Abstract
Purpose
The expression of Annexin A1 (ANXA1) is known to be reduced in human breast cancer; however, the role of ANXA1 expression in the development of breast cancer remains unclear. In this study, we determined the relationship between the expression features of ANXA1 and the prognostic factors of breast cancer.
Methods
Human breast tissues were obtained from patients specimens who had undergone breast surgery or core needle biopsies. The patterns of ANXA1 expression were analyzed by immunohistochemical staining in relation to histopathological diagnosis, clinical characteristics and outcomes.
Results
One hundred eighty-two cases were included and the mean age of the patients was 46.34 ± 11.5 years. A significant loss of ANXA1 expression was noted in both ductal carcinoma in situ (DCIS) and invasive carcinomas compared to normal breast tissues (p<0.001) and benign breast diseases (p<0.001). There was a significant alteration in ANXA1 expression according to hormone receptor status (p<0.001), cancer intrinsic type (p<0.001), and nuclear grade (p=0.004) in invasive cancer. In a univariate analysis, ANXA1 positivity tended to be related with poor breast cancer-related survival (p=0.062); however, the same results was not realized in multivariate results (p=0.406). HER2 overexpression and TNM staging were significantly associated with relapse-free survivals (RFS) in the multivariate analysis (p=0.037, p=0.048, respectively). In particular, in node-positive patients (p=0.048), HER2 overexpressed patients (p=0.013), and non-triple negative breast cancer patients (p=0.002), ANXA1 overexpression was correlated with poor RFS.
Conclusion
Although significant loss of ANXA1 expression was noted in breast cancer including DCIS and invasive carcinoma, in cases of invasive cancer, overexpression of ANXA1 was related to unfavorable prognostic factors. And these results imply that ANXA1 plays dualistic roles and is involved in variable mechanisms related to cancer development and progression.
Keywords: Annexin A1, Breast neoplasms
INTRODUCTION
Clinically, breast cancer develops through sequential stages from normal ductal epithelium to hyperplasia, ductal carcinoma in situ (DCIS), invasive cancer, and metastatic carcinoma. Although extensive research has focused on identifying the molecular changes involved in carcinogenesis, the mechanisms underlying the development of breast cancer remain unclear [1]. Various reports have demonstrated that the expression of Annexin A1 (ANXA1) is ordinarily regulated in the mammary gland during the developmental period. Although the molecular action of ANXA1 is not yet fully understood, ANXA1 appears to take part in intracellular signaling and cell differentiation. ANXA1 is a 37-kDa calcium- and phospholipid-binding protein of the annexin superfamily, and has been detected in miscellaneous organisms, including vertebrates, invertebrates, and plants [2]. Further, ANXA1 is an important mediator in glucocorticoid-regulated inflammatory responses that modulates the activation of innate immune cells, such as neutrophils and macrophages [3], and it is known to have an association with dexamethasone-induced cell growth arrest [4]. The ANXA1 protein is partially located in the nucleus of endothelial cells, which indicates that it has a role in an intracellular signaling pathway. Because ANXA1 is distinctively expressed in the mammary gland during embryonic development [5-7], we postulate an association between ANXA1 and breast cancer development. Decreased expression of ANXA1 has been consistently reported at both the RNA and protein levels in breast cancer [8,9]; however, the role of ANXA1 expression in tumor initiation or progression has remained unclear. In this study, we specified the patterns of ANXA1 expression in various breast diseases including benign tumors and malignancies and then analyzed the relationship with prognostic factors and survivals of ANXA1 in invasive breast cancer.
METHODS
Patients
Between April 2005 and December 2007, human breast tissue samples from benign or malignant lesions were retrieved from patients who had undergone breast surgery or core-needle biopsy at the Ewha Medical Center. Normal breast tissues were obtained from non-tumorous areas of breast cancer patients more than 10 cm from the primary cancer. All of the patients who were enrolled in this study gave written informed consent in accordance with the Declaration of Helsinki. The study was reviewed and approved by the local institutional review committees.
