Abstract
We have recently demonstrated that the glutamate transporter activator riluzole paradoxically enhanced glutamate-induced glutamate release from cultured astrocytes. We further showed that both riluzole and the α2δ subunit ligand gabapentin activated descending inhibition in rats by increasing glutamate receptor signaling in the locus coeruleus and hypothesized that these drugs share common mechanisms to enhance glutamate release from astrocytes. In the present study, we examined the effects of riluzole and gabapentin on glutamate uptake and release and glutamate-induced Ca2+ responses in primary cultures of astrocytes. Riluzole and gabapentin facilitated glutamate-induced glutamate release from astrocytes and significantly increased glutamate uptake, the latter being completely blocked by the non-selective glutamate transporter blocker DL-threo-β-benzyloxyaspartic acid (DL-TBOA). Riluzole and gabapentin also enhanced the glutamate-induced increase in intracellular Ca2+ concentrations. Some α2δ subunit ligands, pregabalin and L-isoleucine, enhanced the glutamate-induced Ca2+ response, whereas another, 3-exo-aminobicyclo[2.2.1]heptane-2-exo-carboxylic acid (ABHCA), did not. The enhancement of glutamate-induced intracellular Ca2+ response by riluzole and gabapentin was blocked by the DL-TBOA and an inhibitor of Na+/Ca2+ exchange, 2-[2-[4-(4-nitrobenzyloxy)phenyl]ethyl]isothiurea (KB-R7943). Gabapentin’s enhancement of Ca2+ increase was specific to glutamate stimulation, as it was not mimicked with stimulation by ATP. These results suggest that riluzole and gabapentin enhance Na+-glutamate co-transport through glutamate transporters, induce subsequent Ca2+ influx via the reverse mode of Na+/Ca2+ exchange, and thereby facilitate Ca2+-dependent glutamate release by glutamate in astrocytes. The present study also demonstrates a novel target of gabapentinoid action in astrocytes other than α2δ subunits in neurons.
Keywords: riluzole, gabapentin, astrocytes, glutamate transporters
1. Introduction
In the central nervous system, astrocytes regulate extracellular glutamate concentration via two types of glutamate transporters, glutamate transporter-1 (GLT-1) and glutamate-aspartate transporter (GLAST) (Anderson and Swanson, 2000). Under physiological conditions, glutamate transporters take up glutamate from the extracellular space. However, in pathological conditions such as ischemia, astrocytes release large amounts of glutamate via reverse transport, and this increased extracellular glutamate participates in neurotoxicity (Malarkey and Parpura, 2008).
Riluzole, a neuroprotective drug approved for amyotrophic lateral sclerosis (Brooks, 2009), activates GLT-1 and GLAST to enhance glutamate uptake (Frizzo et al., 2004; Fumagalli et al., 2008), and reduces extracellular glutamate concentrations in the spinal cord (Coderre et al., 2007). In contrast to this glutamate lowering effect in the spinal cord, we recently demonstrated that a local injection of riluzole into the locus coeruleus resulted in activation of noradrenergic neurons to induce descending inhibition in rats, possibly via facilitation of glutamate-induced glutamate release from astrocytes (Hayashida et al., 2010). However, the mechanisms by which riluzole might activate glutamate-induced glutamate release from astrocytes is unknown.
Gabapentin inhibits pain transmission via an interaction with α2δ subunits of voltage-gated Ca2+ channels (Gee et al., 1996). This inhibitory mechanism contrasts with our recent demonstration that gabapentin, like riluzole, activated locus coeruleus neurons via glutamatergic signaling and induced subsequent spinal noradrenaline release in rats and humans (Hayashida et al., 2007; Hayashida et al., 2008). We therefore hypothesized that riluzole and gabapentin may share common mechanisms for glutamate regulation in astrocytes.
Glutamate increases intracellular Ca2+ in astrocytes via activation of Ca2+ permeable ionotropic glutamate receptors, which respond to AMPA, and metabotropic glutamate receptors, which release Ca2+ from internal stores through 1,4,5-inositol-trisphosphate signaling (Hansson et al., 2000; Verkhratsky and Kirchhoff, 2007). In some astrocytes, co-transport of sodium ions and glutamate by glutamate transporters results in Ca2+ influx via the reverse mode of Na+/Ca2+ exchange (Kirischuk et al., 1997; Rojas et al., 2007), which in turn leads to glutamate release from astrocytes via Ca2+-dependent mechanisms (Malarkey and Parpura, 2008). By this mechanism, glutamate uptake by astrocytes can paradoxically result in glutamate release. We therefore hypothesized that riluzole and gabapentin enhance Ca2+-dependent glutamate release from astrocytes by activation of glutamate transporters and subsequent Ca2+ influx via the reverse mode of Na+/Ca2+ exchange. The present study examined whether riluzole, gabapentin, and other α2δ subunit ligands increase glutamate uptake through glutamate transporters, enhance glutamate-induced intracellular Ca2+ response by activation of glutamate transporters and subsequent Ca2+ influx via the reverse mode of Na+/Ca2+ exchange, and thereby facilitate glutamate-induced glutamate release in primary cultured astrocytes.
