Abstract
We have previously shown that the anti-cancer agent 2-methoxyestradiol (2ME) induces hyperpermeability across endothelial monolayers. Here, we show that both microtubule disruptor, 2ME, and microtubule stabilizer, paclitaxel (taxol), increase vascular lung permeability in vitro and in vivo. Simultaneous application of 2ME and taxol alleviates 2ME-induced endothelial barrier dysfunction, which is evident by the decreased Evans Blue Dye accumulation in lung tissue and increased transendothelial resistance across monolayers. 2ME significantly increases the level of p38 and MLC phosphorylation in both endothelial monolayers and murine lungs; this increase is suppressed in the presence of taxol. Taxol treatment leads to an immediate and sustained increase in tubulin acetylation in human pulmonary artery endothelial cells (HPAEC). Surprisingly, 2ME treatment also increases tubulin acetylation; however, the on-set of this process is delayed and co-insides with the stage of a partial barrier restoration in HPAEC monolayer. Inhibition of histone deacetylase 6 (HDAC6) with tubacin increases tubulin acetylation level, suppresses 2ME-induced HSP27 and MLC phosphorylation, and decreases 2ME-induced barrier dysfunction, suggesting barrier-protective and/or barrier-restorative role for tubulin acetylation in vascular endothelium.
Keywords: taxol, 2-methoxyestradiol, barrier dysfunction, tubulin acetylation
2-methoxyestradiol is a microtubule (MT)-disrupting agent shown to have antiproliferative effects on various cancer lines in vitro, and anti-tumor and antiangiogenic effects in preclinical models of cancer in vivo (Mueck and Seeger, 2010). Evaluation of 2ME in clinical trials for the treatment of several cancers is presently in progress (Verenich and Gerk, 2010). Paclitaxel (taxol) is a MT-stabilizing chemotherapeutic agent used for the treatment of several malignancies (Baird et al., 2010; Bonomi, 2007; Sakamoto et al., 2009; Saloustros et al., 2008). Although the effects of 2ME and taxol on MTs are seemingly opposite, these drugs were shown to potentiate each other’s anti-proliferative and anti-tumor effects in vitro and in vivo (Sweeney et al., 2001; Han et al., 2005; Ricker et al., 2004; Chen et al., 2009). Interestingly, both MT-disrupting and MT-stabilizing drugs down-regulate the level of proangiogenic hypoxia-inducible factor-1 (HIF-1), suggesting the importance of functional microtubular network for signaling through HIF-1 (Escuin et al., 2005). These results provided a strong rationale for testing the combination of 2ME and taxanes in clinical trials. The initial assessment of the combination therapy of 2ME with docetaxel (taxatere) was undertaken; however, systemic exposure to 2ME remained below the expected therapeutic range due to the low bio-availability of the current 2ME form (James et al., 2007).
Although deemed comparatively safe (Briasoulis and Pavlidis, 2001; Mueck and Seeger, 2010), both 2ME and taxanes applications were noted to be associated with certain vascular side effects. Severe hypersensitivity reactions characterized by flushing, chest discomfort, respiratory distress and pulmonary edema were reported in response to taxol (Kris et al., 1986; Weiss et al., 1990; Cormio et al., 1999; Price and Castells, 2002). Grades 3-4 dyspnea, edema, and angioedema were reported in 2ME clinical trials (Dahut et al., 2006; Matei et al., 2009; Rajkumar et al., 2007).
We have demonstrated recently that 2ME causes barrier dysfunction in the pulmonary endothelial cell monolayer (Bogatcheva et al., 2007). This effect was found to be linked to p38 activation and Rho kinase (ROCK)-dependent myosin light chain (MLC) phosphorylation, induced by initial MT disruption. Stabilization of MTs with taxol suppressed 2ME-induced endothelial hyperpermeability in vitro. The objective of the current study was to determine whether taxol would be able to suppress 2ME-induced vascular leakage in vivo and to delineate barrier-disruptive signaling pathways affected by taxol in 2ME-challenged pulmonary endothelium. Here, we analyzed the effect of taxol on 2ME-induced p38 activation and MLC phosphorylation. We also assessed the role of MT acetylation in the regulation of endothelial barrier.
Materials and Methods
Materials
2ME was purchased from Sigma (St. Louis, MO). Paclitaxel (taxol) and antiprotease cocktail were purchased from Merck (Whitehouse Station, NJ). Tubacin was from Enzo Life Sciences (Plymouth Meeting, PA). The antibody recognizing myosin light chains (MLC), diphosphorylated MLC, p38, phosphorylated p38, and acetylated lysine were from Cell Signaling (Beverly, MA). Antibodies against acetylated, polyglutamylated, and tyrosin-tubulin, as well as beta-actin, were from Sigma. VE-cadherin antibody was from Cayman Chemical (Ann Arbor, MI). Beta-tubulin antibody was from Covance (Princeton, New Jersey). All reagents used for immunofluorescent staining were obtained from Invitrogen (Carlsbad, CA).
Cell culture
HPAEC were purchased from Lonza (Walkerville, MD) and used at passages 5-7. They were cultured in media containing 5% FBS and maintained at 37°C in a humidified atmosphere of 5%CO2-95% air.
Measurement of transendothelial permeability
Transendothelial electrical resistance (TER) was measured using the highly sensitive biophysical assay with an electrical cell-substrate impedance sensor (ECIS) (Applied Biophysics, Troy, NY) as described previously (Bogatcheva et al., 2007). HPAEC were grown to confluence on gold microelectrodes to the resistance of approximately 1,000 Ohms. Media were changed to basal media 1h prior the experiment.
