Abstract
The role of taurine in regulating glucose-induced nitrosative stress has been examined in human Schwann cells, a model for understanding the pathogenesis of diabetic neuropathy. Exposure to high glucose increased nitrated proteins (1.56 fold p<0.05), inducible nitric oxide synthase (iNOS) and neuronal NOS (nNOS) mRNA expression (1.55 fold and 2.2 fold respectively, p<0.05 both), phospho-p38 MAPK (1.32 fold, p<0.05) abundance and decreased Schwann cell growth (11 ± 2%, p<0.05). Taurine supplementation prevented high-glucose induced iNOS and nNOS mRNA upregulation, reduced nitrated proteins and phospho-p38 MAPK (56 ± 11% and 45 ± 18% (p<0.05 both) respectively) and restored Schwann cell growth to control levels. High glucose and taurine treatment alone reduced phospho-p42/44 MAPK and phospho-AKT to below detectable levels. Treatment of human Schwann cells with donors of nitric oxide and peroxynitrite reduced taurine transporter (TauT) expression (by 35 ± 5% and 29 ± 7% respectively p<0.05 both) as well as the maximum velocity of taurine uptake (TauT Vmax). NOS inhibition prevented glucose-mediated TauT mRNA downregulation, and restored TauT Vmax. These data demonstrate an important role for taurine in the prevention of nitrosative stress in human Schwann cells, which may have important implications for the development and treatment of diabetic neuropathy.
Keywords: Hyperglycemia and Nitric oxide in Schwann cells
Introduction
Peripheral diabetic neuropathy (DN) affects up to 50% of those with diabetes and is a leading cause of lower limb amputations. Hyperglycemia induced oxidative/nitrosative stress is a major cause of microvascular complications including DN. Accumulation of nitrated proteins (3-NT), a footprint of reactive nitrogen species (RNS) injury, has been observed in the peripheral nerve (Cheng, C. and Zochodne, D. W. 2003), spinal cord and dorsal root ganglion (DRG) in animal models of both type 1 and type 2 diabetes mellitus (Obrosova, I., 2008). In vitro studies using cultured human Schwann cells (HSC) exposed to high glucose have demonstrated nitrated proteins accumulation along with increased iNOS expression (Obrosova, I., et al. 2005). Nitric oxide (NO) can be generated by three nitric oxide synthase (NOS) isoforms; neuronal NOS (nNOS) inducible NOS (iNOS) and endothelial NOS (eNOS). An important pathophysiological role for NO in DN, is supported by the salutary effects of iNOS and nNOS gene deletion in diabetic mice which demonstrate improved nerve conductivity and reduced hypoalgesia compared to the diabetic wild-type (Vareniuk, I., et al. 2008; Vareniuk, I., et al. 2009). iNOS in particular has been implicated in the development of diabetes complications via inflammatory pathways such as NFκB and TGF-α (Empl, M., et al. 2001; Powell, L. A., et al. 2004; Ramana, K. V., et al. 2003)
Taurine is a sulfur-containing, free amino acid that has multiple putative metabolic functions including that of an antioxidant (Obrosova, I., et al. 2001). Taurine depletion has been demonstrated in blood samples of patients with type 1 and type 2 diabetes as well as in the peripheral nerve (Stevens, M. J., et al. 1993), lens (Malone, J. I., et al. 1993) and mesangial cells (Trachtman, H., et al. 1993) of diabetic rodents. Taurine supplementation has been shown to attenuate oxidative stress in many of these tissues (Obrosova, I., et al. 2009). Taurine also has roles as an osmolyte, calcium modulator (Li, F., et al. 2005) and neurotransmitter (Bravenboer, B., et al. 1992). Intracellular taurine concentration is maintained by the Na+Cl−-dependent taurine transporter (TauT) which is regulated by glucose, oxidative stress and changes in osmolarity. Disruption of taurine transport has been identified as an important pathway leading to its intracellular depletion (Nakashima, E., et al. 2005; Stevens, M. J., et al. 1999). In models of diabetic neuropathy, taurine supplementation has been shown to reduce reactive oxygen species (ROS), lipid peroxidation, poly(ADP-ribose) accumulation (Askwith, T., et al. 2009), attenuate functional deficits and ameliorate thermal and mechanical hyperalgesia (Li, F., Obrosova, I. G., et al 2005; Li, F., et al. 2006). However, the effects of taurine supplementation on nitrated proteins and NOS have not been explored.
The pathophysiology of diabetic neuropathy remains controversial with some authorities believing that SC dysfunction plays a primary role in the development of this condition. Autopsy studies and nerve biopsy material from patients with diabetic neuropathy identify lesions involving peripheral axons, loss of large and small myelinated nerve fibres and onion bulb formation due to successive demyelination and remyelination (Thomas, P.K and Lascelles, R. G. 1965), all suggesting SC as the primary abnormality, a finding which has also been re-emphasised by Mizisin et al (Mizisin., et al. 1998) .. In diabetes, nerve demyelination and the resultant impairment of nerve conduction velocity are thought to reflect SC dysfunction. Glucose uptake into SC is insulin-independent and the enzyme aldose reductase which catalyses the reduction of glucose to sorbitol, the first step in polyol pathway of glucose metabolism, is highly expressed in SCs (Ludvigson, M. A. and Sorenson, R. L. 1980), and has been implicated in the development of oxidative/nitrosative stress and glucose-toxicity (Maekawa, K., et al. 2001; Suzuki, T., et al. 1999). Together these make SC vulnerable to hyperglycemic toxicity, and SC have been identified as the critical focus of oxidative/nitrosative stress in diabetic models.. Increases in nitrated proteins and iNOS have both been consistently observed in SC in response to high glucose (Obrosova, I., et al. 2005), but the effects on nNOS are more varied (Adeghate, E., et al. 2003; Ii, M., et al. 2005; Okada, S., et al. 2008; Onozato, M. L., et al. 2002; Park, J. W., et al. 2006; Yabuki, A., et al. 2006).
P38 and JNK/SAPK (c-Jun NH2-terminal kinase/stress activated protein kinase) are mitogen activated protein kinases (MAPK) stimulated by oxidative and osmotic stress as well as inflammation. Both p38 and JNK/SAPK are activated by RNS and glucose and are able to mediate inflammatory responses and iNOS expression. Recently the MAPK pathways have been considered as transducers linking high glucose with biochemical dysfunction, observed with complications of diabetes. In particular, increases in p38 MAPK have been observed in DRG neurons (Purves, T., et al. 2001) and sciatic nerve (Price, S. A., et al. 2004) of streptozotocin-diabetic (STZ-D) rats as well as hyperglycemia-treated immortalized SC (Almhanna, K., et al. 2002) and sural nerves from patients with advanced DN undergoing lower-limb amputations (Purves, T., et al. 2001). Evidence also links increased p38 with chronic pain in DN. Inhibition of aldose reductase reduces p38 activation (Price, S. A., et al.2004), improves nerve conduction and reduces hyperalgesia in diabetic animal models.