2. Materials and Methods
2.1. Astrocyte cultures
Primary astrocyte cultures were prepared from the cerebral cortices of neonatal rats between postnatal days 1 and 2. Cerebral cortices were mechanically dissociated in ice-cold Hank’s buffered salt solution (HBSS, pH=7.2) by fire-polished glass pipettes and centrifuged at 300×G for 5 min. Tissues were re-dissociated in ice-cold HBSS and the procedure was repeated two times using smaller pipette tip diameters. Cells were first seeded onto T-50 flasks and incubated in Dulbecco’s modified Eagle’s medium, containing 10% fetal bovine serum, 100 units/ml penicillin/streptomycin, 2 mM L-glutamine, and 10 ng/ml epidermal growth factor (EGF, BD Biosciences, Bedford, MA, USA) to maintain GLT-1 and GLAST protein expression in astrocytes (Zelenaia et al., 2000), at 37°C and 5% CO2. After one week, astrocytes were reseeded onto poly-d-lysine-coated glass coverslips (35 mm diameter) at a density of 4×104 cells for Ca2+ imaging experiments and in 24-well plates at a density of 104 cells/well for glutamate uptake/release experiments, then cultured for 6 days. In the present study, cells consisted of >98% of flat polygonal astrocytes with positive immunostaining for glial fibrillary acidic protein.
2.2. Ca2+ imaging
Ca2+ imaging was performed according to our previous study in neurons (Hayashida et al., 2006) with modifications for cultured astrocytes. Astrocytes were incubated with 5 μM Fura-2 AM (Molecular Probes, Eugene, OR, USA) and 0.01% Pluronic F-127 (Molecular Probes) for 30 min at 37 °C, washed with HEPES buffer containing (in mM): 140 NaCl, 4 KCl, 2 CaCl2, 1.2 MgSO4, 10 glucose, and 10 HEPES, pH=7.4, and then left at room temperature in a dark environment for 20 min. Coverslips were mounted on a chamber equipped with a pressure valve perfusion system (ALA-VM8, ALA Scientific Instruments, New York, NY, USA) and viewed through an inverted microscope. Fura-2 fluorescence was recorded at 510 nm during alternating excitation at 340 and 380 nm at 1 Hz using a monochromater (PTI Deltascan, Photon Technology International, South Brunswick, NJ, USA). Only cells responding to 300 μM ATP at the end of experiment were included in analysis.
2.3. Glutamate uptake and release
For glutamate uptake experiments, astrocytes were incubated with HEPES buffer for 40 min with a change to fresh buffer at 20 min and treated with test drugs for 5 min at 37 °C. Astrocytes were incubated with 1 μM glutamate (combination of both tritiated and unlabeled glutamate) containing test drugs for 1 min at 37 °C, quickly washed twice, and then lysed with 0.4% Triton X-100 for 10 min. Amount of radioactivity in lysates was measured by scintillation spectrometry (LS6500, Beckman Coulter Inc., Fullerton, CA, USA).
For glutamate release experiments, astrocytes were incubated with Dulbecco’s modified Eagle’s medium containing 1 μM glutamate (combination of both tritiated and unlabeled glutamate) for 1 hr at 37 °C, and washed twice with HEPES buffer. Astrocytes were then incubated with test drugs alone for 5 min, followed by unlabeled 10 μM glutamate in the presence of test drug for 5 min at 37 °C. Supernatants were collected and astrocytes were lysed. The amount of radioactivity in supernatants and lysates was measured as described above.
2.4. Drugs
DL-threo-β-benzyloxyaspartic acid (DL-TBOA, Tocris Bioscience, Ellisville, MO, USA) and 2-[2-[4-(4-nitrobenzyloxy)phenyl]ethyl]isothiurea (KB-R7943, Tocris Bioscience) were dissolved in DMSO and diluted with HEPES buffer. Riluzole (Sigma Chemical CO, St. Louis, MO, USA), gabapentin (Tronto Research Chemicals Inc., North York, ON, USA), pregabalin (gifted from Pfizer Inc., New York, NY, USA), L-isoleucine (Sigma Chemical CO), and 3-exo-aminobicyclo[2.2.1]heptane-2-exo-carboxylic acid (ABHCA, Acros organics, New Jersey, USA) were dissolved in distilled water, and then diluted with HEPES buffer.
2.5. Data analysis
Data from glutamate uptake and release experiments are normally distributed and presented as mean ± S.E.M. Data from Ca2+ imaging experiments were not normally distributed and presented as 25th, 50th, and 75th percentiles of Fura-2 fluorescence ratio response. Differences among groups were determined using two-way analysis of variance (ANOVA) or ANOVA on ranks as appropriate. P<0.05 was considered significant.
3. Results
3.1. Glutamate uptake and release
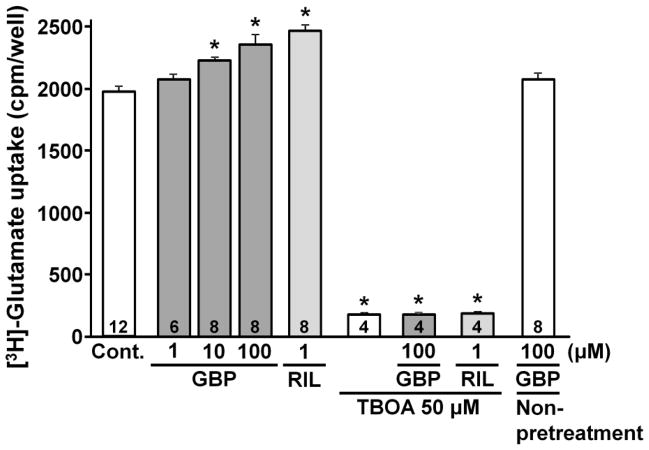
For glutamate uptake, we selected relatively low concentration of glutamate (1 μM) to minimize glutamate-induced glutamate release. As previously shown (Frizzo et al., 2004), riluzole (1 μM) significantly enhanced [3H]-glutamate uptake in astrocytes (Fig. 1, P<0.01). The concentration of riluzole in the uptake experiment was determined from the previous study (Frizzo et al., 2004). Gabapentin pretreatment also increased [3H]-glutamate uptake in a concentration-dependent manner. However, when acutely administered, gabapentin (100 μM) did not affect [3H]-glutamate uptake, consistent with a previous observation (Su et al., 1995). The non-selective glutamate transporter blocker DL-TBOA (100 μM) strongly reduced [3H]-glutamate uptake (P<0.001) and abolished the enhancement of [3H]-glutamate uptake by riluzole and gabapentin.
