Abstract
BACKGROUND
Alcohol dependence is associated with neurocognitive deficits related to neuropathological changes in structure, metabolism, and function of the brain. Impairments of motor functioning in alcoholics have been attributed to well-characterized neuropathological brain abnormalities in cerebellum.
METHODS
Using functional magnetic resonance imaging (fMRI), we studied in vivo the functional connectivity between cerebellar and cortical brain regions. Participants were 10 uncomplicated chronic alcoholic patients studied after 5–7 days of abstinence when signs of withdrawal had abated, and 10 matched healthy controls. We focused on regions of prefrontal, frontal, temporal, and parietal cortex that exhibited an fMRI response associated with non-dominant hand finger tapping in the patients but not in the controls. We predicted that fronto-cerebellar functional connectivity would be diminished in alcoholics compared to controls.
RESULTS
Functional connectivity in a circuit involving premotor areas (Brodmann Area 6) and Lobule VI of the superior cerebellum was reduced in the patients compared to the controls. Functional connectivity was also reduced in a circuit involving prefrontal cortex (Brodmann Area 9) and Lobule VIII of the inferior cerebellum. Reductions in connectivity were specific to fronto-cerebellar circuits and were not found in other regions examined.
CONCLUSIONS
Our findings show a pattern in recently abstinent alcoholic patients of specific deficits in functional connectivity and recruitment of additional brain regions for performance of a simple finger tapping task. A small sample, differences in smoking, and a brief abstinence period preclude definitive conclusions, but this pattern of diminished fronto-cerebellar functional connectivity is highly compatible with the characteristic neuropathological lesions documented in alcoholics, and may reflect brain dysfunction associated with alcoholism.
Keywords: Alcohol, alcoholism, functional MRI, motor, connectivity
Introduction
Alcohol dependence is associated with deficits in a broad range of neurocognitive functions (Bowden 1990, Eckardt and Martin 1986, Fein et al. 2006, Oscar-Berman et al. 2004, Parsons and Nixon 1998, Sullivan et al. 2000). Although the neuropathological underpinnings of these deficits are incompletely understood, evidence from brain autopsy studies indicates that the cerebellum is most susceptible to brain injury associated with chronic alcohol consumption and associated malnutrition (Harper 1998). Findings from brain magnetic resonance imaging and spectroscopic studies suggest that damage due to chronic alcohol exposure in cerebellar regions is also associated with frontal lobe abnormalities (Adams et al. 1995, Martin et al. 1992, Parks et al. 2002, Seitz et al. 1999, Sullivan 2003, Sullivan et al. 2003). For example, glucose utilization as measured with positron emission tomography is slightly different in chronic alcoholic patients compared to controls in brain regions including the cerebellum (Adams et al. 1995, Gilman et al. 1990), and normal correlations between glucose utilization in frontal and cerebellar regions are diminished in these patients (Martin et al. 1992). Moreover, morphometric analyses of MR images in alcohol-naive high risk subjects also implicate cerebellum and frontal lobe (Benegal et al. 2007, Hill et al. 2007). Some diffusion tensor imaging studies also suggest that white matter changes in alcoholism, while rather widespread, show some proclivity for the frontal lobes (Pfefferbaum et al. 2006, Yeh et al. 2009). A recent review suggests that dysfunction of cortico-thalamo-cerebellar circuitry may be a major predisposing factor in alcoholism (Tessner and Hill 2010). Though it remains to be determined whether all of the identified deficits are precursors or consequences of alcohol consumption and related lifestyle factors, these reports highlight that alcohol dependence is associated with anatomical and functional abnormalities of frontal and cerebellar regions of the brain.
Impairment of performance on finger-tapping tasks is representative of neurocognitive deficits in alcoholic patients (Parks et al. 2010, Parks et al. 2003, Welch et al. 1997). Achieved finger tapping speed is correlated with performance on cognitive tasks that measure frontal lobe functions, especially in alcoholics with significant neurocognitive deficits, supporting the presence of significant interactions between frontal and cerebellar brain regions (Welch et al. 1997). Alcoholic patients also have exhibited less strongly lateralized functional magnetic resonance imaging (fMRI) responses to self-paced finger tapping, with the spatial extent of fMRI activation being greater for a given motor response compared to controls (Parks et al. 2003). This was more apparent during use of the non-dominant hand compared to the dominant hand, and indeed lesser BOLD signal differences between dominant and non-dominant hand were observed in the patient group. Similar findings were noted during an externally paced finger-tapping task, where alcoholic patients have shown increased fMRI responses in frontal and parietal lobes as well as areas of activation not present in controls (Parks et al. 2010). Since finger movements involve frontal and cerebellar systems, these results taken together suggest that finger tapping is a simple and easily implemented task that interrogates the integrity of fronto-cerebellar circuits.
In this study, we examined the functional connectivity of fronto-cerebellar circuits in uncomplicated chronic alcoholic patients. Specifically, we examined those brain regions that we had previously reported (Park et al. 2010) were required by alcoholic patients for simple finger tapping but were not activated in control subjects performing the same task. Some of the regions uniquely activated in alcoholics were in the frontal lobe. and therefore, we reasoned the connectivity of these regions to cerebellum may be differently configured than in controls. A different degree of linkage between cerebellum and frontal cortex may partially explain the mechanism of motor performance deficits observed in alcoholics. We studied patients after detoxification and 5–7 days of abstinence to avoid acute effects of alcohol consumption or withdrawal on brain functioning and hemodynamics. Connectivity measures were based on low frequency signals in residual time series data after removing task-related responses. Our specific hypothesis was that fronto-cerebellar functional connectivity would be diminished in alcoholics compared to controls, a reasonable prediction based on the high prevalence of cerebellar lesions observed at autopsy in alcoholics.
