Abstract
Background
The transforming growth factor (TGF)-β superfamily comprises cytokines such as TGF-β and Bone Morphogenetic Proteins (BMPs), which have a critical role in a multitude of biological processes. In breast cancer, high levels of TGF-β are associated with poor outcome, whereas inhibition of TGF-β-signaling reduces metastasis. In contrast, BMP-7 inhibits bone metastasis of breast cancer cells.
Methods
In this study, we investigated the effect of BMP-7 on TGF-β-induced invasion in a 3 dimensional invasion assay.
Results
BMP-7 inhibited TGF-β-induced invasion of the metastatic breast cancer cell line MCF10CA1a, but not of its premalignant precursor MCF10AT in a spheroid invasion model. The inhibitory effect appears to be specific for BMP-7, as its closest homolog, BMP-6, did not alter the invasion of MCF10CA1a spheroids. To elucidate the mechanism by which BMP-7 inhibits TGF-β-induced invasion, we analyzed invasion-related genes. BMP-7 inhibited TGF-β-induced expression of integrin αvβ3 in the spheroids. Moreover, targeting of integrins by a chemical inhibitor or knockdown of integrin β3 negatively affected TGF-β-induced invasion. On the other hand, overexpression of integrin β3 counteracted the inhibitory effect of BMP7 on TGF-β-induced invasion.
Conclusion
Thus, BMP-7 may exert anti-invasive actions by inhibiting TGF-β-induced expression of integrin β3.
Electronic supplementary material
The online version of this article (doi:10.1007/s13402-011-0058-0) contains supplementary material, which is available to authorized users.
Keywords: Breast cancer, Invasion, TGF-β, BMP-7, Integrin β3
Introduction
Breast cancer is one of the major causes of death in women. However, most mortality and morbidity does not arise from the primary tumor, but from distant metastasis. High morbidity is especially caused by bone metastasis. In order to metastasize a cancer cell must shed many of its epithelial characteristics, invade the surrounding tissue to enter the circulation, subsequently survive in the circulation, extravasate and proliferate in the metastatic niche [1]. Invasion is therefore a key step in the metastatic cascade. Several classes of proteins have been shown to play a role in this process, such as the integrin family of adhesion receptors. Integrins, which consist of an α and β subunit, are important for adhesion of cells to the extracellular matrix [2]. Cancer cells express specific integrin combinations, whose signaling favors migration and invasion [3]. Integrin αvβ3 is strongly expressed by breast cancer cells, especially by cells residing in bone metastasis [4]. Its expression promotes bone metastasis of breast cancer cells [5, 6], whereas inhibition of αvβ3 diminishes their osteotropism [7].
Transforming Growth Factor (TGF)-β has a dual role in carcinogenesis. In the early stages it has a growth inhibitory and pro-apoptotic effect, whereas at the later stages of cancer, TGF-β promotes invasion and metastasis [8, 9]. In line with its stimulatory role in cancer progression, TGF-β is frequently overexpressed in breast cancer and its expression correlates with poor prognosis and metastasis [10–12]. Moreover, studies in mouse models have demonstrated that inhibition of TGF-β-signaling in breast cancer cells reduces metastasis [13–16].
TGF-β and Bone Morphogenetic Proteins (BMPs) signal via comparable mechanisms. Upon binding of the ligand, the type II receptor phosphorylates the type I receptor. The type I receptor, in turn, phosphorylates the receptor-regulated (R)- Sma and Mad related gene product (Smad). Phosphorylated R-Smads form heteromeric complexes with the common mediator Smad (Co-Smad) Smad4. These complexes then translocate to the nucleus, where they regulate transcription of target genes in collaboration with other transcription factors [17]. In most cell types, TGF-β signals through the TGF-β receptor type II (TβRII) and the TGF-β type I receptor, also termed activin receptor-like kinase 5 (ALK5). ALK5 mediates the phosphorylation of Smad2 and Smad3 [18]. BMPs signal through their specific BMP receptor type II (BMPRII) and BMP type I receptors (BMPRI) which induce the activation of Smad1, Smad5 and Smad8 [19]. In addition to the previously described Smad pathways, receptor activation results in activation of several other non-Smad signaling pathways, for example Mitogen Activated Protein Kinase (MAPK) pathways [20].
TGF-β is thought to be pro-invasive by inducing epithelial-to-mesenchymal transition (EMT). During this process, carcinoma cells acquire a more motile, mesenchymal phenotype [21]. In normal breast and kidney epithelial cells BMP-7 can inhibit TGF-β-induced EMT [22]; BMP-7 has also been shown to inhibit EMT and bone metastasis in breast and prostate tumors [23, 24]. However, the effect of BMP-7 on tumor invasion is unknown.
Previously, we have set up a spheroid invasion model to study TGF-β-induced invasion [25, 26]. In this assay, spheroids made from MCF10A series of cell lines invade in response to TGF-β. The MCF10A cell lines originate from MCF10A1, a spontaneously immortalized breast cell line [27]. This cell line was transformed with oncogenic RAS, resulting in MCF10AT (hereafter referred to as M-II), which forms premalignant lesions in mice that upon grafting progressed into carcinoma [28]. From these carcinomas MCF10CA1a was isolated (hereafter referred to as M-IV), which metastatasizes to the lung [29]. Using these cell lines in the invasion assay, we have shown the importance of Smad signaling for TGF-β-induced invasion [25]. In the current paper, we have analyzed the role of BMP-7. We show that BMP-7 can inhibit TGF-β-induced invasion through inhibition of TGF-β-induced integrin β3 expression.
