Abstract
Extracellular signal-regulated kinase (ERK1/2) has been implicated in modulating drug seeking behavior and is a target of alcohol and other drugs of abuse. Given that the discriminative stimulus (subjective/interoceptive) effects of drugs are determinants of abuse liability and can influence drug seeking behavior, we examined the role of ERK1/2 in modulating the discriminative stimulus effects of alcohol. Using drug discrimination procedures, rats were trained to discriminate a moderate intragastric (IG) alcohol dose (1 g/kg) versus water (IG). Following an alcohol (1 g/kg) discrimination session phosphorylated ERK1/2 (pERK1/2) immunoreactivity (IR) was significantly elevated in the amygdala, but not the nucleus accumbens. Therefore, we hypothesized that intra-amygdala inhibition of ERK1/2 would disrupt expression of the discriminative stimulus effects of alcohol. However, intra-amygdala or accumbens administration of the MEK/ERK1/2 inhibitor U0126 (1 and 3 μg) had no effect on the discriminative stimulus effects of the training dose of alcohol (1 g/kg). Contrary to our hypothesis, intra-amygdala infusion of U0126 (3 μg) potentiated the discriminative stimulus effects of a low alcohol dose (0.5 g/kg) and had no effect following nucleus accumbens infusion. Importantly, site-specific inhibition of pERK1/2 in each brain region was confirmed. Therefore, the increase in pERK1/2 IR in the amygdala following systemic alcohol administration may be reflective of the widespread effects of alcohol on the brain (activation/inhibition of brain circuits), whereas the site specific microinjection studies confirmed functional involvement of intra-amygdala ERK1/2. These findings show that activity of the ERK signaling pathway in the amygdala can influence the discriminative stimulus effects of alcohol.
Keywords: drug discrimination, MEK, MAPK, alcoholism, discriminative stimulus, ethanol, drinking, interoceptive, subjective, alcohol
1. Introduction
Alcohol drinking can be impacted by a variety of factors. A likely contributor to alcohol drinking and seeking behaviors is the interoceptive or subjective drug effects, such as the feeling of “drunkenness” or “lightheadedness” that accompanies alcohol consumption. In both clinical and preclinical studies, these interoceptive effects can serve as discriminative stimuli, such that the subject uses these cues to distinguish between drug and non-drug conditions. These interoceptive drug effects provide drug-specific feedback to the organism and can promote drug seeking behavior [1, 2]. Therefore, examination of the neural mechanisms that regulate these cues is critical to better understand how drugs gain control over behavioral processes in addiction. Although the contribution of neurotransmitter receptor systems (e.g., γ-aminobutyric acid (GABA) and N-Methyl-D-aspartic acid (NMDA)) have been well-characterized [3, 4], the potential cell signaling mechanisms that might underlie the expression of the discriminative stimulus (interoceptive) effects of alcohol have not been characterized.
There is growing interest in the extracellular signal-regulated kinase (ERK) as a molecular mechanism of drug seeking and taking behavior [5–10]. ERK is a member of the mitogen-activated protein kinase (MAPK) family. Activation of two closely related isoforms of ERK (ERK1 and ERK2, or ERK1/2), via phosphorylation by the upstream kinase MEK1/2, can result in changes in gene transcription, and consequently induce long-term changes in neural and behavioral functions [11–16]. Accordingly, there is a growing literature demonstrating involvement of this pathway in neural plasticity and learning and memory processes [17, 18]. Further, ERK1/2 is activated in vivo by acute administration of several drugs of abuse, including alcohol [19–24]. Interestingly, these studies show drug-induced ERK1/2 activation in limbic brain regions known to modulate the discriminative stimulus effects of alcohol, such as the nucleus accumbens and the amygdala [25–30]. However, it is unknown if ERK/MAPK signaling in these key limbic structures influences the discriminative stimulus effects of alcohol.
Accordingly, the purpose of this study was to examine the potential role of ERK/MAPK signaling in modulating the discriminative stimulus effects of alcohol. In male rats trained to discriminate between a moderate dose of alcohol (1 g/kg, IG) vs. water in a two-lever discrimination task, we first examined brain regional response to alcohol as indexed by phosphorylated (i.e., activated) ERK1/2 (pERK1/2) immunoreactivity. We focused on the nucleus accumbens and amygdala given their known roles in regulating the discriminative stimulus effects of alcohol. We hypothesized that in the brain region(s) that showed sensitivity to alcohol (as measured by changes in pERK1/2 IR), ERK1/2 activity may functionally modulate the discriminative stimulus effects of alcohol. To address this hypothesis, discrimination trained rats were implanted with bilateral cannulae aimed at the amygdala and nucleus accumbens for site-specific administration of the MEK/ERK1/2 inhibitor 1,4-Diamino-2,3-dicyano-1,4-bis[2-aminophenylthio]butadiene (U0126) to assess the functional role of ERK1/2 activity in these regions in modulating the expression of the discriminative stimulus effects of alcohol.
2. Materials and Methods
2.1. Animals
Male Long Evans rats (Harlan Sprague Dawley, Indianapolis, IN) were individually housed in Plexiglas cages. Rats were handled and weighed daily for one week before lever press training began. Rats were fed approximately 16 g of food daily for the duration of the study such that weights were maintained at approximately 330–340 g. Water was available continuously in the home cage. The colony room was maintained on a 12-h light/dark cycle and experiments were conducted during the light portion of the cycle. Animals were under continuous care and monitoring by veterinary staff from the Division of Laboratory Animal Medicine (DLAM) at UNC-Chapel Hill. All procedures were also carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and institutional guidelines.
2.2. Alcohol Discrimination Training and Testing Procedures
Discrimination training
Rats were trained on a two-lever alcohol discrimination task. The same training procedures and conditioning chambers described in [26, 29, 31, 32] were used. Briefly, immediately following intragastic gavage (IG) administration alcohol (1 g/kg) or water rats were placed in the chambers and after a 10 min delay the house light was illuminated and both levers were extended into the chamber (beginning of the 15-min session). IG administration of alcohol results in rapid brain alcohol concentrations [33], and the 10 min time point corresponds to the ascending limb of peak blood and brain alcohol concentrations [33, 34]. Following alcohol administration, completion of an FR10 on the alcohol-appropriate lever resulted in the availability of the sucrose (10%, w/v) solution. Similarly, following water administration, completion of an FR10 on the water-appropriate lever resulted in sucrose delivery. During both alcohol and water sessions, responses on the inappropriate lever were recorded but produced no programmed consequences. Prior to the start of two-lever discrimination training, rats experienced 16 errorless learning sessions, in which only the appropriate lever (i.e., right or left) was present for the alcohol or water session. Water and alcohol training days varied on a double alternation schedule (W, W, A, A, …). Training sessions continued until the percentage of alcohol- and water-appropriate lever press responses emitted prior to the first reinforcer, and during the entire session was >80% for 8 out of 10 consecutive days. Once these criteria were met, testing began.