Materials and Methods
Ethics Statement
All participants in this study gave written informed consent to the study procedures in a protocol approved by the Vanderbilt University Medical Center (VUMC) Institutional Review Board.
Participants
Ten right-handed uncomplicated chronic alcohol-dependent patients and ten healthy controls matched for age and gender participated in this study. Participant characteristics are listed in Table 1. This sample has been described in detail in our previous publication (Parks et al. 2010). Patients were recruited from the Vanderbilt Addiction Center within 24 hours of voluntary admission for alcohol detoxification and rehabilitation and were scanned after 5–7 days of abstinence when signs of acute withdrawal had completely abated. Alcohol consumption has acute effects on brain hemodynamics that could affect group comparisons in fMRI studies (Luchtmann et al. 2010). The delay prior to scanning was intended to minimize the effect of this variable on our findings, though it may not completely preclude residual effects and is still too brief to permit study of longer term recovery. A senior psychiatrist (PRM) interviewed all patients and reviewed their medical histories and laboratory tests to determine that they were free of any other Axis I diagnoses (Diagnostic and Statistical Manual of Mental Disorders, DSM-IV: American Psychiatric Association, 2000). Patients met DSM-IV criteria for alcohol dependence with physiologic dependence; were between 18 and 70 years of age; and had an estimated pre-morbid Full Scale Intelligence Quotient of at least 85. Patients consumed alcohol according to their customary pattern and intensity until approximately 48 hours prior to admission. Alcoholic patients were excluded from participation if they had a history within the last 6 months of any other DSM-IV substance dependence (except nicotine), or a major severe psychiatric disorder prior to the onset of alcohol dependence or during periods of abstinence of 6 months or more. Patients were excluded if they had any neurological disorders not directly related to alcoholism or any contraindications to MR examination.
Table 1. Participant characteristics.
Variables are reported as mean and (standard deviation).
| Variable | Alcoholic (n=10) | Control (n=10) |
|---|---|---|
| Age (years) | 43 (12) | 40 (13) |
| Gender (% male) | 70% | 70% |
| Education (years) | 14 (2) * | 16 (2) |
| Estimated IQ | 106 (8) | 112 (6) |
| Average drinks per day | 9.6 (3.5) ** | 0.2 (0.3) |
| Total years drinking | 18 (7) ** | 9 (12) |
| Cigarette smoker (%) | 90% ** | 20% |
p<0.05;
p<0.01 comparing alcoholic and control groups.
Ten right-handed healthy controls were recruited from the Vanderbilt campus by Internet postings, as well as by word of mouth. They were enrolled in the study if they had no history of alcohol or substance abuse or any other psychiatric disorder; no known neurological disorders, including head trauma; no medical condition known to affect brain functioning; no medications known to affect brain functions within one week of testing; no alcohol use within 24 hours prior to study; and no known contraindications to MR procedures. Controls were matched to patients for age and gender, but not cigarette smoking.
Image acquisition and task
Images were acquired at 3 Tesla. A high-resolution T1 weighted anatomical image with 1 mm3 isotropic resolution was acquired for each subject. Functional MRI data used a 2D BOLD-weighted gradient echo EPI sequence: TR 2000 ms, TE 35ms, 80×80 matrix reconstructed at 128×128, FOV 240 mm in plane, 28 4.5-mm slices with a 0.45 mm gap, 150 volumes per run. Subjects were instructed to tap fingers in response to visual cues. The cues were presented via video and consisted of a leftward arrow in the left visual hemifield, or a rightward arrow in the right hemifield, to indicate which hand was to be used. The arrow flashed at 2 Hz. Tapping was performed in 20 second blocks alternating with a 20-sec cursor that cued no movement. Responses were made via a button under the index fingers. Two 5-minute runs each contained three task conditions (left tapping, right tapping, and fixation). Condition order was counterbalanced.
Image preprocessing
Preprocessing of the functional images consisted of slice timing correction, motion correction, registration to the MNI152 template space, and spatial smoothing. These operations were performed using the SPM5 software (Wellcome Department of Imaging Neuroscience, University College London). Data for each slice were shifted in time to align temporally with the inferior-most slice. Then each volume was rigidly coregistered with the others, within run and between runs, to reduce the effects of head motion and produce an estimate of motion amplitude. Each subject’s high-resolution anatomical image was rigidly coregistered with the subject’s functional images, then non-linearly registered to the MNI template image using a mutual information criterion. The nonlinear transformation’s spatial basis functions had minimum period 25 mm. The nonlinear registration was then applied to the functional images, which were then smoothed with an 8 mm FWHM Gaussian kernel.
Data quality assessment
Head motion in every subject was less than 3 mm of translation and less than 3 degrees of rotation relative to the first volume. Maximum extent of head motion did not differ between patients and controls in most comparisons (p > 0.08, except second run translation p=0.03). Visual inspection of the intensity distributions of the residual variance images revealed no group differences in noise. The residual variance was not different between groups (p>0.58).
