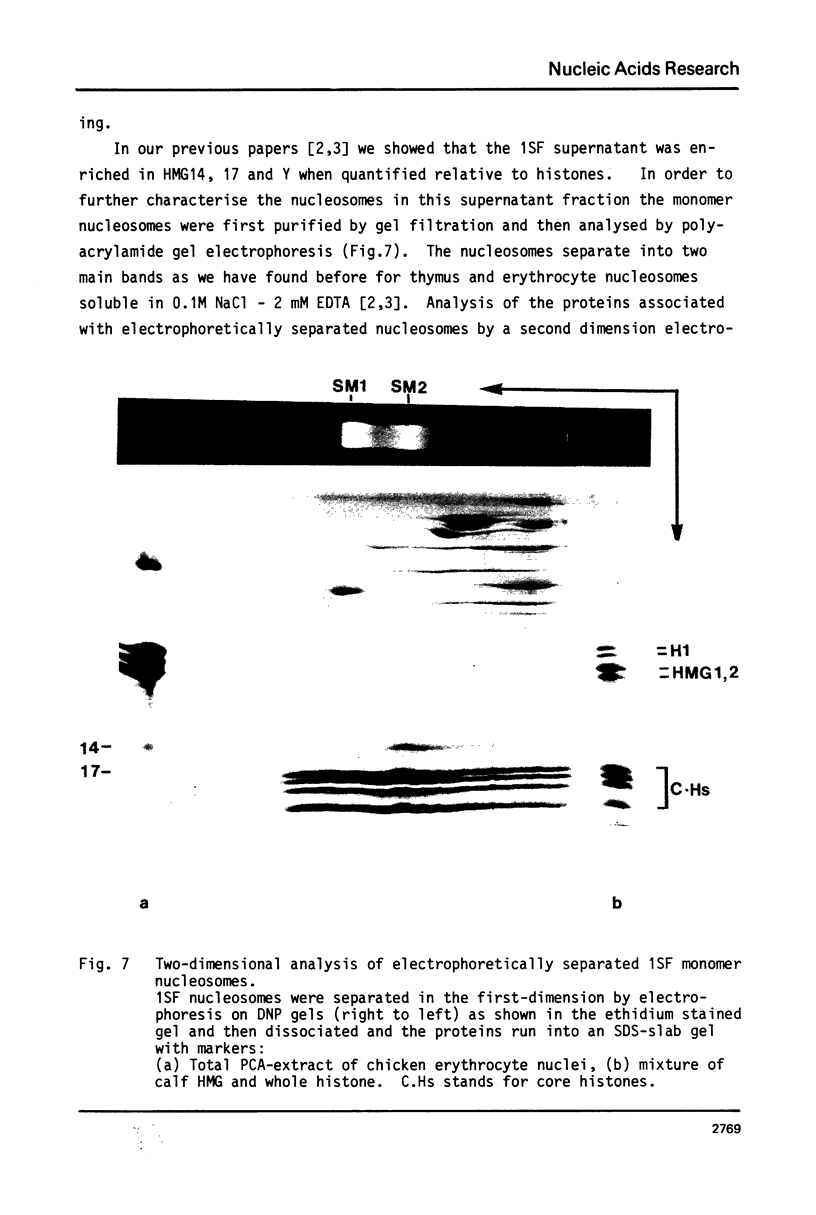
Nucleosomes released from oviduct nuclei during brief micrococcal nuclease digestions are enriched in transcribed sequences (bloom K.S. and Anderson, J.N. (1978) Cell, 15, 141-150). Such nucleosomes released into this 1Sf supernatant fraction are enriched in proteins HMG14, 17 and a third lower molecular weight protein which we show in this paper to be related to HMG14 and 17. This protein, which we call HMGY, runs as a doublet on polyacrylamide gels. A similar doublet is present in smaller quantities in chicken erythrocyte nuclei. Monomer nucleosomes in the 1SF supernatant have been separated by polyacrylamide gel electrophoresis into two main bands. The slower moving band contains the three HMG proteins HMG14, 17 and Y but lacks histone H1.
