Abstract
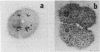
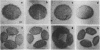
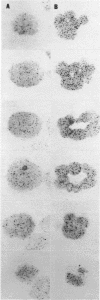
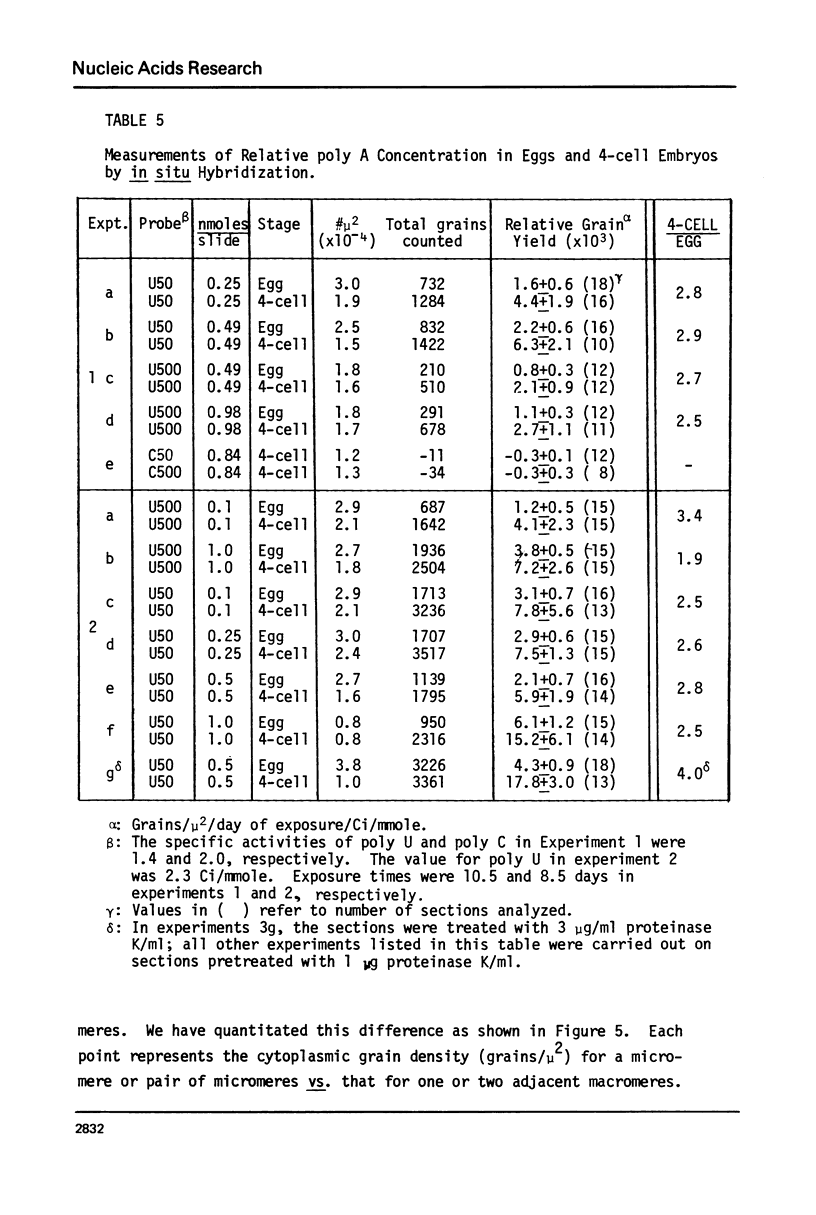
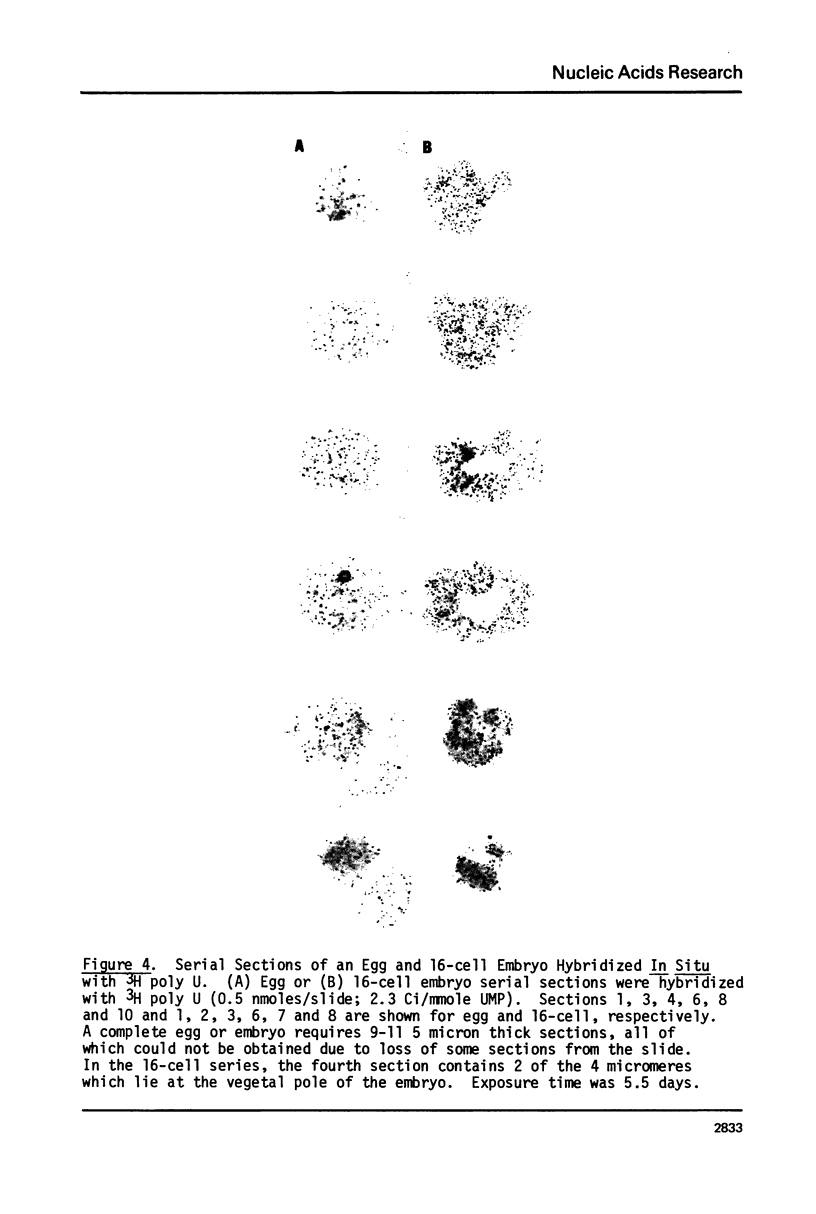
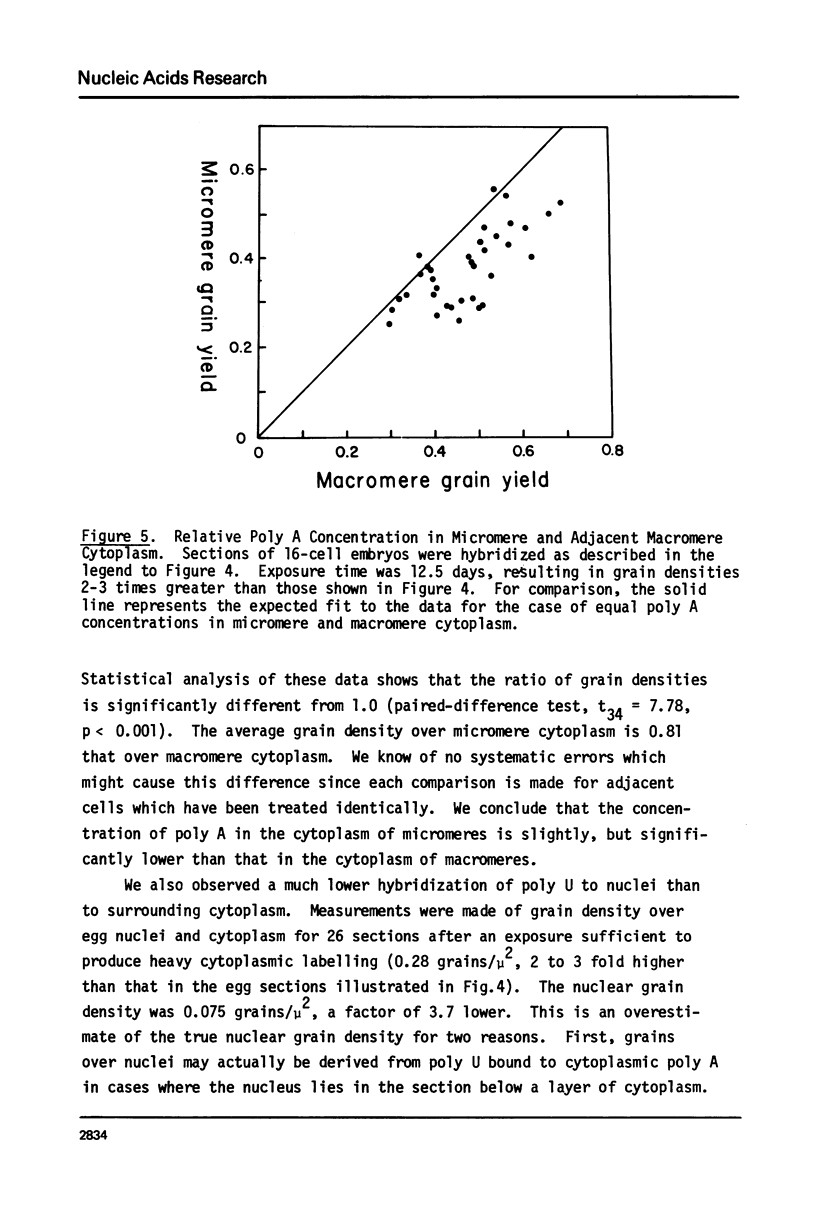
We present an improved procedure for detecting poly A tracts in situ by hybridization of 3H poly U. Glutaraldehyde fixation achieves significantly higher retention of RNA and better morphologic preservation than does Carnoy's. A dramatic increase in signal to noise is obtained by prehybridization treatment of glutaraldehyde-fixed sections with proteinase K and acetic anhydride. Measurement of the increase in poly A concentration after fertilization by solution titration and by in situ hybridization are in excellent agreement indicating that in situ measurements yield accurate relative estimates of local RNA concentrations in sections. Examination of the grain density distribution in section of sea urchin eggs and cleaving embryos reveals no major cytoplasmic localization of poly A+ RNA, although nuclei show much less labelling and micromeres of 16-cell embryos have a small, but significant, reduction in poly A concentration.
