Abstract
Dyskeratosis congenita(DC) is an inherited bone marrow failure syndrome associated with characteristic mucocutaneous features and a variable series of other somatic abnormalities. The disease is heterogeneous at the genetic and clinical levels. Determination of the genetic basis of DC has established that the disease is caused by a number of genes, all of which encode products involved in telomere maintenance, either as part of telomerase or as part of the shelterin complex that caps and protects telomeres. There is overlap at the genetic and clinical levels with other, more common conditions, including aplastic anemia (AA), pulmonary fibrosis (PF)and liver cirrhosis. Although part of the spectrum of disorders known to be associated with DC it has emerged that mutations in telomere maintenance genes can lead to the development of AA and PF in the absence of other DC features. Here we discuss the genetics of DC and its relationship to disease presentation.
Keywords: Bone marrow failure, telomerase, dyskerin, short telomeres, anticipation
Introduction
Dyskeratosis congenita (DC) is an inherited bone marrow failure syndrome. The disease was first described by Zinsser in 1906 ((1)) and recognized as a clinical entity by Engman (1926) (2) and Cole (1930) (3). In the past DC was clinically characterized by the classic diagnostic triad of reticular skin pigmentation, nail dystrophy and mucosal leukoplakia. It is now known that bone marrow failure is frequently found in DC patients and is the most common cause of death(4, 5). Patients often present with various other somatic abnormalities and are prone to the development of immune deficiency, pulmonary complications and malignancy. The disease is heterogeneous at the genetic level. As well as the most common X-linked form, autosomal recessive and dominant forms occur. In the last decade great progress has been made in the genetics and molecular biology of DC with about half of DC patients now assigned to mutations in known genes(6, 7). This work has established that DC is a disease caused primarily by defects in telomere maintenance since all causative mutations found have been in genes whose products function in telomerase activity or assembly or in telomere integrity. In addition patients at presentation nearly all have very short telomeres that are below the first percentile in length compared with healthy age-matched controls (Figure 1). Tracking mutations in these genes in families has uncovered more heterogeneity in DC than previously appreciated, and some mutations originally found in DC are now known to be causative in some cases of idiopathic aplastic anemia, idiopathic pulmonary fibrosis and other conditions.
Fig.1. DC patients have short telomeres.
The graph shows telomere length in 24 DC patients at presentation and telomere length of healthy controls. All patients shown have dyskeratosis congenita and a mutation in a known DC gene. Telomere lengths were measured by flow cytometric fluorescence in situ hybridization in peripheral blood mononuclear cells. The 1st, 90th, 25th, 50th, 75th, 90th, 99th percentiles of 243 healthy controls between the ages of 1 day and 94 years are shown.
DKC1
The most common reported form of DC is the X-linked form. Classical linkage analysis using a collection of X-linked DC families established the DKC1 gene, encoding the protein dyskerin, as the gene responsible for the X-linked disease(8, 9). Dyskerin is a 58kD nucleolar protein that is associated with small nucleolar RNAs (snoRNAs) in H/ACA snoRNP complexes (10, 11). These complexes function mainly in modifying specific uridine residues in newly synthesized ribosomal RNA (rRNA). H/ACA snoRNPs consist of one snoRNA and 4 proteins, dyskerin, NOP10, NHP2 and GAR1(12). The snoRNA acts as a guide RNA and guides the complex to the target pseudouridine by base sequence complementarity while dyskerin is the active pseudouridine synthase enzyme(13–15). The other proteins are responsible for assembly and stability of the complex, some members of which modify spliceosomal snRNAs or catalyze cleavage reactions of rRNA precursors. A related snoRNP family, C/D snoRNPs, use a different set of snoRNAs and 4 different proteins to modify specific rRNA bases by methylation(16). The rRNA modification role of dyskerin is highly conserved in evolution and it was assumed when DKC1 was identified as the culprit gene in DC that a defect in pseudouridylation or ribosome biogenesis was responsible. However the situation is more complex. In vertebrates, but not in simpler animals, dyskerin binds to telomerase RNA, the RNA that is an integral part of the telomerase enzyme and acts as a template for the synthesis of the TTAGGG repeats that are found at the telomeres of all chromosomes(17). The telomerase RNA (TERC) contains a H/ACA snoRNA like sequence at its 3’end that binds dyskerin and the 3 other H/ACA proteins(18)(Figure 2). It seems that the H/ACA snoRNA complex has been adopted in vertebrates to help in the assembly of telomerase in Cajal bodies (19–21)and its translocation to the telomeres. Telomerase RNPs and a subset of snoRNPs that act upon spliceosomal snRNAs are localized in Cajal bodies and the RNAs contain a specific sequence motif, the CAB box, that is necessary for import into Cajal bodies – these RNAs are sometimes known as ScaRNAs(20, 22, 23).