HPAEC imaging
For immunofluorescence experiments, HPAEC monolayers were plated on gelatin-covered coverslips and grown to confluence. Media was changed to the basal media 1 hour prior the experiment. Before immunostaining, cells were briefly washed with phosphate-buffered saline (PBS) and fixed in PBS solution of 4% formaldehyde. After permeabilization with 0.25% Triton X-100 and blocking, cells were stained with VE-cadherin-specific antibodies, and Alexa594-conjugated fluorescent secondary antibodies. After mounting in anti-fade mounting media, the coverslips were viewed and photographed with Zeiss Axio Observer video imaging system using Zeiss Axiovision software.
Animal experiments
19-23g male C57BL/6N mice were purchased from Charles River Laboratories (Wilmington, MA) or Harlan (Indianapolis, IN). 2ME, taxol, or tubacin dissolved in DMSO/propylenglycol mixture (1/24, v/v) was diluted 1.5 times with saline immediately prior to the injection. All injections were administered to ketamine (150mg/kg of body weight)/acetopromazine (15mg/kg)-anesthetized animals. 2ME, taxol, 2ME/taxol mixture, tubacin, or 2ME/tubacin mixture were administered intravenously via the jugular vein in the final volume not exceeding 60 μL. For wet/dry lung weight assessment, animals were terminated without further manipulation. For Evans Blue Dye (EBD)-albumin extravasation assessment, EBD-albumin conjugate (0.5% EBD in 4% BSA saline solution) was administered in the tail vein (30mg/kg) 1 hour prior animal sacrifice. All animal studies conformed to NIH guidelines. The experimental procedure was approved by the Georgia Health Sciences University Institutional Animal Care and Use Committee.
Measurement of lung permeability
In anesthetized animal, the chest cavity was opened. For wet/dry lung weight assessment, lungs were excised, blotted, weighed, and dried for 24h at 65°C. Weight was documented, and lungs were allowed to dry for another 24h, after which the weight was assessed again to assure the complete drying of the tissue. For EBD extravasation assessment, lungs were washed from blood by injecting saline/EDTA via the right ventricle and collected for further analysis. The right lung was used for protein analysis, and the left lung was homogenized in formamide to extract EBD (18h, 60°C). The homogenate was spun for 30 min at 5000g; the optical density of the supernatant was determined at 620nm and 750nm. The extravasated EBD concentration was calculated using a standard curve and normalized to lung weight (Moitra et al., 2007) .
Western immunoblotting
To obtain cell lysates, cells were grown in 12-well or 6-well plates; media was changed to basal media 1h prior the experiment. After pretreatment and stimulation, cells were rinsed with ice-cold PBS and lysed with PBS containing 1% SDS, antiprotease cocktail and 20mM NaF. After freezing-thawing and aspiration through 25 g needle, samples were supplemented with Western blot loading buffer and boiled.
To obtain lung extracts, dry-blotted lungs were snap-frozen in liquid nitrogen and stored at −80°C until homogenization. The lungs were pulverized using Besson tissue pulverizer and lysed with PBS containing 1% SDS, antiprotease cocktail and 20mM NaF. After freezing-thawing in liquid nitrogen and sequential aspiration through 18g and 23g needles, protein concentration was assessed with BCA kit (Pierce). Samples were equalized in protein, supplemented with Western blot loading buffer and boiled.
Protein extracts were separated on 4-20% gels and transferred to nitrocellulose membrane. After staining with specific antibodies, membranes were developed and scanned using Kodak MI imaging system.
Statistical analysis was performed on the data pooled from parallel experiments using one-way ANOVA test; results with p<0.05 were considered significantly different.
Results
Effect of 2ME, taxol, and their combination on endothelial permeability
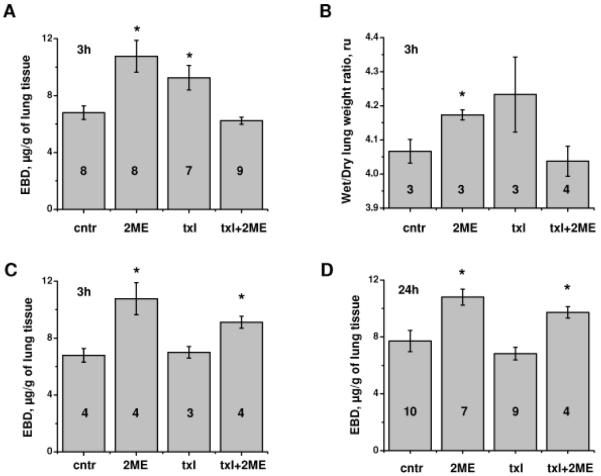
In a previous study (Bogatcheva et al., 2007), we showed that application of taxol alleviated 2ME-induced barrier dysfunction in HPAEC monolayers. Here, we determine how taxol affects 2ME-induced vascular leakage in murine lung tissue. To detect changes in pulmonary vascular permeability, we assessed the process of EBD permeation from vascular lumen to the pulmonary interstitium. Intravenous administration of 10 mg/kg 2ME increased accumulation of EBD and water in the lung 3h post-injection, confirming the loss of barrier function in pulmonary endothelium (Fig 1A, B). Intravenous administration of 0.8 mg/kg taxol also resulted in marked increase in EBD accumulation (Fig 1A); increase in water accumulation did not reach significant level (Fig 1B). Lower concentration of taxol (0.4 mg/kg) did not induce permeability in lung (Fig 1C). Whereas 2ME effect on lung permeability was clearly seen 24h post-administration (Fig 1D), the effect of 0.8 mg/kg taxol was more transient in nature (Fig 1A, D). Importantly, simultaneous administration of 0.8 mg/kg, but not 0.4mg/kg, taxol suppressed 2ME-induced EBD accumulation in lung 3h post-administration (Fig 1A, C), suggesting counteraction of taxol and 2ME effects on vascular leakage. The alleviating effect of 0.8 mg/kg taxol on 2ME-induced murine vascular leakage was no longer seen 24h post-administration, concomitant with the negligible effect of taxol on barrier permeability seen at this time point (Fig 1D).