P42/44 MAPK and Akt are mediators of cell growth and proliferation, Akt also being able to stimulate glucose uptake and eNOS activation in endothelial cells (Zdychova, J. and Komers, R. 2005). Despite being transducers of growth and cell proliferation, p42/44 MAPK have been implicated in diabetic complications. P42/44 MAPK can be activated by ROS and RNS (Liaudet, L., et al. 2009) and activation is increased in models of diabetes (Tomlinson, D. R. and Gardiner, N. J. 2008); in addition inhibition of p42/44 MAPK has attenuated glucose-mediated iNOS activation (Yuan, Z., et al. 2009). Discordant data exist with regards to the effect of RNS on Akt since peroxynitrite has been observed to both increase as well as decrease Akt activation depending on the model used (Liaudet, L., et al. 2009)
In this report we explore the effects of high glucose and taurine on RNS, iNOS and nNOS in HSC as well as the effects on activated forms of p38, p42/44 MAPK, SAPK/JNK and Akt. We demonstrate that taurine reduces RNS and NOS upregulation in glucose-exposed HSC and explored the potential mechanisms involved.
Methods
Cell culture
The primary adult HSC strain (ScienCell, Carlsbad, California) a commercially available cell strain derived from a young healthy male adult. Identity was confirmed by immunohistochemical staining with Schwann cell markers S-100, GFAP (glial fibrillary acid protein) and CD90 performed by ScienCell. They have been previously used in (Askwith, T., et al. 2009; Obrosova, I., et al. 2005). Cells were cultured at 37°C in a 5% CO2, humidified atmosphere in Dulbecco’s modified Eagles’ media (DMEM) (Lonza) supplemented with 10% FBS (fetal bovine serum), 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine. Twenty four hours prior to treatment media was changed, and replaced with DMEM supplemented with 5% fetal bovine calf serum (BCS), containing high (10 and 30 mM) or normal (5 mM) glucose and other experimental conditions and passaged as required. Passages 6-9 were used in all experiments.
For measurements with 100 μM α-lipoic acid (ALA) and the aldose reductase inhibitor (ARI), 10 μM Sorbinil, reagent stocks were dissolved in DMSO and treatments were compared to control with vehicle added. ALA is a potent free radical scavenger which has been shown to have therapeutic benefits in both animal and clinical models of DN, The concentration of ALA was used based upon other in vitro studies such as (Vincent, A. M., et al. 2005). For measurements with taurine supplementation, 250 μM was used. In diabetes both intracellular and plasma taurine concentrations are reduced. In human and animal models, dietary taurine supplementation restores plasma and intracellular taurine content. Similarly in cell culture models, exogenous taurine supplementation restores intracellular taurine content (Nandhini, A. T., et al. 2004). This restoration cannot be explained by Na+ independent taurine uptake, which is passive. Antioxidants including taurine restore TauT mRNA expression as well as Na+K+ATPase activity, which maintains the Na+ gradient down which taurine is transported (Nandhini, A. T., et al. 2004). Therefore a combination of these two factors could restore intracellular taurine content and explain the profound effect with modest taurine supplement.
Western Blotting
HSC were grown for 7 days in experimental conditions. At 7 days cells were washed in Hank’s balanced salt solution (HBSS) and lysed in 2% SDS containing 1% protease inhibitor cocktail (Sigma). Protein concentration was measured using bicinchoninic acid (BCA). 40μg of sample were separated electrophoretically on a 10% SDS-polyacrylamide gel and electrotransferred onto a polyvinylidene difluoride (PVDF) membrane. The blots were blocked with 3% milk and incubated with primary antibodies (mouse nitrated proteins 1:1000 (Millipore), rabbit p38 MAPK, phospho-p38 MAPK (Thr180/Tyr182), p42/44 MAPK, phospho-p42/p44 MAPK (Thr202/Tyr204), SAPK/JNK phospho-SAPK/JNK (Thr185/Tyr185), Akt and phospho-Akt (Ser 473) [1:1000, Cell Signaling all, except p42/44 MAPK 1:2000 Cell Signaling]) prepared in 3% milk containing 0.05% Tween-20 + 0.05% sodium azide overnight at 4°C. Blots were probed with secondary antisera conjugated to horseradish peroxidase (anti rabbit IgG 1:4000; GE healthcare) and visualization was performed with ECL detection reagents (Roche). To confirm equal loading, membranes were stripped and re-probed with rabbit anti-actin (1:200; Sigma). For nitrated protein western blot the total of all bands were analyzed and for p38, p42/44, JNK/SAPK and Akt corresponding bands were quantified by densitometry using a Syngene Ingenious system and analyzed using Gene Tools software.
Quantitative reverse transcription Polymerase Chain Reaction (Q-RT-PCR)
Q-RT-PCR on HSC RNA was conducted using the Invitrogen SuperScript® III Platinum® One-Step qRT-PCR Kit according to manufacturer’s instructions. Certified Fam-labeled lux primers (Invitrogen) specific for nNOS in exon 12, iNOS at the boundary between exons 18 and 19 and the TauT boundary between exons 1 and 2 (5′TCTTGGAGATCATCATAGG′3 and 3′CGGCATTACAACGGAGG′5) Certified Joe labeled β-actin primer (Invitrogen) as the housekeeping gene, were used to which the data was normalised. Preliminary studies demonstrated the expression of β-actin was unchanged in any of the treatment groups (data not shown). Results were analyzed using SDS software using the ΔΔCT method and results expressed as relative quantity (RQ).
Taurine uptake
Measurements of taurine uptake were performed as previously described (Askwith, T., et al. 2009). Briefly, uptake of 6.9 nM [3H] taurine was performed over 30 min at 37°C with increasing concentrations of unlabelled taurine (1-50 μM). Maximum velocity (Vmax) and Michaelis-Menten constant (Km) were obtained by non-linear regression analysis on the Michaelis-Menten plot, performed using Prism software.
Measurements of Schwann cell viability
For measurements of cell viability, HSC were grown for 7 days in media supplemented as described above. On day 5 cells were plated onto poly-L-lysine coated wells. After culture for 7 days propidium iodide (PI) (10 μg/ml) was added and incubated with the cells at 37°C, 5% CO2 for 10 mins. Dead cells were assayed at excitation 488 nm, emission 562-588 nm using a plate reader. For total cell number cells were washed in PBS and lysed by addition of Triton X-100 containing 10 μg/ml PI for 10min at 37°C, 5% CO2 and assayed at 544 nm, emission 612 nm.
Results
Effect of high glucose on nNOS and iNOS mRNA expression
Changes in nNOS and iNOS mRNA expression were measured in HSC by qRT-PCR after exposure to 5, 7.5, 10, 15, 22 and 30 mM glucose for 7 days (figure 1). Expression of iNOS was significantly increased at glucose concentrations >15 mM, with an 1.82 fold increase at 30 mM glucose (p<0.05). nNOS expression was increased at glucose concentrations >10 mM with a maximal 2.2 fold increase at 30 mM glucose (p<0.05). No increase was found when the osmotic control, 25 mM raffinose, was used in place of glucose.
Figure 1.
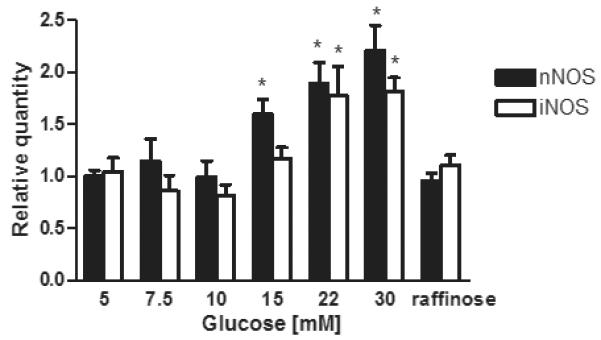
Concentration dependent effects of chronic glucose exposure on the expression of iNOS and nNOS mRNAs
iNOS and nNOS mRNA expression was measured by q-RT-PCR using TauT specific primers. Data expressed as relative quantity of 6 separate experiments mean ± SEM p<0.05. * p<0.05 vs. 5mM glucose †p<0.05 vs. respective glucose
Effect of high glucose on nitrated proteins
Western blotting of cells incubated for 7 days in 5, 10 and 30 mM glucose were performed using antisera to nitrated proteins. The data are shown in figure 2. The abundance of nitrated proteins, assessed by densitometry was increased by 1.46 fold in 10 mM and by 1.56 in 30 mM glucose (p<0.05) compared to 5 mM glucose.