Confirming discriminative stimulus control by alcohol
In both experiments, once the training criteria were met (60.1±4.1 two-lever discrimination training sessions), discriminative stimulus control by alcohol was verified by conducting a cumulative alcohol dose (0 – 1.7 g/kg, IG) substitution curve [26, 29, 30, 35]. Test sessions were similar to training sessions except that they were 2 min in duration (after the 10 min delay). During test sessions, completion of an FR10 on either lever resulted in sucrose reinforcement delivery to assess the possible effects of treatments on overall response rate. For Experiment 2 (Section 2.4), these test sessions were interspersed with training sessions only if performance during 3 out of the previous 4 training sessions met the accuracy criteria and testing occurred no more than twice per week.
2.3. Experiment 1
Identification of amygdala and nucleus accumbens response to alcohol as indexed by pERK1/2 IR
Discrimination-trained rats were administered water or alcohol (1 g/kg, IG; n=6/group) and placed in the chambers for a standard test session. Upon completion of the session, rats were returned to the homecage. Approximately 90 min after the water/alcohol administration, rats were deeply anesthetized with pentobarbital and perfused with 0.1 M PBS, followed by 4% paraformaldehyde, 4°C; pH=7.4. The brains were removed from the skull and placed in the same fixative solution for approximately 24 h before being washed with PBS and sliced coronally on a vibratome into 40 μm sections. Tissue was stored (−20°C) in cryoprotectant until immunohistochemistry processing.
pERK1/2 immunohistochemistry (IHC)
Free-floating sections were blocked in PBS/0.1% Triton-X/10% goat serum, and then incubated in PBS/0.1% Triton X/3% goat serum, and rabbit anti-phospho-p44/42 MAP kinase (1:400; Cell Signaling Technology, Inc., MA) for 16 h, at 4° C with agitation. Sections were then incubated in secondary antibody for 1 h using the Dako EnVision Kit (Dako, Carpinteria, CA). Immunoreactivity was detected with nickel-enhanced diaminobenzidine (Dako EnVision Kit) as a chromagen. Sections were then counterstained with toluidine blue (0.012%), mounted, dried and coverslipped. For consistency of staining across subjects, the brain tissue from each experiment was processed simultaneously.
pERK1/2 immunohistochemical quantification
Immunoreactivity (IR) was quantified as described in [5, 26, 29, 36]. Briefly, pERK1/2 IR (i.e., pixel density/mm2; optical density) was visualized using an Olympus CX41 light microscope (Olympus America, Center Valley, PA) and image analysis software (Bioquant Nova Advanced Image Analysis; R&M Biometric, Nashville, TN). The microscope, camera, and software were background corrected and normalized to preset light levels to ensure fidelity of data acquisition. Analysis was conducted by a researcher blind to treatment conditions. Data were acquired from a minimum of two sections/brain region/animal and the data were averaged to obtain a single value per subject. The brain regions examined were the nucleus accumbens (shell and core; AP +1.7 to 1.0 mm) and the amygdala (central nucleus - CeA, basolateral - BLA, dorsolateral - LaDL; AP −1.8 to −2.5).
2.4. Experiment 2
Effects of intra-amygdala and intra-accumbens ERK1/2 inhibition on the discriminative stimulus effects of alcohol
To determine the functional involvement of ERK1/2 activity on the discriminative stimulus effects of alcohol (1 g/kg, IG), discrimination-trained rats (n=11) were implanted with bilateral guide cannulae (26-gauge; Plastics One, VA) that terminated 2 mm above the amygdala (CeA) and the nucleus accumbens (core), as described in detail in [26, 29]. The coordinates (in mm) for the amygdala and nucleus accumbens were AP −1.9, ML +4.2, DV −6.5 and AP +1.7, ML +1.5, DV −5.5 (DV measurements from skull), respectively [37].
Site specific bilateral microinjections were made with 1.0 μl Hamilton syringes connected to 33-gauge injectors (Plastics One, VA) extending 2 mm below the guide cannulae. The infusions were delivered by a pump (Harvard Apparatus, MA) at a volume of 0.5 μl/side across 1 min. The injector remained in place for 1.5 min after the infusion to allow for diffusion. The MEK/ERK1/2 inhibitor U0126 was microinfused into the amygdala (0, 1, 3 μg/0.5 μl/side) or the nucleus accumbens (0, 1, 3 μg/0.5 μl/side). After the 1.5 min diffusion period, rats were placed in the home cage for 30 min. This 30 min pretreatment interval was chosen based on previous work showing decreased phosphorylated ERK1/2 protein utilizing similar infusion parameters [8, 9]. After the pretreatment interval, rats received the alcohol training dose (1 g/kg, IG), and were placed in the chamber for a test session. For the first tests, rats received a sham injection and a vehicle injection in the nucleus accumbens and the same order in the amygdala. Then, in order to determine if ERK1/2 activity is required for alcohol discrimination, U0126 (0, 1 or 3 μg) was administered in the amygdala or nucleus accumbens in randomized order 30 min prior to systemic administration of the alcohol training dose (1.0 g/kg, IG). Next, the ability of MEK/ERK inhibition to modulate the discriminative stimulus effects of a lower dose of alcohol (0.5 g/kg, IG) was assessed. U0126 (0 or 3 μg/0.5μl/side) was microinfused into the amygdala or nucleus accumbens 30 min before alcohol (0.5 g/kg, IG) administration. Finally, to determine whether MEK/ERK1/2 inhibition produces alcohol-like discriminative stimulus effects, U0126 (sham and 3 μg/0.5μl/side) was tested alone as described in both brain regions; however, water was administered before the test session. For this final assessment, rats received a sham infusion in each region in place of vehicle (aCSF/DMSO) to minimize the number of infusions, given that a confirmational terminal infusion would be required.
Effects of U0126 on pERK1/2 IR
To confirm that the microinjection procedures decreased ERK1/2 phosphorylation, approximately 2 weeks following the final test, rats were divided into two groups, such that U0126 was tested in one group in the amygdala (n=5) and in the nucleus accumbens for the other group (n=6). Rats in both groups, received a unilateral microinjection of aCSF/DMSO vehicle on one side (e.g., left) and U0126 (3 μg) on the other side (e.g., right). 30 min later rats were perfused, brain tissue was processed for pERK1/2 IR and quantified as described in Experiment 1 (Section 2.3). Tissue was also stained with cresyl violet to confirm cannulae placements. Only the data from rats with cannulae determined to be in the target brain regions were used in the analyses.
2.5. Drugs
For intragastric gavage (IG) administration, alcohol (95% w/v) was diluted in distilled water to a concentration of 20% (v/v) and administered in various volumes to obtain cumulative doses of 0.1, 0.3, 1.0, and 1.7 g/kg (for the initial cumulative substitution curve). A corresponding volume of water to the 1 g/kg alcohol training dose was used for water IG administration. The 1 g/kg training dose was selected given that it is a moderate alcohol dose that has been widely studied, is detectable by the animals given that it can serve as a discriminative stimulus, and does not produce motor deficits. 1,4-Diamino-2,3-dicyano-1,4-bis[2-aminophenylthio]butadiene (U0126), a specific inhibitor of MEK, an upstream regulator of ERK1/2 activity [38, 39], was dissolved in 50% DMSO/artificial cerebrospinal fluid (aCSF; in mM, 147 NaCl, 1.3 anhydrous CaCl2, 0.9 anhydrous MgCl2, 4.0 KCl; pH=7.0–7.2) vehicle. The dose range of U0126 and the strategy of testing the same ranges in each brain region was based on previous reports [40–44] and on pilot experiments performed in our laboratory.