Full text
PDF






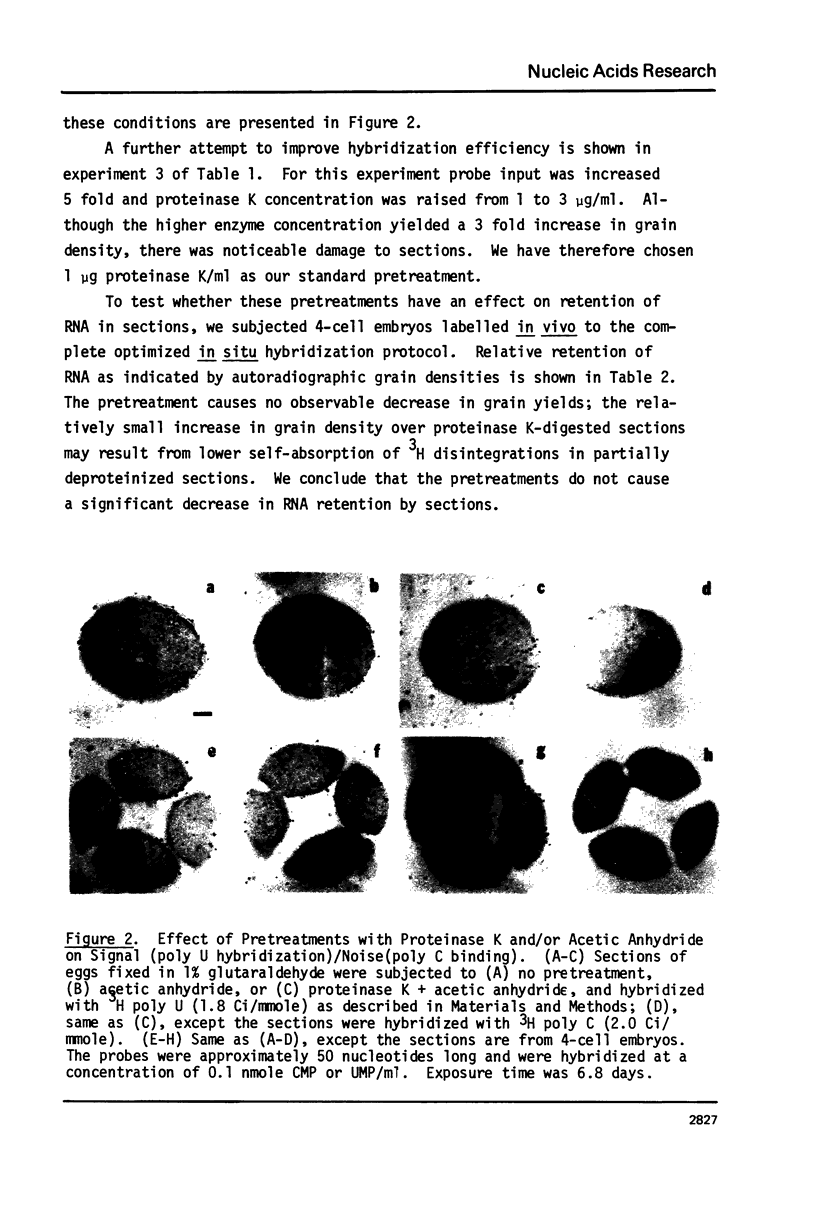
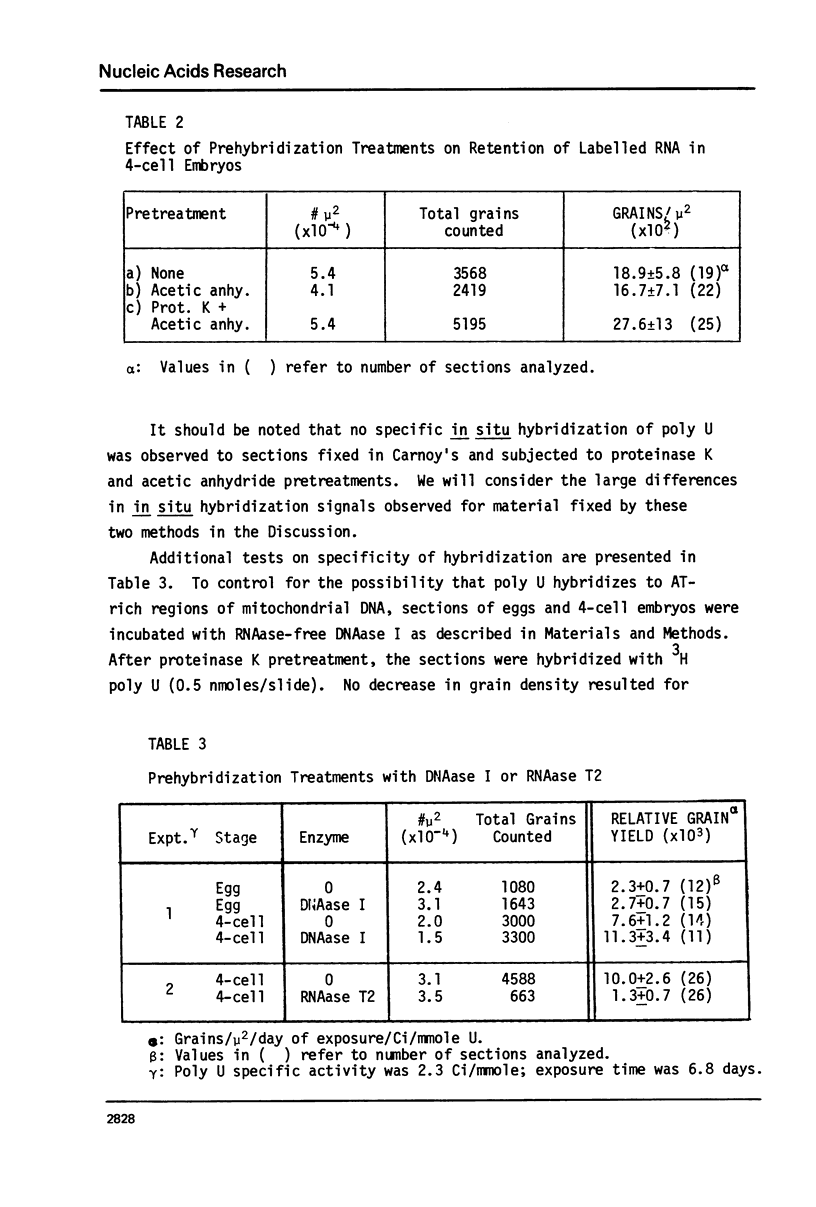

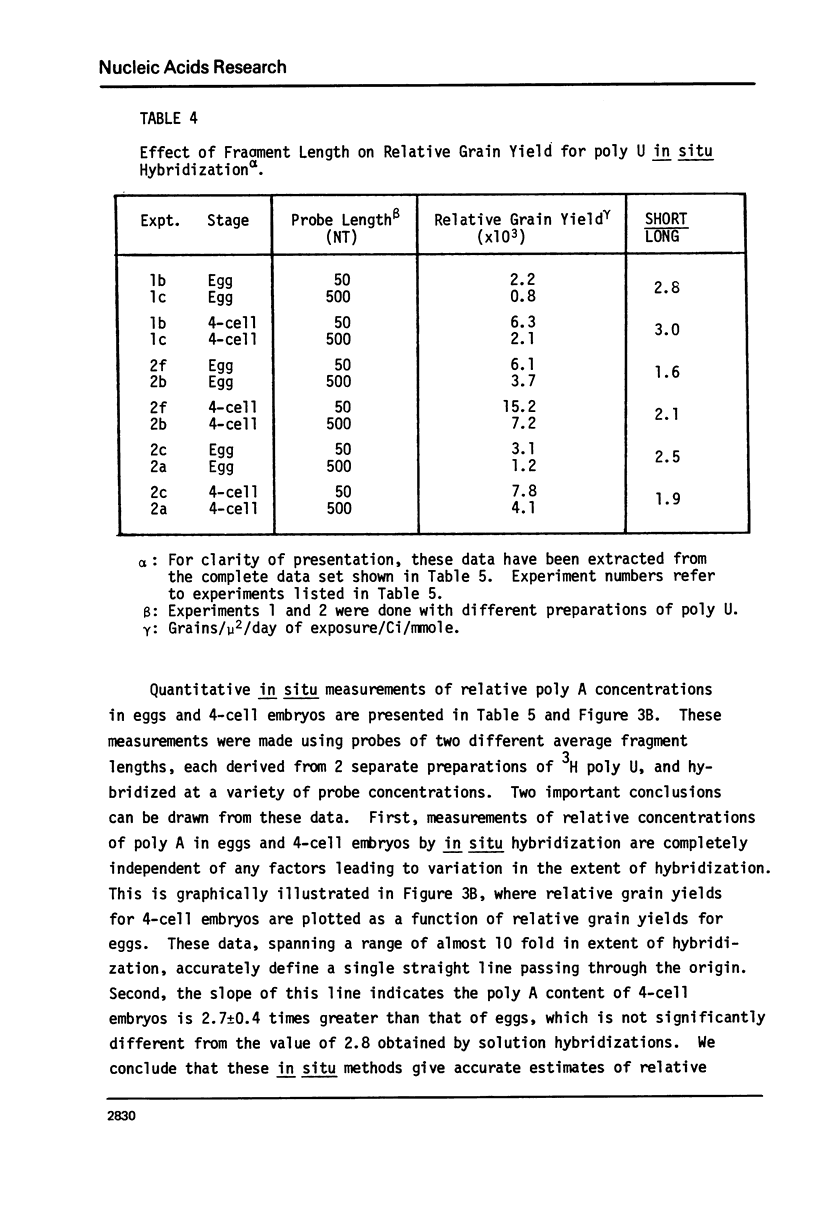
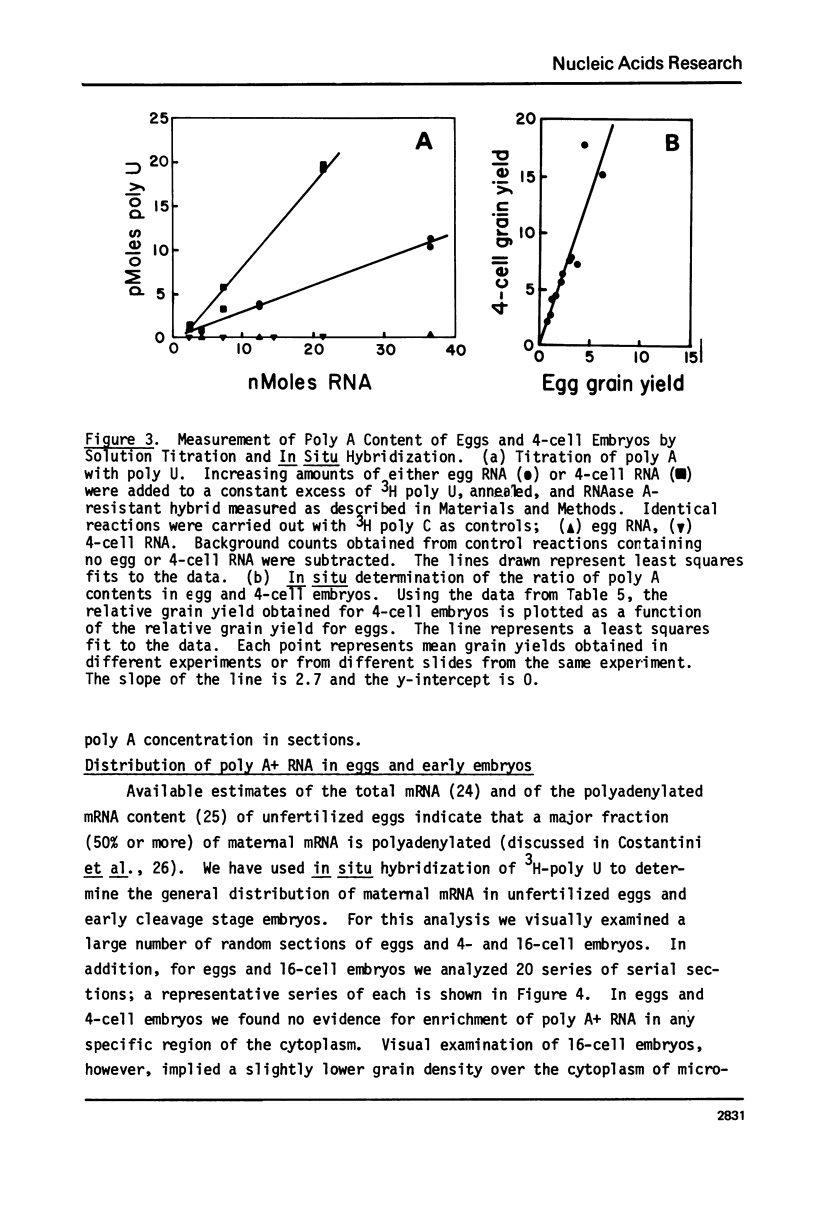






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ada G. L., Humphrey J. H., Askonas B. A., McDevitt H. O., Nossal G. J. Correlation of grain counts with radioactivity (125I and tritium) in autoradiography. Exp Cell Res. 1966 Mar;41(3):557–572. doi: 10.1016/s0014-4827(66)80106-4. [DOI] [PubMed] [Google Scholar]
- Brahic M., Haase A. T. Detection of viral sequences of low reiteration frequency by in situ hybridization. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6125–6129. doi: 10.1073/pnas.75.12.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst B. P. Simultaneous synthesis, translation, and storage of mRNA including histone mRNA in sea urchin eggs. Dev Biol. 1980 Sep;79(1):139–148. doi: 10.1016/0012-1606(80)90079-2. [DOI] [PubMed] [Google Scholar]