Activation analysis
BOLD responses to finger tapping were measured as previously described (Parks et al. 2010). Individual subject BOLD responses were calculated by fitting a voxelwise general linear model with regressors predicting BOLD response for the left and right finger tapping conditions, using a dual gamma hemodynamic response model. Parametric maps of BOLD response for right and left tapping conditions relative to fixation were calculated from contrasts of the regression parameters. Group-level statistical parametric maps were obtained from the individual subject maps of the contrasts of left tapping versus rest and right tapping versus rest. A second-level general linear model was used to compare patients and controls in the whole brain. In that analysis, reported previously (Parks et al. 2010), we found that during left hand (non-dominant) finger tapping, controls showed no regions of activation greater than the patients (contrasting controls>patients). However, alcoholic patients showed BOLD responses in areas of right parietal, right premotor, right prefrontal, and left temporal cortex where controls showed no statistically significant responses (contrasting patients>controls, excluding voxels where controls>0). These four areas (shown in Figure 1A and Table 2) were used as the seed regions for subsequent connectivity analysis. The seed regions were centered on the peak voxels from the statistical map comparing BOLD responses to finger tapping in patients versus controls, and included all significant voxels from the patient versus control contrast (p<0.001) within 8 mm of the peak.
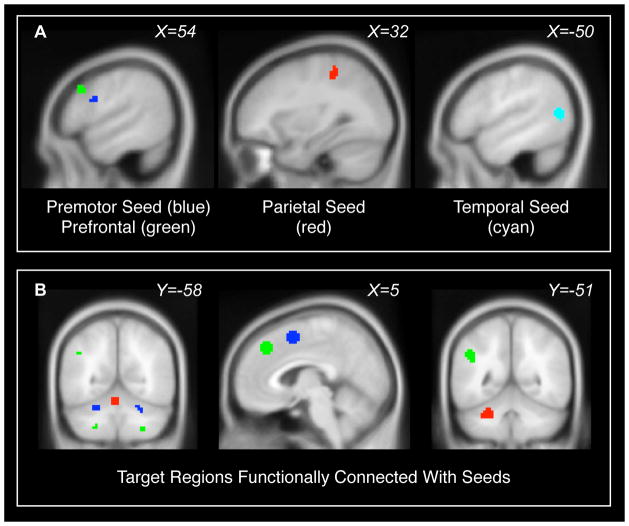
Figure 1. Regions of interest.
(A) Seed regions were areas where patients exhibited fMRI responses to finger tapping but controls did not. Seed regions were located in right premotor cortex (blue), right prefrontal cortex (green), right parietal cortex (red), and left temporal cortex (cyan). Seed regions are further described in Table 2. (B) Additional target regions of interest in cerebellum, preSMA, medial frontal cortex, and left parietal lobe were defined from the connectivity maps of the seeds, using voxels that exhibited significant intrinsic functional connectivity during the finger-tapping experiment (including both patients and controls, p<0.001 uncorrected). These are color-coded according to the seed region that produced them. The target regions for the middle temporal seed are not shown; they overlap with the target regions for the premotor seed. Target regions are further described in Table 4.
Table 2. Seed regions for connectivity analysis, where patients exhibited fMRI responses to non-dominant hand finger tapping that were not present in controls.
The seed regions exhibited a higher fMRI response to finger tapping in patients compared to controls (p<0.05 corrected), but no response in the controls (p>0.05 uncorrected). The seeds included all voxels within 8 mm of the peak where the patient-control difference was significant at p<0.001 uncorrected.
| Seed Region | Peak Voxel MNI Coords (mm) |
|---|---|
| R Premotor | 60, 9, 27 |
| R Prefrontal | 48, 21, 36 |
| R Parietal | 33, −36, 54 |
| L Temporal | −51, −66, 9 |
Connectivity analysis
Seed region connectivity maps
The seed regions for connectivity analysis were the four areas shown in Figure 1A and Table 2, derived from the comparison between patients and controls as described above. These regions exhibited finger tapping activation in patients but not controls, and we focused on these to determine if differences in functional connectivity would explain or relate to the observed differences in activation. Our goal was to measure the seed regions’ connectivity during the finger tapping scans with signals driven by the task’s block design removed. To accomplish this, the time series of each seed region was extracted from the preprocessed images. A new design matrix was created to model this time series: each task block (left or right hand tapping) was modeled with a separate regressor created by convolving a boxcar with a canonical hemodynamic response. Additional regressors were added to account for global signal, drift, and head motion. The residual time series after fitting this model was low-passed filtered to remove signals above 0.1 Hz. This filtered residual seed region time series was then included as an additional predictor in the individual subject general linear model that had been used for the activation analysis. The map of the parameter estimate for this new predictor, calculated for each run of each subject, was the connectivity map for the seed region, with any effects of stimulus, head motion, global signal, and high-frequency noise removed. These connectivity maps were averaged over the two runs within each subject, then combined at the group level to create statistical parametric maps (T) that showed voxels exhibiting functional connectivity with each seed over all subjects disregarding group.
Target regions
Target regions of interest were defined in the cerebellum to investigate specific hypotheses regarding fronto-cerebellar connectivity. The target regions were defined from the seed region connectivity maps, including voxels within 8 mm of local maxima that exhibited functional connectivity at a voxelwise threshold of p<0.001. The target regions are shown in Figure 1B, and their coordinates are listed in Tables 3 and 4. We used the same procedure to select control target regions to verify that group differences in connectivity were specific to cerebellum. The arbitrary control regions were dorsal medial frontal cortex and left parietal cortex for the right prefrontal seed, and preSMA for the right premotor seed.
Table 3. Functional connectivity was lower in alcohol-dependent patients than in matched controls.