Materials and methods
Cell culture
MCF10AT (M-II) and MCF10CA1a (M-IV) were obtained from Dr. Fred Miller (Barbara Ann Karmanos Cancer Institute, Detroit, USA) and maintained as described [25].
Invasion spheroid assays
The invasion spheroid assay was performed essentially as described in [25]. Briefly, 103 cells were allowed to form spheroids and spheroids were embedded in a 1:1 collagen:methocel mixture onto a collagen coated plate. Recombinant human TGF-β3 (a generous gift of Dr. K. Iwata, OSI Pharmaceuticals, Inc, New York, USA), BMP-6, BMP-7 (both a kind gift of K. Sampath, Creative Biomolecules, Inc, Hoptinton, USA), isotype control antibody (Santa Cruz) or human BMP-7 neutralizing antibody (R&D Systems) were added directly into the collagen:methocel mixture at 4°C. After 30 min incubation at 37°C medium was added on top of the collagen, either containing no solvent, DMSO or the integrin inhibitor GLPG0187 (Galapagos NV, Mechelen, Belgium). Invasion was monitored during the next 2 days and quantified by measuring the area using ImageJ or Adobe Photoshop.
Western blot analysis
2 × 105 cells were seeded onto a six well plate and 1 day later starved for 16 h in medium containing 2.5% horse serum (Gibco), 10 ng/ml epidermal growth factor (EGF) (Upstate), 50 ng/ml cholera toxin (Calbiochem), 0.25 μg/ml hydrocortisone (Sigma), 5 μg/ml insulin (Sigma), 100 U/ml penicillin and 50 μg/ml streptomycin (Gibco). After starvation, cells were stimulated, washed and lysed in RIPA buffer or sample buffer. Lysates were subjected to SDS-PAGE & Western blotting. Phosphorylation of Smad1/5/8 was detected by anti phospho-Smad1 antibody as described before [30]. Phospho-Smad2 antibody was purchased from Cell Signaling. Total Smad1 antibody was from Zymed.
Transcriptional reporter assay
1 × 105 cells were seeded onto a 24-well plate and 1 day later transfected for 4 h with the reporters CAGA12-Luc [31] or BRE-Luc [32] using either Lipofectamine 2000 or LTX (Invitrogen). An expression plasmid for β-galactosidase was co-transfected and used to correct for transfection efficiency. One day after transfection, cells were serum starved overnight in medium containing 2.5% horse serum (Gibco), 10 ng/ml epidermal growth factor (EGF) (Upstate), 50 ng/ml cholera toxin (Calbiochem), 0.25 μg/ml hydrocortisone (Sigma), 5 μg/ml insulin (Sigma), 100 U/ml penicillin and 50 μg/ml streptomycin (Gibco) and subsequently stimulated for 8 h. Luciferase and β-galactosidase activity were determined as previously described [31]. Each transfection was carried out in triplicate and representative experiments are shown.
RNA isolation, cDNA synthesis and quantitative PCR
RNA isolation, cDNA synthesis and quantitative PCR (Q-PCR) were performed as previously described [25]. The following primers were used: acidic ribosomal phosphoprotein (ARP) forward 5′- CACCATTGAAATCCTGAGTGATGT -3′ and reverse 5′- TGACCAGCCGAAAGGAGAAG -3′; matrix metalloproteinase 2 (MMP2) forward 5′-AGATGCCTGGAATGCCAT-3′ and reverse 5′-GGTTCTCCAGCTTCAGGTAAT-3′; integrin αv forward 5′-CAGGCTTGCAACCCATTCTT-3′ and reverse 5′-GAATGTGAGCCTGTCGACTAATGTT-3′; integrin β3 forward 5′-ACCTGCTTGCCCATGTTTG-3′ and reverse 5′-GCGGGTCACCTGGTCAGTTA-3′. All samples were analyzed in duplicate for each primer set. Gene expression levels were determined with the comparative ∆Ct method using ARP as reference and the non-stimulated condition was set to 1. Relative expression levels are presented as mean ± S.D.
Lentiviral transduction
Constructs TRCN0000003234,TRCN0000003235, TRCN0000003236 and TRCN0000003237 from the Mission library (Sigma) were used to target integrin β3 with construct SHC001 as a negative control. Cells were transduced in the presence of 4 μg/ml polybrene (Sigma) overnight. After recovery cells were selected and maintained in growth medium containing 0.5 μg/ml puromycin. For overexpression of integrin β3, M-IV cells were transduced with a lentiviral integrin β3 expression construct [33], kindly provided by Dr. Deborah Novack, Washington University, St. Louis, USA.
Statistical analysis
All results are expressed as the mean ± S.D. Statistical differences were examined by one-way ANOVA followed by Bonferroni’s multiple comparison test. P < 0.05 was considered as statistically significant.
Results
BMP-7 inhibits TGF-β-induced invasion in the metastatic cell line M-IV, but not in the premalignant M-II cell line
BMP-7 inhibits TGF-β-induced EMT of both normal breast cells [22], as well as of invasive, osteotropic breast and prostate cancer cells, which is associated with inhibition of bone metastasis [23, 24]. To investigate whether BMP-7 also has an inhibitory effect on TGF-β-induced invasion, we analysed its effect on M-IV in a spheroid invasion assay. The response of this metastatic cell line was compared to a precursor cell line, the RAS-transformed cell line M-II and not M-I, since RAS has been reported to enhance TGF-β-induced responses [34]. In the absence of TGF-β BMP-7 had no inhibitory effect on the invasion of M-II and M-IV cells, while TGF-β induced invasion of both cell types (Fig. 1a–d). However, BMP-7 strongly inhibited the TGF-β- induced invasion of the metastatic M-IV cells, but not of the premalignant M-II cells (Fig. 1a–d). This suggests that BMP-7 specifically inhibits TGF-β pro-invasive mechanisms exploited by metastatic cells.