2.6. Data Analyses
Response accuracy was expressed as the percentage of alcohol-appropriate lever presses upon delivery of the first reinforcer. Response rate (responses/min) was analyzed for the entire session and provided an index of locomotor ability. Complete expression of the discriminative stimulus effects of alcohol (i.e., full substitution) was defined as >80% choice of the alcohol lever upon completion of the first FR10 during test sessions. One-way repeated measures analysis of variance (RM ANOVA) were used to analyze response accuracy and response rate data, with Student-Newman-Keuls post hoc analyses. Paired or unpaired t-tests were used for two group comparisons. Statistical significance was declared at P≤0.05.
3. Results
3.1. Confirmation of discriminative stimulus control by alcohol
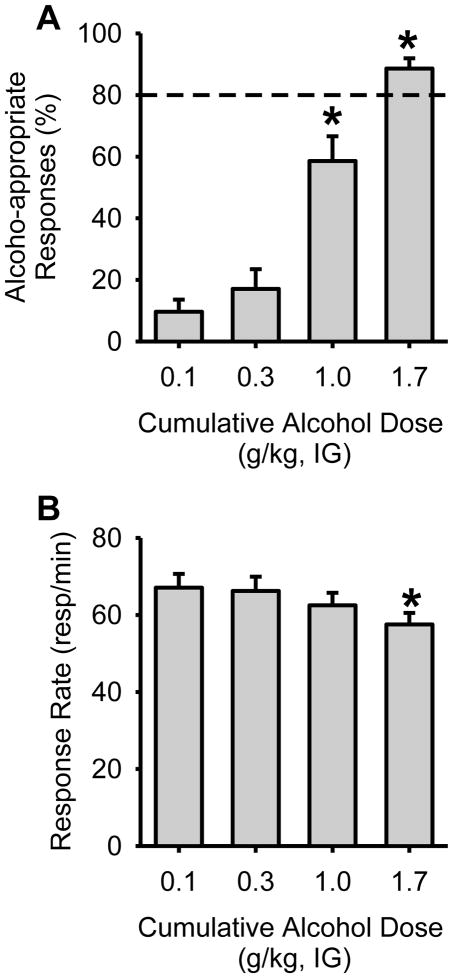
After training criteria were met, alcohol discriminative stimulus control was evaluated in each rat tested in Experiments 1 and 2, by determining a cumulative alcohol substitution curve (Figure 1). Analyses are representative of all rats used in this study (n=23). A one-way RM ANOVA confirmed that alcohol-appropriate responding increased as a function of alcohol test dose [F(3,66)=43.9, p<0.001], with 1.7 g/kg resulting in full substitution (>80%) for the alcohol training dose (i.e., produced discriminative stimulus effects similar to the training dose; Figure 1A). The training dose (1.0 g/kg), which generally produces >90% alcohol-appropriate responding during regular training sessions tends to produce approximately 60% alcohol-appropriate responding during cumulative dosing procedures [26, 29, 30]. A significant decrease in response rate was observed at the highest alcohol dose (1.7 g/kg; F(3,66)=10.4, p<0.001; Figure 1B). These results demonstrate dose-dependent discriminative stimulus control by alcohol.
Figure 1. Confirmation of discriminative stimulus control by alcohol.
(A) Before testing, discriminative stimulus control was confirmed in all rats by testing a cumulative alcohol substitution curve (n=23). Dose-dependent substitution for the 1 g/kg alcohol dose was observed, demonstrating that the training procedures established reliable stimulus control. (B) Response rate was reduced by the highest alcohol dose (1.7 g/kg). Horizontal dashed lines (>80%) denote full substitution for the discriminative stimulus effects of alcohol. Graphed values are expressed as mean ± s.e.m. *p<0.05 vs. 0.1 g/kg alcohol (Student Newman Keuls post hoc).
3.2. Experiment 1
Identification of amygdala and nucleus accumbens response to alcohol as indexed by pERK1/2 IR
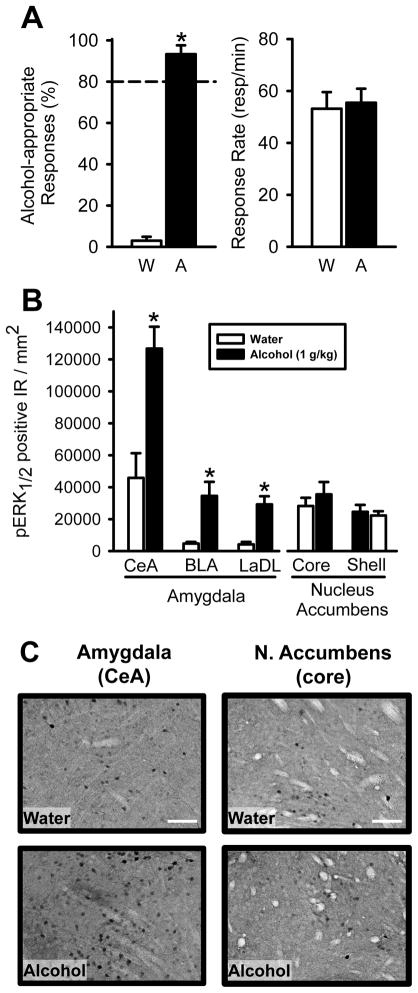
On the terminal test session, rats in both the Water and Alcohol groups demonstrated accurate performance as demonstrated by low alcohol-appropriate responding after water (IG) administration and high alcohol-appropriate responding after alcohol (IG) administration (Figure 2A, left panel). Response rates did not differ between the groups (Figure 2A, right panel).
Figure 2. Alcohol administration increases pERK1/2 IR in specific nuclei of the amygdala.
(A) Robust discrimination performance and similar response rates on the final test session for rats administered water or the alcohol training dose (1 g/kg; n=6/group). Horizontal dashed lines (>80%) denote full substitution for the discriminative stimulus effects of alcohol. (B) Animals were sacrificed approximately 90 min following alcohol/water administration. Significant elevations in pERK1/2 positive IR were observed in the subnuclei of the amygdala (CeA, BLA, and LaDL) in the alcohol group relative to the Water group. In contrast, no changes in pERK1/2 IR were observed in the nucleus accumbens core or shell. (C) Representative photomicrographs (20X) of pERK1/2 IR in the CeA (central portion of the CeA shown in photomicrograph) and (D) nucleus accumbens core (medial to the anterior commissure shown in photomicrograph) after water and alcohol (1 g/kg, IG) administration. Scale bars, 100 μm. Graphed values are expressed as mean ± s.e.m. *p<0.05 vs. water (t-test).
Analysis of pERK1/2 IR in the amygdala subnuclei (CeA, BLA, LaDL) showed a significant increase in pERK1/2 IR following alcohol (1 g/kg) administration (ps<0.05; Figure 2B, C). No alcohol-induced differences were detected in the nucleus accumbens (core, shell).
3.3. Experiment 2
Effects of intra-amygdala and intra-accumbens ERK1/2 inhibition on the discriminative stimulus effects of alcohol
Baseline accuracy performance (mean±S.E.M.) from the alcohol and water session preceding the start of testing was 91.4±3.3 and 14.2±5.8% alcohol-appropriate responses, respectively. The corresponding response rates (mean±S.E.M.) for the alcohol and water session were 59.2±3.5 and 63.8±3.0 responses/min.