Tests of the a priori hypothesis regarding functional connectivity in fronto-parietal circuits are reported. Connectivity for each region pair is expressed as a regression coefficient with 95% confidence interval shown in parentheses. The seed regions were selected because they showed finger tapping activation in patients but not in controls. The target regions were selected because they showed positive functional connectivity with the corresponding seed on average over all subjects regardless of group (p<0.001 uncorrected).
| Region 1 (Seed) | Region 2 (Target) | Controls | Patients | Difference, Controls - Patients | Region 2 MNI Coords (mm) |
|---|---|---|---|---|---|
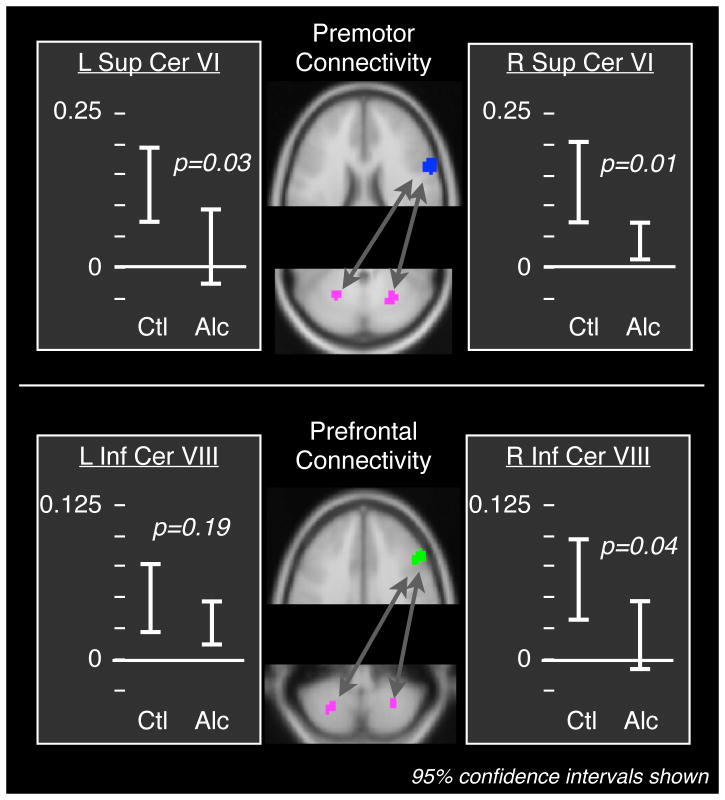
| Right Premotor | R Cerebellum VI | 0.14 (0.07,0.21) | 0.04 (0.01,0.08) | 0.10 (0.03,0.17), p=0.01 | 24, −63, −27 |
| L Cerebellum VI | 0.14 (0.07,0.20) | 0.04 (−0.03,0.10) | 0.10 (0.01,0.18), p=0.03 | −27, −57, −27 | |
| Right Prefrontal | R Cerebellum VIII | 0.06 (0.03,0.10) | 0.02 (−0.01,0.05) | 0.04 (0.00,0.08), p=0.04 | 30, −60, −48 |
| L Cerebellum VIII | 0.05 (0.02,0.08) | 0.03 (0.01,0.05) | 0.02 (−0.01,0.05), p=0.19 | −27, −63, −48 |
Table 4. Alcoholism-related differences in connectivity were specific to fronto-cerebellar circuits.
Post hoc tests of connectivity in circuits other than fronto-parietal are reported to serve as a control analysis. Connectivity for each region pair is expressed as a regression coefficient with 95% confidence interval shown in parentheses. The seed regions were selected because they showed finger tapping activation in patients but not in controls. The target regions were selected because they showed positive functional connectivity with the corresponding seed on average over all subjects regardless of group (p<0.001 uncorrected).
| Region 1 (Seed) | Region 2 (Target) | Controls | Patients | Difference, Controls - Patients | Region 2 MNI Coords (mm) |
|---|---|---|---|---|---|
| Right Premotor | PreSMA | 0.13 (0.09,0.17) | 0.11 (0.05,0.17) | 0.02 (−0.05,0.09), p=0.53 | 9, 5, 54 |
| Right Prefrontal | Med. Dorsal Prefrontal | 0.14 (0.10,0.18) | 0.12 (0.06,0.18) | 0.03 (−0.04,0.09), p=0.44 | 3, 36, 42 |
| Left Parietal | 0.05 (0.02,0.07) | 0.05 (0.01,0.09) | 0.00 (−0.05,0.04), p=0.84 | −45, −51, 33 | |
| Right Parietal | L Cerebellum VI | 0.11 (0.02,0.20) | 0.15 (0.07,0.22) | −0.04 (−0.15,0.07), p=0.48 | −27, −48, −33 |
| Cerebellar Vermis | 0.11 (0.02,0.20) | 0.14 (0.07,0.21) | −0.03 (−0.14,0.08), p=0.61 | −3, −63, −18 | |
| Left Temporal | R Cerebellum VI | 0.08 (0.03,0.13) | 0.08 (0.03,0.13) | 0.00 (−0.07,0.06), p=0.91 | 24, −60, −27 |
| L Cerebellum VI | 0.09 (0.03,0.14) | 0.09 (0.04,0.14) | 0.00 (−0.07,0.06), p=0.90 | −24, −57, −24 |
Statistical analysis
The outcome of interest was the difference in connectivity between patients and controls; to test for this, we used a two-sample T test for each combination of seed and target regions (results are presented in Table 3 for the a priori hypothesis and Table 4 for control regions). The connectivity value in each case was the parameter estimate for the seed time series regressor, averaged within the target region. We also calculated 95% confidence intervals for the connectivity values in each subject group. Because of the small sample size, we limited connectivity analysis to the a priori regions of interest and did not perform whole brain comparisons.
Results
Our goal was to measure the functional connectivity of brain regions recruited for finger tapping by alcoholic patients but not controls, with particular emphasis on the cerebellum. Connectivity measures were based on low frequency signals in residual time series data after removing task-related responses.