Fig. 1.

BMP-7, but not BMP-6, inhibits TGF-β-induced invasion in M-IV, but not in M-II. a–d Spheroids were allowed to invade collagen in the presence of BMP-7 (100 ng/ml), TGF-β (5 ng/ml) or TGF-β & BMP-7. a Pictures of representative spheroids of M-II. b Quantification of invasion of M-II experiments shown in (a). c Pictures of representative spheroids of M-IV. d Quantification of invasion of M-IV experiments shown in (c). e Spheroids of M-IV were allowed to invade collagen in the presence of BMP-6 (100 ng/ml), TGFβ (5 ng/ml) or TGF-β & BMP-6. Pictures of representative spheroids are shown. f Quantification of invasion of experiments shown in (e). The results are expressed as mean ± S.D. of at least three spheroids per condition. One representative out of three independent experiments is shown. Significance *** p < 0.001
BMP-7 does not affect proliferation in M-IV cells
To rule out that the observed effects of BMP-7 on TGF-β-induced invasion are due to effects on cell growth, we performed proliferation assays. As reported previously [25], TGF-β has a minor growth-inhibitory effect on M-IV. BMP-7 on its own did not affect cell proliferation (Supplementary Figure 1). BMP-7 also did not affect TGF-β-induced growth inhibition. Thus, the inhibitory effect of BMP-7 on TGF-β-induced invasion is not due to effects on the growth of these cells.
BMP-7, but not BMP-6, inhibits TGF-β-induced invasion in M-IV
To assess whether other BMPs are capable of inhibiting TGF-β-induced invasion, we studied the effect of BMP-6, which is the closest homolog of BMP-7 and shares 73% identity on the amino acid level. In contrast to BMP-7, BMP-6 did not affect the TGF-β-induced invasion of M-IV cells, and also did not inhibit basal invasion. (Fig. 1e, f). These data indicate that the ability of BMP-7 to inhibit TGF-β-induced invasion is specific for BMP-7.
Noggin induces invasion in M-IV through blocking of BMP-7
Previous studies of our group have shown that the basal invasion of M-IV cells is dependent on TGF-β-signaling [25]. However we did not observe an inhibitory effect of BMP-7 addition on the basal invasion (Fig. 1c, d). One explanation for this might be that BMP-7 is already present at saturating levels under these conditions. Indeed, we found that M-IV cells express BMP-7 mRNA and that the horse serum used in the assays contained BMP-7 protein (data not shown). Therefore, we tested the effect of a natural antagonist of BMP-2, 4 and 7, Noggin. As shown in Fig. 2a and b, Noggin enhanced basal invasion 2.0-fold in M-IV. In the M-II cell line, in which TGF-β-induced invasion is not affected by BMP-7, addition of Noggin also had no effect on basal invasion (Supplementary Figure 2). Thus, inhibition of BMP-2, 4 and 7 induces invasion in M-IV cells, but not in M-II cells.
Fig. 2.

Noggin enhances basal invasion through inhibition of BMP-7. Spheroids of M-IV were allowed to invade collagen in the absence or presence of Noggin (200 ng/ml). a Pictures of representative spheroids. b Quantification of spheroids of experiments shown in (a). c Spheroids of M-IV were allowed to invade into collagen in the presence of control antibody or BMP-7 blocking antibody (2 μg/ml). Representative pictures are shown. d Quantification of spheroids of experiments shown in (c). The results are expressed as mean ± S.D. of at least four spheroids per condition. One representative out of three independent experiments is shown. Significance *** p < 0.001
Since Noggin inhibits BMP-2, 4 and 7, we next determined if blockade of BMP-7 can also induce basal invasion of M-IV cells. For this, we added BMP-7 neutralizing antibodies to the spheroid invasion system. These antibodies enhanced basal invasion 1.8-fold compared to the control antibody (Fig. 2c, d). This indicates that the induction of invasion by Noggin is mainly due to inhibition of BMP-7. Moreover, it suggests that TGF-β and BMP-7 also antagonize each other under basal conditions.
Both BMP-6 and BMP-7 induce Smad signaling
Next, we asked whether if the different effects of BMP-6 and BMP-7 on TGF-β-induced invasion in M-IV cells could be due to the possibility that BMP-7 is more potent in inducing BMP-signaling than BMP-6. To examine this, we analyzed activation of BMP-Smad signaling by two means. First, we analyzed whether BMP-6 and BMP-7 differ in their ability to induce phosphorylation of Smad1. However, Western blot analysis showed that Smad1 phosphorylation is induced to the same extent by both BMPs in M-IV cells, whereas the total levels of Smad1 were not affected (Fig. 3a). Second, we investigated transcriptional activation of BMP-Smads, by using a luciferase-reporter driven by BMP Responsive Elements (BRE) [32]. Both BMP-6 and BMP-7 were found to activate the reporter ∼30 fold and thus efficiently induced BMP-Smad transcriptional activity in M-IV cells (Fig. 3b). Taken together, these results indicate that BMP-6 and BMP-7 do not differ in their ability to induce Smad signaling in this cell line.