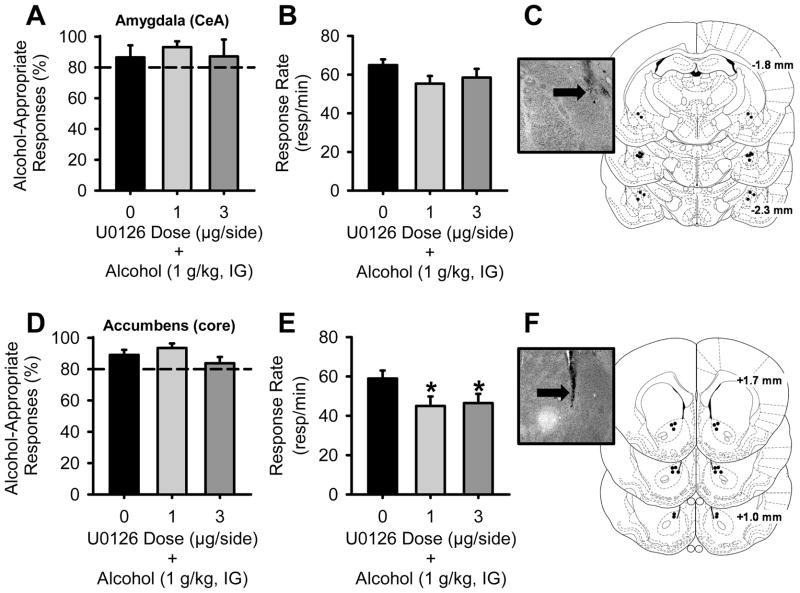
First, we sought to determine if inhibition of ERK1/2 phosphorylation would alter the discriminative stimulus effects of the training dose of alcohol. Administration of the MEK/ERK1/2 inhibitor U0126 (0 – 3 μg) in the amygdala (Figure 3A) or the nucleus accumbens (Figure 3D) did not alter the discriminative stimulus effects of alcohol (1 g/kg, IG). Response rate was not altered by intra-amygdala U0126 administration (Figure 3B), but a significant reduction was observed following U0126 administration in the nucleus accumbens [RM ANOVA; F(2,16)=4.18, p=0.04], with both doses reducing response rate relative to vehicle administration (ps<0.04; Figure 3E). For each brain region, there was one rat with a clogged cannula such that drug testing could not occur, and one with incorrect cannulae placement (resulting in n=9/brain region; and n=4 for the amygdala group and n=5 for the nucleus accumbens group for the terminal U0126 test).
Figure 3. MEK/ERK1/2 inhibition in the amygdala or nucleus accumbens does not alter the discriminative stimulus effects of alcohol (1 g/kg, IG).
(A) Intra-amygdala infusion of the MEK/ERK1/2 inhibitor U0126 (n=9) 30 min prior to alcohol (1 g/kg, IG) did not alter the discriminative stimulus effects of alcohol or (B) response rate. (C) Illustration depicting accurate amygdala injector placements aimed at the CeA and a corresponding photomicrograph showing an injector tract (arrow). (D) Intra-accumbens infusion of U0126 (n=9) also did not alter the discriminative stimulus effects of alcohol, but (E) produced significant response rate reductions. (F) Illustration depicting accurate nucleus accumbens injector placements aimed at the core and a corresponding photomicrograph showing an injector tract (arrow). Horizontal dashed lines (>80%) denote full substitution for the discriminative stimulus effects of alcohol. Graphed values are expressed as mean ± s.e.m. *p<0.05 vs. vehicle (Student Newman Keuls post hoc).
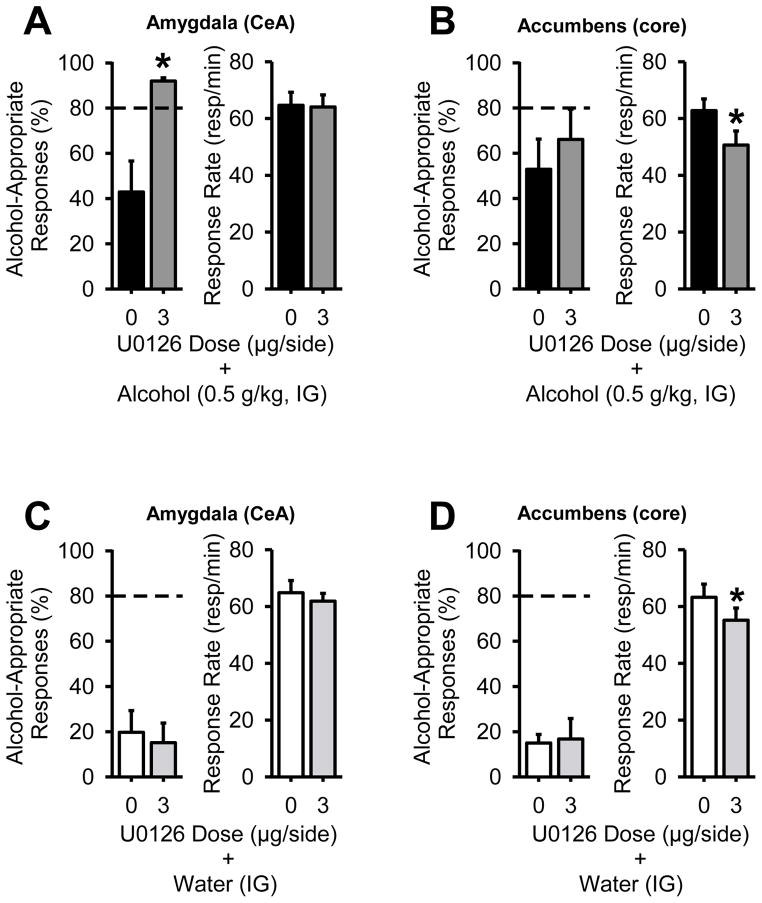
Given the lack of effect of U0126 on modulating the discriminative stimulus effects of the alcohol training dose (1 g/kg), which could reflect a drug or behavioral ceiling effect, we next sought to determine if U0126 infusion might alter the discriminative stimulus effects of a lower alcohol dose (0.5 g/kg, IG; e.g., able to detect bidirectional effects). In the amygdala, U0126 (3 μg) pretreatment significantly increased the percent of alcohol-appropriate responding [paired t-test; t(8)=3.84, p=0.004], and did not alter response rate (Figure 4A), indicating potentiation of the discriminative stimulus effects of the low alcohol dose. In contrast, intra-accumbens U0126 administration did not alter alcohol-appropriate responding, but produced a significant reduction in response rate [Figure 4B; paired t-test; t(8)=2.75, p=0.03].
Figure 4. Intra-amygdala MEK/ERK1/2 inhibition in the amygdala potentiates the discriminative stimulus effects of a low alcohol dose (0.5 g/kg, IG).