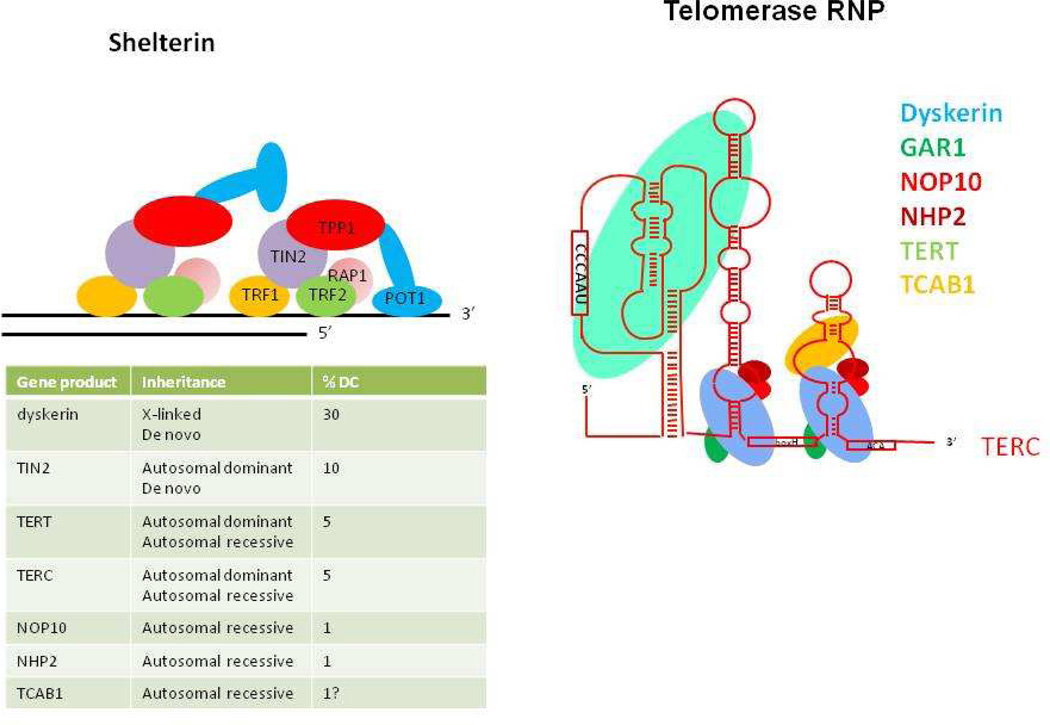
Fig. 2. The gene products known to be defective in dyskeratosis congenita.
TERT, telomerase reverse transcriptase and TERC, the telomerase RNA containing the template for telomere synthesis are core components of telomerase. Dyskerin, NOP10 and NHP2 are associated with telomerase in the telomerase RNP and are thought to be important for assembly and stability. Along with GAR1 these three proteins are also found in H/ACA snoRNPs and scaRNPs. There are 2 copies of the H/ACA complex in each telomerase RNP (112, 113). TCAB1 is important for the assembly of telomerase, it’s localization in Cajal bodies and its translocation to its site of action at the telomere. TIN2 is one of 6 proteins making up shelterin, a protein complex that protects telomeres from degredation by exonucleases and from the cell’s DNA repair machinery. The estimates of the proportion of DC patients in each group is based on(111) and (93). The frequency of TERT and hTR are probably underestimated to ascertainment bias.