Immunohistochemistry
The patterns of ANXA1 expression were examined by immunohistochemical (IHC) staining. Breast tissues were formalin-fixed and paraffin-embedded using standard methods. Monoclonal anti-ANXA1 antibody (catalog no. 610067; BD Transduction Laboratories, Lexington, USA), a specific antibody for ANXA1, has been suitable for immunochemical use on a variety of formalin-fixed and paraffin-embedded tissues of bovine and human origin [10,11]. Monoclonal anti-ANXA antibody was added as a 100 mL aliquot at a concentration of 1 mg/mL. For isotype control monoclonal antibody (mAb), we used a purified mouse IgG1.kappa (MOPC 21) from Sigma Chemical (St. Louis, USA). Tissue sections were cut in 4 µm slices and mounted on protein-coated glass slides. After dewaxing in xylene and rehydration in a series of alcohols, the staining procedure was carried out as follows. All the linking and labeling reagents, substrates, and chromogens were supplied from BioGenex (San Ramon, USA) as a super-sensitive immunodetection system. All procedures were carried out at room temperature. Before the mouse mAb was applied, a sufficient amount of protein blocking reagent (normal goat serum), which completely covered each tissue section, was placed on the slides for 20 minutes. After blotting around each section, primary mAb (1:100 dilution) was applied to the slides, incubated for 60 minutes, followed by the addition of prediluted, biotinylated anti-immunoglobulin for 20 minutes, and a labeling agent (alkaline phosphatase-labeled streptavidin) for 20 minutes [12]. For the chromagen, one tablet of Fast Red substrate was dissolved in 5 mL of naphtol phosphate-substrate solution, and levamisole was added to the substrate solution to block endogenous alkaline phosphatase activity. Enough the chromogen substrate solution was added to cover each section entirely, and the slides were incubated for 10-30 minutes, or until acceptable color intensity developed. Finally, the slides were counterstained with Mayer's hematoxylin solution and mounted with aqueous mounting medium (BioGenex). Each tissue section was analysed and scored by a pathologist. ANXA1 expression was scored as follows: the staining intensity was evaluated from 0 to 3 (representing negative to strong staining, respectively) and % cells in each intensity was obtained. The overall score was determined as follows: overall score=[(% cells with visual score 1)×1]+[(% cells with visual score 2)×2]+[(% cells with visual score 3)×3]; expression was positive if the score was more than 70. The hormone receptors (HR) (either., estrogen receptor or progesterone receptor) were considered positive when ≥10% of positive tumor cells had nuclear staining. Membranous staining for HER2 was scored as follows: 0, no staining or membranous staining in <10% of the cells; 1+, faint, incomplete staining in 10% of the cells; 2+, weak-to-moderate complete staining in 10% of the cells; and 3+, strong complete staining in 10% of the cells. HER2 overexpression was defined as a score of 3+. p53 was considered positive if >10% of the cells were stained with strong intensity.
Statistical analysis
Statistical analysis was performed with SPSS version 17.0 (SPSS Inc., Chicago, USA). Pearson's χ2 test was used to determine the correlation between variables, and the Cox proportional regression hazard model was employed with several variables. Kaplan-Meier curves were plotted from breast cancer-related survival (BCRS) and relapse-free survival (RFS) data. Univariate and multivariate p-values were two-sided and were considered to be significant when they were equal to or less than 0.05.