- Capco D. G., Jeffery W. R. Differential distribution of poly(A)-containing RNA in the embryonic cells of Oncopeltus fasciatus. Analysis by in situ hybridization with a [3H]poly(U) probe. Dev Biol. 1978 Nov;67(1):137–151. doi: 10.1016/0012-1606(78)90305-6. [DOI] [PubMed] [Google Scholar]
- Capco D. G., Jeffery W. R. Origin and spatial distribution of maternal messenger RNA during oogenesis of an insect, Oncopeltus fasciatus. J Cell Sci. 1979 Oct;39:63–76. doi: 10.1242/jcs.39.1.63. [DOI] [PubMed] [Google Scholar]
- Clissold P. M., Arnstein H. R., Chesterton C. J. Quantitation of globin mRNA levels during erythroid development in the rabbit and discovery of a new beta-related species in immature erythroblasts. Cell. 1977 Jun;11(2):353–361. doi: 10.1016/0092-8674(77)90052-6. [DOI] [PubMed] [Google Scholar]
- Costantini F. D., Britten R. J., Davidson E. H. Message sequences and short repetitive sequences are interspersed in sea urchin egg poly(A)+ RNAs. Nature. 1980 Sep 11;287(5778):111–117. doi: 10.1038/287111a0. [DOI] [PubMed] [Google Scholar]
- Darnbrough C., Ford P. J. Cell-free translation of messenger RNA from oocytes of Xenopus laevis. Dev Biol. 1976 Jun;50(2):285–301. doi: 10.1016/0012-1606(76)90152-4. [DOI] [PubMed] [Google Scholar]