It is not known precisely how the mutations in dyskerin lead to telomere shortening but in both humans and mouse cells the mutations lead to lower levels of TERC(24–26). Presumably the lower levels of TERC are not sufficient to maintain telomere length in the stem cells that give rise to blood, skin and other renewable tissues causing telomere attrition and eventually stem cell exhaustion. This simple scheme is supported by the fact that stem cells, unlike other somatic cells, do contain telomerase activity and that DC is a progressive disease, getting worse with age. The tissues affected in DC, at least in its classical form tend to be those that require constant renewal by stem cell activity such as blood, skin, fingernails, the gut lining etc. An intriguing finding is the presence of DNA damage foci at the telomeres in mouse cells carrying Dkc1 mutations that are pathogenic in humans(27). This might suggest that the presence of mutant dyskerin in the active telomerase complex (28) may cause a transient capping defect at telomeres that may lead to telomere loss. Although there are no indications that ribosome biogenesis is affected in DC patients, studies of mice with Dkc1 mutations or perturbed expression suggest that defects in ribosome biogenesis or pseudouridylation may contribute to the DC phenotype(26, 29–31).
Mutations in DKC1 cause DC with varying severity, centred around a classical disease course where boys appear normal at birth, develop the characteristic triad of mucocutaneous features in the first decade, bone marrow failure in the second decade and succumb to death from the complications of bone marrow failure, bleeding and infection, in their third decade(5, 32). However presentation can vary widely. While some males with DKC1 mutations can show intrauterine growth delay, immune deficiency and cerebellar aplasia, a syndrome that was first described as Hoyeraal Hreidarrson syndrome others can be almost asymptomatic (33–35). This point, which highlights the influence of other genetic and/or environmental factors on the penetrance of DC mutations is well illustrated by an analysis of patients carrying the A353V mutation, which occurs in about 40% of X-linked DC cases in the registry held in London. While 4 of 28 cases had HH, 8 presented at age 15 or over with classical mucocutaneous features of DC but not with aplastic anemia (defined as a cytopenia of 2 or more lineages) (6)
The mutations in DKC1 are almost all point mutations causing single amino acid substitutions. Many of the mutations are de novo but patients can also inherit the mutation from their mother who is usually an asymptomatic heterozygous carrier(36). The mutations are clustered in 2 regions of the gene encoding the PUA RNA binding domain and a region near the N-terminus. In the 3-d structure of the dyskerin ortholog from archae, Cbf5, these 2 regions are close together suggesting the mutations all affect the same function of dyskerin(37–39). This region may be involved in the binding of RNAs or, more intriguingly, in the binding of an as yet unidentified factor, the identification of which may explain why DC causing mutations can specifically target telomerase function whilst having only a mild or no effect on ribosome synthesis. Exceptions to this general rule are a 2kb deletion that removes the last exon of the gene producing a protein truncated by 21 amino acids(40), a splice site mutation(36) and a putative promoter mutation(41).
TERC
Classical linkage analysis of a large multi-generation family in which autosomal dominant DC was segregating found linkage to chromosome 3q. Because defects in telomerase had been implicated in the pathogenesis of the X-linked form of DC the gene encoding the RNA component of telomerase, TERC, located in 3q was selected as a candidate gene and found to have a deletion of the 3’ 74 bases of the TERC coding region(42). Mutations in TERC were subsequently found in 2 other DC families in the original report and have since been found to be present in about 4% of DC families as well as in patients with other conditions (The Telomerase Database http://telomerase.asu.edu/).
TERC is one of the core components of telomerase, along with the telomerase reverse transcriptase enzyme, TERT(43). TERC is intimately associated with TERT and contains the template that the TERT uses to add TTAGGG telomere repeats onto the 3’end of telomeres after they have been replicated during the S phase of the cell cycle(44). Curiously TERC is widely expressed while TERT expression in humans is restricted to embryonic cells, stem cells, lymphocytes, and cancer cells(45, 46). DNA polymerase can only synthesize DNA in a 5’ to 3’ direction with the inevitable consequence that at the ends of chromosomes one strand will not be completely replicated. Without telomerase extending the 3’ end, telomeres would shorten with each cycle of replication, as indeed they do in cells that do not express telomerase. Shortened telomeres cause chromosome instability that can lead to cell senescence or cell death, by the activation of a p53 dependent signaling pathway(47). This is most likely the pathogenetic pathway that leads to DC in the X-linked and the autosomal dominant form with mutated dyskerin, or haploinsufficiency for TERC, rendering telomerase incapable of maintaining telomere length with resulting cell death and exhaustion of the stem cell pool needed to supply blood cells and other cells in tissues that need constant renewal.