(A) In the same rats, amygdala infusion of U0126 30 min prior to alcohol (0.5 g/kg, IG; n=9) potentiated the discriminative stimulus effects of alcohol, without altering response rate. (B) In contrast, intra-accumbens infusion of U0126 did not alter the discriminative stimulus effects of the low alcohol dose (0.5 g/kg, IG; n=9), but significantly reduced response rate, indicating differential involvement of the two brain regions in regulating the discriminative stimulus effects of alcohol. To evaluate whether MEK/ERK1/2 inhibition alone produced alcohol-like discriminative stimulus effects, the rats were administered U0126 30 min prior to water (IG). (C) Intra-amygdala U0126 did not produce alcohol-like effects (i.e., low alcohol-appropriate responses) and did not alter response rate. (D) Similarly, intra-accumbens U0126 did not produce alcohol-like effects, but significantly reduced response rate. Horizontal dashed lines (>80%) denote full substitution for the discriminative stimulus effects of alcohol. Graphed values are expressed as mean ± s.e.m. *p<0.05 vs. vehicle (paired t-test).
To determine whether U0126 produced alcohol-like effects alone, the inhibitor was administered before water. Intra-amygdala and accumbens administration of U0126 did not produce alcohol-like discriminative stimulus effects, as evidenced by low alcohol-appropriate responding (Figure 4C, 4D). Differential sensitivity to the motor impairing effects of U0126 was again noted, as U0126 significantly reduced response rate in the accumbens [paired t-test: t(8)=2.52, p=0.04], but not the amygdala (Figure 4C, 4D).
Effects of U0126 on pERK1/2 IR
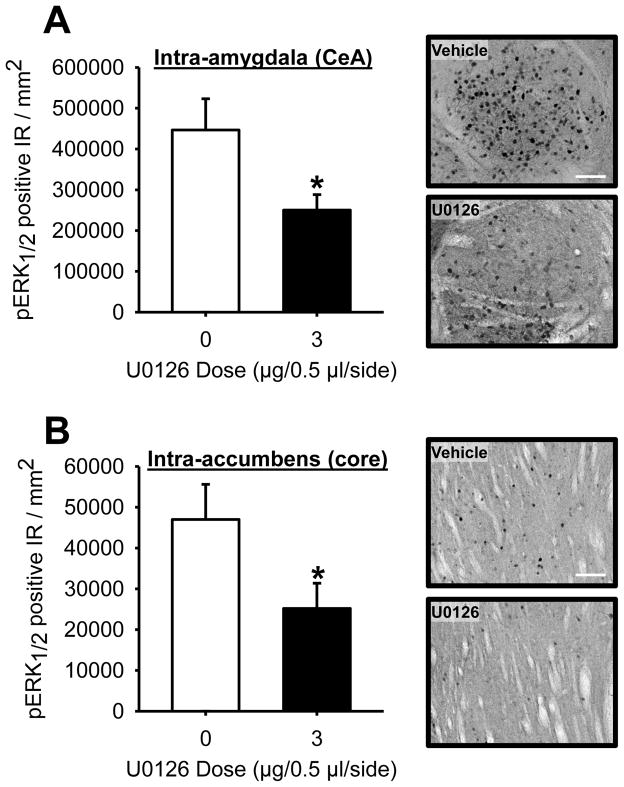
Intra-amygdala U0126 administration significantly decreased ERK1/2 activity as measured by pERK1/2 IR. The reduction was specific to the CeA (Figure 5A; paired t-test: t(3)=3.86, p=0.03), as no change in pERK1/2 IR was evident in the BLA (Veh: 12,328.6±2,515.1 pERK1/2 positive IR/mm2; U0126: 13,119.6±4,942.1 pERK1/2 positive IR/mm2) or LaDL (Veh: 13,775.1±2344.4 pERK1/2 positive IR/mm2; U0126: 20,677.7±5,903.8 pERK1/2 positive IR/mm2). This confirms the function of MEK/ERK1/2 inhibitor and the CeA-specific inhibition is consistent with previous findings in which microinjections were targeted at the CeA [9]. Intra-accumbens administration of U0126 showed a similar decrease in pERK1/2 in the nucleus accumbens core (Figure 5B; paired t-test: t(4)=3.79, p=0.02), but not the shell (Veh: 41,430.6±8,400.7; U0126: 40,140.7±9,375.9). These data confirm that U0126 effectively inhibited ERK1/2 activity in both target brain regions.
Figure 5. Confirmation of decreased pERK1/2 IR following U0126 administration.
Following the completion of testing, rats received a unilateral infusion of vehicle and U0126 and were sacrificed 30 min later. (A) Intra-amygdala U0126 (3 μg; n=4) significantly reduced pERK1/2 IR in the CeA. Representative photomicrographs (20X) showing decreased pERK1/2 IR following U0126 administration in the CeA. Pictures (central portion of the CeA) are taken proximal to the injector tracts to avoid artifacts associated with tissue penetration from the microinjections. (B) Similarly, intra-accumbens U0126 (3 μg; n=5) administration significantly reduced pERK1/2 in the core. (D) Representative photomicrographs (20X) showing decreased pERK1/2 IR following U0126 administration in the nucleus accumbens core (medial to the anterior commissure). Scale bars, 100 μm. Pictures are taken proximal to the injector tracts to avoid artifacts associated with tissue damage from the microinjections.
4. Discussion
In the present study, alcohol administration induced a significant increase in phosphorylated ERK1/2 (pERK1/2) IR in subregions of the amygdala, but not the nucleus accumbens, in alcohol discrimination-trained rats. Based on these results, we hypothesized that site specific inhibition of ERK1/2 in the amygdala would disrupt the expression of the discriminative stimulus effects of alcohol; however, contrary to our hypothesis MEK/ERK1/2 inhibition in the amygdala or the nucleus accumbens did not alter the expression of the discriminative stimulus effects of the alcohol training dose (1 g/kg). In fact, intra-amygdala ERK1/2 inhibition potentiated the discriminative stimulus effects of a low alcohol dose (0.5 g/kg); no change following intra-accumbens administration was observed. Differential sensitivity to ERK1/2 inhibition was also evidenced by significant reductions in response rate following U0126 administration in the nucleus accumbens, but not the amygdala.