Figure 2.
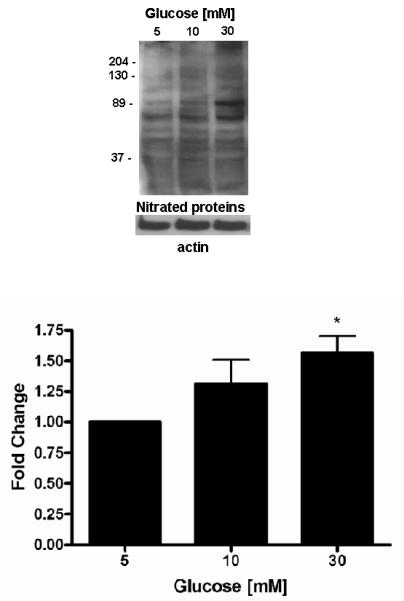
Effect of high glucose on nitrated proteins
A representative Western blot of 3-NT is shown. Equal protein was loading confirmed by β-actin. Quantitation was performed by densitometry and data expressed as mean ± SEM of 5 separate experiments. * p<0.05 vs. 5 mM glucose † p<0.05 vs. respective glucose.
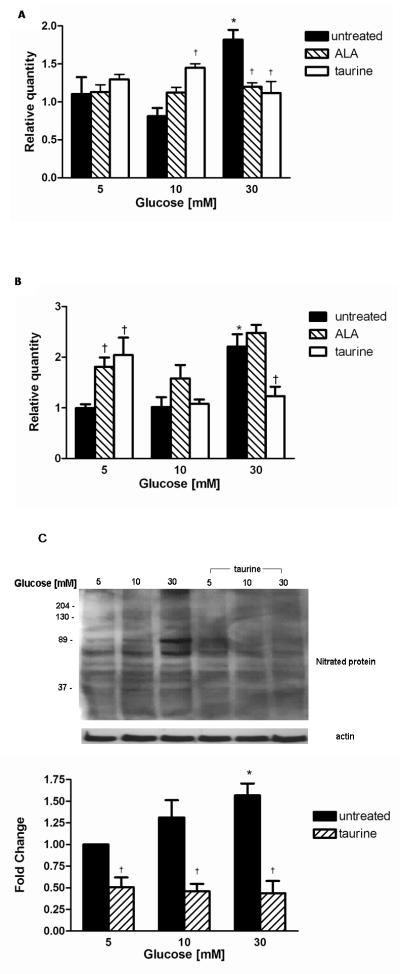
Effect of taurine on nNOS, iNOS and nitrated proteins
To understand the effect of taurine and oxidative stress on nitrated proteins, the effect of taurine co-treatment on iNOS, nNOS expression and 3-NT were explored in normal (5 mM) and high (10-30 mM) glucose. Changes in iNOS and nNOS mRNA expression induced by glucose were compared to co-treatment with ALA.
In 5 mM glucose, neither taurine nor ALA was found to affect iNOS expression (figure 3A). Interestingly, in 10 mM glucose, taurine co-treatment increased iNOS expression 1.45 fold (p<0.05), compared to untreated 10 mM glucose. The increase observed with ALA not achieving statistical significance.
Figure 3.
Effect of treatment with 100 μM ALA and 250 μM taurine on (A) iNOS and (B) nNOS mRNA expression in normal and high glucose. Data expressed as relative quantity of 6 separate experiments mean ± SEM * p<0.05 vs. 5 mM glucose †p<0.05 vs. respective glucose
Effect of high glucose and taurine treatment on nitrated proteins(C). Representative Western blots of 3-NT protein shown. Equal protein loading confirmed by β-actin. Quantitation was performed by densitometry and data expressed as mean ± SEM of 5 separate experiments. * p<0.05 vs. 5 mM glucose † p<0.05 vs. respective glucose.
In 30 mM glucose, taurine supplementation entirely prevented the upregulation of iNOS which was similar to the response of ALA co-treatment.
The corresponding changes in nNOS expression are shown in figure 3B. In 5 mM glucose both taurine and ALA increased nNOS expression by 2.3 fold and 1.8 fold respectively (p<0.05) compared to untreated SC. In 30 mM glucose the effects of ALA and taurine differed: Taurine decreased nNOS expression by 44 ± 18% to levels similar to 5 mM glucose, whereas ALA co-treatment did not affect nNOS expression.
Co-treatment with 250 μM taurine reduced 3-NT significantly to below control with reductions at 5, 10 and 30 mM of 50 ± 10%, 54 ± 7% and 56 ± 11%, respectively (p<0.05 all) compared to respective untreated glucose concentration figure 3C.
Effect of polyol pathway flux on iNOS and nNOS expression
Flux through the polyol pathway increases oxidative/nitrosative stress and depletes intracellular taurine. To explore the effect of increased polyol pathway flux on iNOS and nNOS expression, HSC incubated in 5, 10 and 30 mM glucose, were treated with the ARI, sorbinil (10 μM) (figure 4). iNOS expression was increased by ARI in 5 and 10 mM glucose by 1.74 fold and 1.72 fold respectively (p<0.05 both) compared to 5 mM glucose. At 30 mM glucose however, ARI reduced glucose-stimulated iNOS expression by 38 ± 10% (p<0.05) compared to 30 mM glucose.
Figure 4.
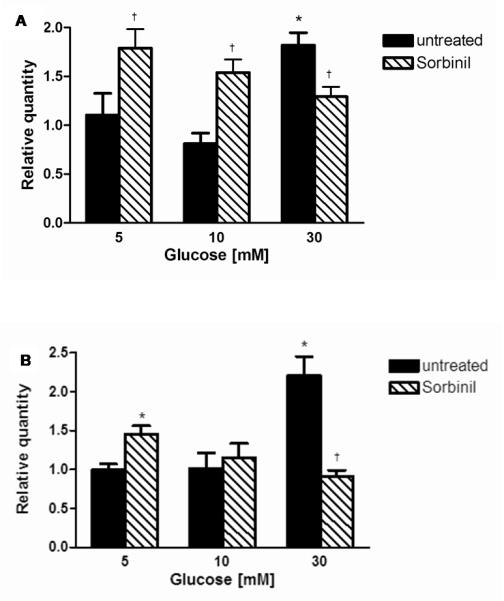
Effect of the aldose reductase inhibition with 10 μM Sorbinil on (A) iNOS and (B) nNOS mRNA expression. Data expressed as relative quantity of 6 separate experiments mean ± SEM * p<0.05 vs. 5 mM glucose †p<0.05 vs. respective glucose
In parallel to the effects of taurine, nNOS expression (figure 4B) was increased by ARI by 1.45 fold at 5 mM glucose (p<0.05). At 30 mM glucose ARI prevented the glucose-mediated increase of nNOS.