Fig. 3.

BMP-6 and BMP-7 induce BMP-Smad signaling, but do not affect TGF-β Smad signaling. a Cells were treated with the indicated ligands (BMP-6 and BMP-7 at 100 ng/ml, TGF-β at 5 ng/ml) for 15 min, lysed and subjected to SDS-PAGE and Western blotting. Blots were incubated with phospho-Smad2, phospho-Smad1 and Smad1 antibodies. Total ERK was used as a loading control. b Cells were transfected with the BRE-Luc reporter construct, starved and subsequently stimulated for 8 h with BMP-6 or 7 (both at 100 ng/ml). Relative luciferase activity (RLU) is expressed as mean ± S.D. of triplicate cultures. c Cells were transfected with the CAGA12-Luc reporter construct, starved and subsequently stimulated for 8 h with the indicated ligands (BMP-6 and BMP-7 at 100 ng/ml, TGF-β at 5 ng/ml). Relative luciferase activity (RLU) is expressed as mean ± S.D. of triplicate cultures. One representative out of at least three independent experiments is shown
BMP-7 does not affect TGF-β-induced Smad signaling
To investigate the molecular mechanism by which BMP-7 inhibits TGF-β-induced invasion, we analyzed the effects of BMP-7 and BMP-6 on TGF-β Smad signaling. First, we analyzed whether BMP-7 had an inhibitory effect on the phosphorylation of Smad2. However, both BMP-7 and BMP-6 did not affect TGF-β-induced Smad2 phosphorylation (Fig. 3a). Second, we studied the effect of BMP-7 on TGF-β-induced Smad3 transcriptional activity. To this end, we used a luciferase reporter containing 12 repeats of the Smad3 binding sequence CAGA [31]. TGF-β induced the activity of this reporter ∼55-fold, but both BMP-7 and BMP6 did not significantly affect the TGF-β-induced reporter activity (Fig. 3c). Thus, BMP-7 has no effect on TGF-β-induced Smad signaling in M-IV cells.
BMP-7 inhibits TGFβ-induced integrin αvβ3 expression
We next examined the effect of BMP-7 on the expression of TGF-β target genes involved in invasion. As we previously identified a critical role for matrix metalloproteinase 2 (MMP2) in TGF-β-induced invasion [25], we first analyzed if TGF-β-induced MMP2 expression is affected by BMP-7 in the spheroid model system. BMP-6 stimulation served as a negative control. However, as shown in Fig. 4a, BMP-7 (and BMP-6) did not affect TGF-β-induced expression of MMP2 in M-IV spheroids in collagen. Since integrin αvβ3 has recently been shown to enhance TGF-β-induced EMT and inhibition of integrin αvβ3 reduces metastasis [7], we subsequently examined the expression of integrin αv and β3. Importantly, TGF-β potently induced the expression of both subunits in the collagen-embedded spheroids, and BMP-7, but not BMP-6, inhibited the TGF-β-induced expression of both αv and β3 (Fig. 4b and c). Also, BMP-7 did not inhibit TGF-β-induced integrin αv and β3 expression in M-II (Supplementary Figure 3a, b). Thus, the inhibitory action of BMP-7 on TGF-β-induced collagen invasion of M-IV spheroids might be mediated through inhibition of TGF-β-induced integrin αvβ3 expression.
Fig. 4.
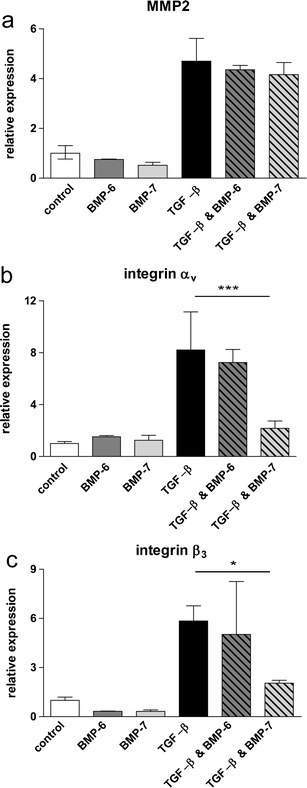
BMP-7 inhibits integrin αvβ3 expression, but does not affect MMP2 expression in M-IV. Spheroids were embedded with BMP-6 (100 ng/ml), BMP-7 (100 ng/ml), TGF-β (5 ng/ml), TGF-β & BMP-6 or TGF-β & BMP-7 for 24 h. MMP2 (a), integrin αv (b) and integrin β3 (c) mRNA expression was analyzed by Q-PCR . One representative out of three independent experiments is shown. Significance *** p < 0.001 * p < 0.05
Integrin β3 is necessary for TGF-β-induced invasion
To determine if integrin αvβ3 is important for TGF-β-induced invasion, we investigated if inhibition of αv integrins blocks TGF-β-induced invasion in the spheroid model system. For this, we used a non-peptide integrin inhibitor, GLPG0187. This molecule exhibits high affinitiy for αvβ1, αvβ3, αvβ5, αvβ6, αvβ8 as well as α5β1 in in vitro competitive binding assays (Supplementary Table 1). Treatment of M-IV spheroids with GLPG0187 inhibited TGF-β-induced invasion (Fig. 5a, b). This indicates that integrin αvβ3 could be necessary for TGF-β-induced invasion of M-IV cells.
Fig. 5.