Figure 1.
Effect of 2ME, taxol, and their co-administration on murine pulmonary permeability. (A, B) Mice subjected to intravenous administration of vehicle control, 10 mg/kg 2ME, 0.8 mg/kg taxol, and simultaneous administration of 2ME and taxol for 3h were analyzed for EBD-albumin (A) or water (B) accumulation in the lung tissue. C) Mice subjected to intravenous administration of vehicle control, 10 mg/kg 2ME, 0.4 mg/kg taxol, and simultaneous administration of 2ME and taxol for 3h were analyzed for EBD-albumin accumulation in the lung tissue. D) Mice subjected to intravenous administration of vehicle control, 10 mg/kg 2ME, 0.8 mg/kg taxol, and simultaneous administration of 2ME and taxol for 24h were analyzed for EBD-albumin accumulation in the lung tissue. Shown are mean±SEM, number of animals is indicated on the figure. *p<0.05 are considered significantly different.
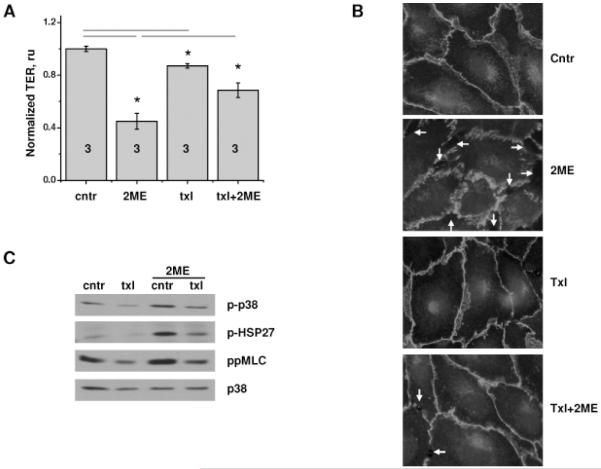
In vitro application of taxol concentration roughly equivalent to 0.8 mg/kg resulted in a decrease in transendothelial resistance of HPAEC monolayers; however, this decrease was considerably lower than the decrease caused by 2ME (Fig 2A). We failed to detect significant changes in the junctional organization of taxol-treated monolayers (Fig 2B); in contrast, 2ME-treated monolayers manifested re-organization of junctional VE-cadherin and marked gap formation. Pretreatment of HPAEC monolayers with taxol suppressed 2ME-induced decrease in TER and gap formation (Fig 2A, B).
Figure 2.
Effect of 2ME, taxol, and their co-administration on HPAEC. A-B) HPAEC monolayers grown on gold microelectrodes (A) or glass coverslips (B) were pretreated with vehicle control or 10 μM taxol for 30 min, and then challenged with vehicle control or 20 μM 2ME for 15 min. (A) TER was normalized to the point preceding taxol addition. Shown are mean±SEM, number of monolayers is indicated on the figure. *p<0.05 are considered significantly different. (B) Immunofluorescent staining with anti-VE-cadherin antibody; gaps in monolayer are indicated with arrows. C) HPAEC monolayers pretreated with vehicle control or 10 μM taxol for 30 min were challenged with 20 μM 2ME for 20min, extracted and analyzed with anti-phospho-p38, anti-phospho-HSP27, anti-diphosphoMLC antibodies. p38 staining was used as loading control.
Phosphorylation of p38 and MLC in 2ME-, taxol-, and 2ME/taxol combination-treated endothelium
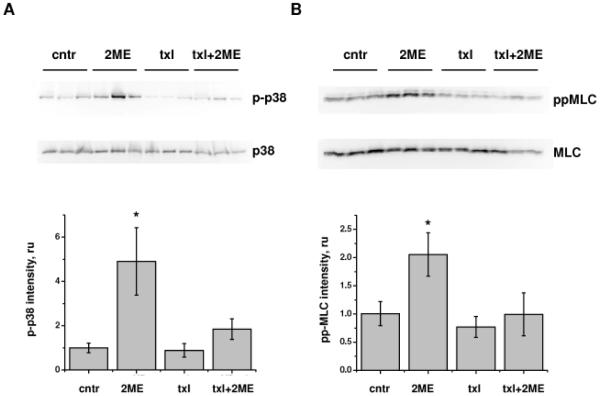
We have shown earlier that activation of p38-dependent and ROCK-dependent pathways plays a significant role in 2ME-induced hyperpermebaility of HPAEC monolayers. We have also shown that MLC phosphorylation in 2ME-treated cells occurs via ROCK-dependent but MLC kinase-independent mechanism (Bogatcheva et al., 2007). Here, we demonstrate that in human pulmonary endothelium, activation of p38/HSP27 and ROCK/MLC cascades in response to 2ME can be significantly suppressed by taxol pretreatment (Fig. 2C). In order to determine whether activation of p38/HSP27 and ROCK/MLC cascades takes part in the response of murine pulmonary tissue to 2ME, we analyzed lung extracts of mice subjected to 2ME injections. We found that pulmonary tissues of 2ME-treated animals manifest significant increases in p38 and MLC phosphorylation with no appreciable changes to the p38 or MLC protein level (Fig 3A, B). Whereas taxol application did not increase phospho-p38 or diphospho-MLC content, taxol co-administration with 2ME suppressed the 2ME-induced increase in p38 and MLC phosphorylation, evident of counteraction of 2ME-induced signaling pathways by taxol.