The seed regions for connectivity analyses are shown in Figure 1A, with coordinates listed in Table 2. The right prefrontal seed exhibited functional connectivity with Lobule VIII in inferior cerebellum bilaterally. The right premotor and left temporal seeds exhibited connectivity with Lobule VI in superior cerebellum bilaterally. The right parietal seed exhibited connectivity with Lobule VI in left superior cerebellum and with superior cerebellar vermis. Target regions of interest were defined from the connectivity maps of the seed regions. We focused on the cerebellum: the right prefrontal seed exhibited connectivity with Lobule VIII in bilateral inferior cerebellum; the right premotor seed with Lobule VI in bilateral superior cerebellum; the right parietal seed with left Lobule VI and cerebellar vermis; and the left temporal seed with Lobule VI bilaterally. We also included preSMA, medial frontal, and left parietal regions as controls. The target regions are shown in Figure 1B, and their coordinates are listed in Tables 3 and 4. The target regions were derived from the connectivity maps averaged over all subjects regardless of group.
Fronto-cerebellar functional connectivity was lower in alcohol-dependent patients than in the matched healthy controls. As fronto-cerebellar connectivity was our primary interest, our first statistical analyses were of connectivity between the right premotor and prefrontal seeds and the cerebellar target regions. The seed regions had exhibited increased fMRI response to finger tapping in patients, as described above. The target cerebellar regions of interest were defined from the connectivity map of the corresponding seed. Connectivity between seed and target regions was generally significant within each group (p<0.05) as indicated by the 95% confidence intervals reported in Tables 3 and 4. The exceptions were connectivity between the right premotor seed and Lobule VI, and connectivity between the right prefrontal seed and Lobule VIII, in the patient group. Premotor-cerebellar connectivity was significantly weaker in patients than controls for both right (p=0.01) and left (p=0.03) Lobule VI in superior cerebellum (Table 3, Figure 2). Prefrontal-cerebellar connectivity was significantly weaker in patients for right Lobule VIII in inferior cerebellum (p=0.04), and tended to be weaker for left Lobule VIII (p=0.19).
Figure 2. Fronto-cerebellar functional connectivity was lower in alcoholic patients than in matched healthy controls.
The seed regions are indicated in blue (premotor) and green (prefrontal); the cerebellar target regions are magenta. The alcoholic patients exhibited a significant reduction in connectivity between premotor cortex and Lobule VI in superior cerebellum, and between prefrontal cortex and Lobule VIII in inferior cerebellum (see Table 3). Connectivity between a number of other regions did not differ between patients and controls (Table 4), suggesting that the result was specific to fronto-cerebellar circuits.
Diminished connectivity in alcohol-dependent patients was specific to the fronto-cerebellar circuits. To verify this, we followed up the previously described fronto-cerebellar analysis by measuring connectivity between the right premotor seed and the preSMA; between the right prefrontal seed and the dorso-medial frontal gyrus; between the right prefrontal seed and the left parietal cortex; between the right parietal seed and the cerebellum; and between the left temporal seed and the cerebellum. Each comparison region was defined from the all-subject connectivity maps in the same way as the other target regions. No differences in connectivity were found between the patients and the controls in these non-fronto-cerebellar circuits (p>=0.44, Table 4).
Discussion
Our main finding was a reduction of functional connectivity specifically in fronto-cerebellar neural circuits in uncomplicated detoxified alcohol-dependent patients compared to age- and gender-matched controls. Connectivity in alcoholics was reduced between prefrontal cortex (Brodmann Area 9) and Lobule VIII of inferior cerebellum, and between premotor areas (Brodmann Area 6) and Lobule VI of superior cerebellum. The reduction was specific to the fronto-cerebellar circuits: the connectivity between prefrontal and premotor cortex and several non-cerebellar control regions was not different between alcoholic patients and controls, nor was the connectivity between parietal cortex and cerebellum or temporal cortex and cerebellum. These findings add to prior work by demonstrating that compromised interactions between cerebellar and frontal regions may be an important aspect of the brain impairments associated with chronic alcohol dependence.
A number of neuroimaging studies have identified structural or functional abnormalities in frontal or cerebellar regions in alcoholics. For example, reductions in frontal lobe glucose metabolic rates correlate with cognitive performance (Adams et al. 1995); glucose metabolism is abnormal in frontal, parietal, and cerebellar regions (Martin et al. 1992); the concentrations of N-acetyl-aspartate and choline change in cerebellum (Seitz et al. 1999) and tend to normalize with abstinence (Martin et al. 1995, Parks et al. 2002); and there are behavior-correlated gray matter volume differences in nodes of fronto-cerebellar circuits, including the cerebellar hemispheres as well as prefrontal cortex, frontal cortex, and parietal cortex (Sullivan et al. 2003). Neuroanatomic tracer studies in non-human primates indicate the existence of a circuit between motor cortex and anterior cerebellum, distinct from a circuit between prefrontal cortex and posterior cerebellum (Sullivan and Pfefferbaum 2005). FMRI functional connectivity patterns of the human cerebellum reflect this distinction (Krienen and Buckner 2009, O’Reilly et al. 2010). Both of these fronto-cerebellar circuits showed deficits in connectivity in alcoholic patients in this study. Also, a recent study has shown decreased connectivity of cerebellar circuits with posterior cingulate in alcoholic patients (Chanraud et al. 2011).