Integrin αvβ3 is necessary for TGF-β-induced invasion. a Spheroids were allowed to invade into collagen in the presence of DMSO or GLPG0187 (80 nM) with or without TGF-β (5 ng/ml) Representative pictures of spheroids are shown. b Quantification of experiment shown in (a). c Cells were transduced with lentivirus encoding shRNA against integrin β3 and control lentivirus.. Spheroids of these cells were allowed to invade in the absence or presence of TGF-β (5 ng/ml). Representative pictures of spheroids are shown. d Quantification of invasion shown in (c). At least four spheroids per condition. were quantified. One representative out of three independent experiments is shown. Significance ** p < 0.01
To further establish if integrin β3 is necessary for TGF-β-induced invasion, we performed RNAi-mediated knockdown experiments. M-IV cells were transduced with viruses encoding 4 different shRNA constructs against integrin β3. These constructs inhibited TGF-β-induced integrin β3 expression at the mRNA level (Supplementary Figure 4a). TGF-β-induced invasion was indeed inhibited by knockdown of integrin β3 (Fig. 5c,d), indicating that downregulation of integrin β3 by BMP-7 contributes to BMP-7-mediated inhibition of invasion.
Overexpression of integrin β3 rescues TGF-β-induced invasion from BMP-7 inhibition
To demonstrate that the inhibition of integrin β3 expression by BMP-7 is critical for the inhibition of TGF-β-induced invasion, we used a lentiviral vector that overexpresses integrin β3 [33]. As shown in Supplementary Figure 4b, M-IV cells transduced with this virus exhibit higher levels of integrin β3 protein compared to wildtype (WT) cells. The overexpression of integrin β3 did not affect TGF-β-induced invasion, but completely counteracted the BMP-7 inhibitory effect (Fig. 6). Taken together, these data suggest that BMP-7 inhibits TGF-β-induced invasion by inhibiting TGF-β-induced integrin β3 expression.
Fig. 6.
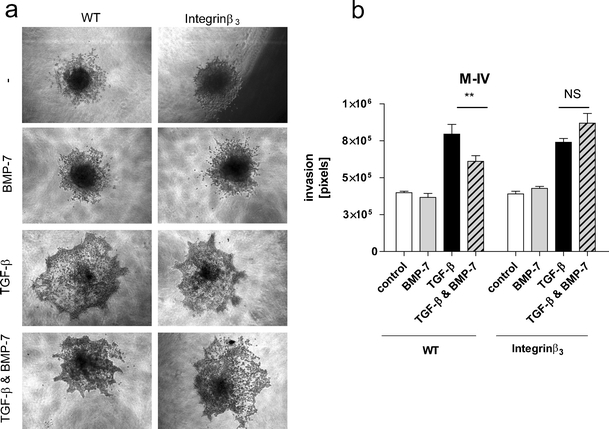
Integrin β3 overexpression rescues TGF-β-induced invasion from BMP-7 inhibition. Cells were transduced with lentivirus containing an integrin β3 overexpression construct. Integrin β3 overexpressing cells or wildtype (WT) cells were allowed to invade in BMP-7 (100 ng/ml), TGF-β (5 ng/ml) or TGF-β & BMP-7. a Representative pictures of spheroids are shown. b Quantification of spheroid experiment shown in (a). The results are expressed as mean ± S.D. of at least four spheroids per condition. One representative out of three independent experiments is shown. Significance ** p < 0.01 NS not significant
Discussion
In order to understand how BMP-7 exerts its anti-metastatic effects reported before [23, 24], we investigated whether BMP-7 interferes with TGF-β-induced invasion of human breast cancer cells. We showed that BMP-7 inhibits TGF-β-induced invasion in the metastatic breast cancer cell line M-IV through inhibition of integrin β3 expression. In addition, we showed that BMP-7 specifically inhibits the invasion of this metastatic cell line, and not the invasion of its premalignant precursor, M-II. Moreover, the inhibitory effect was specific for BMP-7, as its closest homolog, BMP-6 did not have any effect on TGF-β-induced invasion. To our knowledge, this is the first report that shows that BMP-7 inhibits TGF-β-induced invasion.
Since the antagonism between TGF-β and BMP-7 has been described before [22–24, 35–37], the question arises whether the process that we describe occurs in other cell types as well. BMP-7 inhibits bone metastasis of the MDA-MB231 breast cancer cell line [23] and PC3 prostate cancer cell line [24]. However, in these cell lines BMP-7 inhibited TGF-β/Smad signaling [23, 24], whereas under our experimental conditions we did not observe an inhibitory effect of BMP-7 on the TGF-β-Smad pathway. On the other hand, BMP-7 inhibited tumor progression of the uveal melanoma cell line OCM-1 without inhibiting the TGF-β-Smad pathway [37]. Thus, BMP-7 is able to inhibit the tumor-promoting effects of TGF-β through either Smad- and non-Smad- mechanisms, depending on the cellular context.
Our data indicate that the inhibitory effect of BMP-7 on breast cancer invasion in part can be explained by inhibition of TGF-β-induced expression of integrin β3. Integrin β3 is induced by TGF-β and enhances EMT in a feed-forward loop by inducing Src [38]. It is therefore tempting to speculate that the inhibition of TGF-β-induced integrin β3 expression by BMP-7 might also play a role in the observed antagonistic effects between TGF-β and BMP-7 in EMT.