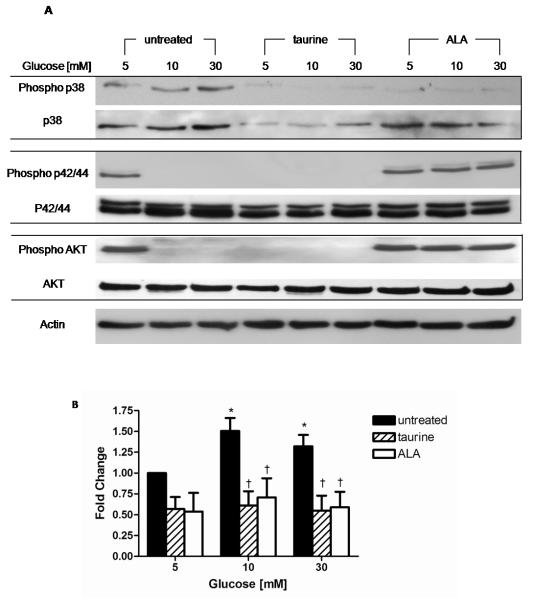
Effect of high glucose on p38, p42/44, JNK/SAPK and Akt
Figure 5 illustrates the effects of high glucose on total and phosphorylated p38, p42/44 MAPK, JNK/SAPK and Akt were examined by western blot and compared with the effects of simultaneous treatment with taurine and ALA. Compared to 5 mM glucose phospho-p38 MAPK was increased by 1.5 fold and by 1.32 fold in 10 and 30 mM glucose, respectively (p<0.05). Taurine reduced phospho-p38 expression by 50% in 5, 10 and 30 mM glucose, a finding which was comparable to ALA. There were no detectable changes in the abundance of total JNK/SAPK or phospho-JNK/SAPK by either high glucose or co-treatment with taurine or ALA. Phospho-SAPK was not detectable in any of the treatment groups.
Figure 5.
Effect of high glucose and treatment with taurine and ALA on MAPK family members and Akt. (A) Representative western blots shown, equal protein loading confirmed by β-actin. Quantitation of phospho-p38 (B) performed by densitometry and normalized by β-actin. Data expressed as mean ± SEM of 5 separate experiments * p<0.05 vs. 5mM glucose † p<0.05 vs. respective glucose.
The abundance of phosphorylated p42/44 MAPK and Akt were reduced in 10 and 30 mM glucose to below the level of detection, with no changes in total p42/44 MAPK or Akt. Taurine co-treatment in 5 mM glucose also reduced phospho p42/44 MAPK and phospho AKT expression to undetectable levels, and at higher glucose concentrations in the presence of taurine, expression remained undetectable. ALA in 5 mM glucose had no discernable effect on phospho-p42/44 MAPK but in higher glucose concentrations ALA preserved levels of both phospho-p42/44 MAPK and Akt to levels observed in 5 mM glucose.
Effect of high glucose on Schwann cell growth and death
Measurements of HSC death using PI demonstrated that neither high glucose, nor taurine or ALA co-treatment increased the proportion of dead cells in the cultures. Assessments of DNA however, showed that glucose reduced HSC growth dose-dependently by 6 ± 2% (p=0.132) and 11 ± 2% (p<0.05) at 10 and 30 mM glucose respectively (Table 1). Co-treatment with either taurine or ALA ablated the high-glucose induced reduction.
Table 1.
Effects of high glucose, taurine and ALA on DNA content
| SC growth % control |
|||
|---|---|---|---|
| Glucose [mM] | 5 | untreated | 100.0 ± 3.5 100.0 ± 1.5 100.0 ± 3.6 |
| taurine | |||
| ALA | |||
| 10 | untreated | 94.4 ± 1.8 101.1 ± 1.8 102.4 ± 1.6 |
|
| taurine | |||
| ALA | |||
| 30 | untreated | 89.0 ± 1.8* 100.4 ± 1.3† 103.7 ± 2.1† |
|
| taurine | |||
| ALA |
Total levels of DNA were measured using the PI method. Data expressed as relative quantity of 6 independent experiments as mean ± SEM p<0.05.
p<0.05 vs. untreated 5 mM glucose
p<0.05 vs. untreated 30 mM glucose
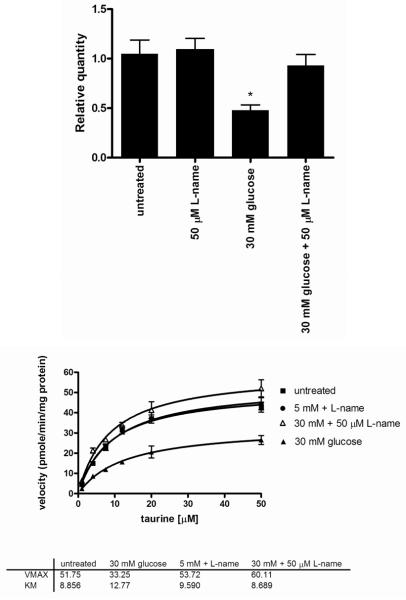
Effect of NO donors on TauT expression and taurine transport
The effect of NO on TauT expression was measured by incubating HSC with increasing concentrations of two NO donors, 3-morpholino-sydnonimine (SIN-1) and sodium nitroprusside (SNP) for 24 hours (figure 6). Both donors decreased TauT expression between 10 and 100 μM. The reduction of TauT expression was maximal in 10 μM SIN-1 which resulted in a 35 ± 5 % decrease in TauT mRNA expression (p<0.05). SNP also reduced TauT mRNA by up to 29 ± 7%.
Figure 6.
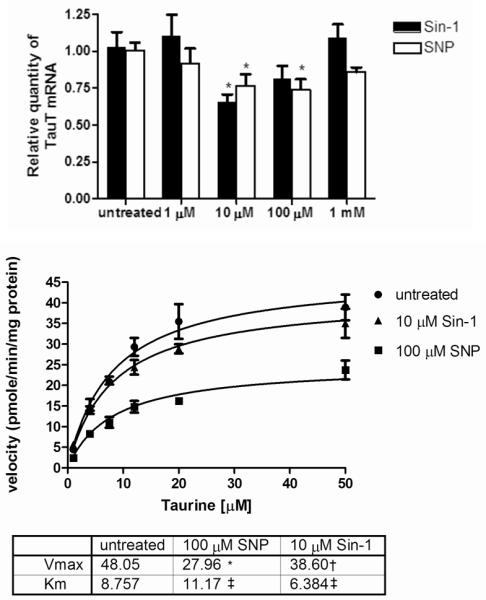
Effect of nitric oxide and peroxynitrite on TauT expression and taurine uptake
A) TauT expression was measured by q-RT-PCR. Data expressed as relative quantity of 6 separate experiments mean ± SEM p<0.05. * P<0.05 vs. untreated
B) Kinetic plots obtained by measuring taurine uptake in SC. Michaelis-Menten plots for Na+ dependent taurine uptake are shown. Vmax and Km were obtained by non-linear regression using Prism software* p<0.01 vs. untreated †p<0.05 vs. untreated ‡ p>0.2 vs. untreated
Measurements of TauT kinetics indicated that 10 μM SIN-1 reduced TauT Vmax from 48 ± 4 pmoles/min/mg protein to 39 ± 2 pmoles/min/mg protein (p<0.05), whereas 100 μM SNP reduced TauT Vmax to 28 ± 2 pmoles/min/mg protein (p<0.005). No significant change in the Km was observed for either donor.