Figure 3.
Effect of 2ME, taxol, and their co-administration on p38 and MLC phosphorylation in murine lung. Lungs of mice subjected to intravenous administration of vehicle control, 10 mg/kg 2ME, 0.8 mg/kg taxol, and simultaneous administration of 2ME and taxol for 3h were extracted and analyzed with phospho-p38 and p38 antibodies (A) or diphospho-MLC and MLC antibodies (B). Normalized phospho-p38 and diphospho-MLC intensities were expressed as fold of control. Shown are mean±SEM, *p<0.05 are considered significantly different.
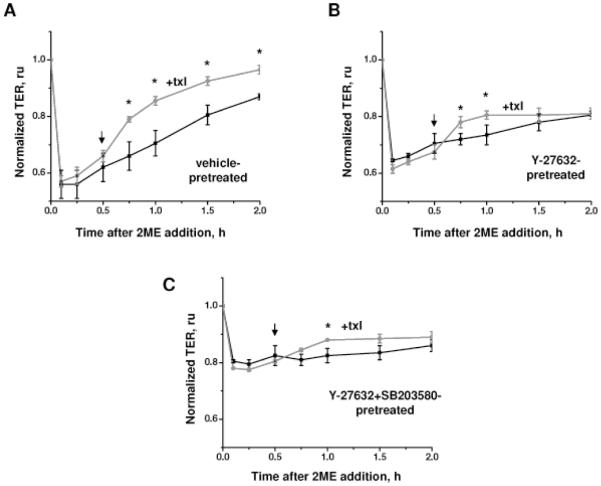
We next analyzed whether pharmacological inhibition of p38 or ROCK pathway would attenuate the alleviating effect of taxol on 2ME-induced permeability. When applied after 2ME, taxol facilitates the restoration of transendothelial resistance which follows an initial loss of endothelial barrier (Fig 4A). In Y-27632-pretreated cells, taxol-induced alleviation is less marked and is observed during a shorter period of time (Fig 4B). In cells pretreated with both Y-27632 and SB 203580, the alleviating effect of taxol is significantly suppressed (Fig 4C).
Figure 4.
Effect of ROCK and p38 inhibition on taxol-mediated alleviation of 2ME-induced endothelial barrier dysfunction. HPAEC monolayers grown on gold microelectrodes were pretreated with vehicle control (A), 3 μM Y-27632 (B), or 3 μM Y-27632 and 10 μM SB 203580 (C), and challenged with 10 μM 2ME. 5 μM taxol was applied at the time point indicated by arrow. Shown are mean±SEM (n=3), *p<0.05 are considered significantly different.
Effect of taxol and 2ME on tubulin acetylation
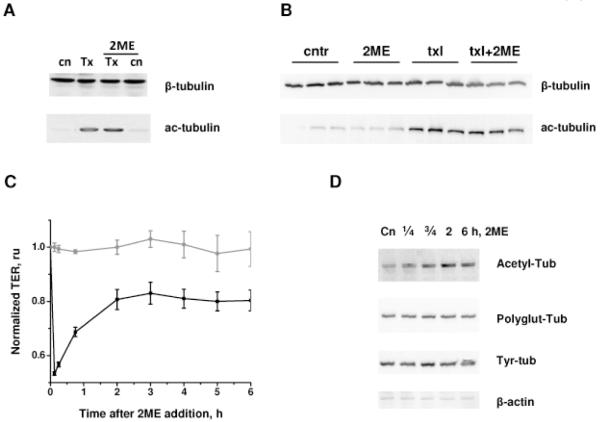
To determine the relationship between microtubule rearrangement and tubulin posttranslational modification, we analyzed the effects of taxol and 2ME on tubulin state in endothelium. Taxol is known to stabilize MTs, whereas stable MTs are known to become acetylated (Hammond et al., 2008). How acetylation affects MT stability remains a controversial subject (Palazzo et al., 2003). Thus, the important question to answer is whether tubulin acetylation is involved in the regulation of barrier function. We analyzed acetyl-tubulin content in HPAEC monolayers (Fig 5A) and lungs (Fig. 5B) and found that taxol significantly increases tubulin acetylation. The increase in acetylation was not reversed by 2ME-mediated MT destabilization in HPAEC monolayers or murine pulmonary tissue. Surprisingly, analysis of the tubulin acetylation state in 2ME-treated cells revealed that 2ME does not decrease tubulin acetylation level. On the contrary, long exposures to 2ME increased tubulin acetylation, which was not accompanied by alterations of other tubulin posttranslational modifications such as detyrosinilation and polyglutamylation (Fig 5D). Time course comparison of the tubulin acetylation versus 2ME-induced changes in TER revealed that an increase in tubulin acetylation corresponds to the partial recovery of the barrier function after 2ME-induced barrier dysfunction (Fig 5C). These data did not contradict the hypothesis that tubulin acetylation may play positive role in the stabilization of MTs and thereby the endothelial barrier, and prompted further investigation into the subject.
Figure 5.