As mentioned before we did not detect a significant effect of BMP-7 on TGF-β Smad signaling, although we have shown that TGF-β-induced invasion is dependent on Smad3 and Smad4 [25]. Since TGF-β-induced integrin β3 expression has been reported to be dependent on p38, but not on Smads [39], the inhibitory effect of BMP-7 on TGF-β-induced invasion might be mediated via non-Smad pathways. However, we could not find a significant effect of BMP-7 on (TGF-β-induced) p38 phosphorylation that could explain the inhibition of BMP-7 on TGF-β-induced integrin β3 expression (data not shown). It is therefore likely that the effect is mainly due to BMP-7 transcriptional targets, such as transcription factors or microRNAs, that interfere with TGF-β-induced integrin β3 expression.
One striking finding is that BMP-7 is able to inhibit TGF-β-induced invasion, whereas BMP-6 is not, despite the fact that they share 73% amino acid homology and are able to bind to the same receptors [40–43]. Generally, BMP-6 is regarded as more potent than BMP-7 in inducing bone differentiation [40, 44, 45]. However, BMP-7 is inhibited by Noggin, whereas BMP-6 is resistant to inhibition by Noggin due to an additional lysine at residue 60 [45]. On the other hand, we found that BMP-6 and BMP-7 induce Smad1 phosphorylation and BMP-Smad transcriptional activity to the same extent in MIV cells. Possibly, BMP-6 or BMP-7 have different effects on non-Smad signaling pathways. However, we did not observe any significant differences between BMP-7 and BMP-6 on (TGF-β-induced) p38 and ERK phosphorylation in MIV cells (data not shown).
BMP-7 did not inhibit TGF-β-induced invasion of the premalignant precursor cell line M-II. Interestingly, overexpression of a dominant-negative TGF-β-receptor also had differential effects on these two cell lines in vivo [46]. It is likely that upon tumor progression the response to TGF-β is altered in such a manner that BMP-7 is able to inhibit certain TGF-β responses. On the genetic level M-IV has an extra copy of the long arm of chromosome 1, a type of alteration often observed in breast cancer [29]. A detailed survey of the genetic changes in the MCF10A series of cell lines revealed several genetic changes in M-IV compared to M-II, as well as differences in global gene expression [47]. It is likely that the ability of BMP-7 to inhibit TGF-β-induced invasion is highly dependent on these additional genetic changes.
In conclusion, we have shown that BMP-7 inhibits TGF-β-induced invasion through inhibition of TGF-β-mediated integrin β3 expression. These data reinforce the rationale to either use BMP-7 or target integrin β3 in breast cancer metastasis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOC 1.65 MB)
Acknowledgements
We thank our colleagues for valuable discussion. We thank M. van Dinther for excellent technical assistance, Ken Iwata (OSI Pharmaceuticals, New York, USA) and Kuber Sampath (Creative Biomolecules, Inc, Hopkinton, USA) for reagents and Fred Miller (Barbara Ann Karmanos Cancer Institute, Detroit, USA) for the cell lines. This work was supported by the Tumor Host Genomics (518198), Centre for Biomedical Genetics and Swedish Cancerfonden (09 0773).
Ethical standards
All experiments comply with the current laws of the country in which they were performed.
Conflict of interest
B.H. is employed at Galapagos SASU, which has a financial interest in GLPG0187.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- ALK
Activin receptor-like kinase
- BMP
Bone Morphogenetic Protein
- EMT
Epithelial to mesenchymal transition
- M-II
MCF10AT
- M-IV
MCF10CA1a
- MMP
Matrix metalloproteinase
- Smad
Sma and Mad related gene product
- TGF-β
Transforming growth factor-β
Footnotes
Hildegonda P. H. Naber and Eliza Wiercinska contributed equally.
References
- 1.Poste G, Fidler IJ. The pathogenesis of cancer metastasis. Nature. 1980;283:139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- 2.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat. Rev. Canc. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 3.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat. Rev. Mol. Cell. Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 4.Liapis H, Flath A, Kitazawa S. Integrin αvβ3 expression by bone-residing breast cancer metastases. Diagn. Mol. Pathol. 1996;5:127–135. doi: 10.1097/00019606-199606000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Pecheur I, Peyruchaud O, Serre CM, Guglielmi J, Voland C, Bourre F, Margue C, Cohen-Solal M, Buffet A, Kieffer N, Clezardin P. Integrin αvβ3 expression confers on tumor cells a greater propensity to metastasize to bone. FASEB J. 2002;16:1266–1268. doi: 10.1096/fj.01-0911fje. [DOI] [PubMed] [Google Scholar]
- 6.Sloan EK, Pouliot N, Stanley KL, Chia J, Moseley JM, Hards DK, Anderson RL. Tumor-specific expression of αvβ3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Canc. Res. 2006;8:R20. doi: 10.1186/bcr1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y, Bachelier R, Treilleux I, Pujuguet P, Peyruchaud O, Baron R, Clement-Lacroix P, Clezardin P. Tumor αvβ3 integrin is a therapeutic target for breast cancer bone metastases. Canc. Res. 2007;67:5821–5830. doi: 10.1158/0008-5472.CAN-06-4499. [DOI] [PubMed] [Google Scholar]