The reduction in fronto-cerebellar functional connectivity may reflect alcoholism-related neuronal injury. Cerebellar deficits are known concomitants of alcohol dependence, related to nutritional deficits associated with chronic alcohol consumption (Martin et al. 2003). These include reduced cerebellar volume, decreased cell density, and neurochemical changes (Sullivan et al. 2003, Sullivan and Pfefferbaum 2005). Deficits in prefrontal cortex include reduced volume in alcoholic patients (Sullivan and Pfefferbaum 2005). Deficits in motor cortex have been less apparent, but there may be mild neuronal loss (Harper and Kril 1989, Kril et al. 1997) – but see Fabricius et al. (2007) – or alterations in neuron size and arborization (Harper and Corbett 1990). FMRI responses in motor cortex are affected in alcoholics (Parks et al. 2010, Parks et al. 2003), possibly reflecting neurocognitive inefficiencies due to pre-existing or alcohol-related impairments. A recent report of reduced functional connectivity in alcohol-naïve youth with a family history of alcohol abuse in fronto-cerebellar circuits adjacent to the ones we studied (Herting et al. 2010) supports our findings and also suggests that the abnormalities described herein may have their origins prior to the start of drinking. Hence, it will be important to determine whether the reduced connectivity we have observed normalizes or persists with longer periods of abstinence (Parks et al. 2002).
This study examined a small sample of uncomplicated alcoholics. The control group was matched for sex and age, but the generalizability of the findings may nonetheless be limited. Smoking and alcohol abuse raise methodological concerns in neuroimaging studies in general, but are especially problematic for efforts to isolate the effects of either agent as the two disorders tend to co-occur (Meyerhoff et al. 2006). Ninety percent of the patients in our sample were smokers, comparable to smoking prevalence among alcoholics in our region; whereas 20% of the controls were smokers, similar to the prevalence of smoking among non-alcoholics (Reich et al. 2008). Nicotine dependence is associated with changes in brain function and connectivity in neuroimaging studies (Azizian et al. 2009, Dager and Friedman 2000, Friedman et al. 2008, McClernon and Gilbert 2004). Additionally, nicotine may affect cognition differently in recovering alcoholics compared to non-alcoholic controls (Nixon et al. 2007). Smoking was not the focus of this study, but we cannot rule out smoking history as a confounding variable. However, the findings of (Herting et al. 2010) in youth who did not likely smoke to the degree of our sample suggest that our findings in adults are unlikely to be fully explained by differences in smoking between patients and controls. It is also unlikely that our observations can be explained by gender differences due to gender matching of the groups. Nevertheless, future studies are needed to determine how interactions of gender with alcohol dependence affect fronto-cerebellar connectivity, as important gender differences have been documented in alcoholism (Greenfield 2002, Schulte et al. 2009, Sullivan et al. 2010) as well as brain connectivity (Gong 2011, Tomasi and Volkow 2011).
Our data analysis approach had some limitations. First, functional connectivity was measured in the residuals of fMRI time series data after removing task effects. Connectivity measured this way is similar to resting state connectivity, but not always equivalent (Fair et al. 2007) because of the underlying cognitive orientation and the inability to perfectly remove stimulus responses. The connectivity results may therefore be too specific to the motor tasks to compare directly with resting state results in other studies. Measuring connectivity from the resting segments between blocks as has been previously suggested (Fair et al. 2007, Hampson et al. 2002) was not applicable for this experiment because of the short 20-second periods. However, we removed stimulus responses as cleanly as possible, and the similarities between our seed region connectivity maps and previous maps of resting-state connectivity with the cerebellum (Krienen and Buckner 2009) are reassuring. Second, the regions of interest were defined using the same fMRI data later used to calculate functional connectivity. However, the seed region definition was based on the differences between finger tapping blocks and rest blocks modeled with a set of regressors in a general linear model. The connectivity measures were calculated on the residuals. This means that, by construction, there was no correlation between the seed-defining signals and the signals used to calculate functional connectivity. The target regions were defined based on group-agnostic connectivity maps. For these reasons we do not expect that the patient/control comparisons in connectivity values were biased by the region selection procedures, and the absence of any global connectivity differences supports this. Third, because of the limited sample size, we analyzed only a few anatomical regions chosen a priori based on our hypothesis regarding recognized neuropathology in alcoholics. The functional deficits in alcoholism may be broader in extent than we have demonstrated, and may reflect changes in large-scale brain networks and their interactions. Recent observation of reduced connectivity in alcoholism between the posterior cingulate and cerebellum during rest and during a working memory task suggest that connectivity differences may also involve the large-scale network known as the default-mode network (Chanraud et al. 2011). Nevertheless, our connectivity findings are consistent with what independent neuropathological observations would predict, and suggest that dysfunction of the fronto-cerebellar network may be particularly relevant in alcoholism.
Our findings together demonstrate a pattern in recently abstinent alcoholic patients of specific deficits in functional connectivity and recruitment of additional brain regions for performance of a simple finger-tapping task. Diminished connectivity of prefrontal and premotor cortex with cerebellum may reflect the pattern of neuronal damage in alcoholism that might be predicted from the common occurrence of characteristic neuropathological lesions in the cerebellum among alcoholics. The small sample, differences in smoking, and brief abstinence period preclude definitive conclusions from our data, but this pattern of diminished functional connectivity is highly compatible with the characteristic neuropathological lesions documented in alcoholics.
Acknowledgments
PRM, MHP, and BPR conceived of the study and planned the analytic approach. MKN recruited and screened participants and administered neuropsychological batteries. BPR and SBK performed connectivity analyses of the imaging data. BPR drafted the manuscript, and all authors provided critical review. The Vanderbilt Institute for Clinical and Translational Research (VICTR) Clinical Research Center assisted with the study. Funding was provided by the National Institutes of Health: R01AA014969 (PRM, MKN, BPR); U01AA14939 (MHP); R01EB000461 (SBK); UL1RR024975 (VICTR).