Effect of 2ME, taxol, and their co-administration on tubulin acetylation. A) HPAEC monolayers pretreated with vehicle control or 10 μM taxol for 30 min, and then challenged with vehicle control or 20 μM 2ME for 20 min were extracted and analyzed by Western blot with anti-acetyl-tubulin and anti-beta-tubulin antibodies. B) Lungs of mice subjected to intravenous administration of vehicle control, 10 mg/kg 2ME, 0.8 mg/kg taxol, and simultaneous administration of 2ME and taxol for 3h were extracted and analyzed with anti-acetyl-tubulin and anti-beta-tubulin antibodies. C) HPAEC monolayers grown on gold microelectrodes were challenged with vehicle control (circles) or 20 μM 2ME (squares) for the time indicated. TER was normalized to the point preceding 2ME addition; shown are mean±SEM (n=3). D) HPAEC monolayers challenged with vehicle control or 20 μM 2ME for the time indicated were extracted and analyzed with anti-acetyl-tubulin, anti-polyglutamylated tubulin, anti-tyrosinilated tubulin and anti-beta-actin antibodies.
Effect of HDAC6 inhibitor on 2ME-induced endothelial hyperpermeability
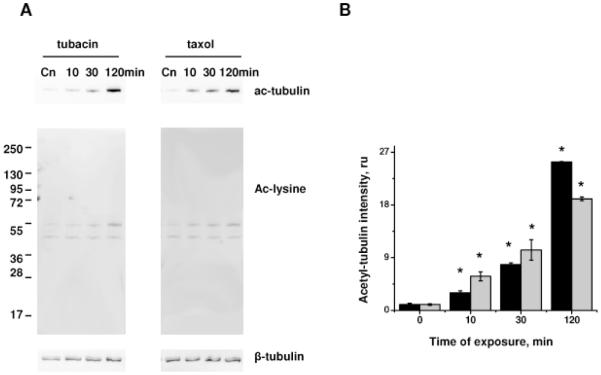
To analyze the role of tubulin acetylation in barrier regulation, we inhibited the enzyme involved in tubulin deacetylation, histone deacetylase (HDAC). The small molecule inhibitor, tubacin, was recently shown to be specific to HDAC6 and to inhibit predominantly the tubulin deacetylation process (Haggarty et al., 2003). Using antibodies specific for acetylated tubulin and acetylated lysine, we next demonstrated that tubacin treatment increased the acetylation level of tubulin (55kDa) with little or no effect on the acetylation level of another major protein substrate (~47-48kDa) (Fig 6A). Taxol treatment also induced specific tubulin acetylation, but required shorter incubation times for the substantial increase to occur (Fig 6A, B).
Figure 6.
Effect of tubacin and taxol on tubulin acetylation. HPAEC monolayers treated with 5 μM tubacin or 10 μM taxol for the time indicated were extracted and analyzed by Western blot with anti-acetyl-tubulin and anti-acetylated lysine antibodies (A). Beta-tubulin staining was used as a loading control. B) Normalized aceto-tubulin intensities (black columns, tubacin; grey columns, taxol) were expressed as fold of control. Shown are mean±SEM, *p<0.05 are considered significantly different.
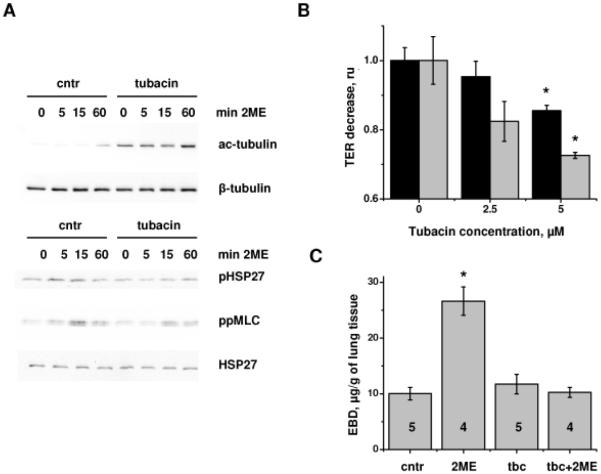
1h pretreatment of HPAEC monolayers with tubacin increased the basal level of tubulin acetylation and potentiated 2ME-induced increase in the tubulin acetylation level (Fig 7A). Analysis of the phosphorylation level of HSP27 and MLC revealed that tubacin pretreatment significantly suppressed the 2ME-induced increase in the phospho-HSP27 and diphospho-MLC level. Accordingly, tubacin reduced 2ME-induced decrease in TER (Fig 7B) and 2ME-induced lung vascular hyperpermeability (Fig 7C), suggesting that an increase in tubulin acetylation can alleviate barrier dysfunction induced by MT-disrupting agents.
Figure 7.
Tubacin pretreatment suppresses 2ME-induced phosphorylation of HSP27 and MLC and alleviates 2ME-induced barrier dysfunction. A) HPAEC monolayers pretreated with vehicle control or 5μM tubacin for 1h, and then challenged with vehicle control (0 min) or 20 μM 2ME for the time indicated were extracted and analyzed by Western blot with anti-acetyl-tubulin, anti-beta-tubulin, anti-phospho-HSP27, anti-HSP27, and anti-diphospho-MLC antibodies. B) HPAEC monolayers grown on gold microelectrodes were pretreated with tubacin or vehicle control for 1h, then challenged with 20 μM 2ME for 15min (black columns) or 60 min (grey columns). TER decrease was presented as fold of control (2ME-induced TER decrease in the absence of tubacin). Shown are mean±SEM (n=3). C) Mice subjected to intravenous administration of vehicle control, 15 mg/kg 2ME, 0.3 mg/kg tubacin, and simultaneous administration of 2ME and tubacin for 3h were analyzed for EBD-albumin accumulation in the lung tissue. Shown are mean±SEM, number of animals is indicated on the figure. *p<0.05 are considered significantly different.