- 8.Massagué J. TGF-β in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akhurst RJ, Derynck R. TGF-β signaling in cancer–a double-edged sword. Trends Cell Biol. 2001;11:S44–S51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 10.Desruisseau S, Palmari J, Giusti C, Romain S, Martin PM, Berthois Y. Determination of TGF-β1 protein level in human primary breast cancers and its relationship with survival. Br. J. Canc. 2006;94:239–246. doi: 10.1038/sj.bjc.6602920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghellal A, Li C, Hayes M, Byrne G, Bundred N, Kumar S. Prognostic significance of TGF-β1 and TGF-β3 in human breast carcinoma. Anticancer. Res. 2000;20:4413–4418. [PubMed] [Google Scholar]
- 12.Sheen-Chen SM, Chen HS, Sheen CW, Eng HL, Chen WJ. Serum levels of transforming growth factor-β1 in patients with breast cancer. Arch. Surg. 2001;136:937–940. doi: 10.1001/archsurg.136.8.937. [DOI] [PubMed] [Google Scholar]
- 13.Deckers M, van Dinther M, Buijs J, Que I, Lowik C, van der Pluijm G, ten Dijke P. The tumor suppressor Smad4 is required for transforming growth factor-β-induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells. Canc. Res. 2006;66:2202–2209. doi: 10.1158/0008-5472.CAN-05-3560. [DOI] [PubMed] [Google Scholar]
- 14.Kang Y, He W, Tulley S, Gupta GP, Serganova I, Chen CR, Manova-Todorova K, Blasberg R, Gerald WL, Massagué J. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc. Natl. Acad. Sci. U.S.A. 2005;102:13909–13914. doi: 10.1073/pnas.0506517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, Massagué J, Mundy GR, Guise TA. TGF-β signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Investig. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petersen M, Pardali E, van der Horst G, Cheung H, van den Hoogen C, van der Pluijm G, ten Dijke P. Smad2 and Smad3 have opposing roles in breast cancer bone metastasis by differentially affecting tumor angiogenesis. Oncogene. 2010;29:1351–1361. doi: 10.1038/onc.2009.426. [DOI] [PubMed] [Google Scholar]
- 17.Wrana JL. Crossing Smads. Sci. STKE. 2000;2000:re1. doi: 10.1126/stke.2000.23.re1. [DOI] [PubMed] [Google Scholar]
- 18.ten Dijke P, Hill CS. New insights into TGF-β-Smad signalling. Trends Biochem. Sci. 2004;29:265–273. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 19.ten Dijke P, Korchynskyi O, Valdimarsdottir G, Goumans MJ. Controlling cell fate by bone morphogenetic protein receptors. Mol. Cell. Endocrinol. 2003;211:105–113. doi: 10.1016/j.mce.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Moustakas A, Heldin CH. Non-Smad TGF-β signals. J. Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 21.Xu J, Lamouille S, Derynck R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R. BMP-7 counteracts TGF-β1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat. Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 23.Buijs JT, Henriquez NV, van Overveld PG, van der Horst G, Que I, Schwaninger R, Rentsch C, ten Dijke P, Cleton-Jansen AM, Driouch K, Lidereau R, Bachelier R, Vukicevic S, Clezardin P, Papapoulos SE, Cecchini MG, Lowik CW, van der Pluijm G. Bone morphogenetic protein-7 in the development and treatment of bone metastases from breast cancer. Canc. Res. 2007;67:8742–8751. doi: 10.1158/0008-5472.CAN-06-2490. [DOI] [PubMed] [Google Scholar]
- 24.Buijs JT, Rentsch CA, van der Horst G, van Overveld PG, Wetterwald A, Schwaninger R, Henriquez NV, ten Dijke P, Borovecki F, Markwalder R, Thalmann GN, Papapoulos SE, Pelger RC, Vukicevic S, Cecchini MG, Lowik CW, van der Pluijm G. BMP-7, a putative regulator of epithelial homeostasis in the human prostate, is a potent inhibitor of prostate cancer bone metastasis in vivo. Am. J. Pathol. 2007;171:1047–1057. doi: 10.2353/ajpath.2007.070168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.E. Wiercinska, H.P.H. Naber, E. Pardali, G. van der Pluijm, H. van Dam, P. ten Dijke, The TGF-β/Smad pathway induces breast cancer cell invasion through the up-regulation of matrix metalloproteinase 2 and 9 in a spheroid invasion model system. Breast Canc. Res. Treat. 128(3), 657–666 (2010) [DOI] [PubMed]
- 26.Buijs JT, Henriquez NV, van Overveld PG, van der Horst G, ten Dijke P, van der Pluijm G. TGF-β and BMP-7 interactions in tumour progression and bone metastasis. Clin. Exp. Metastasis. 2007;24:609–617. doi: 10.1007/s10585-007-9118-2. [DOI] [PubMed] [Google Scholar]
- 27.Soule HD, Maloney TM, Wolman SR, Peterson WD, Jr, Brenz R, McGrath CM, Russo J, Pauley RJ, Jones RF, Brooks SC. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Canc. Res. 1990;50:6075–6086. [PubMed] [Google Scholar]
- 28.Strickland LB, Dawson PJ, Santner SJ, Miller FR. Progression of premalignant MCF10AT generates heterogeneous malignant variants with characteristic histologic types and immunohistochemical markers. Breast Canc. Res. Treat. 2000;64:235–240. doi: 10.1023/A:1026562720218. [DOI] [PubMed] [Google Scholar]