References
- Adams KM, Gliman S, Koeppe RA, Kluin KJ, Junck L, Johnson-Greene D, Berent S, Dede D, Kroll P. Correlation of neuropsychological function with cerebral metabolic rate in subdivisions of frontal lobes of older alcoholic patients measured with [18F] fluorodeoxyglucose and positron emission tomography. Neuropsychology. 1995;9:275–280. [Google Scholar]
- Azizian A, Monterosso J, O’Neill J, London ED. Magnetic resonance imaging studies of cigarette smoking. Handb Exp Pharmacol. 2009:113–43. doi: 10.1007/978-3-540-69248-5_5. [DOI] [PubMed] [Google Scholar]
- Benegal V, Antony G, Venkatasubramanian G, Jayakumar PN. Gray matter volume abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addict Biol. 2007;12:122–32. doi: 10.1111/j.1369-1600.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- Bowden SC. Separating cognitive impairment in neurologically asymptomatic alcoholism from Wernicke-Korsakoff syndrome: is the neuropsychological distinction justified? Psychol Bull. 1990;107:355–66. doi: 10.1037/0033-2909.107.3.355. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Pfefferbaum A, Sullivan EV. Disruption of functional connectivity of the default-mode network in alcoholism. Cereb Cortex. 2011 doi: 10.1093/cercor/bhq297. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dager SR, Friedman SD. Brain imaging and the effects of caffeine and nicotine. Ann Med. 2000;32:592–9. doi: 10.3109/07853890009002029. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, Martin PR. Clinical assessment of cognition in alcoholism. Alcohol Clin Exp Res. 1986;10:123–7. doi: 10.1111/j.1530-0277.1986.tb05058.x. [DOI] [PubMed] [Google Scholar]
- Fabricius K, Pakkenberg H, Pakkenberg B. No changes in neocortical cell volumes or glial cell numbers in chronic alcoholic subjects compared to control subjects. Alcohol Alcohol. 2007;42:400–6. doi: 10.1093/alcalc/agm007. [DOI] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Torres J, Price LJ, Di Sclafani V. Cognitive performance in long-term abstinent alcoholic individuals. Alcohol Clin Exp Res. 2006;30:1538–44. doi: 10.1111/j.1530-0277.2006.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman L, Turner JA, Stern H, Mathalon DH, Trondsen LC, Potkin SG. Chronic smoking and the BOLD response to a visual activation task and a breath hold task in patients with schizophrenia and healthy controls. Neuroimage. 2008;40:1181–94. doi: 10.1016/j.neuroimage.2007.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S, Adams K, Koeppe RA, Berent S, Kluin KJ, Modell JG, Kroll P, Brunberg JA. Cerebellar and frontal hypometabolism in alcoholic cerebellar degeneration studied with positron emission tomography. Ann Neurol. 1990;28:775–85. doi: 10.1002/ana.410280608. [DOI] [PubMed] [Google Scholar]
- Gong G, He Y, Evans AC. Brain connectivity: Gender makes a difference. Neuroscientist. 2011 Apr 28; doi: 10.1177/1073858410386492. doi: 10.1177/1073858410386492. [DOI] [PubMed] [Google Scholar]
- Greenfield SF. Women and alcohol use disorders. Harv Rev Psychiatry. 2002;10:76–85. doi: 10.1080/10673220216212. [DOI] [PubMed] [Google Scholar]
- Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Hum Brain Mapp. 2002;15:247–62. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C. The neuropathology of alcohol-specific brain damage, or does alcohol damage the brain? J Neuropathol Exp Neurol. 1998;57:101–10. doi: 10.1097/00005072-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Harper C, Corbett D. Changes in the basal dendrites of cortical pyramidal cells from alcoholic patients--a quantitative Golgi study. J Neurol Neurosurg Psychiatry. 1990;53:856–61. doi: 10.1136/jnnp.53.10.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Kril J. Patterns of neuronal loss in the cerebral cortex in chronic alcoholic patients. J Neurol Sci. 1989;92:81–9. doi: 10.1016/0022-510x(89)90177-9. [DOI] [PubMed] [Google Scholar]
- Herting MM, Fair D, Nagel BJ. Altered fronto-cerebellar connectivity in alcohol-naive youth with a family history of alcoholism. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.10.030. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Muddasani S, Prasad K, Nutche J, Steinhauer SR, Scanlon J, McDermott M, Keshavan M. Cerebellar volume in offspring from multiplex alcohol dependence families. Biol Psychiatry. 2007;61:41–7. doi: 10.1016/j.biopsych.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19:2485–97. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–98. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Luchtmann M, Jachau K, Tempelmann C, Bernarding J. Alcohol induced region-dependent alterations of hemodynamic response: implications for the statistical interpretation of pharmacological fMRI studies. Exp Brain Res. 2010;204:1–10. doi: 10.1007/s00221-010-2277-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PR, Gibbs SJ, Nimmerrichter AA, Riddle WR, Welch LW, Willcott MR. Brain proton magnetic resonance spectroscopy studies in recently abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:1078–82. doi: 10.1111/j.1530-0277.1995.tb00992.x. [DOI] [PubMed] [Google Scholar]
- Martin PR, Rio D, Adinoff B, Johnson JL, Bisserbe JC, Rawlings RR, Rohrbaugh JW, Stapleton JM, Eckardt MJ. Regional cerebral glucose utilization in chronic organic mental disorders associated with alcoholism. J Neuropsychiatry Clin Neurosci. 1992;4:159–67. doi: 10.1176/jnp.4.2.159. [DOI] [PubMed] [Google Scholar]