Generally disease associated with TERC mutations tends to be milder than the X-linked disease(6, 32). A very low frequency of TERC alterations were found in a cohort of children with severe aplastic anemia and MDS who had received a bone marrow transplant (48). Parents of children with dyskeratosis congenita, who carry the same mutation as their affected child, are sometimes asymptomatic or have mild anemia. Studies on families with TERC mutations showed that in these families disease severity increased and the age of disease onset decreased in successive generations, a phenomenon known as genetic anticipation(49). These observations, along with the fact that short telomeres were a common feature of aplastic anemia(50) led investigators to screen patients presenting with idiopathic aplastic anemia for TERC mutations and 1–2% of patients had such mutations (51–53). Several studies showed that in these families (and families with TERT mutations) age specific telomere lengths are shorter in later generations(49, 54, 55). This implies that a child with aplastic anemia or dyskeratosis congenita inherits the mutated gene and pre-shortened telomeres from its affected parent and both are needed for disease development. Anticipation also takes place in mice deficient in either TERC or TERT. In this case laboratory mice completely deficient for TERC or TERT are healthy in the first generation, likely because laboratory mice have very long telomeres (about 50kb compared with 10kb for humans) and it takes several generations before telomeres become short enough to cause problems. In later generations, or after breeding with short telomere wild strains, these mice display abnormalities similar to those found in human DC, notably decreased proliferation of blood cells, poor wound healing, gut abnormalities and increased cancer incidence(56). It is interesting to consider whether mice or humans who inherit short telomeres in the absence of a pathogenic telomerase mutation show any abnormalities. In mice such animals do show short telomeres and defective germ cell development or other degenerative defects(57, 58). In humans data is sparse but shorter than expected telomeres have been observed in children from a TERC family who did not inherit the mutated gene(54).
TERC mutations causing DC include large and small deletions, insertions and single and multiple point mutations (The Telomerase Database http://telomerase.asu.edu/). The TERC molecule contains 3 major domains(59), the pseudoknot domain contains the template region and is thought to be important in dimer formation, the CR4 – CR5 region contains which contains sequences that bind TERT and the 3’ sca RNA domain which is important for assembly and localization and contains the sca motif also present in scaRNAs that are targeted to Cajal bodies(20, 22). Though mutations are found in all domains there are more in the pseudoknot domain than in the others. Because the mutations include large deletions it is assumed that the mechanism of deficiency is haploinsufficiency, ie. 50% of the normal amount of TERC is not sufficient to prevent a level of telomere erosion in some cells that leads to pathological consequences. This is supported by in vitro reconstitution experiments where equal amounts of mutant and wild type TERC transfected into TERC and TERT null cells usually produce 50% of the activity of an equivalent amount of wild type TERC(60–62). Since there is good evidence that TERT acts as a dimer (28, 63, 64)it is conceivable that some mutants may act to suppress telomerase activity by a dominant negative mechanism but this is not the general rule. Two mutations that may act as dominant negatives were reported by Marrone et al(65). These were G178A and C180T and they both gave rise to aplastic anemia as de novo mutations. With the usual mutations that act through haploinsufficiency the mutation has to pass through several generations before telomeres are short enough to produce a clinical phenotype (anticipation, see below). Interestingly both these mutations are located in the pseudoknot in a region of TERC that is thought to facilitate formation of a homodimer by trans-base pairing (66, 67). However the 2 mutations did not show a dominant negative effect when co-tranfected into telomerase negative cells along with wild type TERC and telomerase activity measured in the lysate(65).