RESULTS
Cilinicopathologic characteristics of patients and immunohistochemical results of ANXA1
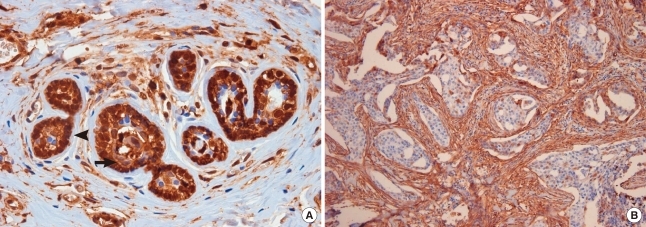
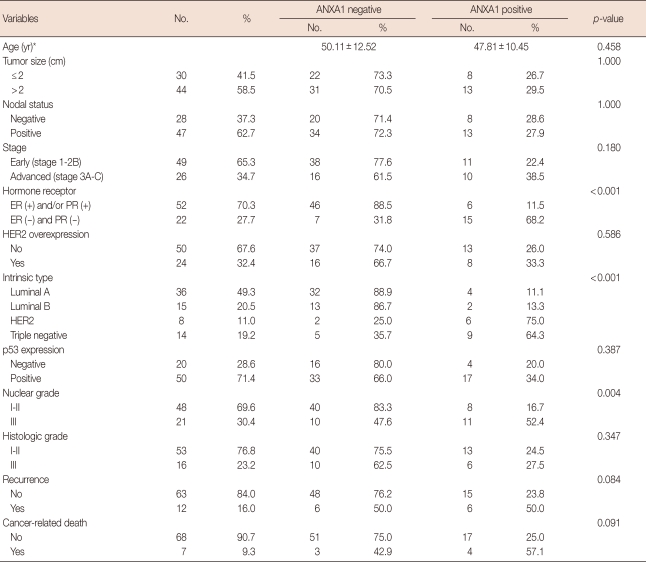
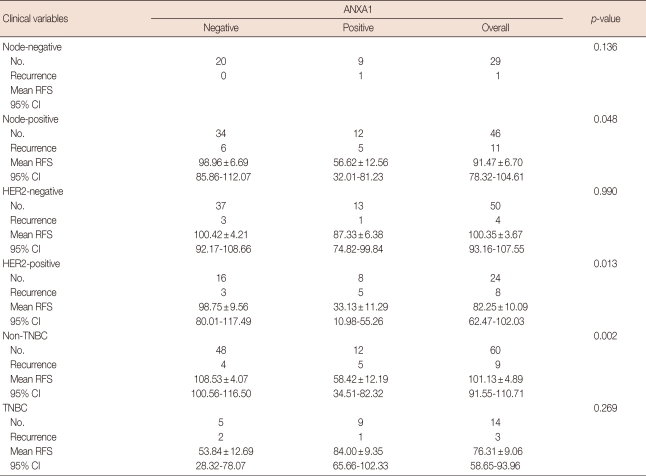
One hundred eighty-two cases were enrolled in this study, and the mean age of all female patients was 46.34±11.5 years. ANXA1 was strongly expressed in myoepithelial cells compared to epithelial cells in normal breast tissues (p<0.001) (Figure 1A). ANXA1 positivity was 87.7% in normal tissues, 53.2% in benign diseases, 14.3% in DCIS, and 30.9% in invasive carcinoma. A significant loss of ANXA1 expression was observed both in DCIS (n=7) and invasive carcinoma (n=75, Figure 1B) compared to normal epithelium (n=73, p<0.001) and benign breast diseases (n=27, p<0.001) including intraductal papilloma (n=10), sclerosinng adenosis (n=4), atypical ductal hyperplasia (n=2), fibroadenoma (n=6), and fibrocystic change (n=5). There was no difference between the ANXA1 expression of DCIS and invasive carcinoma (p=1.000). In the analysis within invasive breast carcinoma, there was no significant difference in ANXA1 expression according to age (p= 0.458), tumor size (p=1.000), nodal status (p=1.000), HER2 overexpression (p=0.586), p53 expression (p=0.387), histologic grade (p=0.347), or recurrence (p=0.084). ANXA1 expression was significantly correlated with unfavorable prognostic factors including HR negativity (p<0.001), HER2 type and triple negative breast cancer (TNBC) (p<0.001), and high nuclear grade (p=0.004) (Table 1). Althougth the data has not been shown in a table, there was no difference in ANXA1 expression among histologic subtypes of invasive breast cancer (p=0.520).
Figure 1.