Fig. 1.
[3H]-glutamate uptake in astrocytes is presented as a total radioactivivity (cpm) per well. Cells were pretreated with buffer (control), gabapentin (GBP, 1–100 μM) or riluzole (RIL, 1 μM) in the presence or absence of TBOA (50 μM) for 5 min and glutamate uptake was performed for 1 min in the presence of test drugs. In non-pretreatment group, GBP (100 μM) was co-applied with glutamate without the pretreatment. Number in each column represents sample size. *P<0.01 vs. control.
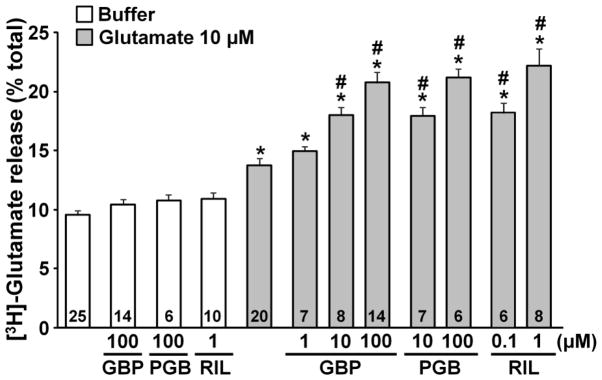
Riluzole, gabapentin, and the other α2δ subunit ligand pregabalin, none of which affected basal [3H]-glutamate release, significantly enhanced glutamate-induced [3H]-glutamate release from astrocytes (Fig. 2, P<0.01). Since TBOA (50 μM) alone significantly increased extracellular [3H]-glutamate concentration (16.4 ± 1.0 %, n=6) compared to the buffer control (9.5 ± 0.4 %, n=25, P<0.01), the current study could not examine whether blockade of glutamate transporters affects gabapentin and riluzole effects.
Fig. 2.
Effects of gabapentin, pregabalin and riluzole on glutamate-induced glutamate release from astrocytes. Cells were treated with buffer or glutamate (10 μM) for 5 min in the presence of buffer alone, gabapentin (GBP, 1–100 μM), pregabalin (PGB, 10–100 μM), or riluzole (RIL, 0.1–1 μM). [3H]-glutamate release is presented as a percentage of total radioactivivity. Number in each column represents sample size. *P<0.01 vs. buffer alone. #P<0.01 vs. glutamate alone.
3.2. Intracellular Ca2+ response
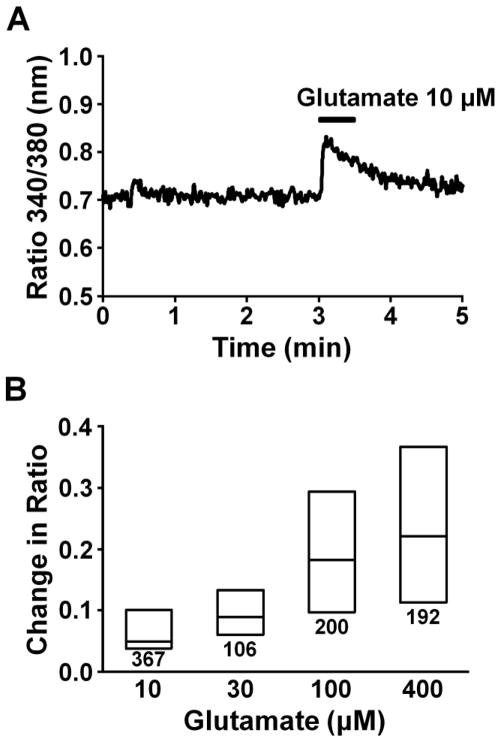
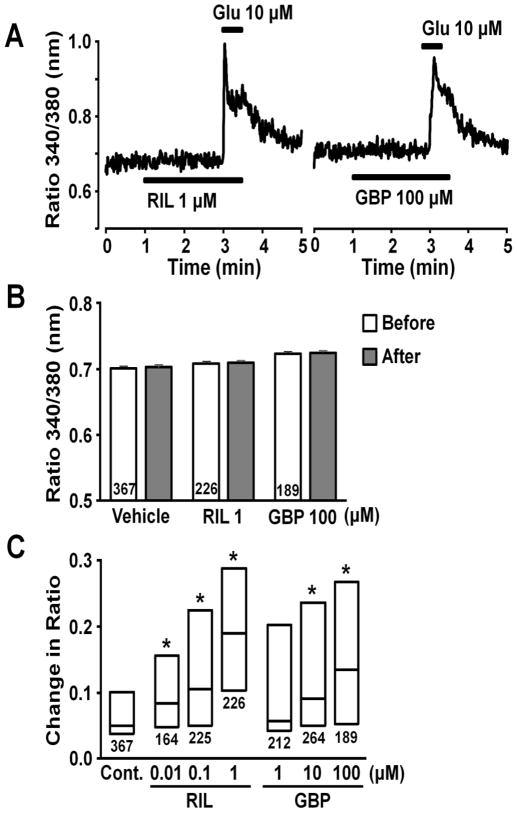
Figure 3A depicts a representative intracellular Ca2+ response in an astrocyte evoked by glutamate (10 μM), as reflected in change in ratio of Fura-2 emission with excitation at two different wavelengths. Figure 3B shows the concentration-dependency of glutamate on this increase in Fura-2 ratio. We chose 10 μM glutamate for the subsequent experiments, since this concentration of glutamate induced both glutamate release and Ca2+ response in astrocytes. Figure 4A depicts representative intracellular Ca2+ responses in astrocytes evoked by glutamate (10 μM) after perfusion with riluzole or gabapentin. Riluzole and gabapentin, neither