Effect of NOS inhibitor on TauT expression and taurine transport
We have previously demonstrated that high glucose reduces TauT expression and taurine transport into HSC (Askwith, T., et al. 2009). To determine whether this effect involves NO, HSC were exposed for 7 days to 30 mM glucose in the presence and absence of 50 μM of the NOS inhibitor N-nitro-L-arginine methyl ester (L-NAME).
Treatment with L-NAME had no effect on TauT mRNA expression in 5 mM glucose, however in 30 mM glucose, L-NAME completely prevented the reduction in TauT expression (figure 7). Similarly measurements of TauT kinetics demonstrated that L-NAME had no effect on TauT kinetics in normal glucose concentrations, however in 30 mM glucose L-NAME restored TauT Vmax from 33 ± 3 pmoles/min/mg protein to 60 ± 4 pmoles/min/mg protein (p<0.05).
Figure 7.
Effects of L-NAME on high glucose-induced downregulation of (A) TauT mRNA expression and (B) TauT kinetics
Discussion
Accumulation of nitrated proteins has been observed in the tissues sensitive to diabetic complications. In this study we found that exposure of HSC to pathophysiological concentrations of glucose increased 3-NT and iNOS mRNA which is consistent with previous reports (Obrosova, I., et al. 2005). Upregulation of iNOS in response to high glucose has been observed in the retina, heart, vascular endothelium, smooth muscle, HSC cells, human retina cells and coronary artery endothelial cells (Vareniuk, I., et al. 2008). The development of experimental neuropathy is reduced in iNOS knockout diabetic mice. We have extended these findings by demonstrating that nNOS expression is also regulated by pathophysiological glucose concentrations. In vivo, NO release from nNOS is thought to aid synaptic transmission (Kiss, J. P. and Vizi, E. S. 2001). Studies exploring the effects of diabetes on nNOS expression have yielded variable results; for example, nNOS expression is increased in the retina (Takeda, M., et al. 2001) and gastroduodenal tract (Adeghate, E., et al. 2003) of STZ-D rats and in the kidney cortex of Otsuka Long Evans Tokushima Fatty rats (Yabuki, A., et al. 2006), but is decreased in the kidney cortex of STZ-D rats (Okada, S., et al. 2008) and high glucose-treated immortalized mouse SC (Ii, M., et al. 2005). However, the importance of nNOS-derived NO in the pathogenesis of DN has been demonstrated in nNOS knockout mice which are protected from neuropathy, albeit to a lesser extent, than iNOS knockout mice (Vareniuk, I., et al. 2009).
We found that taurine regulated iNOS and nNOS expression as well as nitrated proteins in both 5 mM glucose and at pathophysiologically elevated levels of glucose. Taurine inhibits oxidative stress, restores DRG neuron Ca2+ signaling (Li, F., et al. 2005) and reduces glycation in animal models of diabetes (Nandhini, T. A. and Anuradha, C. V. 2003; Schaffer, S. W., et al. 2009). In HSC, taurine reduces glucose-induced increases in oxidative stress, lipid peroxidation and poly(ADP-ribosyl)ated proteins (Askwith, T., et al. 2009). It is unclear whether all the beneficial effects of taurine are mediated by an antioxidant mechanism, or due to other metabolic properties (Schaffer, S. W., et al. 2009). Therefore in order to explore the potential mechanisms of action of taurine, we compared the effects of taurine with that of the potent antioxidant ALA in this communication.
Taurine reduced nitrated protein abundance by 50% in HSC at all glucose concentrations, an effect typical of an antioxidant. In 30 mM glucose, taurine restored iNOS and nNOS mRNA expression to levels observed in 5 mM glucose. The response of iNOS to taurine was similar to that of ALA, consistent with an antioxidant effect. In parallel, an antioxidant action would seem to account for the upregulation of nNOS gene expression by taurine and ALA in 5 mM glucose. However in 30 mM glucose the response of nNOS differed, because although taurine prevented high-glucose mediated nNOS upregulation, ALA treatment was without effect. This suggests that at high glucose concentrations, the effect of taurine (and indeed ARI) on nNOS is unlikely to be mediated by an antioxidant action, but could be mediated by carbonyl scavenging or perhaps by restoring Ca2+ signaling. Ca2+ influx mediated by glutamate binding to NMDA receptors activates nNOS (Forstermann, U., et al. 1998; Park, J. W., et al. 2006) and since taurine has been shown to attenuate excessive Ca2+ flux in diabetes (Li, F., et al. 2005), normalization of Ca2+ signaling by taurine in HSC may underpin its effect on nNOS expression.
Under conditions of sustained high glucose, flux through the polyol pathway is increased which contributes to both oxidative and nitrosative stress (Edwards, J. L., et al. 2008). ARI prevents deficits of taurine transport in HSC and replete intracellular taurine in complications prone diabetic tissues such as the nerve and retina (Askwith, T., et al. 2009). We found that the ARI sorbinil prevented the high (30 mM) glucose-induced increase in both iNOS and nNOS expression, suggesting the increase in NOS expression is at least in part, AR-mediated. At lower glucose concentrations however, only iNOS expression was significantly increased by ARI. Although the mechanisms of the effect of ARI on iNOS are unclear, the close parallelism of the effects of ARI and taurine suggest that taurine intracellular repletion could be an important component of this response. Since both taurine (in 10 mM glucose) and ARI (in 5 and 10 mM glucose) increased iNOS expression at lower glucose concentrations it is also possible that an osmotic effect of depleting intracellular sorbitol results in increased iNOS expression, at 5 and 10 mM glucose whereas an the reduction of iNOS by these agents at 30 mM glucose may be primarily mediated by an antioxidant action.
The effects of taurine and ALA were compared on glucose-induced changes in MAPK family members and Akt. MAPKs have been identified as transducers linking high glucose to biochemical deficits in diabetes. P38 mediates responses to osmotic stress including the regulation of genes such as AR and taurine. Together with SAPK/JNK these are thought to be activated by oxidative stress, RNS and increased polyol pathway flux in the peripheral nerves of diabetic rodents and patients (Purves, T., et al. 2001). ARI-sensitive phosphorylation and nuclear migration of p38 MAPK has been demonstrated in DRG sensory neurons of diabetic rodents and specific inhibition of p38 prevents Na+ channel phosphorylation and nerve conduction slowing. This study demonstrates a clear increase in phospho-p38 MAPK, and a trend to an increase in total p38. However, consistent with the report of Yuan et al 2009 (Yuan, Z., et al. 2009) no changes were seen in SAPK/JNK and only phospho-JNK was detectable. To our knowledge, this is the first communication to assess the effect of taurine on the MAPK pathway, demonstrating that taurine reduced phospho-p38, a response mirroring that of 3-NT. Finally, activation of sensory neurons DRG p38 MAPK has been invoked in the development of hyperalgesia (Daulhac, L., et al. 2006) and thus the salutary effect of taurine to prevent hyperalgesia in diabetic rodents could be mediated via this mechanism.