Immunohistochemistry for Annexin A1 (ANXA1) expression. (A) ANXA1 is strongly expressed in myoepithelium (arrowhead) compared to epithelium (arrow) in normal breast tissue (× 400). (B) There is a significant ANXA1 loss in invasive ductal carcinoma (× 100).
Table 1.
Clinicopathologic characteristics related to Annexin A1 expression of 75 patients with invasive breast cancer
ANXA1=Annexin A1; HR=hormone receptor; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.
*Mean±SD.
Relationship between ANXA1 expression and clinical outcomes
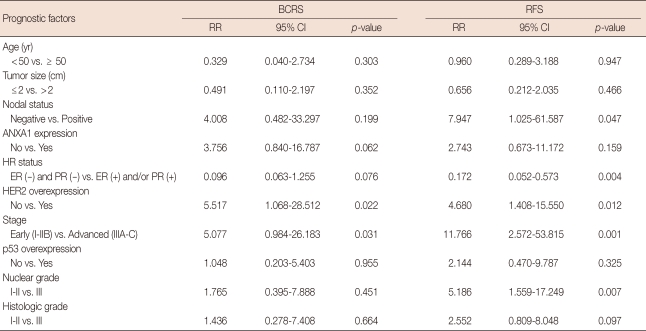
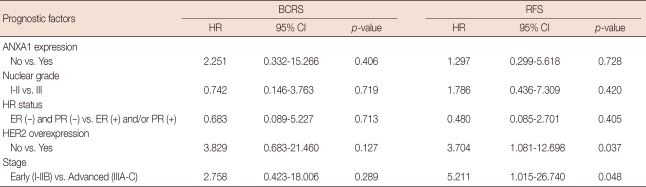
The median follow-up duration was 63.0±24.08 months (range, 6.0-117.0) for invasive ductal carcinoma (n=75). In a univariate analysis, HER2 overexpression (p=0.022) and advanced stage (p=0.031) were correlated with poor BCRS (Table 2). Although there was no statistical significance, ANXA1 expression tended to be associated with unfavorable BCRS (p= 0.062). In relapse-free survival (RFS), nodal status (p=0.047), HR status (p=0.004), HER2 overexpression (p=0.012), TNM stage (p=0.001), and nuclear grade (p=0.007) had the statistical significance. In contrast, there was no significant difference in BCRS and RFS according to ANXA1 expression in the multivariate analysis (Table 3). HER2 positivity and advanced stage were associated with poor RFS in multivariate analysis (p=0.037 and p=0.048, respectively).
Table 2.
Univariate Cox regression for breast cancer-related survival (BCRS) and relapse-free survival (RFS) in 75 patients with invasive breast cancer
RR=relative risk; CI=confidence interval; ANXA1=Annexin A1; HR=hormone receptor; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.
Table 3.
Multivariate analysis of prognosis factors for breast cancer-related survival (BCRS) and relapse-free survival (RFS) in patients with in invasive breast cancer (Cox's proportional hazards model)
HR=hazard ratio; CI=confidence interval; ANXA1=Annexin A1; HR=hormone receptor; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2.