of which affected basal Fura-2 ratio (Fig. 4B), enhanced the increase evoked by glutamate in a concentration-dependent manner (Fig. 4C). We then tested effects of other α2δ subunit ligands glutamate-induced Ca2+ response (Fig. 5). Pregabalin and L-isoleucine enhanced the glutamate-induced Ca2+ response in a concentration-dependent manner. However, another α2δ subunit ligand ABHCA, which has a similar α2δ binding affinity to gabapentin (Lynch et al., 2006), failed to alter the glutamate-induced Ca2+ response. In contrast to gabapentin’s effect on glutamate stimulation, the intracellular Ca2+ response evoked by 10 μM ATP (25th, 50th, and 75th percentile; 0.36, 0.41, and 0.46, n=173) was not altered by 100 μM gabapentin (25th, 50th, and 75th percentile; 0.34, 0.40, and 0.46, n=172).
Fig. 3.
(A) A representative intracellular Ca2+ response in an astrocyte stimulated with glutamate, presented as change in Fura-2 fluorescence ratio. (B) Concentration response of glutamate (10–400 μM)-induced Fura-2 response. Each box represents the 25th, 50th, and 75th percentiles of Fura-2 fluorescence ratio response. Number under each box represents sample size.
Fig. 4.
(A) Representative intracellular Ca2+ responses in astrocytes stimulated with glutamate (Glu) after perfused with gabapentin (GBP) or riluzole (RIL), presented as change in Fura-2 fluorescence ratio. (B) Effects of RIL and GBP on basal Fura-2 fluorescence ratio. Basal values were determined from the average ratio for 1 min before and after starting vehicle, RIL, or GBP perfusion. Number in each column represents sample size. (C) Effects of RIL (0.01–1 μM) and GBP (1–100 μM) on glutamate-induced intracellular Fura-2 response. Each box represents the 25th, 50th, and 75th percentiles of Fura-2 fluorescence ratio response. Number under each box represents sample size. *P<0.05 vs. control (10 μM glutamate alone).
Fig. 5.
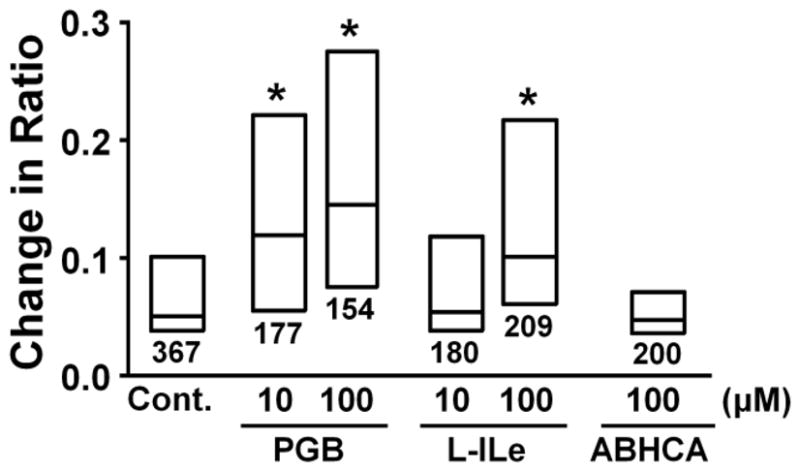
Effects of pregabalin (PGB, 10–100 μM), L-isoleucine (L-ILe, 10–100 μM), and ABHCA (100 μM) on glutamate-induced intracellular Fura-2 response. Each box represents the 25th, 50th, and 75th percentiles of Fura-2 fluorescence ratio response. Number under each box represents sample size. *P<0.05 vs. control (10 μM glutamate alone).