- Dubroff L. M., Nemer M. Developmental shifts in the synthesis of heterogeneous nuclear RNA classes in the sea urchin embryo. Nature. 1976 Mar 11;260(5547):120–124. doi: 10.1038/260120a0. [DOI] [PubMed] [Google Scholar]
- Dubroff L. M., Nemer M. Molecular classes of heterogeneous nuclear RNA in sea urchin embryos. J Mol Biol. 1975 Jul 5;95(3):455–476. doi: 10.1016/0022-2836(75)90203-x. [DOI] [PubMed] [Google Scholar]
- Ernst S. G., Hough-Evans B. R., Britten R. J., Davidson E. H. Limited complexity of the RNA in micromeres of sixteen-cell sea urchin embryos. Dev Biol. 1980 Sep;79(1):119–127. doi: 10.1016/0012-1606(80)90077-9. [DOI] [PubMed] [Google Scholar]
- GROSS P. R., MALKIN L. I., MOYER W. A. TEMPLATES FOR THE FIRST PROTEINS OF EMBRYONIC DEVELOPMENT. Proc Natl Acad Sci U S A. 1964 Mar;51:407–414. doi: 10.1073/pnas.51.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godard C. M., Jones K. W. Improved method for detection of cellular transcripts by in situ hybridization: detection of poly (A) sequences in individual cells. Histochemistry. 1980;65(3):291–300. doi: 10.1007/BF00493178. [DOI] [PubMed] [Google Scholar]