- 29.Santner SJ, Dawson PJ, Tait L, Soule HD, Eliason J, Mohamed AN, Wolman SR, Heppner GH, Miller FR. Malignant MCF10CA1 cell lines derived from premalignant human breast epithelial MCF10AT cells. Breast Canc. Res. Treat. 2001;65:101–110. doi: 10.1023/A:1006461422273. [DOI] [PubMed] [Google Scholar]
- 30.Persson U, Souchelnytskyi S, Franzen P, Miyazono K, ten Dijke P, Heldin CH. Transforming growth factor (TGF-β)-specific signaling by chimeric TGF-β type II receptor with intracellular domain of activin type IIB receptor. J. Biol. Chem. 1997;272:21187–21194. doi: 10.1074/jbc.272.34.21187. [DOI] [PubMed] [Google Scholar]
- 31.Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM. Direct binding of Smad3 and Smad4 to critical TGF-β-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–3100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korchynskyi O, ten Dijke P. Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J. Biol. Chem. 2002;277:4883–4891. doi: 10.1074/jbc.M111023200. [DOI] [PubMed] [Google Scholar]
- 33.Zhao H, Kitaura H, Sands MS, Ross FP, Teitelbaum SL, Novack DV. Critical role of β3 integrin in experimental postmenopausal osteoporosis. J. Bone Miner. Res. 2005;20:2116–2123. doi: 10.1359/JBMR.050724. [DOI] [PubMed] [Google Scholar]
- 34.Oft M, Peli J, Rudaz C, Schwarz H, Beug H, Reichmann E. TGF-β1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Gene. Dev. 1996;10:2462–2477. doi: 10.1101/gad.10.19.2462. [DOI] [PubMed] [Google Scholar]
- 35.Fuchshofer R, Yu AH, Welge-Lussen U, Tamm ER. Bone morphogenetic protein-7 is an antagonist of transforming growth factor-β2 in human trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2007;48:715–726. doi: 10.1167/iovs.06-0226. [DOI] [PubMed] [Google Scholar]
- 36.Luo DD, Phillips A, Fraser D. Bone morphogenetic protein-7 inhibits proximal tubular epithelial cell Smad3 signaling via increased SnoN expression. Am. J. Pathol. 2010;176:1139–1147. doi: 10.2353/ajpath.2010.090459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Notting I, Buijs J, Mintardjo R, van der Horst G, Vukicevic S, Lowik C, Schalij-Delfos N, Keunen J, van der Pluijm G. Bone morphogenetic protein-7 inhibits tumor growth of human uveal melanoma in vivo. Investig. Ophthalmol. Vis. Sci. 2007;48:4882–4889. doi: 10.1167/iovs.07-0505. [DOI] [PubMed] [Google Scholar]
- 38.Galliher AJ, Schiemann WP. β3 integrin and Src facilitate transforming growth factor-β mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Canc. Res. 2006;8:R42. doi: 10.1186/bcr1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pechkovsky DV, Scaffidi AK, Hackett TL, Ballard J, Shaheen F, Thompson PJ, Thannickal VJ, Knight DA. Transforming growth factor β1 induces αvβ3 integrin expression in human lung fibroblasts via a β3 integrin-, c-Src-, and p38 MAPK-dependent pathway. J. Biol. Chem. 2008;283:12898–12908. doi: 10.1074/jbc.M708226200. [DOI] [PubMed] [Google Scholar]
- 40.Ebisawa T, Tada K, Kitajima I, Tojo K, Sampath TK, Kawabata M, Miyazono K, Imamura T. Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J. Cell Sci. 1999;112(Pt 20):3519–3527. doi: 10.1242/jcs.112.20.3519. [DOI] [PubMed] [Google Scholar]
- 41.Rosenzweig BL, Imamura T, Okadome T, Cox GN, Yamashita H, ten Dijke P, Heldin CH, Miyazono K. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc. Natl. Acad. Sci. U. S. A. 1995;92:7632–7636. doi: 10.1073/pnas.92.17.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.ten Dijke P, Yamashita H, Sampath TK, Reddi AH, Estevez M, Riddle DL, Ichijo H, Heldin CH, Miyazono K. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J. Biol. Chem. 1994;269:16985–16988. [PubMed] [Google Scholar]
- 43.Yamashita H, ten Dijke P, Huylebroeck D, Sampath TK, Andries M, Smith JC, Heldin CH, Miyazono K. Osteogenic protein-1 binds to activin type II receptors and induces certain activin-like effects. J. Cell Biol. 1995;130:217–226. doi: 10.1083/jcb.130.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedman MS, Long MW, Hankenson KD. Osteogenic differentiation of human mesenchymal stem cells is regulated by bone morphogenetic protein-6. J. Cell. Biochem. 2006;98:538–554. doi: 10.1002/jcb.20719. [DOI] [PubMed] [Google Scholar]
- 45.Song K, Krause C, Shi S, Patterson M, Suto R, Grgurevic L, Vukicevic S, van Dinther M, Falb D, ten Dijke P, Alaoui-Ismaili MH. Identification of a key residue mediating bone morphogenetic protein (BMP)-6 resistance to noggin inhibition allows for engineered BMPs with superior agonist activity. J. Biol. Chem. 2010;285:12169–12180. doi: 10.1074/jbc.M109.087197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang B, Vu M, Booker T, Santner SJ, Miller FR, Anver MR, Wakefield LM. TGF-β switches from tumor suppressor to prometastatic factor in a model of breast cancer progression. J. Clin. Investig. 2003;112:1116–1124. doi: 10.1172/JCI18899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kadota M, Yang HH, Gomez B, Sato M, Clifford RJ, Meerzaman D, Dunn BK, Wakefield LM, Lee MP. Delineating genetic alterations for tumor progression in the MCF10A series of breast cancer cell lines. PLoS One. 2010;5:e9201. doi: 10.1371/journal.pone.0009201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 1.65 MB)