- Martin PR, Singleton CK, Hiller-Sturmhofel S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health. 2003;27:134–42. [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Gilbert DG. Human functional neuroimaging in nicotine and tobacco research: basics, background, and beyond. Nicotine Tob Res. 2004;6:941–59. doi: 10.1080/14622200412331337394. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, Tizabi Y, Staley JK, Durazzo TC, Glass JM, Nixon SJ. Smoking comorbidity in alcoholism: neurobiological and neurocognitive consequences. Alcohol Clin Exp Res. 2006;30:253–64. doi: 10.1111/j.1530-0277.2006.00034.x. [DOI] [PubMed] [Google Scholar]
- Nixon SJ, Lawton-Craddock A, Tivis R, Ceballos N. Nicotine’s effects on attentional efficiency in alcoholics. Alcohol Clin Exp Res. 2007;31:2083–91. doi: 10.1111/j.1530-0277.2007.00526.x. [DOI] [PubMed] [Google Scholar]
- O’Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex. 2010;20:953–65. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Kirkley SM, Gansler DA, Couture A. Comparisons of Korsakoff and non-Korsakoff alcoholics on neuropsychological tests of prefrontal brain functioning. Alcohol Clin Exp Res. 2004;28:667–75. doi: 10.1097/01.alc.0000122761.09179.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks MH, Dawant BM, Riddle WR, Hartmann SL, Dietrich MS, Nickel MK, Price RR, Martin PR. Longitudinal brain metabolic characterization of chronic alcoholics with proton magnetic resonance spectroscopy. Alcohol Clin Exp Res. 2002;26:1368–80. doi: 10.1097/01.ALC.0000029598.07833.2D. [DOI] [PubMed] [Google Scholar]
- Parks MH, Greenberg DS, Nickel MK, Dietrich MS, Rogers BP, Martin PR. Recruitment of additional brain regions to accomplish simple motor tasks in chronic alcohol-dependent patients. Alcohol Clin Exp Res. 2010;34:1098–109. doi: 10.1111/j.1530-0277.2010.01186.x. [DOI] [PubMed] [Google Scholar]
- Parks MH, Morgan VL, Pickens DR, Price RR, Dietrich MS, Nickel MK, Martin PR. Brain fMRI activation associated with self-paced finger tapping in chronic alcohol-dependent patients. Alcohol Clin Exp Res. 2003;27:704–11. doi: 10.1097/01.ALC.0000062759.14944.CF. [DOI] [PubMed] [Google Scholar]
- Parsons OA, Nixon SJ. Cognitive functioning in sober social drinkers: a review of the research since 1986. J Stud Alcohol. 1998;59:180–90. doi: 10.15288/jsa.1998.59.180. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiol Aging. 2006;27:994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Reich MS, Dietrich MS, Finlayson AJ, Fischer EF, Martin PR. Coffee and cigarette consumption and perceived effects in recovering alcoholics participating in Alcoholics Anonymous in Nashville, Tennessee, USA. Alcohol Clin Exp Res. 2008;32:1799–806. doi: 10.1111/j.1530-0277.2008.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte MT, Ramo D, Brown SA. Gender differences in factors influencing alcohol use and drinking progression among adolescents. Clin Psychol Rev. 2009;29:535–47. doi: 10.1016/j.cpr.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz D, Widmann U, Seeger U, Nagele T, Klose U, Mann K, Grodd W. Localized proton magnetic resonance spectroscopy of the cerebellum in detoxifying alcoholics. Alcohol Clin Exp Res. 1999;23:158–63. [PubMed] [Google Scholar]
- Sullivan EV. Compromised pontocerebellar and cerebellothalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcohol Clin Exp Res. 2003;27:1409–19. doi: 10.1097/01.ALC.0000085586.91726.46. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Harding AJ, Pentney R, Dlugos C, Martin PR, Parks MH, Desmond JE, Chen SH, Pryor MR, De Rosa E, Pfefferbaum A. Disruption of frontocerebellar circuitry and function in alcoholism. Alcohol Clin Exp Res. 2003;27:301–9. doi: 10.1097/01.ALC.0000052584.05305.98. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180:583–94. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rose J, Pfefferbaum A. Mechanisms of postural control in alcoholic men and women: biomechanical analysis of musculoskeletal coordination during quiet standing. Alcohol Clin Exp Res. 2010;34:528–37. doi: 10.1111/j.1530-0277.2009.01118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Lim KO, Pfefferbaum A. Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: relationships to changes in brain structure. Neuropsychology. 2000;14:178–88. [PubMed] [Google Scholar]
- Tessner KD, Hill SY. Neural circuitry associated with risk for alcohol use disorders. Neuropsychol Rev. 2010;20:1–20. doi: 10.1007/s11065-009-9111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Gender differences in brain functional connectivity density. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch LW, Cunningham AT, Eckardt MJ, Martin PR. Fine motor speed deficits in alcoholic Korsakoff’s syndrome. Alcohol Clin Exp Res. 1997;21:134–9. [PubMed] [Google Scholar]
- Yeh PH, Simpson K, Durazzo TC, Gazdzinski S, Meyerhoff DJ. Tract-Based Spatial Statistics (TBSS) of diffusion tensor imaging data in alcohol dependence: abnormalities of the motivational neurocircuitry. Psychiatry Res. 2009;173:22–30. doi: 10.1016/j.pscychresns.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]