TERT
Following the discovery of TERC mutations in DC and aplastic anemia several groups tested patients with unknown mutations for mutations in TERT, the telomerase reverse transcriptase. The situation here was not so simple since TERT is a large gene with several genetic polymorphisms in different human populations. Nevertheless families in which TERT mutations were segregating with dyskeratosis congenita or aplastic anemia were found(55, 68, 69). Patients in these studies had pathogenic mutations in TERT by different combinations of criteria including absence of the mutation in a cohort of healthy controls, segregation with the disease, decreased telomerase activity in cell lysates and the presence of short telomeres. Patients with moderate or severe aplastic anemia, dyskeratosis congenita and Hoyeraal Hreidarrson syndrome were included. Generally disease due to TERT mutations seems to be somewhat different from that caused by mutations in TERC. Penetrance seems to be lower, with more asymptomatic or mildly affected mutation carriers and the disease seems more heterogeneous (see below). This could be because fewer TERT than TERC mutations are null, meaning the residual telomerase activity in the patients cells will be generally higher with TERT than TERC mutations. Part of this heterogeneity and low penetrance may be due to the anticipation phenomenon discussed above, which applies to TERT mutations as well as TERC mutations. Further insight into this anticipation with TERT mutations was provided by a study of a cohort of patients with Cri-du chat syndrome(70), a multi-system disorder due to a 5p- deletion. All these children are haploinsufficient for TERT and yet they have normal blood counts and normal telomere lengths. However a longitudinal study showed evidence that their rate of telomere shortening is increased compared with healthy controls. Thus in early generations carrying a de novo TERT mutations telomeres may not shorten sufficiently to cause disease but shorter than normal telomeres may be passed on to the next generation along with, in some cases, the pathogenic mutation. Later generations may therefore develop very short telomeres and bone marrow failure. Homozygotes or compound heterozygotes for TERT mutations can develop severe disease.
Examination of a 3 generation pedigree with dyskeratosis congenita showing anticipation and telomere shortening revealed that some members of early generations did not have severe bone marrow failure but showed a mild anemia, manifested by a high MCV (mean cell volume) (55). Several of these family members had pulmonary fibrosis (PF), a severe progressive lung disease known to be a late complication of DC. This led the Johns Hopkins group to screen a set of idiopathic PF patients for mutations in TERT and TERC(71). The result of this was that of 73 patients screened 6 (8%) had mutations in either TERT (5) or TERC (1). At the same time a second group of investigators, using linkage studies on 2 large families with multiple cases of PF, mapped the responsible gene to interval 5p15(72). The interval contained TERT which was selected as a candidate because it was known to cause AD DC and PF is known to develop in a number of DC patients. Both families contained TERT mutations that segregated with the disease. Further TERT mutations were found in other families with PF (3/44) and in individuals with idiopathic PF (1/44). In addition a mutation in TERC, previously found in severe aplastic anemia, was present in one of the 44 PF families. Both studies have shown that short telomeres are a feature of PF patients with telomerase mutations. In most cases PF develops in older individuals who do not show the classical features of dyskeratosis congenita but who often show a mild anemia. It is likely that PF cases develop in individuals who have inherited a telomerase mutation but who do not have sufficiently short telomeres to develop the mucocutaneous features and BMF characteristic of DC. In many pedigrees their descendants, with further telomere shortening, may develop severe aplastic anemia and dyskeratosis congenita. Liver disease, including liver cirrhosis is often present in individuals in pedigrees segregating for TERT mutations. The penetrance here appears to be lower than for aplastic anemia or PF and, though it is difficult to make a fine distinction, it seems that short telomeres may increase the susceptibility to liver disease caused by other known risk factors such as alcohol and hepatitis C(73). This suggests that there may be a common mechanism by which short telomeres promote the development of fibrotic diseases in the lung and the liver.
In the case of some TERT mutations investigators have concluded that some mutations may cause susceptibility to certain conditions but do not contribute directly to disease development. A case in point is the SNP rs35719940, a non-synonymous C/T change causing a Ala 1062 to Thr substitution. A careful study of the ethnic distribution of this polymorphism by Calado led to the conclusion that the minor T allele is mainly restricted to white people (15 / 477 whites and in a separate group 3/183 white blood donors were heterozygous, allele frequency, 0.014). The only other individuals with the T allele were 2/122 Pakistanis and 1/10 Hispanics; all other Asian and African individuals tested were negative(73). The T allele has been reported to be present in 8/133 Brazilian, 2/89 American and 10/372 Canadian individuals with de novo AML respectively giving gene frequencies of 0.03, 0.012 and 0.013(74). In addition the T allele is found in 6 (5 heterozygous and 1 homozygous)/134 individuals with liver cirrhosis (gene frequency 0.026) (75). While these figures are suggestive that this mutation may contribute to disease susceptibility the ethnic distribution of the mutation makes the interpretation difficult and more data is required for a definite relationship to be established.