Discussion
Taxol and 2ME are low toxicity anti-cancer agents already in use or currently undergoing testing for the treatment of several malignancies (Briasoulis and Pavlidis, 2001; Mueck and Seeger, 2010). Mechanistically, they exert their anti-cancer actions primarily through affecting disassembly (taxol) or assembly (2ME) of MTs, consequently resulting in mitotic arrest and cell death. However, involvement of MT dynamics in cells functions other than cell division provide a molecular basis for the multifaceted effects of MT-affecting drugs. In endothelium, MT disruption was known to activate cell contraction and induce endothelial hyperpermeability (Birukova et al., 2004b). To the contrary, MT stabilization was thought to serve as a barrier-preserving mechanism, based on the facts that taxol pretreatment alleviated endotoxin-induced lung injury (Mirzapoiazova et al., 2007), and suppressed thrombin-, TNF-, and TGF-induced endothelial permeability (Petrache et al., 2003; Birukova et al., 2004a; Birukova et al., 2005). However, multiple reports of taxol-induced pulmonary toxicity implied opposite, barrier-destabilizing, effects of taxol (Kris et al., 1986; Weiss et al., 1990; Cormio et al., 1999; Price and Castells, 2002). Assuming that mechanisms of taxol-induced endothelial permeability are dramatically different from the mechanisms employed by the majority of edemagenic agents, it is conceivable to expect that when applied simultaneously, taxol is able to counteract barrier disruption induced by LPS, TNF, TGF and thrombin.
The present study revealed that both 2ME and taxol applications induced leakage in murine pulmonary vasculature and increased permeability across human pulmonary endothelial monolayer. In our experiments, murine lung endothelium seemed to be more susceptible to taxol-induced barrier dysfunction than human pulmonary artery endothelium. Importantly, in both experimental systems simultaneous application of 2ME and taxol resulted in the reduction of vascular leakage, rather than potentiation of permeability. This was consistent with the taxol-mediated suppression of p38 and ROCK/MLC cascades, induced by 2ME. These cascades control the contractile state of endothelium, thereby regulating the integrity of endothelium and its barrier properties (Bogatcheva and Verin, 2008). In HPAEC monolayers, where p38 and ROCK/MLC cascades were pharmacologically inhibited, taxol-mediated alleviation of 2ME-induced barrier dysfunction was significantly suppressed, suggesting that p38 and ROCK are key signaling enzymes differentially regulated by 2ME and taxol.
Our data demonstrate that simultaneous application of 2ME with taxol can alleviate 2ME-induced barrier dysfunction. Together with the data of literature showing a synergistic anti-cancer action of taxol and 2ME (Sweeney et al., 2001; Han et al., 2005; Ricker et al., 2004; Chen et al., 2009), our data continue to characterize the potential effects of taxol/2ME combinatory application and to provide a novel insight into the potential use of MT-affecting drug therapies.
From the mechanistic point of view, MT dynamics are seen as a pivotal module of the complex signaling network that regulates barrier function (Bogatcheva and Verin, 2008; Lee and Gotlieb, 2003). The process of MT acetylation, reflective of MT stability (Hammond et al., 2008), was long implied to be involved in barrier regulation (Birukova et al., 2004a; Petrache et al., 2003); however, only now, with the development of the specific HDAC6 inhibitors, the role of MT acetylation is starting to be addressed. A recent study demonstrated that an increase in MT acetylation is associated with the alleviation of thrombin-induced barrier dysfunction and the suppression of MLC phosphorylation (Saito et al., 2011). Therefore, taxol-induced counteraction of 2ME effects can be achieved, among other mechanisms, via an increase in MT acetylation occurred after MT stabilization. Assessment of the effect of HDAC6 inhibitor on 2ME-induced barrier dysfunction let us suggest that MT acetylation is likely to contribute to the barrier-protective mechanisms evoked by taxol. Consistent with the previous report (Saito et al., 2011), we have shown that increased MT acetylation is concomitant with the suppression of barrier-disruptive pathways, namely MLC and HSP27 phosphorylation.
Surprisingly, endothelial cells treated with 2ME displayed a late-phase increase in MT acetylation. As MT deacetylation in response to 2ME was noted in cancer cells in vitro and in vivo (Gokmen-Polar et al., 2005; Kang et al., 2006; Kirches and Warich-Kirches, 2009), one will have to assume that endothelial cell MT dynamics are considerably different from the MT dynamics in cancer cells. Importantly, in our study an increase in the MT acetylation level coincided with a partial recovery of the endothelial barrier from 2ME-induced dysfunction, further suggesting that MT acetylation is a barrier-protective or barrier-restorative event.
In conclusion, our findings indicate that application of taxol attenuates 2ME-induced permeability in vitro and in vivo via the suppression of ROCK/MLC and p38 pathways and enhancement of MT acetylation. Our results warrant further investigation of the combined effects of 2ME/taxol or 2ME/HDAC6 inhibitor to ensure the possibility of the safer anti-cancer therapies.
Acknowledgements
This work was supported by AHA SDG 0930038N (NB), R01 HL-080675 (AV), R01 HL-083327 (AV), and R01 HL-067307 (AV). The authors are grateful to Mr. Gregory Daglis, Ms. Nurgul Moldobaeva, and Dr. Kyung-Mi Kim for the technical assistance with some of the experiments, and to Ms. Jenna Gallops for the help with manuscript preparation.