Phosphorylation of both Akt and p42/44 MAPK signaling intermediates were inhibited by incubation with 10 mM and 30 mM glucose compared with 5 mM glucose. ALA prevented the glucose-mediated reduction of phospho-p42/44 MAPK and phospho-Akt suggesting that ROS are responsible for this inhibition. Taurine treatment however failed to restore the glucose-mediated reduction of either phospho-p42/44 MAPK or phospho-Akt and reduced levels in 5 mM glucose. These data are compatible with an effect of taurine, reported in other cell lines, to increase phosphatase activities. Taurine increased activities of p42/44 MAPK phosphatase in human retinal pigment epithelial cells and protein tyrosine phosphatase in rat vascular smooth muscle cells which are responsible for the dephosphorylation of MAPK and Akt respectively (Lornejad-Schafer, M. R., et al. 2009; Yoshimura, H., et al. 2005). In vitro glucose-exposed Schwann cells display many metabolic abnormalities such as depletion of arachidinyl-containing phospholipids, oxidative-nitrosative stress, poly(ADP-ribose)polymerase (Obrosova, I. G., et al. 2005;Askwith, T., et al. 2009) and 12/15-lipoxygenase activation all of which are found in the diabetic peripheral nerve (Stavniichuk, R., et al. 2010). Transgenic mice over-expressing aldose reductase show more severe nerve conduction velocity deficit and oxidative stress under hyperglycemic stress (Song, Z., et al. 2003), demonstrating the importance of Schwann cell deficits on diabetic neuropathy.
Changes in pAKT are further indication of these metabolic deficits and further demonstrate SC dysfunction induced by glucose-exposure. Akt is an essential mediator of glucose uptake, regulating Glut4as well as glycogen synthesis. Akt phosphorylation is reduced in models of both Type 1 and Type 2 diabetes and it is possible this could contribute to secondary insulin resistance in type 1 patients. What is unclear, however, is whether the reduction in Akt phosphorylation is secondary to insulin resistance/impaired insulin secretion, or due to prolonged hyperglycaemia or a consequence of prolonged hyperglycaemia such as ROS and RNS (Liaudet, L., et al. 2009) as well as glucosamine, a product of the hexosamine pathway, reduce Akt activation (Du, X. L., et al. 2001; Schleicher, E. D. and Weigert, C. 2000). The response in these isolated cultures suggests the effect on Akt is independent on insulin and a direct result of glucose toxicity. The effect of ALA indicates this effect is ROS mediated and the effect of taurine consistent with upregulation of phosphatases. Ultimately the importance of taurine mediated, down-regulation of pAKT and the lack of effect in high glucose is unclear.
Previously we have demonstrated that high glucose exposure reduced TauT expression and taurine transport in HSC (Askwith, T., et al. 2009). In order to determine the functional consequences of increased NOS on taurine transport in HSC we studied the effect of NO and RNS donors on TauT. Both Sin-1 and SNP reduced TauT mRNA expression as well as taurine transport while NOS inhibition with L-NAME restored the glucose-induced down-regulation of TauT mRNA. These data indicate that upregulation of NOS and increased RNS production may detrimentally disrupt taurine transport in HSC.
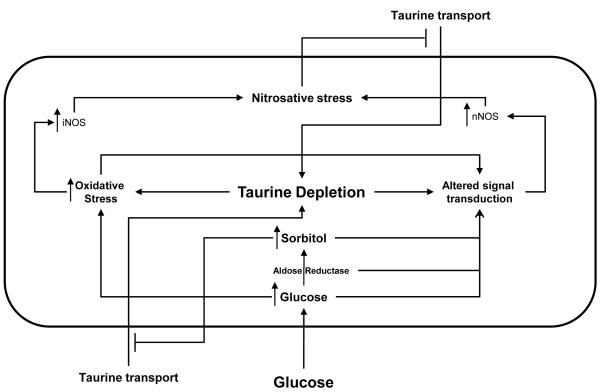
In conclusion, as illustrated in figure 8, taurine can abrogate high glucose-mediated accumulation of nitrated proteins, p38 activation, impaired growth and increased NOS expression in HSC. Taurine appears to play an important role in the regulation of nNOS in particular, with a mechanism of action which is at least partially independent of an antioxidant effect. Finally, high glucose-mediated TauT down-regulation in HSC appears to be at least partially-mediated by glucose-induced increase in NO and RNS.
Figure 8.
Model of the interrelationships of taurine and oxidative/nitrosative stress in Schwann cells in diabetes.
High intracellular glucose increases intracellular sorbitol content and nitrosative stress. Both deplete taurine by reducing taurine uptake. Taurine depletion in turn increases oxidative stress and iNOS and nNOS expression, further increasing nitrosative stress resulting in a feed-forward cycle.
Highlights.
Effects of high glucose and taurine on RNS, iNOS and nNOS in Human Schwann Cells.
Important role for taurine in the prevention of nitrosative stress in HSC cells
Mechanism of action which is partially independent of an antioxidant effect
Taurine reduces RNS and NOS upregulation in glucose-exposed HSC
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Adeghate E, al-Ramadi B, Saleh AM, Vijayarasathy C, Ponery AS, Arafat K, Howarth FC, El-Sharkawy T. Increase in neuronal nitric oxide synthase content of the gastroduodenal tract of diabetic rats. Cell Mol.Life Sci. 2003;60:1172–1179. doi: 10.1007/s00018-003-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almhanna K, Wilkins PL, Bavis JR, Harwalkar S, Berti-Mattera LN. Hyperglycemia triggers abnormal signaling and proliferative responses in Schwann cells. Neurochem.Res. 2002;27:1341–1347. doi: 10.1023/a:1021671615939. [DOI] [PubMed] [Google Scholar]
- 3.Askwith T, Zeng W, Eggo MC, Stevens MJ. Oxidative Stress and Dysregulation of the Taurine Transporter in High Glucose-Exposed human Schwann Cells: Implications for Pathogenesis of Diabetic Neuropathy. Am.J.Physiol Endocrinol.Metab. 2009;297(3):E620–8. doi: 10.1152/ajpendo.00287.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bravenboer B, Kappelle AC, Hamers FP, van BT, Erkelens DW, Gispen WH. Potential use of glutathione for the prevention and treatment of diabetic neuropathy in the streptozotocin-induced diabetic rat. Diabetologia. 1992;35:813–817. doi: 10.1007/BF00399926. [DOI] [PubMed] [Google Scholar]
- 5.Cheng C, Zochodne DW. Sensory neurons with activated caspase-3 survive long-term experimental diabetes. Diabetes. 2003;52:2363–2371. doi: 10.2337/diabetes.52.9.2363. [DOI] [PubMed] [Google Scholar]
- 6.Daulhac L, Mallet C, Courteix C, Etienne M, Duroux E, Privat AM, Eschalier A, Fialip J. Diabetes-induced mechanical hyperalgesia involves spinal mitogen-activated protein kinase activation in neurons and microglia via N-methyl-D-aspartate-dependent mechanisms. Mol.Pharmacol. 2006;70:1246–1254. doi: 10.1124/mol.106.025478. [DOI] [PubMed] [Google Scholar]