- Godard C., Jones K. W. Detection of AKR MuLV-specific RNA in AKR mouse cells by in situ hybridization. Nucleic Acids Res. 1979 Jun 25;6(8):2849–2861. doi: 10.1093/nar/6.8.2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding J. D., MacDonald R. J., Przybyla A. E., Chirgwin J. M., Pictet R. L., Rutter W. J. Changes in the frequency of specific transcripts during development of the pancreas. J Biol Chem. 1977 Oct 25;252(20):7391–7397. [PubMed] [Google Scholar]
- Hayashi S., Gillam I. C., Delaney A. D., Tener G. M. Acetylation of chromosome squashes of Drosophila melanogaster decreases the background in autoradiographs from hybridization with [125I]-labeled RNA. J Histochem Cytochem. 1978 Aug;26(8):677–679. doi: 10.1177/26.8.99471. [DOI] [PubMed] [Google Scholar]
- Jeffery W. R., Capco D. G. Differential accumulation and localization of maternal poly(A)-containing RNA during early development of the ascidian, Styela. Dev Biol. 1978 Nov;67(1):152–166. doi: 10.1016/0012-1606(78)90306-8. [DOI] [PubMed] [Google Scholar]
- John H. A., Patrinou-Georgoulas M., Jones K. W. Detection of myosin heavy chain mRNA during myogenesis in tissue culture by in vitro and in situ hybridization. Cell. 1977 Oct;12(2):501–508. doi: 10.1016/0092-8674(77)90126-x. [DOI] [PubMed] [Google Scholar]
- Jones K. W., Bishop J. O., Brito-da-Cunha A. Complex formation between poly-r (U) and various chromosomal loci in Rhynchosciara. Chromosoma. 1973;43(4):375–390. doi: 10.1007/BF00406744. [DOI] [PubMed] [Google Scholar]
- Kaufman S. L., Gallo R. C., Miller N. R. Detection of virus-specific RNA in simian sarcoma-leukemia virus-infected cells in in situ hybridization to viral complementary DNA. J Virol. 1979 May;30(2):637–641. doi: 10.1128/jvi.30.2.637-641.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell I. H., Maxwell F., Hahn W. E. Removal of RNase activity from DNase by affinity chromatography on agarose coupled aminophenylphosphoryl-uridine-2' (3')-phosphate. Nucleic Acids Res. 1977 Jan;4(1):241–246. doi: 10.1093/nar/4.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher A., Humphreys T. Activation of maternal mRNA in the absence of poly(A) formation in fertilised sea urchin eggs. Nature. 1974 May 10;249(453):138–139. doi: 10.1038/249138a0. [DOI] [PubMed] [Google Scholar]