The TERT protein consists of an N-terminal region containing a TEN (telomerase essential) and a TRBD (RNA binding) region, a RT (reverse transcriptase) domain and a C-terminal domain. TERT mutations are distributed throughout the length of the coding region with a slight clustering in the reverse transcriptase domain (The Telomerase Database http://telomerase.asu.edu/). There is no obvious correlation between those that cause PF, AA or DC, possibly because whether mutations cause these conditions may depend on the inherited telomere length and other genetic and environmental factors as well as the nature of the mutation.
NOP10 and NHP2
Autosomal recessive dyskeratosis congenita sometimes occurs in consanguineous marriages. In these cases homozygosity mapping can be used to identify parts of the genome that are derived from the same ancestor and it is predicted that the disease causing gene will be within one of these regions. The region will be homozygous in all affected children and will not be homozygous in non-affected children or in either of the non asymptomatic parents, making the approach highly sensitive in large families. Using such an approach Walne and colleagues determined that the genetic cause of diseases in a series of consanguineous families were varied but they found a homozygous mutation in NOP10 in a large family(76). The family contained 3 affected children who all had the classical mucocutaneous features of DC while only 1 had bone marrow failure. Affected individuals were homozygous for a R34W mutation, had very short telomeres and low levels of TERC. Heterozygotes, who were asymptomatic, had significantly shorter telomeres and lower levels of TERC than healthy controls, but in neither case was the effect as severe as in the affected children.
Using a candidate gene approach the same group found 2 families with AR DC due to mutations in the NHP2 gene(77). One case was homozygous for a Y139H mutation and the other was a compound heterozygote (V126M and X154Arg, which led to a protein extended by 52 amino acids). The first patient had nail dystrophy, thrombocytopenia, growth and mental retardation and other abnormalities associated with DC while the second presented at age 12 with classical DC with pancytopenia. Both patients had short telomeres and low TERC levels.
Both NOP10 and NHP2 are small proteins that, like dyskerin, are present in both the telomerase complex and in H/ACA snoRNPs. The R34W mutation in NOP10 and the Y139H and V126M mutations in NHP2 all alter highly conserved residues. It is notable that dyskerin, NOP10 and NHP2 all bind to the RNA component of the snoRNP (TERC, snoRNA or scaRNA) and experimental knockdown(76, 77) causes a decrease in TERC levels. In an in vitro transcription translation system the pathogenic mutations in NOP10 and NHP2 severely affected assembly of the telomerase snoRNP complex (78). The fourth component of mature snoRNPs is the protein GAR1, in which no DC mutations have been found and whose knockdown does not affect TERC levels. There are two other proteins that are involved in snoRNP assembly but are not present in the mature complex, NAF1 and SHQ1 (79); knockdown of both of these decreases the steady state levels of TERC (80, 81). It remains to be seen if these proteins are mutated in DC.
TINF2
Savage et al (82) used linkage analysis to find the mutation responsible for DC in a large family with variable clinical expression and no mutations in TERT, TERC or DKC1; patients with disease in the family had very short telomeres. This resulted in the discovery of a heterozygous K280E mutation in the gene TINF2, which encodes TIN2, a component of the shelterin complex that is associated with telomere repeats at the telomeres. The investigators then sequenced the TINF2 gene in 8 other DC probands whose causative gene was not known and found mutations in 4 of them, 3 had a R282H changes and one had a R282S mutation. All of these probands presented when below 4 years of age with severe DC-like features and very short telomeres. One of them had been diagnosed with Revesz syndrome(83), characterized by bilateral retinopathy, developmental delay, the DC triad and cerebellar hypoplasia. Walne et al then carried out a large study of 175 uncharacterized DC patients and found 33 of them had TINF2 mutations, mostly de novo(84). 21 of the mutations affected a single residue R282 changing it to H(14) or C(7). The majority of patients had very severe disease, with most presenting below the age of 10 and all had very short telomeres. Subsequent studies have confirmed these findings showing TINF2 mutations cause severe disease with very short telomeres and with mutations tightly clustered in and around amino acid 282 (85, 86). Curiously, although usually highly penetrant and severe, rare individuals carrying one of these mutations have been found that are silent carriers(82) or present with some DC features but not aplastic anemia(84).