The differential brain regional sensitivity to MEK/ERK1/2 inhibition in relation to the modulation of alcohol discrimination is consistent with the IHC results in which the amygdala, but not the nucleus accumbens showed a response to alcohol as measured by increased pERK1/2 IR. This alcohol-induced ERK1/2 activation is consistent with other work in which alcohol administration and other drugs of abuse, have been shown to induce phosphorylation of ERK1/2 in the central amygdala [19–24]. The increase in pERK1/2 IR in the central amygdala following alcohol (1 g/kg, IG) administration is most in line with a study by Ibba et al. (2009) that showed an increase in pERK1/2 in this nucleus using the same alcohol dose and route of administration (1 g/kg, IG). Under another route of administration, Pandey et al. (2008) report a similar alcohol (1 g/kg, IP)-induced increase in ERK1/2 phosphorylation following an anxiety assessment (elevated plus-maze). The similarity between the findings of the present work and these other studies is interesting given marked differences in the experimental protocols, and suggests the generality of the effect. For example, in the present study, rats received intermittent (double alternation schedule) exposure to alcohol (1 g/kg, IG) during the extensive behavioral training required by drug discrimination methods; whereas, in the Ibba et al. (2009) and Pandey et al. (2008) studies, the alcohol-induced increase in pERK1/2 was observed following a single exposure to alcohol in alcohol naïve animals. This may suggest that pERK1/2 response to alcohol in the central amygdala is not altered by a history of alcohol exposure; however, continuous exposure to alcohol (i.e., alcohol vapor exposure) has been shown to decrease pERK1/2 levels in the central amygdala [45]. Another major difference between the studies is the timing of the alcohol injection. In the present study, the alcohol-induced increase in pERK1/2 was evident 90 min following alcohol administration compared to 15 min [20] and approximately 1 h [21], which could suggest an enduring alcohol-induced change. Also, in the present study, pERK1/2 IR was compared between discrimination-trained rats administered water or alcohol prior to the 2 min behavioral test, not to a naïve group. Therefore, it is possible that exposure to and/or performance in the discrimination task alone activated ERK1/2 expression in the central amygdala, and that alcohol-administration further potentiated that level. This is plausible given that the MAPK pathway is critically involved in learning and memory-associated plasticity processes [13, 17], and that the ERK pathway specifically in the amygdala has been shown to regulate memory consolidation [46] and the expression of drug-related cue learning [8, 9, 47]. Indeed, rats trained with the -opioid receptor agonist (U-50,488H) as a discriminative stimulus have greater pERK1/2 levels in the central amygdala relative to drug-matched controls (i.e., nondiscrimination-trained) [48], suggestive of a learning-induced adaptation in pERK1/2. However, there is evidence to suggest that the ERK pathway is not involved in modulating alcohol-related associative learning (as measured by alcohol-induced conditioned place preference) [49]. It will be of interest to directly assess the possibility of a discrimination learning-induced activation of pERK1/2 and to examine total ERK1/2 expression in future work using both a drug-matched control group and a drug-naïve group [50].
In the present work, pERK1/2 IR in the nucleus accumbens (core and shell) was not altered following alcohol (1 g/kg, IG) administration. This is in contrast to results by Ibba et al. (2009) in which pERK1/2 IR was increased in both the nucleus accumbens core and shell following alcohol administration (0.5, 1, 2 g/kg, IG). In addition to the procedural differences in the studies (discussed above) which could potentially account for the findings, it is also possible that alcohol-induced pERK1/2 activation has a shorter time course and thus was missed in the present work. That is, in the present work, brains were collected approximately 90 min after alcohol administration vs. 15 min in the Ibba et al. (2009) study. Importantly, the absence of an alcohol-induced change in pERK1/2 IR in the nucleus accumbens supports the behavioral data showing lack of a functional role of accumbens ERK1/2 in modulating the discriminative stimulus effects of alcohol.
In general, systemically and intra-amygdala and accumbens administered compounds that reduce excitatory neurotransmission, such as GABAA agonists or NMDA antagonists tend to substitute for alcohol (i.e., produce alcohol-like effects) or potentiate the effects of alcohol (i.e., effects are more alcohol-like) in discrimination tasks [3, 25, 27, 28, 51–58]. Therefore, the finding that intra-amygdala U0126 administration (3 μg) potentiated effects of the low alcohol dose (0.5 g/kg, IG) raises the possibility that inhibition of MEK/ERK1/2 activity specifically in this brain region may have contributed to decreased neuronal excitability. Indeed, ERK1/2 inhibition in the central amygdala has been shown to decrease neuronal excitability induced by reactive phosphorylation oxygen species [59]. Further, while U0126 (3 μg) significantly inhibited ERK1/2 phosphorylation in the CeA (Figure 5A), this dose administered alone was not sufficient to produce alcohol-like discriminative stimulus effects. It will be of interest for future work to assess whether higher doses of U0126 administered alone would induce alcohol-like effects or whether the presence of alcohol/interaction with alcohol (as shown in the present work) is necessary for the potentiation of the discriminative stimulus effects of alcohol.
Interestingly, while intra-accumbens U0126 decreased ERK1/2 phosphorylation in that region (Fig 5B), MEK/ERK1/2 inhibition did not alter the expression of the discriminative stimulus effects of the low alcohol dose (0.5 g/kg, IG) or the alcohol training dose (1 g/kg, IG). This pattern of results suggests a dissociation between the functional role of ERK1/2 in the amygdala and the nucleus accumbens in the modulation of the discriminative stimulus effects of alcohol. Further, intra-accumbens U0126 pretreatment before alcohol (1 and 0.5 g/kg) and water, resulted in response rate reductions, which confirm that the compound was behaviorally active following accumbens infusion, and provides further evidence of differential sensitivity between the two brain regions given that intra-amygdala U0126 did not alter response rates at any dose tested. Higher doses of U0126 were not tested in the nucleus accumbens given that response rates were reduced by the highest dose tested (3 μg); however, it is plausible that a higher dose of the MEK/ERK1/2 inhibitor could potentiate the discriminative stimulus effects of the low alcohol dose, at a dose that induces a response rate reduction. Regardless, this pattern of results would be consistent with the findings of the present work, showing differential sensitivity to ERK1/2 inhibition.
An interesting observation is that under vehicle conditions pERK1/2 positive IR in the central amygdala were approximately 10-fold higher (Experiment 2; Figure 5A) than the nucleus accumbens core. This difference in pERK1/2 IR was not observed in Experiment 1 where the two regions showed fairly similar pERK1/2 IR following Water administration (Figure 2B), which is consistent with previous work showing similar pERK1/2 levels between the CeA and nucleus accumbens following saline injection [23]. While it is difficult to compare the immunohistochemistry results across experiments given the different procedural experience prior to sacrifice (i.e., water discrimination session vs. vehicle microinjection), pERK1/2 IR in the nucleus accumbens following vehicle infusion is surprisingly similar to Experiment 1 (suggesting the lack of a vehicle effect), as is pERK1/2 IR in the BLA and LaDL across the two experiments. Therefore, this data pattern suggests that the microinjection protocol may have led to an activation of pERK1/2 in the CeA, which would be in line with previous work showing activation of ERK1/2 in this region following stress exposure [60].
The alcohol-induced increase in pERK1/2 IR in the amygdala led to the hypothesis that the expression of the discriminative stimulus effects of alcohol (1 g/kg) are associated, in part, with increased ERK1/2 phosphorylation in this region. However, inhibition of ERK1/2 activation in the amygdala by U0126, potentiated the discriminative stimulus effects of alcohol, which was contrary to this hypothesis. A possible explanation for this apparent discrepancy is that systemically administered alcohol can have widespread effects such that multiple brain circuits can be activated/inhibited which could impact phosphorylation of ERK1/2 in the central amygdala (and other regions). Overall, while this initial experiment identified the amygdala as a region that shows a response in ERK1/2 phosphorylation following an alcohol discrimination session, the site specific microinjection studies were able to confirm the functional involvement of ERK1/2 in the amygdala on the discriminative stimulus effects of alcohol.
In summary, the present findings show that the expression of the discriminative stimulus effects of alcohol is regulated, in part, by inhibition of the phosphorylation of ERK1/2 in the CeA. Given that interoceptive or discriminative stimulus drug cues have the potential to impact drug seeking and taking behavior, it is possible that changes to the interoceptive effects of consumed alcohol, as evident after ERK inhibition, may impact alcohol drinking. Indeed, future work will be necessary to pursue this possibility; however, the novel findings of this work show that activity of the ERK signaling pathway in the amygdala can influence the expression of the interoceptive/discriminative stimulus effects of alcohol.
Research Highlights.