- Minty A. J., Birnie G. D., Paul J. Gene expression in Friend erythroleukemia cells following the induction of hemoglobin synthesis. Exp Cell Res. 1978 Aug;115(1):1–14. doi: 10.1016/0014-4827(78)90395-6. [DOI] [PubMed] [Google Scholar]
- Moar M. H., Jones K. W. Detection of virus-specific DNA and RNA base-sequences in individual cells transformed or infected by adenovirus type 2. Int J Cancer. 1975 Dec 15;16(6):998–1007. doi: 10.1002/ijc.2910160613. [DOI] [PubMed] [Google Scholar]
- Neer A., Baran N., Manor H. In situ hybridization analysis of polyoma DNA replication in an inducible line of polyoma-transformed cells. Cell. 1977 May;11(1):65–71. doi: 10.1016/0092-8674(77)90317-8. [DOI] [PubMed] [Google Scholar]
- Pelc S. R., Welton M. G. Quantitative evaluation of tritium in autoradiography and biochemistry. Nature. 1967 Dec 2;216(5118):925–927. doi: 10.1038/216925a0. [DOI] [PubMed] [Google Scholar]
- Rodgers W. H., Gross P. R. Inhomogeneous distribution of egg RNA sequences in the early embryo. Cell. 1978 Jun;14(2):279–288. doi: 10.1016/0092-8674(78)90114-9. [DOI] [PubMed] [Google Scholar]