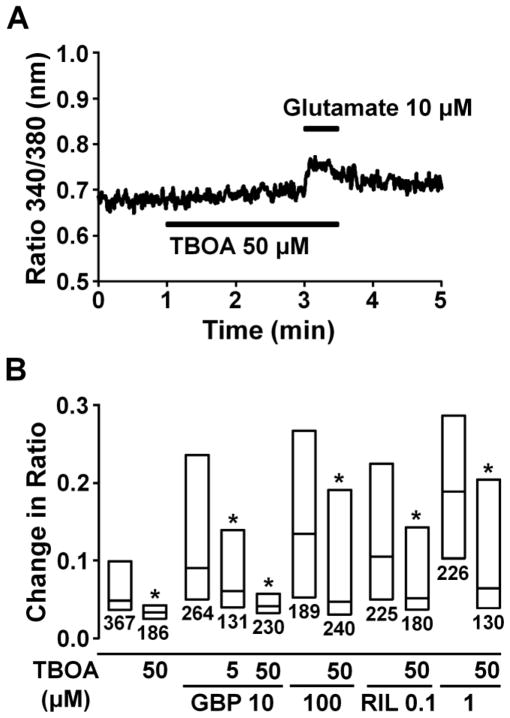
DL-TBOA (50 μM) alone did not affect the basal Fura-2 ratio but significantly reduced the glutamate-induced Fura-2 ratio increase (Fig. 6A and B, P<0.05). The facilitatory effect of gabapentin (10 and 100 μM) and riluzole (0.1 and 1 μM) on the glutamate-induced intracellular Ca2+ response was significantly reduced by DL-TBOA (P<0.05).
Fig. 6.
(A) A representative intracellular Ca2+ concentration response in an astrocyte stimulated with glutamate (10 μM) after perfused with DL-TBOA (50 μM), presented as change in Fura-2 fluorescence ratio. (B) Effects of TBOA (5–50 μM) on gabapentin (GBP, 10 and 100 μM) and riluzole (Ril, 0.1 and 1 μM)-induced enhancement of glutamate-induced intracellular Fura-2 fluorescence ratio response. Each box represents the 25th, 50th, and 75th percentiles of Fura-2 fluorescence ratio response in astrocytes. Number under each box represents sample size. *P<0.05 vs. without TBOA.
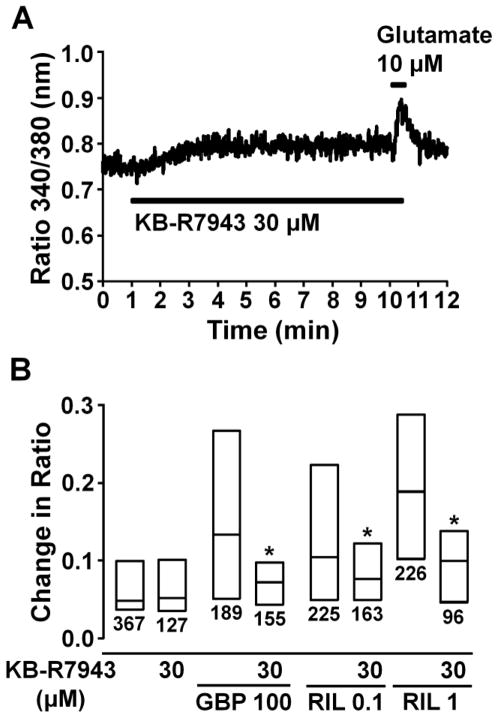
The Na+/Ca2+ exchange inhibitor KB-R7943 (30 μM) alone caused a small increase in the basal Fura-2 ratio within 2–3 min that was sustained during the perfusion, but did not affect the glutamate-induced Ca2+ response (Fig. 7A and B). The facilitatory effects of gabapentin (100 μM) and riluzole (0.1–1 μM) on glutamate-induced intracellular Ca2+ response were significantly reduced by KB-R7943 (P<0.05). Since KB-R7943 (30 μM) alone induced Ca2+ response, we did not test higher concentrations of KB-R7943 in the present study.
Fig. 7.
(A) A representative intracellular Ca2+ concentration response in an astrocyte stimulated with glutamate (10 μM) after perfusion with KB-R7943 (30 μM), presented as change in Fura-2 fluorescence ratio. (B) Effects of KB-R7943 (30 μM) on gabapentin (GBP, 100 μM) and riluzole (Ril, 0.1–1 μM)-induced enhancement of glutamate-induced intracellular Fura-2 fluorescence ratio response. Each box represents the 25th, 50th, and 75th percentiles of Fura-2 fluorescence ratio response. Number under each box represents sample size. *P<0.05 vs. without KB-R7943.
4. Discussion
4.1. Activation of glutamate transporters by riluzole facilitates glutamate release from cultured astrocytes
Glutamate is the most ubiquitous excitatory neurotransmitter in the central nervous system and its regulation by glutamate transporters in astrocytes has been intensely investigated. The current study confirms previous observations in cultured astrocytes that riluzole activates glutamate transporters to enhance glutamate uptake (Frizzo et al., 2004; Fumagalli et al., 2008), and extends these observations by demonstrating that riluzole enhances glutamate-induced increases in intracellular Ca2+ via the reverse mode of Na+/Ca2+ exchange to facilitate glutamate release.
Astrocytes take up glutamate from the extracellular space via glutamate transporters (GLT-1 and GLAST) under physiological conditions, but during pathological states such as ischemia, high extracellular K+ concentrations can increase extracellular glutamate concentrations by reverse transport through these transporters (Malarkey and Parpura, 2008). However, since riluzole did not alter basal glutamate release in the current study, it is unlikely that riluzole induces glutamate release via reverse transport.