Discrimination-trained rats show alcohol (A)-induced pERK1/2 in the amygdala
Intra-amygdala ERK inhibition potentiates low dose A discriminative stimulus (DS)
No change to A training dose DS after intra-amygdala or accumbens ERK inhibition
pERK1/2 specifically reduced after brain injection of ERK inhibitor
Acknowledgments
This work was supported in part by funds from the National Institutes of Health AA016009 (JB), AA019682 (JB), AA011605 (CWH), ABMRF/The Foundation for Alcohol Research (JB), and by the Bowles Center for Alcohol Studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stolerman I. Drugs of abuse: behavioural principles, methods and terms. Trends Pharmacol Sci. 1992;13(5):170–6. doi: 10.1016/0165-6147(92)90059-f. [DOI] [PubMed] [Google Scholar]
- 2.Wise RA, Wang B, You ZB. Cocaine serves as a peripheral interoceptive conditioned stimulus for central glutamate and dopamine release. PLoS ONE. 2008;3(8):e2846. doi: 10.1371/journal.pone.0002846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bienkowski P, et al. Discriminative stimulus properties of ethanol in the rat: differential effects of selective and nonselective benzodiazepine receptor agonists. Pharmacol Biochem Behav. 1997;58(4):969–73. doi: 10.1016/s0091-3057(97)00034-8. [DOI] [PubMed] [Google Scholar]
- 4.Grant KA. Strategies for understanding the pharmacological effects of ethanol with drug discrimination procedures. Pharmacol Biochem Behav. 1999;64(2):261–7. doi: 10.1016/s0091-3057(99)00075-1. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder JP, et al. Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: blockade by the mGluR5 antagonist MPEP. Neuropharmacology. 2008;55(4):546–54. doi: 10.1016/j.neuropharm.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Xu M. Dopamine D1 and D3 receptors are differentially involved in cue-elicited cocaine seeking. J Neurochem. 2010;114(2):530–41. doi: 10.1111/j.1471-4159.2010.06775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faccidomo S, et al. Increased operant responding for ethanol in male C57BL/6J mice: specific regulation by the ERK1/2, but not JNK, MAP kinase pathway. Psychopharmacology (Berl) 2009;204(1):135–47. doi: 10.1007/s00213-008-1444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li YQ, et al. Central amygdala extracellular signal-regulated kinase signaling pathway is critical to incubation of opiate craving. J Neurosci. 2008;28(49):13248–57. doi: 10.1523/JNEUROSCI.3027-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu L, et al. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8(2):212–9. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- 10.Radwanska K, et al. Alcohol relapse induced by discrete cues activates components of AP-1 transcription factor and ERK pathway in the rat basolateral and central amygdala. Neuropsychopharmacology. 2008;33(8):1835–46. doi: 10.1038/sj.npp.1301567. [DOI] [PubMed] [Google Scholar]
- 11.Qi M, Elion EA. MAP kinase pathways. J Cell Sci. 2005;118(Pt 16):3569–72. doi: 10.1242/jcs.02470. [DOI] [PubMed] [Google Scholar]
- 12.Pearson G, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22(2):153–83. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 13.Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14(3):311–7. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5(3):173–83. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- 15.Treisman R. Regulation of transcription by MAP kinase cascades. Curr Opin Cell Biol. 1996;8(2):205–15. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- 16.Grewal SS, York RD, Stork PJ. Extracellular-signal-regulated kinase signalling in neurons. Curr Opin Neurobiol. 1999;9(5):544–53. doi: 10.1016/S0959-4388(99)00010-0. [DOI] [PubMed] [Google Scholar]
- 17.Adams JP, Sweatt JD. Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol. 2002;42:135–63. doi: 10.1146/annurev.pharmtox.42.082701.145401. [DOI] [PubMed] [Google Scholar]
- 18.Mazzucchelli C, et al. Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron. 2002;34(5):807–20. doi: 10.1016/s0896-6273(02)00716-x. [DOI] [PubMed] [Google Scholar]
- 19.Acquas E, et al. Differential effects of intravenous R,S-(+/−)-3,4-methylenedioxymethamphetamine (MDMA, Ecstasy) and its S(+)- and R(−)-enantiomers on dopamine transmission and extracellular signal regulated kinase phosphorylation (pERK) in the rat nucleus accumbens shell and core. J Neurochem. 2007;102(1):121–32. doi: 10.1111/j.1471-4159.2007.04451.x. [DOI] [PubMed] [Google Scholar]
- 20.Ibba F, et al. Ethanol-induced extracellular signal regulated kinase: role of dopamine D1 receptors. Alcohol Clin Exp Res. 2009;33(5):858–67. doi: 10.1111/j.1530-0277.2009.00907.x. [DOI] [PubMed] [Google Scholar]
- 21.Pandey SC, et al. Effector immediate-early gene arc in the amygdala plays a critical role in alcoholism. J Neurosci. 2008;28(10):2589–600. doi: 10.1523/JNEUROSCI.4752-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salzmann J, et al. Importance of ERK activation in behavioral and biochemical effects induced by MDMA in mice. Br J Pharmacol. 2003;140(5):831–8. doi: 10.1038/sj.bjp.0705506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valjent E, et al. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19(7):1826–36. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- 24.Valjent E, et al. Delta 9-tetrahydrocannabinol-induced MAPK/ERK and Elk-1 activation in vivo depends on dopaminergic transmission. Eur J Neurosci. 2001;14(2):342–52. doi: 10.1046/j.0953-816x.2001.01652.x. [DOI] [PubMed] [Google Scholar]
- 25.Besheer J, Cox AA, Hodge CW. Coregulation of ethanol discrimination by the nucleus accumbens and amygdala. Alcohol Clin Exp Res. 2003;27(3):450–6. doi: 10.1097/01.ALC.0000057036.64169.C1. [DOI] [PubMed] [Google Scholar]
- 26.Besheer J, et al. Interoceptive effects of alcohol require mGlu5 receptor activity in the nucleus accumbens. J Neurosci. 2009;29(30):9582–91. doi: 10.1523/JNEUROSCI.2366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hodge CW, Aiken AS. Discriminative stimulus function of ethanol: role of GABAA receptors in the nucleus accumbens. Alcohol Clin Exp Res. 1996;20(7):1221–8. doi: 10.1111/j.1530-0277.1996.tb01116.x. [DOI] [PubMed] [Google Scholar]
- 28.Hodge CW, Cox AA. The discriminative stimulus effects of ethanol are mediated by NMDA and GABA(A) receptors in specific limbic brain regions. Psychopharmacology (Berl) 1998;139(1–2):95–107. doi: 10.1007/s002130050694. [DOI] [PubMed] [Google Scholar]
- 29.Cannady R, et al. Activation of Group II Metabotropic Glutamate Receptors Inhibits the Discriminative Stimulus Effects of Alcohol via Selective Activity Within the Amygdala. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodge CW, et al. The discriminative stimulus properties of self-administered ethanol are mediated by GABA(A) and NMDA receptors in rats. Psychopharmacology (Berl) 2001;154(1):13–22. doi: 10.1007/s002130000619. [DOI] [PubMed] [Google Scholar]