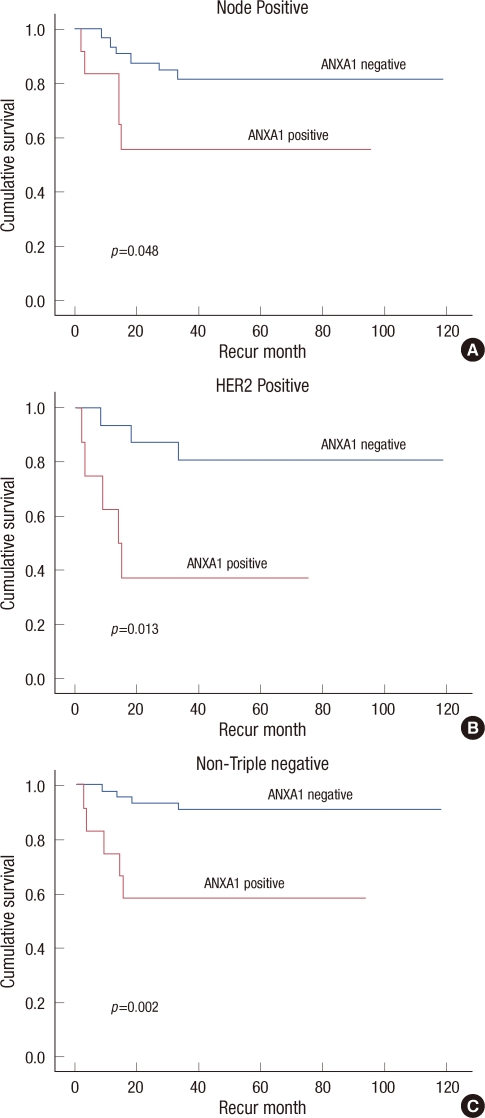
The ANXA1 positive group had significantly poor RFS in the node positive group (p=0.048, Figure 2A), in the HER2 positive group (p=0.013, Figure 2B), and in the non-TNBC group (p=0.002, Figure 2C) compared to the ANXA1 negative group based in a Kaplan-Meier analysis (Table 4, Figure 2).
Figure 2.
Five-year relapse-free survival according to Annexin A1 (ANXA1) expression with clinical variables. ANXA1 positive groups have poor relapse-free survivals in node-positive patients (A), in HER2-positive patients (B), and in non-triple negative breast cancer patients (C).
Table 4.
Comparison of Kaplan-Meier analysis (log rank) for relapse-free survival (RFS) and Annexin A1 overexpression according to clinical variables
ANXA1=Annexin A1; CI=confidence interval; HER2=human epidermal growth factor receptor 2; TNBC=triple negative breast cancer.
DISCUSSION
A reduction in ANXA1 expression in breast cancer has been consistently reported [8,9], a finding which was corroborated by this study. We also identified that ANXA1 expression was more prominent in myoepithelium than in epithelium in normal breast tissue; however, disappearance of myoepithelial cells has been a major hallmark in invasive breast cancer [13]. Shen et al. [1] tried to determine whether the decreased integrated maximal intensity of ANXA1 staining observed in DCIS samples was simply due to a reduction in the number of these cells, or if there was an underlying mechanism involved, such as a decreased level or frequency of cellular ANXA1 protein expression. They examined the expression of ANXA1 in myoepithelial cells from the TMA spots of normal, hyperplastic, and DCIS, and any minor expression was included. They concluded that the proportion of ANXA1 expression decreased, and the proportion of ANXA1 non-expressing areas increased in myoepithelial cells of non-malignant tissues compared to DCIS lesions. However, the distribution of ANXA1 expression in myoepithelial cells did not reveal notable differences between normal and hyperplastic ductal lesions. These results indicated that the loss of ANXA1 expression occurs early during malignant transformation. In this study, we found a significant loss of ANXA1 expression both in DCIS and invasive carcinoma compared to normal epithelium and benign breast diseases which supports previous studies. There have been a number of theories about the potential mechanism of ANXA1 in cancer development. For example, ANXA1 has been linked with reduced cell proliferation involving the regulation of the extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) signal transduction pathway. Forced expression of ANXA1 activates the ERK/MAPK pathway and reduces cell proliferation by disrupting the actin skeleton and ablating cyclin D1 expression [14,15]. Multiple members of the annexin family, including annexins 1, 2, 5, 6, and 7, have been reported to have involvement in the down-regulation of cell proliferation. Therefore ANXA1 has been postulated to have tumor suppressing properties [2,16].