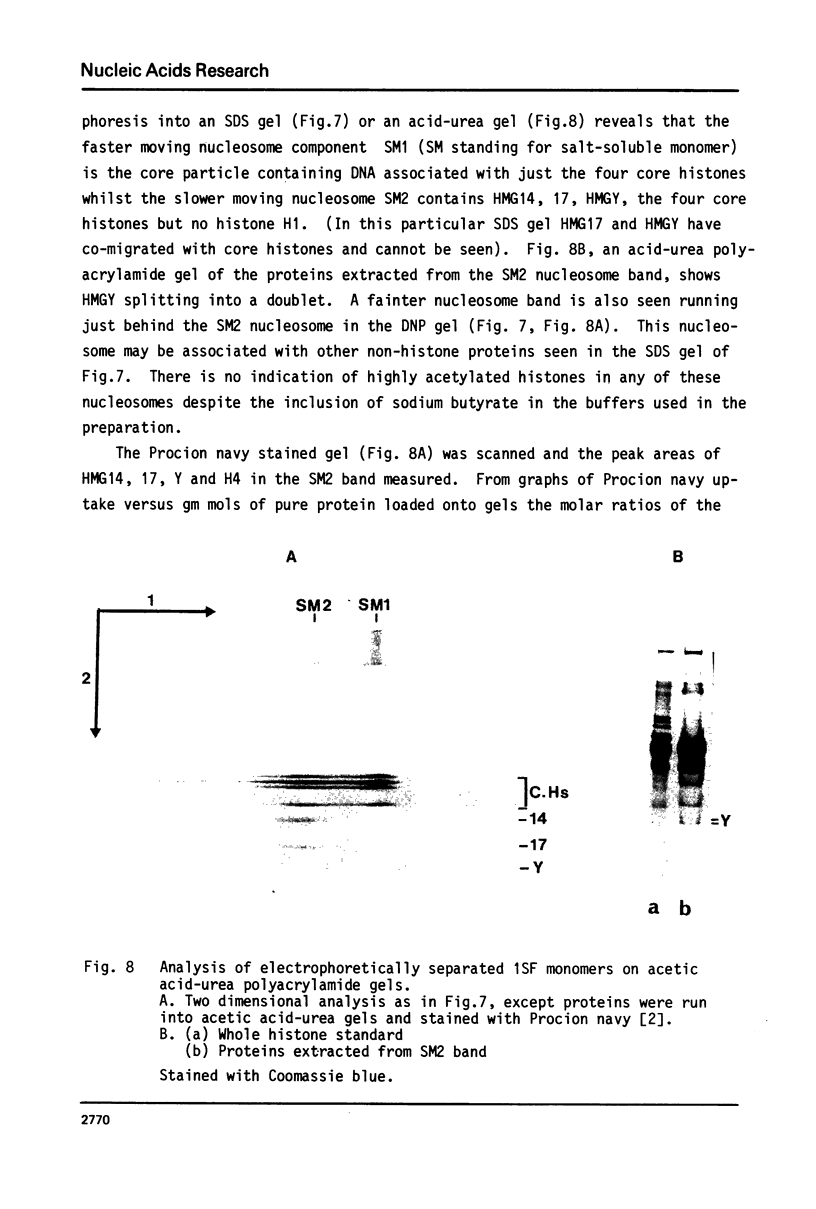
Full text
PDF



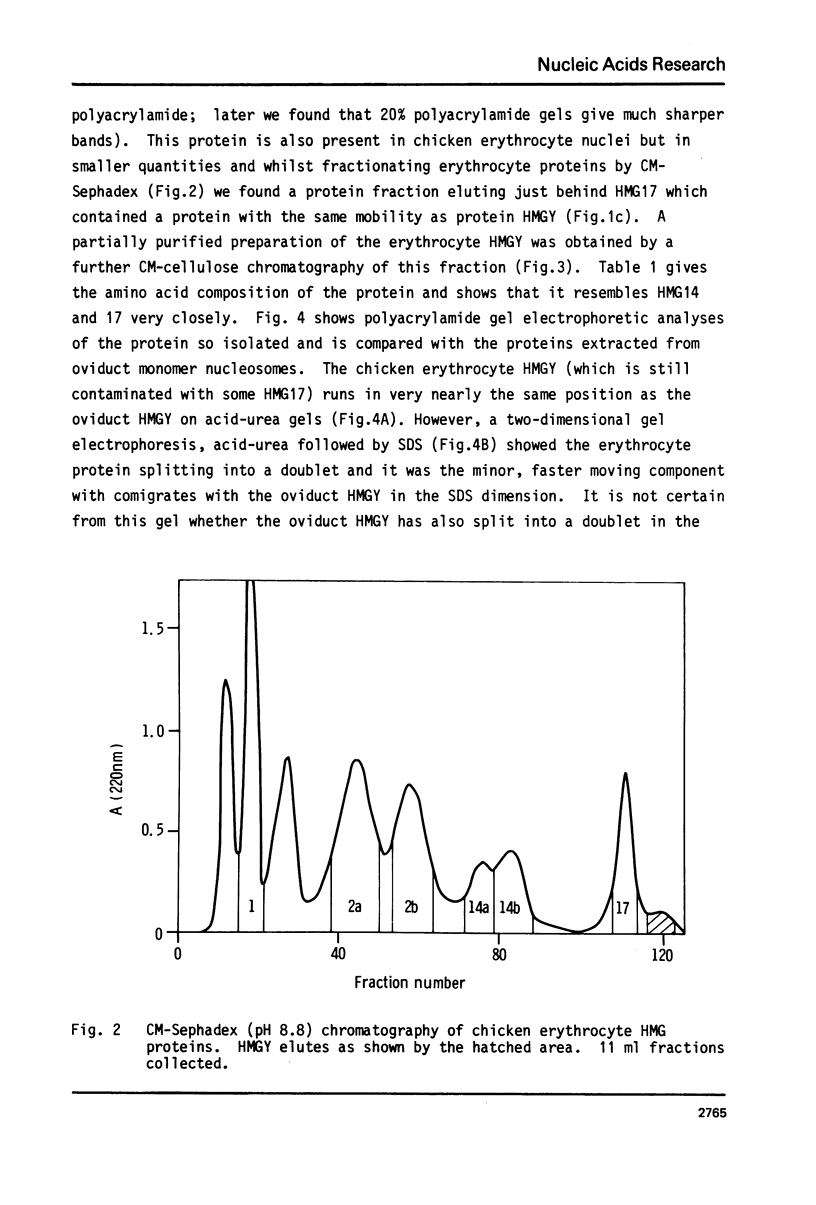
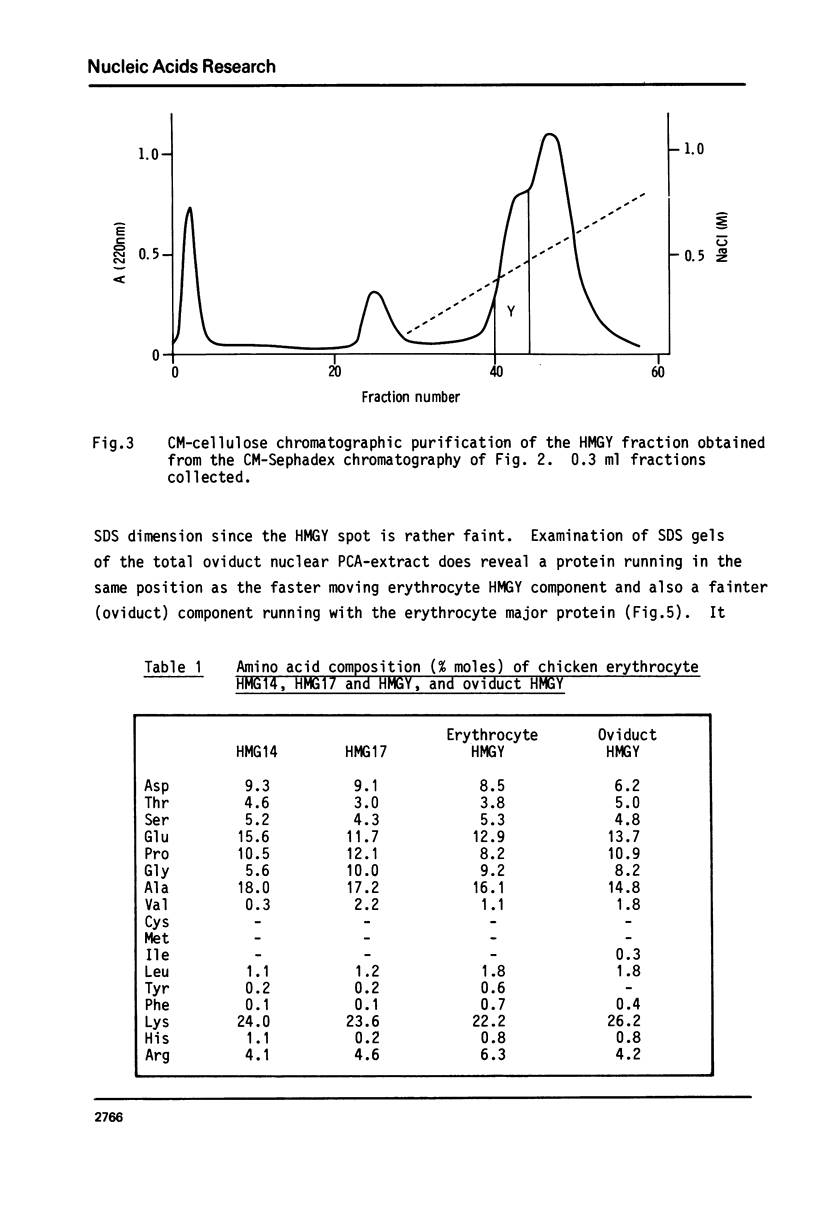
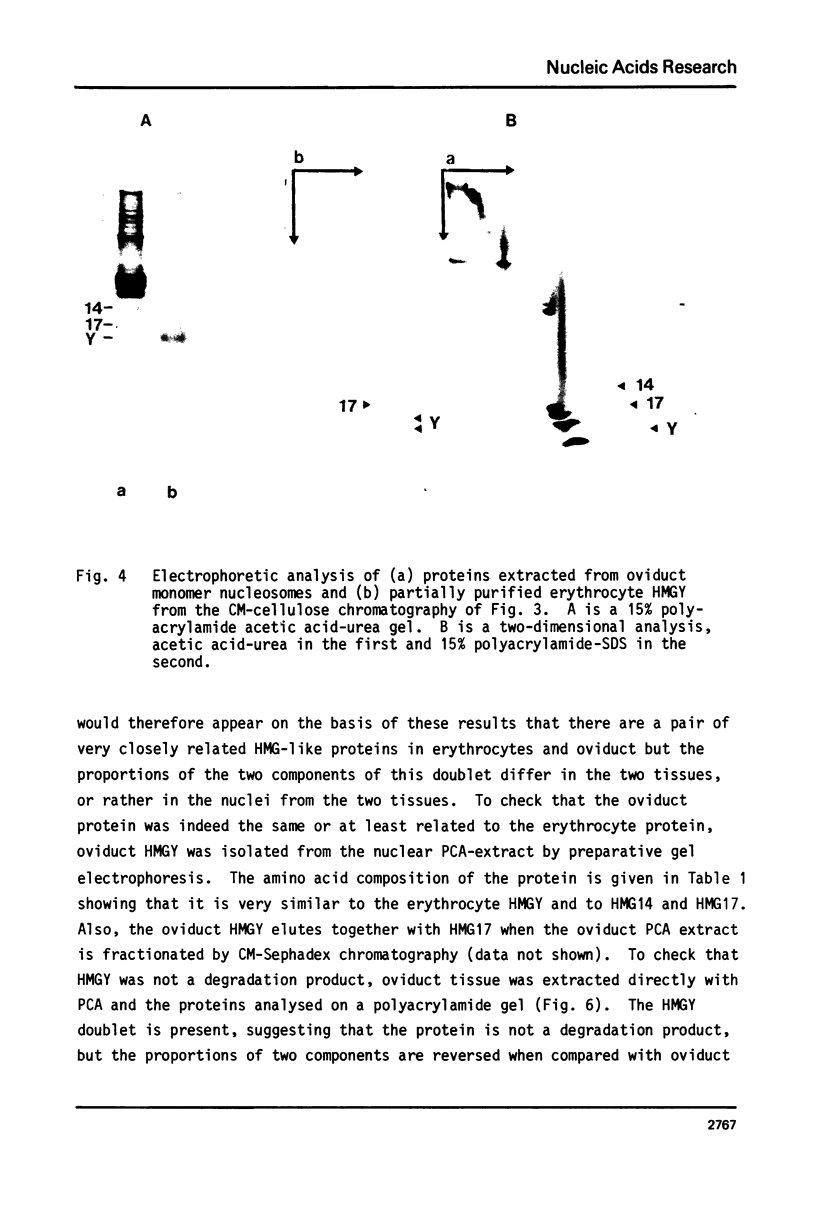
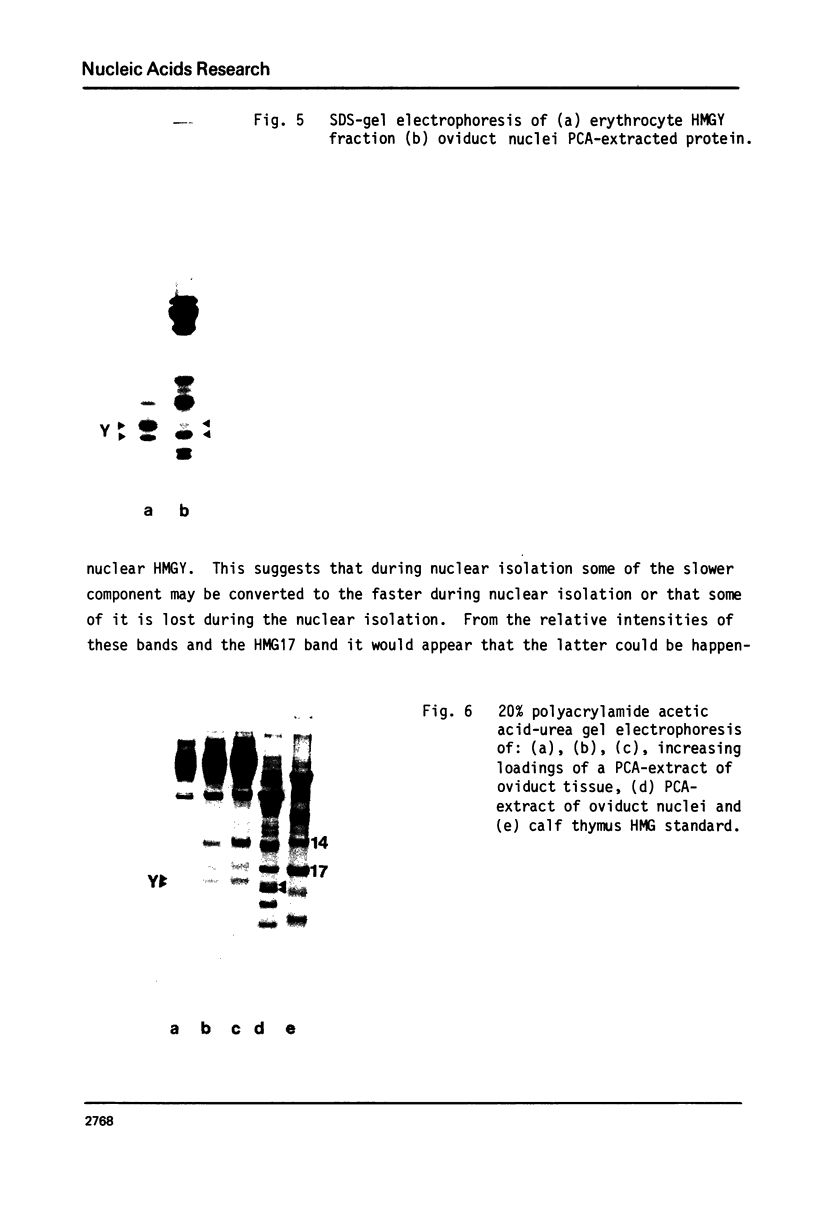





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albanese I., Weintraub H. Electrophoretic separation of a class of nucleosomes enriched in HMG 14 and 17 and actively transcribed globin genes. Nucleic Acids Res. 1980 Jun 25;8(12):2787–2805. doi: 10.1093/nar/8.12.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright S. C., Wiseman J. M., Lange R. A., Garrard W. T. Subunit structures of different electrophoretic forms of nucleosomes. J Biol Chem. 1980 Apr 25;255(8):3673–3684. [PubMed] [Google Scholar]
- Bakayev V. V., Schmatchenko V. V., Georgiev G. P. Subnucleosome particles containing high mobility group proteins HMG-E and HMG-G originate from transcriptionally active chromatin. Nucleic Acids Res. 1979 Nov 24;7(6):1525–1540. doi: 10.1093/nar/7.6.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom K. S., Anderson J. N. Fractionation of hen oviduct chromatin into transcriptionally active and inactive regions after selective micrococcal nuclease digestion. Cell. 1978 Sep;15(1):141–150. doi: 10.1016/0092-8674(78)90090-9. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H., Brown E., Walker J. M., Johns E. W. The isolation of three new high mobility group nuclear proteins. Biochim Biophys Acta. 1980 Jun 26;623(2):329–338. doi: 10.1016/0005-2795(80)90260-3. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H., Mathew C. G., Wright C. A., Venkov C. D., Johns E. W. Analysis of the high mobility group proteins associated with salt-soluble nucleosomes. Nucleic Acids Res. 1979 Dec 11;7(7):1815–1835. doi: 10.1093/nar/7.7.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin G. H., Nicolas R. H., Johns E. W. An improved large scale fractionation of high mobility group non-histone chromatin proteins. Biochim Biophys Acta. 1975 Oct 20;405(2):280–291. doi: 10.1016/0005-2795(75)90094-x. [DOI] [PubMed] [Google Scholar]
- Goodwin G. H., Walker J. M., Johns E. W. Studies on the degradation of high mobility group non-histone chromosomal proteins. Biochim Biophys Acta. 1978 Jun 22;519(1):233–242. doi: 10.1016/0005-2787(78)90076-x. [DOI] [PubMed] [Google Scholar]
- Levinger L., Barsoum J., Varshavsky A. Two-dimensional hybridization mapping of nucleosomes. comparison of DNA and protein patterns. J Mol Biol. 1981 Mar 5;146(3):287–304. doi: 10.1016/0022-2836(81)90389-2. [DOI] [PubMed] [Google Scholar]
- Levy B., Dixon G. H. Partial purification of transcriptionally active nucleosomes from trout testis cells. Nucleic Acids Res. 1978 Nov;5(11):4155–4163. doi: 10.1093/nar/5.11.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardian J. K., Paton A. E., Bunick G. J., Olins D. E. Nucleosome cores have two specific binding sites for nonhistone chromosomal proteins HMG 14 and HMG 17. Science. 1980 Sep 26;209(4464):1534–1536. doi: 10.1126/science.7433974. [DOI] [PubMed] [Google Scholar]
- Mathew C. G., Goodwin G. H., Igo-Kemenes T., Johns E. W. The protein composition of rat satellite chromatin. FEBS Lett. 1981 Mar 9;125(1):25–29. doi: 10.1016/0014-5793(81)80988-x. [DOI] [PubMed] [Google Scholar]
- Mathew C. G., Goodwin G. H., Wright C. A., Johns E. W. The high mobility group proteins and transcribed nucleosomes. Cell Biol Int Rep. 1981 Jan;5(1):37–43. doi: 10.1016/0309-1651(81)90155-7. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H., O'Farrell P. Z. Two-dimensional polyacrylamide gel electrophoretic fractionation. Methods Cell Biol. 1977;16:407–420. doi: 10.1016/s0091-679x(08)60116-8. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Sandeen G., Wood W. I., Felsenfeld G. The interaction of high mobility proteins HMG14 and 17 with nucleosomes. Nucleic Acids Res. 1980 Sep 11;8(17):3757–3778. doi: 10.1093/nar/8.17.3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealy L., Hartley J., Donelson J., Chalkley R., Hutchison N., Hamkalo B. Characterization of a highly repetitive sequence DNA family in rat. J Mol Biol. 1981 Jan 15;145(2):291–318. doi: 10.1016/0022-2836(81)90207-2. [DOI] [PubMed] [Google Scholar]
- Spiker S. A modification of the acetic acid-urea system for use in microslab polyacrylamide gel electrophoresis. Anal Biochem. 1980 Nov 1;108(2):263–265. doi: 10.1016/0003-2697(80)90579-5. [DOI] [PubMed] [Google Scholar]
- Sterner R., Vidali G., Allfrey V. G. Discrete proteolytic cleavage of high mobility group proteins. Biochem Biophys Res Commun. 1979 Jul 12;89(1):129–133. doi: 10.1016/0006-291x(79)90953-7. [DOI] [PubMed] [Google Scholar]
- Todd R. D., Garrard W. T. Two-dimensional electrophoretic analysis of polynucleosomes. J Biol Chem. 1977 Jul 10;252(13):4729–4738. [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Georgiev G. P. Heterogeneity of chromatin subunits in vitro and location of histone H1. Nucleic Acids Res. 1976 Feb;3(2):477–492. doi: 10.1093/nar/3.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. M., Johns E. W. The isolation, characterization and partial sequences of the chicken erythrocyte non-histone chromosomal proteins HMG14 and HMG17. Comparison with the homologous calf thymus proteins. Biochem J. 1980 Feb 1;185(2):383–386. doi: 10.1042/bj1850383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Palter K., Van Lente F. Histones H2a, H2b, H3, and H4 form a tetrameric complex in solutions of high salt. Cell. 1975 Sep;6(1):85–110. doi: 10.1016/0092-8674(75)90077-x. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Groudine M., Weintraub H. Interaction of HMG 14 and 17 with actively transcribed genes. Cell. 1980 Jan;19(1):289–301. doi: 10.1016/0092-8674(80)90410-9. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Weintraub H. Isolation of a subclass of nuclear proteins responsible for conferring a DNase I-sensitive structure on globin chromatin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):630–634. doi: 10.1073/pnas.76.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]