Glutamate primarily acts on AMPA and metabotropic glutamate receptors to increase intracellular Ca2+ in astrocytes (Hansson et al., 2000; Verkhratsky and Kirchhoff, 2007). In addition to this glutamate receptor-mediated Ca2+ response, co-transport of sodium ions with glutamate or kainite through glutamate transporters results in Ca2+ influx via the reverse mode of Na+/Ca2+ exchange in astrocytes in vitro and in situ (Kirischuk et al., 1997; Rojas et al., 2007), consistent with the current observation that blockade of glutamate transporters by TBOA reduced glutamate-induced Ca2+ response in cultured astrocytes. However, we did not observe inhibition of glutamate-induced Ca2+ response by KB-R7943 (30 μM), although we did not test higher concentrations of KB-R7943 in the present study. Further studies, such as direct measurements of Na+/Ca2+ exchange current and/or intracellular Na+ concentration, are required to clarify whether reverse mode of Na+/Ca2+ exchange contributes to glutamate-induced Ca2+ response in astrocytes. Nevertheless, the current study demonstrated that both TBOA and KB-R7943 reduced facilitatory effect of riluzole on glutamate-induced Ca2+ response, suggesting that glutamate transporters and the reverse mode of Na+/Ca2+ exchange are involved in riluzole’s effect. Since riluzole did not affect the basal intracellular Ca2+ level in the current study, it is unlikely that riluzole directly reverse Na+/Ca2+ exchange to induce intracellular Ca2+ response. These results suggest that activation of glutamate transporters by riluzole can facilitate glutamate-induced glutamate release from cultured astrocytes.
In addition to activation of glutamate transporters (Frizzo et al., 2004; Fumagalli et al., 2008), high concentrations of riluzole (>10 μM) inhibit voltage-dependent sodium and Ca2+ channels (Lamanauskas and Nistri, 2008) and glutamate receptors (De Sarro et al., 2000). All of these effects of riluzole would induce inhibition rather than activation in astrocytes and neurons, and would support in vivo observations that systemic administered riluzole reduced extracellular glutamate concentration in the spinal cord and some brain regions in rodents (Coderre et al., 2007; Irifune et al., 2007; Takahashi et al., 2011). However, we have recently demonstrated in rats that riluzole increased glutamate signaling in the locus coeruleus (Hayashida et al., 2010) and we observed facilitation rather than inhibition of glutamate-induced glutamate release by riluzole (1 μM) in the current study. Although the current study added EGF in the culture medium to maintain expression of GLT-1 and GLAST in astrocytes (Zelenaia et al., 2000), we recognize that astrocytes become reactive in the culture condition (Kimelberg et al., 2000) and differ from those in vivo. Further study is required to determine whether effects of riluzole on glutamate regulation in astrocytes differ between the locus coeruleus and spinal cord in vivo.
4.2. Novel target of gabapentinoid action in astrocytes
Gabapentin has a high affinity for α2δ subunits of voltage-gated Ca2+ channels, which modulate the release of excitatory neurotransmitters (Gee et al., 1996). Peripheral nerve injury in rats induces up-regulation of α2δ subunits in the spinal cord (Luo et al., 2002) and gabapentin shows analgesic effects in transgenic mice with up-regulated α2δ subunits but not in normal mice (Li et al., 2006). Although acute inhibition of Ca2+ currents by gabapentin is either very minor or absent (Davies et al., 2007), it does inhibit trafficking of voltage-gated Ca2+ channels to the cell membrane by binding to α2δ subunits (Heblich et al., 2008; Hendrich et al., 2008). These previous results suggest that gabapentin relies on α2δ subunits to reduce neuronal excitation. Interestingly, however, some α2δ subunit ligands including ABHCA fail to produce behavioral analgesia (Lynch et al., 2006), indicating additional mechanisms, other than α2δ interactions, would contribute to the analgesic efficacy of gabapentin.
The current study demonstrates that gabapentin, like riluzole, increases glutamate uptake via TBOA-sensitive glutamate transporters, enhances the glutamate-induced intracellular Ca2+ response via the reverse mode of Na+/Ca2+ exchange, and by this mechanism facilitates glutamate release in cultured astrocytes. On the other hand, co-application of gabapentin with glutamate failed to affect glutamate uptake in astrocytes in the current experiment and previous study (Su et al., 1995). These results suggest that gabapentin activates glutamate transporters in astrocytes via direct or indirect interaction, but not via co-transport with glutamate. Since there is no evidence for the presence of α2δ subunits in astrocytes, α2δ interactions are unlikely to contribute to gabapentin effects in astrocytes. This is further supported by the observation that some α2δ ligands, pregabalin and L-isoleucine, enhance glutamate-induced intracellular Ca2+ response, while another (ABHCA) did not, despite similar α2δ binding affinity of ABHCA as gabapentin (Lynch et al., 2006). In some neurons, pertussis toxin-sensitive G-protein pathways are critical for action of gabapentinoids on ion channels (Bertrand et al., 2003; McClelland et al., 2004). In astrocytes, pertussis toxin itself increases glutamate uptake but reduces noradrenaline-induced facilitation of glutamate uptake in astrocytes (Fahrig, 1993). Further studies are required to examine whether second messenger pathways, such as G-protein pathways, are involved in gabapentin’s action in astrocytes.
Systemically administered gabapentin reduces extracellular glutamate concentration in the spinal cord in rats after peripheral nerve injury (Coderre et al., 2007), likely due to the reduction of excitatory neurotransmitters release via α2δ interactions (Gee et al., 1996; Li et al., 2006; Luo et al., 2002). However, we previously demonstrated in rats after peripheral nerve injury that systemically administered gabapentin activated locus coeruleus neurons, via glutamate-mediated mechanisms, to induce spinal noradrenalin release and that the anti-hypersensitivity effect of intra- locus coeruleus gabapentin was blocked by intra- locus coeruleus AMPA receptor antagonist, indicating an obligatory role for glutamate signaling in gabapentin’s effect in the locus coeruleus (Hayashida et al., 2008), consistent with the current results in astrocytes. The clinical relevance of these laboratory observations was demonstrated in patients with chronic pain that oral administration of gabapentin, in a dose that produces postoperative analgesia, increased noradrenaline concentrations in cerebrospinal fluid (Hayashida et al., 2007). The current study demonstrates that gabapentinoid directly acts on glutamate regulation in astrocytes and the role of this novel target of gabapentinoid action should be tested in vivo.