Abbreviations
- 2ME
2-methoxyestradiol
- EBD
Evans Blue Dye
- HDAC6
histone deacetylase 6
- HPAEC
human pulmonary endothelial cells
- MLC
myosin light chains
- MT
microtubules
- ROCK
Rho kinase
- TER
transendothelial electrical resistance
- VE-cadherin
vascular endothelium cadherin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baird RD, Tan DS, Kaye SB. Weekly paclitaxel in the treatment of recurrent ovarian cancer. Nat Rev Clin Oncol. 2010;7:575–582. doi: 10.1038/nrclinonc.2010.120. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Birukov KG, Adyshev D, Usatyuk P, Natarajan V, Garcia JG, Verin AD. Involvement of microtubules and Rho pathway in TGF-beta1-induced lung vascular barrier dysfunction. J Cell Physiol. 2005;204:934–947. doi: 10.1002/jcp.20359. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Birukov KG, Smurova K, Adyshev D, Kaibuchi K, Alieva I, Garcia JG, Verin AD. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. Faseb J. 2004a;18:1879–1890. doi: 10.1096/fj.04-2328com. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Smurova K, Birukov KG, Usatyuk P, Liu F, Kaibuchi K, Ricks-Cord A, Natarajan V, Alieva I, Garcia JG, Verin AD. Microtubule disassembly induces cytoskeletal remodeling and lung vascular barrier dysfunction: role of Rho-dependent mechanisms. J Cell Physiol. 2004b;201:55–70. doi: 10.1002/jcp.20055. [DOI] [PubMed] [Google Scholar]
- Bogatcheva NV, Adyshev D, Mambetsariev B, Moldobaeva N, Verin AD. Involvement of microtubules, p38, and Rho kinases pathway in 2-methoxyestradiol-induced lung vascular barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2007;292:L487–499. doi: 10.1152/ajplung.00217.2006. [DOI] [PubMed] [Google Scholar]
- Bogatcheva NV, Verin AD. The role of cytoskeleton in the regulation of vascular endothelial barrier function. Microvasc Res. 2008;76:202–207. doi: 10.1016/j.mvr.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomi P. Paclitaxel poliglumex (PPX, CT-2103): macromolecular medicine for advanced non-small-cell lung cancer. Expert Rev Anticancer Ther. 2007;7:415–422. doi: 10.1586/14737140.7.4.415. [DOI] [PubMed] [Google Scholar]
- Briasoulis E, Pavlidis N. Noncardiogenic pulmonary edema: an unusual and serious complication of anticancer therapy. Oncologist. 2001;6:153–161. doi: 10.1634/theoncologist.6-2-153. [DOI] [PubMed] [Google Scholar]
- Chen CH, Lee WJ, Chang TC, Chen RJ, Chien CH, Chow SN. Antiproliferative effects of 2-methoxyestradiol alone and in combination with chemotherapeutic agents on human endometrial cancer cells. Eur J Gynaecol Oncol. 2009;30:275–280. [PubMed] [Google Scholar]
- Cormio G, Di Vagno G, Melilli GA, Cazzolla A, Di Gesu G, Carriero C, Cramarossa D, Loverro G, Selvaggi L. Hypersensitivity reactions in ovarian cancer patients receiving paclitaxel. J Chemother. 1999;11:407–409. doi: 10.1179/joc.1999.11.5.407. [DOI] [PubMed] [Google Scholar]
- Dahut WL, Lakhani NJ, Gulley JL, Arlen PM, Kohn EC, Kotz H, McNally D, Parr A, Nguyen D, Yang SX, Steinberg SM, Venitz J, Sparreboom A, Figg WD. Phase I clinical trial of oral 2-methoxyestradiol, an antiangiogenic and apoptotic agent, in patients with solid tumors. Cancer Biol Ther. 2006;5:22–27. doi: 10.4161/cbt.5.1.2349. [DOI] [PubMed] [Google Scholar]
- Escuin D, Kline ER, Giannakakou P. Both microtubule-stabilizing and microtubule-destabilizing drugs inhibit hypoxia-inducible factor-1alpha accumulation and activity by disrupting microtubule function. Cancer Res. 2005;65:9021–9028. doi: 10.1158/0008-5472.CAN-04-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokmen-Polar Y, Escuin D, Walls CD, Soule SE, Wang Y, Sanders KL, Lavallee TM, Wang M, Guenther BD, Giannakakou P, Sledge GW., Jr. beta-Tubulin mutations are associated with resistance to 2-methoxyestradiol in MDA-MB-435 cancer cells. Cancer Res. 2005;65:9406–9414. doi: 10.1158/0008-5472.CAN-05-0088. [DOI] [PubMed] [Google Scholar]
- Haggarty SJ, Koeller KM, Wong JC, Grozinger CM, Schreiber SL. Domain-selective small-molecule inhibitor of histone deacetylase 6 (HDAC6)-mediated tubulin deacetylation. Proc Natl Acad Sci U S A. 2003;100:4389–4394. doi: 10.1073/pnas.0430973100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JW, Cai D, Verhey KJ. Tubulin modifications and their cellular functions. Curr Opin Cell Biol. 2008;20:71–76. doi: 10.1016/j.ceb.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han GZ, Liu ZJ, Shimoi K, Zhu BT. Synergism between the anticancer actions of 2-methoxyestradiol and microtubule-disrupting agents in human breast cancer. Cancer Res. 2005;65:387–393. [PubMed] [Google Scholar]