On the contrary, some studies have shown that a higher level of ANXA1 in lymph node metastasis in comparison with primary breast cancer [14]. A similar finding was observed by Pencil and Toth [17] in rat metastatic mammary cancer and by Ahn et al. [18] in human breast cancer. Further, ANXA1 expression was found to be down-regulated by breast cancer metastasis suppressor 1 gene in the MDA-MB-435 breast cancer cell line metastatic to the lung in an athymic mouse model [19]. A more recent study showed that membrane ANXA1 had an autocrine/paracrine action in stimulating the migration of SKCO-15 colorectal cancer cells through activation of n-formyl peptide receptors, which is a leukocyte migratory factor [20]. A recent study [21] showed that ANXA1 expression provoked drug resistance in breast cancer cells. The overexpression of ANXA1 induced resistance to anti-cancer drugs, and ANXA1-depleted tumor cells showed increased sensitivity to anticancer drugs. Therefore, these results supported that ANXA1 also has oncogenic potential for breast cancer progression and metastasis via multiple mechanisms. In the current study, ANXA1 expression was significantly correlated with unfavorable prognostic features of breast cancer, such as hormone receptor negativity (p<0.001), positive HER2 status and TNBC type (p<0.001), high nuclear grade (p=0.004). The ANXA1 positivity was also correlated with poor RFS in some groups of invasive breast cancer such as node positive, HER2 positive and non-TNBC. This results could be explained that, in condition with unfavorable factors including node positive and HER2 positive status, ANXA1 might tend to re-express in cancer tissue and which result in more metastatic and aggressive phenotype. Given that this study had a limited number of patients, further study may be required to further support out findings.
Although large scale, solid base studies are required to draw clinically meaningful conclusions, we found a major loss of ANXA1 in breast cancer tissues, including DCIS and invasive carcinoma, relative to normal and benign breast tissues. However, in cases of invasive breast cancer, ANXA1 positivity was significantly correlated with unfavorable prognostic factors. These results implied that ANXA1 plays dualistic roles and mechanisms in cancer development and progression in terms of both tumor suppressive and oncogenic activity.
Footnotes
The authors declare that they have no competing interests.
References
- 1.Shen D, Nooraie F, Elshimali Y, Lonsberry V, He J, Bose S, et al. Decreased expression of annexin A1 is correlated with breast cancer development and progression as determined by a tissue microarray analysis. Hum Pathol. 2006;37:1583–1591. doi: 10.1016/j.humpath.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- 3.D'Acquisto F, Paschalidis N, Sampaio AL, Merghani A, Flower RJ, Perretti M. Impaired T cell activation and increased Th2 lineage commitment in Annexin-1-deficient T cells. Eur J Immunol. 2007;37:3131–3142. doi: 10.1002/eji.200636792. [DOI] [PubMed] [Google Scholar]
- 4.Croxtall JD, Flower RJ. Lipocortin 1 mediates dexamethasone-induced growth arrest of the A549 lung adenocarcinoma cell line. Proc Natl Acad Sci U S A. 1992;89:3571–3575. doi: 10.1073/pnas.89.8.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lozano JJ, Silberstein GB, Hwang S, Haindl AH, Rocha V. Developmental regulation of calcium-binding proteins (calelectrins and calpactin I) in mammary glands. J Cell Physiol. 1989;138:503–510. doi: 10.1002/jcp.1041380309. [DOI] [PubMed] [Google Scholar]
- 6.Horlick KR, Ganjianpour M, Frost SC, Nick HS. Annexin-I regulation in response to suckling and rat mammary cell differentiation. Endocrinology. 1991;128:1574–1579. doi: 10.1210/endo-128-3-1574. [DOI] [PubMed] [Google Scholar]