5. Conclusion
The present study demonstrates that riluzole and gabapentin activate glutamate transporters to induce subsequent Ca2+ influx via the reverse mode of Na+/Ca2+ exchange, and thereby facilitate glutamate-induced glutamate release in cultured astrocytes. The present study also demonstrates a novel site of gabapentinoid action in astrocytes other than α2δ subunits in neurons. Since gabapentin and pregabalin have been recognized as first line drugs for treatment of various chronic pain conditions, this study provides strong rationale for investigating whether astrocytes are important target of gabapentinoid action to reduce chronic pain.
Acknowledgments
This work was supported by grants DA27690 to KH and NS59574 to JE from the National Institutes of Health, Bethesda, Maryland.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Bertrand S, Nouel D, Morin F, Nagy F, Lacaille JC. Gabapentin actions on Kir3 currents and N-type Ca2+ channels via GABAB receptors in hippocampal pyramidal cells. Synapse. 2003;50:95–109. doi: 10.1002/syn.10247. [DOI] [PubMed] [Google Scholar]
- Brooks BR. Managing amyotrophic lateral sclerosis: slowing disease progression and improving patient quality of life. Ann Neurol. 2009;65(Suppl 1):S17–23. doi: 10.1002/ana.21544. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Kumar N, Lefebvre CD, Yu JS. A comparison of the glutamate release inhibition and anti-allodynic effects of gabapentin, lamotrigine, and riluzole in a model of neuropathic pain. J Neurochem. 2007;100:1289–1299. doi: 10.1111/j.1471-4159.2006.04304.x. [DOI] [PubMed] [Google Scholar]
- Davies A, Hendrich J, Van Minh AT, Wratten J, Douglas L, Dolphin AC. Functional biology of the alpha(2)delta subunits of voltage-gated calcium channels. Trends Pharmacol Sci. 2007;28:220–228. doi: 10.1016/j.tips.2007.03.005. [DOI] [PubMed] [Google Scholar]
- De Sarro G, Siniscalchi A, Ferreri G, Gallelli L, De Sarro A. NMDA and AMPA/kainate receptors are involved in the anticonvulsant activity of riluzole in DBA/2 mice. Eur J Pharmacol. 2000;408:25–34. doi: 10.1016/s0014-2999(00)00709-3. [DOI] [PubMed] [Google Scholar]
- Fahrig T. Receptor subtype involved and mechanism of norepinephrine-induced stimulation of glutamate uptake into primary cultures of rat brain astrocytes. Glia. 1993;7:212–218. doi: 10.1002/glia.440070304. [DOI] [PubMed] [Google Scholar]
- Frizzo ME, Dall’Onder LP, Dalcin KB, Souza DO. Riluzole enhances glutamate uptake in rat astrocyte cultures. Cell Mol Neurobiol. 2004;24:123–128. doi: 10.1023/B:CEMN.0000012717.37839.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli E, Funicello M, Rauen T, Gobbi M, Mennini T. Riluzole enhances the activity of glutamate transporters GLAST, GLT1 and EAAC1. Eur J Pharmacol. 2008;578:171–176. doi: 10.1016/j.ejphar.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Gee NS, Brown JP, Dissanayake VU, Offord J, Thurlow R, Woodruff GN. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J Biol Chem. 1996;271:5768–5776. doi: 10.1074/jbc.271.10.5768. [DOI] [PubMed] [Google Scholar]
- Hansson E, Muyderman H, Leonova J, Allansson L, Sinclair J, Blomstrand F, Thorlin T, Nilsson M, Ronnback L. Astroglia and glutamate in physiology and pathology: aspects on glutamate transport, glutamate-induced cell swelling and gap-junction communication. Neurochem Int. 2000;37:317–329. doi: 10.1016/s0197-0186(00)00033-4. [DOI] [PubMed] [Google Scholar]
- Hayashida K, DeGoes S, Curry R, Eisenach JC. Gabapentin activates spinal noradrenergic activity in rats and humans and reduces hypersensitivity after surgery. Anesthesiology. 2007;106:557–562. doi: 10.1097/00000542-200703000-00021. [DOI] [PubMed] [Google Scholar]
- Hayashida K, Obata H, Nakajima K, Eisenach JC. Gabapentin acts within the locus coeruleus to alleviate neuropathic pain. Anesthesiology. 2008;109:1077–1084. doi: 10.1097/ALN.0b013e31818dac9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida K, Parker RA, Eisenach JC. Activation of glutamate transporters in the locus coeruleus paradoxically activates descending inhibition in rats. Brain Res. 2010;1317:80–86. doi: 10.1016/j.brainres.2009.12.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida KI, Bynum T, Vincler M, Eisenach JC. Inhibitory M2 muscarinic receptors are upregulated in both axotomized and intact small diameter dorsal root ganglion cells after peripheral nerve injury. Neuroscience. 2006;140:259–268. doi: 10.1016/j.neuroscience.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Heblich F, Tran Van Minh A, Hendrich J, Watschinger K, Dolphin AC. Time course and specificity of the pharmacological disruption of the trafficking of voltage-gated calcium channels by gabapentin. Channels (Austin) 2008;2:4–9. doi: 10.4161/chan.2.1.6045. [DOI] [PubMed] [Google Scholar]