- Slater D. W., Slater I., Gillespie D. Post-fertilization synthesis of polyadenylic acid in sea urchin embryos. Nature. 1972 Dec 8;240(5380):333–337. doi: 10.1038/240333a0. [DOI] [PubMed] [Google Scholar]
- Szabo P., Elder R., Steffensen D. M., Uhlenbeck O. C. Quantitative in situ hybridization of ribosomal RNA species to polytene chromosomes of Drosophila melanogaster. J Mol Biol. 1977 Sep 25;115(3):539–563. doi: 10.1016/0022-2836(77)90170-x. [DOI] [PubMed] [Google Scholar]
- Venezky D. L., Angerer L. M., Angerer R. C. Accumulation of histone repeat transcripts in the sea urchin egg pronucleus. Cell. 1981 May;24(2):385–391. doi: 10.1016/0092-8674(81)90328-7. [DOI] [PubMed] [Google Scholar]
- Wilt F. H. Polyadenylation of maternal RNA of sea urchin eggs after fertilization. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2345–2349. doi: 10.1073/pnas.70.8.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilt F. H. The dynamics of maternal poly(A)-containing mRNA in fertilized sea urchin eggs. Cell. 1977 Jul;11(3):673–681. doi: 10.1016/0092-8674(77)90084-8. [DOI] [PubMed] [Google Scholar]