- 7.Creutz CE, Kambouris NG, Snyder SL, Hamman HC, Nelson MR, Liu W, et al. Effects of the expression of mammalian annexins in yeast secretory mutants. J Cell Sci. 1992;103(Pt 4):1177–1192. doi: 10.1242/jcs.103.4.1177. [DOI] [PubMed] [Google Scholar]
- 8.Shen D, He J, Chang HR. In silico identification of breast cancer genes by combined multiple high throughput analyses. Int J Mol Med. 2005;15:205–212. [PubMed] [Google Scholar]
- 9.Shen D, Chang HR, Chen Z, He J, Lonsberry V, Elshimali Y, et al. Loss of annexin A1 expression in human breast cancer detected by multiple high-throughput analyses. Biochem Biophys Res Commun. 2005;326:218–227. doi: 10.1016/j.bbrc.2004.10.214. [DOI] [PubMed] [Google Scholar]
- 10.Zokas L, Glenney JR., Jr The calpactin light chain is tightly linked to the cytoskeletal form of calpactin I: studies using monoclonal antibodies to calpactin subunits. J Cell Biol. 1987;105:2111–2121. doi: 10.1083/jcb.105.5.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glenney J, Zokas L. Antibodies to the N-terminus of calpactin II (p35) affect Ca2+ binding and phosphorylation by the epidermal growth factor receptor in vitro. Biochemistry. 1988;27:2069–2076. doi: 10.1021/bi00406a038. [DOI] [PubMed] [Google Scholar]
- 12.Wood GS, Warnke R. Suppression of endogenous avidin-binding activity in tissues and its relevance to biotin-avidin detection systems. J Histochem Cytochem. 1981;29:1196–1204. doi: 10.1177/29.10.7028859. [DOI] [PubMed] [Google Scholar]
- 13.Man YG, Sang QX. The significance of focal myoepithelial cell layer disruptions in human breast tumor invasion: a paradigm shift from the "protease-centered" hypothesis. Exp Cell Res. 2004;301:103–118. doi: 10.1016/j.yexcr.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 14.Alldridge LC, Harris HJ, Plevin R, Hannon R, Bryant CE. The annexin protein lipocortin 1 regulates the MAPK/ERK pathway. J Biol Chem. 1999;274:37620–37628. doi: 10.1074/jbc.274.53.37620. [DOI] [PubMed] [Google Scholar]
- 15.Alldridge LC, Bryant CE. Annexin 1 regulates cell proliferation by disruption of cell morphology and inhibition of cyclin D1 expression through sustained activation of the ERK1/2 MAPK signal. Exp Cell Res. 2003;290:93–107. doi: 10.1016/s0014-4827(03)00310-0. [DOI] [PubMed] [Google Scholar]
- 16.Bastian BC. Annexins in cancer and autoimmune diseases. Cell Mol Life Sci. 1997;53:554–556. doi: 10.1007/s000180050071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pencil SD, Toth M. Elevated levels of annexin I protein in vitro and in vivo in rat and human mammary adenocarcinoma. Clin Exp Metastasis. 1998;16:113–121. doi: 10.1023/a:1021917017109. [DOI] [PubMed] [Google Scholar]
- 18.Ahn SH, Sawada H, Ro JY, Nicolson GL. Differential expression of annexin I in human mammary ductal epithelial cells in normal and benign and malignant breast tissues. Clin Exp Metastasis. 1997;15:151–156. doi: 10.1023/a:1018452810915. [DOI] [PubMed] [Google Scholar]
- 19.Cicek M, Samant RS, Kinter M, Welch DR, Casey G. Identification of metastasis-associated proteins through protein analysis of metastatic MDA-MB-435 and metastasis-suppressed BRMS1 transfected-MDAMB-435 cells. Clin Exp Metastasis. 2004;21:149–157. doi: 10.1023/b:clin.0000024729.19084.f0. [DOI] [PubMed] [Google Scholar]
- 20.Babbin BA, Lee WY, Parkos CA, Winfree LM, Akyildiz A, Perretti M, et al. Annexin I regulates SKCO-15 cell invasion by signaling through formyl peptide receptors. J Biol Chem. 2006;281:19588–19599. doi: 10.1074/jbc.M513025200. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Serfass L, Roy MO, Wong J, Bonneau AM, Georges E. Annexin-I expression modulates drug resistance in tumor cells. Biochem Biophys Res Commun. 2004;314:565–570. doi: 10.1016/j.bbrc.2003.12.117. [DOI] [PubMed] [Google Scholar]