- James J, Murry DJ, Treston AM, Storniolo AM, Sledge GW, Sidor C, Miller KD. Phase I safety, pharmacokinetic and pharmacodynamic studies of 2-methoxyestradiol alone or in combination with docetaxel in patients with locally recurrent or metastatic breast cancer. Invest New Drugs. 2007;25:41–48. doi: 10.1007/s10637-006-9008-5. [DOI] [PubMed] [Google Scholar]
- Kang SH, Cho HT, Devi S, Zhang Z, Escuin D, Liang Z, Mao H, Brat DJ, Olson JJ, Simons JW, Lavallee TM, Giannakakou P, Van Meir EG, Shim H. Antitumor effect of 2-methoxyestradiol in a rat orthotopic brain tumor model. Cancer Res. 2006;66:11991–11997. doi: 10.1158/0008-5472.CAN-06-1320. [DOI] [PubMed] [Google Scholar]
- Kirches E, Warich-Kirches M. 2-methoxyestradiol as a potential cytostatic drug in gliomas? Anticancer Agents Med Chem. 2009;9:55–65. doi: 10.2174/187152009787047725. [DOI] [PubMed] [Google Scholar]
- Kris MG, O’Connell JP, Gralla RJ, Wertheim MS, Parente RM, Schiff PB, Young CW. Phase I trial of taxol given as a 3-hour infusion every 21 days. Cancer Treat Rep. 1986;70:605–607. [PubMed] [Google Scholar]
- Lee JS, Gotlieb AI. Understanding the role of the cytoskeleton in the complex regulation of the endothelial repair. Histol Histopathol. 2003;18:879–887. doi: 10.14670/HH-18.879. [DOI] [PubMed] [Google Scholar]
- Matei D, Schilder J, Sutton G, Perkins S, Breen T, Quon C, Sidor C. Activity of 2 methoxyestradiol (Panzem NCD) in advanced, platinum-resistant ovarian cancer and primary peritoneal carcinomatosis: a Hoosier Oncology Group trial. Gynecol Oncol. 2009;115:90–96. doi: 10.1016/j.ygyno.2009.05.042. [DOI] [PubMed] [Google Scholar]
- Mirzapoiazova T, Kolosova IA, Moreno L, Sammani S, Garcia JG, Verin AD. Suppression of endotoxin-induced inflammation by taxol. Eur Respir J. 2007;30:429–435. doi: 10.1183/09031936.00154206. [DOI] [PubMed] [Google Scholar]
- Moitra J, Sammani S, Garcia JG. Re-evaluation of Evans Blue dye as a marker of albumin clearance in murine models of acute lung injury. Transl Res. 2007;150:253–265. doi: 10.1016/j.trsl.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Mueck AO, Seeger H. 2-Methoxyestradiol--biology and mechanism of action. Steroids. 2010;75:625–631. doi: 10.1016/j.steroids.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Palazzo A, Ackerman B, Gundersen GG. Cell biology: Tubulin acetylation and cell motility. Nature. 2003;421:230. doi: 10.1038/421230a. [DOI] [PubMed] [Google Scholar]
- Petrache I, Birukova A, Ramirez SI, Garcia JG, Verin AD. The role of the microtubules in tumor necrosis factor-alpha-induced endothelial cell permeability. Am J Respir Cell Mol Biol. 2003;28:574–581. doi: 10.1165/rcmb.2002-0075OC. [DOI] [PubMed] [Google Scholar]
- Price KS, Castells MC. Taxol reactions. Allergy Asthma Proc. 2002;23:205–208. [PubMed] [Google Scholar]
- Rajkumar SV, Richardson PG, Lacy MQ, Dispenzieri A, Greipp PR, Witzig TE, Schlossman R, Sidor CF, Anderson KC, Gertz MA. Novel therapy with 2-methoxyestradiol for the treatment of relapsed and plateau phase multiple myeloma. Clin Cancer Res. 2007;13:6162–6167. doi: 10.1158/1078-0432.CCR-07-0807. [DOI] [PubMed] [Google Scholar]
- Ricker JL, Chen Z, Yang XP, Pribluda VS, Swartz GM, Van Waes C. 2-methoxyestradiol inhibits hypoxia-inducible factor 1alpha, tumor growth, and angiogenesis and augments paclitaxel efficacy in head and neck squamous cell carcinoma. Clin Cancer Res. 2004;10:8665–8673. doi: 10.1158/1078-0432.CCR-04-1393. [DOI] [PubMed] [Google Scholar]
- Saito S, Lasky JA, Guo W, Nguyen H, Mai A, Danchuk S, Sullivan DE, Shan B. Pharmacological inhibition of HDAC6 attenuates endothelial barrier dysfunction induced by thrombin. Biochem Biophys Res Commun. 2011;408:630–634. doi: 10.1016/j.bbrc.2011.04.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto J, Matsui T, Kodera Y. Paclitaxel chemotherapy for the treatment of gastric cancer. Gastric Cancer. 2009;12:69–78. doi: 10.1007/s10120-009-0505-z. [DOI] [PubMed] [Google Scholar]
- Saloustros E, Mavroudis D, Georgoulias V. Paclitaxel and docetaxel in the treatment of breast cancer. Expert Opin Pharmacother. 2008;9:2603–2616. doi: 10.1517/14656566.9.15.2603. [DOI] [PubMed] [Google Scholar]
- Sweeney CJ, Miller KD, Sissons SE, Nozaki S, Heilman DK, Shen J, Sledge GW., Jr. The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res. 2001;61:3369–3372. [PubMed] [Google Scholar]
- Verenich S, Gerk PM. Therapeutic promises of 2-methoxyestradiol and its drug disposition challenges. Mol Pharm. 2010;7:2030–2039. doi: 10.1021/mp100190f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RB, Donehower RC, Wiernik PH, Ohnuma T, Gralla RJ, Trump DL, Baker JR, Jr., Van Echo DA, Von Hoff DD, Leyland-Jones B. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8:1263–1268. doi: 10.1200/JCO.1990.8.7.1263. [DOI] [PubMed] [Google Scholar]