- 7.Descarries LM, Cai S, Robitaille R, Josephson EM, Morest DK. Localization and characterization of nitric oxide synthase at the frog neuromuscular junction. J.Neurocytol. 1998;27:829–840. doi: 10.1023/a:1006907531778. [DOI] [PubMed] [Google Scholar]
- 8.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J.Clin.Invest. 2001;108:1341–1348. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edwards JL, Vincent AM, Cheng HL, Feldman EL. Diabetic neuropathy: Mechanisms to management. Pharmacol.Ther. 2008;120:1–34. doi: 10.1016/j.pharmthera.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Empl M, Renaud S, Erne B, Fuhr P, Straube A, Schaeren-Wiemers N, Steck AJ. TNF-alpha expression in painful and nonpainful neuropathies. Neurology. 2001;56:1371–1377. doi: 10.1212/wnl.56.10.1371. [DOI] [PubMed] [Google Scholar]
- 11.Erdamar H, Turkozkan N, Balabanli B, Ozan G, Bircan FS. The relationship between taurine and 3-nitrotyrosine level of hepatocytes in experimental endotoxemia. Neurochem.Res. 2007;32:1965–1968. doi: 10.1007/s11064-007-9395-9. [DOI] [PubMed] [Google Scholar]
- 12.Forstermann U, Boissel JP, Kleinert H. Expressional control of the ‘constitutive’ isoforms of nitric oxide synthase (NOS I and NOS III) FASEB J. 1998;12:773–790. [PubMed] [Google Scholar]
- 13.Ii M, Nishimura H, Kusano KF, Qin G, Yoon YS, Wecker A, Asahara T, Losordo DW. Neuronal nitric oxide synthase mediates statin-induced restoration of vasa nervorum and reversal of diabetic neuropathy. Circulation. 2005;112:93–102. doi: 10.1161/CIRCULATIONAHA.104.511964. [DOI] [PubMed] [Google Scholar]
- 14.Kiss JP, Vizi ES. Nitric oxide: a novel link between synaptic and nonsynaptic transmission. Trends Neurosci. 2001;24:211–215. doi: 10.1016/s0166-2236(00)01745-8. [DOI] [PubMed] [Google Scholar]
- 15.Li F, Abatan OI, Kim H, Burnett D, Larkin D, Obrosova IG, Stevens MJ. Taurine reverses neurological and neurovascular deficits in Zucker diabetic fatty rats. Neurobiol.Dis. 2006;22:669–676. doi: 10.1016/j.nbd.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Li F, Obrosova IG, Abatan O, Tian D, Larkin D, Stuenkel EL, Stevens MJ. Taurine replacement attenuates hyperalgesia and abnormal calcium signaling in sensory neurons of STZ-D rats. Am.J.Physiol Endocrinol.Metab. 2005;288:E29–E36. doi: 10.1152/ajpendo.00168.2004. [DOI] [PubMed] [Google Scholar]
- 17.Liaudet L, Vassalli G, Pacher P. Role of peroxynitrite in the redox regulation of cell signal transduction pathways. Front Biosci. 2009;14:4809–4814. doi: 10.2741/3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lornejad-Schafer MR, Schafer C, Schoffl H, Frank J. Cytoprotective role of mitogen-activated protein kinase phosphatase-1 in light-damaged human retinal pigment epithelial cells. Photochem.Photobiol. 2009;85:834–842. doi: 10.1111/j.1751-1097.2008.00479.x. [DOI] [PubMed] [Google Scholar]
- 19.Ludvigson MA, Sorenson RL. Immunohistochemical localization of aldose reductase. I. Enzyme purification and antibody preparation--localization in peripheral nerve, artery, and testis. Diabetes. 1980;29:438–449. doi: 10.2337/diab.29.6.438. [DOI] [PubMed] [Google Scholar]
- 20.Maekawa K, Tanimoto T, Okada S, Suzuki T, Suzuki T, Yabe-Nishimura C. Expression of aldose reductase and sorbitol dehydrogenase genes in Schwann cells isolated from rat: effects of high glucose and osmotic stress. Brain Res.Mol.Brain Res. 2001;87:251–256. doi: 10.1016/s0169-328x(01)00009-2. [DOI] [PubMed] [Google Scholar]
- 21.Malone JI, Benford SA, Malone J., Jr. Taurine prevents galactose-induced cataracts. J.Diabetes Complications. 1993;7:44–48. doi: 10.1016/1056-8727(93)90023-r. [DOI] [PubMed] [Google Scholar]
- 22.Mizisin AP, Shelton GD, Wagner S, Rusbridge C, Powell HC. Myelin splitting, Schwann cell injury and demyelination in feline diabetic neuropathy. Acta Neuropathol. 1998;95:171–174. doi: 10.1007/s004010050783. [DOI] [PubMed] [Google Scholar]
- 23.Nakashima E, Pop-Busui R, Towns R, Thomas TP, Hosaka Y, Nakamura J, Greene DA, Killen PD, Schroeder J, Larkin DD, Ho YL, Stevens MJ. Regulation of the human taurine transporter by oxidative stress in retinal pigment epithelial cells stably transformed to overexpress aldose reductase. Antioxid.Redox.Signal. 2005;7:1530–1542. doi: 10.1089/ars.2005.7.1530. [DOI] [PubMed] [Google Scholar]
- 24.Nandhini AT, Thirunavukkarasu V, Anuradha CV. Stimulation of glucose utilization and inhibition of protein glycation and AGE products by taurine. Acta Physiol Scand. 2004;181:297–303. doi: 10.1111/j.1365-201X.2004.01287.x. [DOI] [PubMed] [Google Scholar]
- 25.Nandhini TA, Anuradha CV. Inhibition of lipid peroxidation, protein glycation and elevation of membrane ion pump activity by taurine in RBC exposed to high glucose. Clin.Chim.Acta. 2003;336:129–135. doi: 10.1016/s0009-8981(03)00337-1. [DOI] [PubMed] [Google Scholar]
- 26.Obrosova IG. Diabetes and the peripheral nerve. Biochim.Biophys.Acta. 2008;1792:931–940. doi: 10.1016/j.bbadis.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Obrosova IG, Drel VR, Pacher P, Ilnytska O, Wang ZQ, Stevens MJ, Yorek MA. Oxidative-nitrosative stress and poly(ADP-ribose) polymerase (PARP) activation in experimental diabetic neuropathy: the relation is revisited. Diabetes. 2005;54:3435–3441. doi: 10.2337/diabetes.54.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obrosova IG, Fathallah L, Stevens MJ. Taurine counteracts oxidative stress and nerve growth factor deficit in early experimental diabetic neuropathy. Exp.Neurol. 2001;172:211–219. doi: 10.1006/exnr.2001.7789. [DOI] [PubMed] [Google Scholar]
- 29.Obrosova IG, Minchenko AG, Marinescu V, Fathallah L, Kennedy A, Stockert CM, Frank RN, Stevens MJ. Antioxidants attenuate early up regulation of retinal vascular endothelial growth factor in streptozotocin-diabetic rats. Diabetologia. 2001;44:1102–1110. doi: 10.1007/s001250100631. [DOI] [PubMed] [Google Scholar]
- 30.Obrosova IG, Pacher P, Szabo C, Zsengeller Z, Hirooka H, Stevens MJ, Yorek MA. Aldose reductase inhibition counteracts oxidative-nitrosative stress and poly(ADP-ribose) polymerase activation in tissue sites for diabetes complications. Diabetes. 2005;54:234–242. doi: 10.2337/diabetes.54.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okada S, Saito M, Kazuyama E, Hanada T, Kawaba Y, Hayashi A, Satoh K, Kanzaki S. Effects of N-hexacosanol on nitric oxide synthase system in diabetic rat nephropathy. Mol.Cell Biochem. 2008;315:169–177. doi: 10.1007/s11010-008-9804-7. [DOI] [PubMed] [Google Scholar]