- 31.Besheer J, Hodge CW. Pharmacological and anatomical evidence for an interaction between mGluR5- and GABA(A) alpha1-containing receptors in the discriminative stimulus effects of ethanol. Neuropsychopharmacology. 2005;30(4):747–57. doi: 10.1038/sj.npp.1300616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Besheer J, Stevenson RA, Hodge CW. mGlu5 receptors are involved in the discriminative stimulus effects of self-administered ethanol in rats. Eur J Pharmacol. 2006;551(1–3):71–5. doi: 10.1016/j.ejphar.2006.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quertemont E, Green HL, Grant KA. Brain ethanol concentrations and ethanol discrimination in rats: effects of dose and time. Psychopharmacology (Berl) 2003;168(3):262–70. doi: 10.1007/s00213-003-1437-7. [DOI] [PubMed] [Google Scholar]
- 34.Nurmi M, Kiianmaa K, Sinclair JD. Brain ethanol in AA, ANA, and Wistar rats monitored with one-minute microdialysis. Alcohol. 1994;11(4):315–21. doi: 10.1016/0741-8329(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 35.Hiltunen AJ, Jarbe TU. Discriminative stimulus properties of ethanol: effects of cumulative dosing and Ro 15-4513. Behav Pharmacol. 1989;1(2):133–140. [PubMed] [Google Scholar]
- 36.Wilkie MB, et al. Acute ethanol administration rapidly increases phosphorylation of conventional protein kinase C in specific mammalian brain regions in vivo. Alcohol Clin Exp Res. 2007;31(7):1259–67. doi: 10.1111/j.1530-0277.2007.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. London: Academic Press; 1998. [Google Scholar]
- 38.Davies SP, et al. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351(Pt 1):95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Favata MF, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273(29):18623–32. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 40.Grissom NM, Bhatnagar S. The basolateral amygdala regulates adaptation to stress via beta-adrenergic receptor-mediated reductions in phosphorylated extracellular signal-regulated kinase. Neuroscience. 2011;178:108–22. doi: 10.1016/j.neuroscience.2010.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai YT, et al. Activation of amygdaloid PKC pathway is necessary for conditioned cues-provoked cocaine memory performance. Neurobiol Learn Mem. 2008;90(1):164–70. doi: 10.1016/j.nlm.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Mattson BJ, et al. Cocaine-induced CREB phosphorylation in nucleus accumbens of cocaine-sensitized rats is enabled by enhanced activation of extracellular signal-related kinase, but not protein kinase A. J Neurochem. 2005;95(5):1481–94. doi: 10.1111/j.1471-4159.2005.03500.x. [DOI] [PubMed] [Google Scholar]
- 43.Ploski JE, et al. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of pavlovian fear conditioning in the lateral amygdala. J Neurosci. 2008;28(47):12383–95. doi: 10.1523/JNEUROSCI.1662-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiflett MW, et al. Cue-elicited reward-seeking requires extracellular signal-regulated kinase activation in the nucleus accumbens. J Neurosci. 2008;28(6):1434–43. doi: 10.1523/JNEUROSCI.2383-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanna PP, et al. ERK regulation in chronic ethanol exposure and withdrawal. Brain Res. 2002;948(1–2):186–91. doi: 10.1016/s0006-8993(02)03191-8. [DOI] [PubMed] [Google Scholar]
- 46.Schafe GE, et al. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J Neurosci. 2000;20(21):8177–87. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li F, et al. The Activation of NMDA Receptor-ERK Pathway in the Central Amygdala is Required for the Expression of Morphine-Conditioned Place Preference in the Rat. Neurotox Res. 2011 doi: 10.1007/s12640-011-9250-2. [DOI] [PubMed] [Google Scholar]
- 48.Yoshizawa K, et al. Activation of extracellular signal-regulated kinase is critical for the discriminative stimulus effects induced by U-50,488H. Synapse. 2011 doi: 10.1002/syn.20937. [DOI] [PubMed] [Google Scholar]
- 49.Groblewski PA, Franken FH, Cunningham CL. Inhibition of extracellular signal-regulated kinase (ERK) activity with SL327 does not prevent acquisition, expression, and extinction of ethanol-seeking behavior in mice. Behav Brain Res. 2011;217(2):399–407. doi: 10.1016/j.bbr.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Besheer J, et al. Ethanol-induced alterations of c-Fos immunoreactivity in specific limbic brain regions following ethanol discrimination training. Brain Res. 2008;1232:124–31. doi: 10.1016/j.brainres.2008.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ator NA, et al. Drug discrimination analysis of endogenous neuroactive steroids in rats. Eur J Pharmacol. 1993;241(2–3):237–43. doi: 10.1016/0014-2999(93)90208-y. [DOI] [PubMed] [Google Scholar]
- 52.Grant KA, et al. Characterization of the discriminative stimulus effects of GABA(A) receptor ligands in Macaca fascicularis monkeys under different ethanol training conditions. Psychopharmacology (Berl) 2000;152(2):181–8. doi: 10.1007/s002130000510. [DOI] [PubMed] [Google Scholar]
- 53.Helms CM, Rogers LS, Grant KA. Antagonism of the ethanol-like discriminative stimulus effects of ethanol, pentobarbital, and midazolam in cynomolgus monkeys reveals involvement of specific GABA(A) receptor subtypes. J Pharmacol Exp Ther. 2009;331(1):142–52. doi: 10.1124/jpet.109.156810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hundt W, et al. Ethanol and N-methyl-D-aspartate receptor complex interactions: a detailed drug discrimination study in the rat. Psychopharmacology (Berl) 1998;135(1):44–51. doi: 10.1007/s002130050484. [DOI] [PubMed] [Google Scholar]
- 55.Jarbe TU, McMillan DE. Interaction of the discriminative stimulus properties of diazepam and ethanol in pigeons. Pharmacol Biochem Behav. 1983;18(1):73–80. doi: 10.1016/0091-3057(83)90254-x. [DOI] [PubMed] [Google Scholar]
- 56.Schechter MD, et al. The NMDA receptor antagonist MK-801 produces ethanol-like discrimination in the rat. Alcohol. 1993;10(3):197–201. doi: 10.1016/0741-8329(93)90035-m. [DOI] [PubMed] [Google Scholar]
- 57.Shelton KL, Grant KA. Discriminative stimulus effects of ethanol in C57BL/6J and DBA/2J inbred mice. Alcohol Clin Exp Res. 2002;26(6):747–57. [PubMed] [Google Scholar]
- 58.Vivian JA, et al. Characterization of the discriminative stimulus effects of N-methyl- D-aspartate ligands under different ethanol training conditions in the cynomolgus monkey (Macaca fascicularis) Psychopharmacology (Berl) 2002;162(3):273–81. doi: 10.1007/s00213-002-1086-2. [DOI] [PubMed] [Google Scholar]
- 59.Li Z, Ji G, Neugebauer V. Mitochondrial reactive oxygen species are activated by mGluR5 through IP3 and activate ERK and PKA to increase excitability of amygdala neurons and pain behavior. J Neurosci. 2011;31(3):1114–27. doi: 10.1523/JNEUROSCI.5387-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang CH, Huang CC, Hsu KS. Differential roles of basolateral and central amygdala on the effects of uncontrollable stress on hippocampal synaptic plasticity. Hippocampus. 2008;18(6):548–63. doi: 10.1002/hipo.20414. [DOI] [PubMed] [Google Scholar]
- 61.de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75(2):134–43. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]