- Hendrich J, Van Minh AT, Heblich F, Nieto-Rostro M, Watschinger K, Striessnig J, Wratten J, Davies A, Dolphin AC. Pharmacological disruption of calcium channel trafficking by the alpha2delta ligand gabapentin. Proc Natl Acad Sci U S A. 2008;105:3628–3633. doi: 10.1073/pnas.0708930105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irifune M, Kikuchi N, Saida T, Takarada T, Shimizu Y, Endo C, Morita K, Dohi T, Sato T, Kawahara M. Riluzole, a glutamate release inhibitor, induces loss of righting reflex, antinociception, and immobility in response to noxious stimulation in mice. Anesth Analg. 2007;104:1415–1421. doi: 10.1213/01.ane.0000263267.04198.36. table of contents. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Cai Z, Schools G, Zhou M. Acutely isolated astrocytes as models to probe astrocyte functions. Neurochem Int. 2000;36:359–367. doi: 10.1016/s0197-0186(99)00144-8. [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Kettenmann H, Verkhratsky A. Na+/Ca2+ exchanger modulates kainate-triggered Ca2+ signaling in Bergmann glial cells in situ. FASEB J. 1997;11:566–572. doi: 10.1096/fasebj.11.7.9212080. [DOI] [PubMed] [Google Scholar]
- Lamanauskas N, Nistri A. Riluzole blocks persistent Na+ and Ca2+ currents and modulates release of glutamate via presynaptic NMDA receptors on neonatal rat hypoglossal motoneurons in vitro. Eur J Neurosci. 2008;27:2501–2514. doi: 10.1111/j.1460-9568.2008.06211.x. [DOI] [PubMed] [Google Scholar]
- Li CY, Zhang XL, Matthews EA, Li KW, Kurwa A, Boroujerdi A, Gross J, Gold MS, Dickenson AH, Feng G, Luo ZD. Calcium channel alpha2delta1 subunit mediates spinal hyperexcitability in pain modulation. Pain. 2006;125:20–34. doi: 10.1016/j.pain.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo ZD, Calcutt NA, Higuera ES, Valder CR, Song YH, Svensson CI, Myers RR. Injury type-specific calcium channel alpha 2 delta-1 subunit up-regulation in rat neuropathic pain models correlates with antiallodynic effects of gabapentin. J Pharmacol Exp Ther. 2002;303:1199–1205. doi: 10.1124/jpet.102.041574. [DOI] [PubMed] [Google Scholar]
- Lynch JJ, 3rd, Honore P, Anderson DJ, Bunnelle WH, Mortell KH, Zhong C, Wade CL, Zhu CZ, Xu H, Marsh KC, Lee CH, Jarvis MF, Gopalakrishnan M. (L)-Phenylglycine, but not necessarily other alpha2delta subunit voltage-gated calcium channel ligands, attenuates neuropathic pain in rats. Pain. 2006;125:136–142. doi: 10.1016/j.pain.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Malarkey EB, Parpura V. Mechanisms of glutamate release from astrocytes. Neurochem Int. 2008;52:142–154. doi: 10.1016/j.neuint.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland D, Evans RM, Barkworth L, Martin DJ, Scott RH. A study comparing the actions of gabapentin and pregabalin on the electrophysiological properties of cultured DRG neurones from neonatal rats. BMC Pharmacol. 2004;4:14. doi: 10.1186/1471-2210-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas H, Colina C, Ramos M, Benaim G, Jaffe EH, Caputo C, DiPolo R. Na+ entry via glutamate transporter activates the reverse Na+/Ca2+ exchange and triggers Ca(i)2+-induced Ca2+ release in rat cerebellar Type-1 astrocytes. J Neurochem. 2007;100:1188–1202. doi: 10.1111/j.1471-4159.2006.04303.x. [DOI] [PubMed] [Google Scholar]
- Su TZ, Lunney E, Campbell G, Oxender DL. Transport of gabapentin, a gamma-amino acid drug, by system l alpha-amino acid transporters: a comparative study in astrocytes, synaptosomes, and CHO cells. J Neurochem. 1995;64:2125–2131. doi: 10.1046/j.1471-4159.1995.64052125.x. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Murasawa H, Yamaguchi K, Yamada M, Nakatani A, Yoshida M, Iwai T, Inagaki M, Saitoh A. Riluzole rapidly attenuates hyperemotional responses in olfactory bulbectomized rats, an animal model of depression. Behav Brain Res. 2011;216:46–52. doi: 10.1016/j.bbr.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Kirchhoff F. Glutamate-mediated neuronal-glial transmission. J Anat. 2007;210:651–660. doi: 10.1111/j.1469-7580.2007.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenaia O, Schlag BD, Gochenauer GE, Ganel R, Song W, Beesley JS, Grinspan JB, Rothstein JD, Robinson MB. Epidermal growth factor receptor agonists increase expression of glutamate transporter GLT-1 in astrocytes through pathways dependent on phosphatidylinositol 3-kinase and transcription factor NF-kappaB. Mol Pharmacol. 2000;57:667–678. doi: 10.1124/mol.57.4.667. [DOI] [PubMed] [Google Scholar]