- 32.Onozato ML, Tojo A, Goto A, Fujita T, Wilcox CS. Oxidative stress and nitric oxide synthase in rat diabetic nephropathy: effects of ACEI and ARB. Kidney Int. 2002;61:186–194. doi: 10.1046/j.1523-1755.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- 33.Park JW, Park SJ, Park SH, Kim KY, Chung JW, Chun MH, Oh SJ. Up-regulated expression of neuronal nitric oxide synthase in experimental diabetic retina. Neurobiol.Dis. 2006;21:43–49. doi: 10.1016/j.nbd.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Pereira EC, Neto HS, Marques MJ. Immunolocalisation of neuronal nitric oxide synthase at the neuromuscular junction of MDX mice: a confocal microscopy study. J.Anat. 2001;198:663–671. doi: 10.1046/j.1469-7580.2001.19860663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powell LA, Warpeha KM, Xu W, Walker B, Trimble ER. High glucose decreases intracellular glutathione concentrations and upregulates inducible nitric oxide synthase gene expression in intestinal epithelial cells. J.Mol.Endocrinol. 2004;33:797–803. doi: 10.1677/jme.1.01671. [DOI] [PubMed] [Google Scholar]
- 36.Price SA, Agthong S, Middlemas AB, Tomlinson DR. Mitogen-activated protein kinase p38 mediates reduced nerve conduction velocity in experimental diabetic neuropathy: interactions with aldose reductase. Diabetes. 2004;53:1851–1856. doi: 10.2337/diabetes.53.7.1851. [DOI] [PubMed] [Google Scholar]
- 37.Purves T, Middlemas A, Agthong S, Jude EB, Boulton AJ, Fernyhough P, Tomlinson DR. A role for mitogen-activated protein kinases in the etiology of diabetic neuropathy. FASEB J. 2001;15:2508–2514. doi: 10.1096/fj.01-0253hyp. [DOI] [PubMed] [Google Scholar]
- 38.Ramana KV, Friedrich B, Bhatnagar A, Srivastava SK. Aldose reductase mediates cytotoxic signals of hyperglycemia and TNF-alpha in human lens epithelial cells. FASEB J. 2003;17:315–317. doi: 10.1096/fj.02-0568fje. [DOI] [PubMed] [Google Scholar]
- 39.Schaffer SW, Azuma J, Mozaffari M. Role of antioxidant activity of taurine in diabetes. Can.J.Physiol Pharmacol. 2009;87:91–99. doi: 10.1139/Y08-110. [DOI] [PubMed] [Google Scholar]
- 40.Schleicher ED, Weigert C. Role of the hexosamine biosynthetic pathway in diabetic nephropathy. Kidney Int.Suppl. 2000;77:S13–S18. doi: 10.1046/j.1523-1755.2000.07703.x. [DOI] [PubMed] [Google Scholar]
- 41.Song Z, Fu DT, Chan YS, Leung S, Chung SS, Chung SK. Transgenic mice overexpressing aldose reductase in Schwann cells show more severe nerve conduction velocity deficit and oxidative stress under hyperglycemic stress. Mol.Cell Neurosci. 2003;23:638–647. doi: 10.1016/s1044-7431(03)00096-4. [DOI] [PubMed] [Google Scholar]
- 42.Stavniichuk R, Drel VR, Shevalye H, Vareniuk I, Stevens MJ, Nadler JL, Obrosova IG. Role of 12/15-lipoxygenase in nitrosative stress and peripheral prediabetic and diabetic neuropathies. Free Radic.Biol.Med. 2010;49(6):1036–45. doi: 10.1016/j.freeradbiomed.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevens MJ, Hosaka Y, Masterson JA, Jones SM, Thomas TP, Larkin DD. Downregulation of the human taurine transporter by glucose in cultured retinal pigment epithelial cells. Am.J.Physiol. 1999;277:E760–E771. doi: 10.1152/ajpendo.1999.277.4.E760. [DOI] [PubMed] [Google Scholar]
- 44.Stevens MJ, Lattimer SA, Kamijo M, Van HC, Sima AA, Greene DA. Osmotically-induced nerve taurine depletion and the compatible osmolyte hypothesis in experimental diabetic neuropathy in the rat. Diabetologia. 1993;36:608–614. doi: 10.1007/BF00404069. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki T, Mizuno K, Yashima S, Watanabe K, Taniko K, Yabe-Nishimura C. Characterization of polyol pathway in Schwann cells isolated from adult rat sciatic nerves. J.Neurosci.Res. 1999;57:495–503. [PubMed] [Google Scholar]
- 46.Takeda M, Mori F, Yoshida A, Takamiya A, Nakagomi S, Sato E, Kiyama H. Constitutive nitric oxide synthase is associated with retinal vascular permeability in early diabetic rats. Diabetologia. 2001;44:1043–1050. doi: 10.1007/s001250100588. [DOI] [PubMed] [Google Scholar]
- 47.Thomas PK, Lascelles RG. Schwann-cell abnormalities in diabetic neuropathy. Lancet. 1965;1:1355–1357. doi: 10.1016/s0140-6736(65)92154-9. [DOI] [PubMed] [Google Scholar]
- 48.Tomlinson DR, Gardiner NJ. Diabetic neuropathies: components of etiology. J.Peripher.Nerv.Syst. 2008;13:112–121. doi: 10.1111/j.1529-8027.2008.00167.x. [DOI] [PubMed] [Google Scholar]
- 49.Trachtman H, Futterweit S, Bienkowski RS. Taurine prevents glucose-induced lipid peroxidation and increased collagen production in cultured rat mesangial cells. Biochem.Biophys.Res.Commun. 1993;191:759–765. doi: 10.1006/bbrc.1993.1282. [DOI] [PubMed] [Google Scholar]
- 50.Vareniuk I, Pacher P, Pavlov IA, Drel VR, Obrosova IG. Peripheral neuropathy in mice with neuronal nitric oxide synthase gene deficiency. Int.J.Mol.Med. 2009;23:571–580. doi: 10.3892/ijmm_00000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vareniuk I, Pavlov IA, Obrosova IG. Inducible nitric oxide synthase gene deficiency counteracts multiple manifestations of peripheral neuropathy in a streptozotocin-induced mouse model of diabetes. Diabetologia. 2008;51:2126–2133. doi: 10.1007/s00125-008-1136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vincent AM, McLean LL, Backus C, Feldman EL. Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. FASEB J. 2005;19:638–640. doi: 10.1096/fj.04-2513fje. [DOI] [PubMed] [Google Scholar]
- 53.Yabuki A, Tahara T, Taniguchi K, Matsumoto M, Suzuki S. Neuronal nitric oxide synthase and cyclooxygenase-2 in diabetic nephropathy of type 2 diabetic OLETF rats. Exp.Anim. 2006;55:17–25. doi: 10.1538/expanim.55.17. [DOI] [PubMed] [Google Scholar]
- 54.Yoshimura H, Nariai Y, Terashima M, Mitani T, Tanigawa Y. Taurine suppresses platelet-derived growth factor (PDGF) BB-induced PDGF-beta receptor phosphorylation by protein tyrosine phosphatase-mediated dephosphorylation in vascular smooth muscle cells. Biochim.Biophys.Acta. 2005;1745:350–360. doi: 10.1016/j.bbamcr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 55.Yuan Z, Feng W, Hong J, Zheng Q, Shuai J, Ge Y. p38MAPK and ERK promote nitric oxide production in cultured human retinal pigmented epithelial cells induced by high concentration glucose. Nitric.Oxide. 2009;20:9–15. doi: 10.1016/j.niox.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Zdychova J, Komers R. Emerging role of Akt kinase/protein kinase B signaling in pathophysiology of diabetes and its complications. Physiol Res. 2005;54:1–16. doi: 10.33549/physiolres.930582. [DOI] [PubMed] [Google Scholar]