TIN2 is one of 6 proteins that make up shelterin, a complex that protects telomere ends from exonucleases and from the cells DNA repair machinery. Shelterin must also co-operate with telomerase to maintain telomere length and integrity (87). The fact that all the genes causing DC discovered before TINF2 encoded components of telomerase suggest that TIN2 may act in some way to recruit telomerase to the telomere and that is the function that is defective in the mutants. Indeed Abreu et al showed that TIN2 and TPP1 depletion results in loss of detectable telomerase recruitment to the telomere in human cells (88). Yang et al (89) then showed, using a system in which pathogenic TINF2 mutations were overexpressed in human cell lines that this overexpression phenocopied the disease in that telomeres shortened in these cells. They did not detect any changes in telomerase activity, localization of TIN2 or telomere protection. Their experiments revealed that TIN2 participated in TPP1-dependent recruitment of telomerase activity and the mutant proteins were deficient in their ability to associate with TERC. It was recently found that TIN2 binds a protein called HP1γ, a protein that binds to telomeres in S phase and is involved in cohesion, the physical association of newly replicated sister chromatids via interaction with protein complexes called cohesins (90). These investigators showed that the HP1γ binding site in TIN2 is required for sister telomere cohesion and that mutations in this binding site can interfere with telomere length maintenance. Intriguingly the HP1 binding site is in the same place as the cluster of TIN2 mutations that cause DC, and moreover the pathogenic mutations block HP1 binding. The authors then showed that DC patient cells are defective in sister telomere cohesion. These results suggest that deficient HP1 binding and defective telomere cohesion may be important in disease pathogenesis. The authors noted that the in vitro telomere shortening resulting from overexpressing pathogenic TINF2 mutations in human cells was modest compared with the drastic shortening that must occur in patients’ cells. They speculated that telomere shortening during the replicative cycle may not be involved in the pathogenesis and cited recent work suggesting that in early embryogenesis telomere maintenance is achieved by recombination, a process more likely to depend on telomere cohesion (91,92). They put forward the intriguing idea that it is this embryonic phase of telomere maintenance that may be defective in DC caused by TINF2 mutations.
TCAB1
Zhong et al (2011) (93)opted to sequence the TCAB1 gene (19) in a cohort of DC patients that had tested negative for mutations in all known DC genes. TCAB1, also known as WDR79 (94) and WRAP53 (95) is a WD40 repeat-containing protein that binds the CAB box (a motif found in scaRNAs including TERC). The CAB motif (21) is required for trafficking to Cajal bodies, nuclear sites of assembly of scaRNPs including TERC (19, 94). Telomerase assembly in Cajal bodies is an important step in the translocation of telomerase to its site of activity (96) at the telomere and TCAB1 inhibition causes telomere shortening(19). Of 16 patients with classical DC or DC-like features, all with telomeres below the first percentile in length, 2 were found to be compound heterozygotes for TCAB1 mutations(93). All residues altered in the mutant proteins were highly conserved in evolution. Expression of epitope tagged wild type and mutant proteins in HeLa cells showed that decreased levels of the mutant proteins accumulated in cells and that their site of accumulation was altered with more in the cytoplasm and less in the nucleus and very little in the Cajal body compared to wild type. Similar results were obtained comparing EBV transformed lymphocytes from patients with normal controls. In in vitro experiments and in patient derived cells TCAB1 depletion, or the presence of the pathogenic mutants led to failure of either dyskerin or TERC to accumulate in Cajal bodies. Instead TERC appeared to localize to nucleoli. These studies represent a third pathogenic pathway whereby DC is caused by failure of telomerase trafficking.
In the two families in which TCAB1 mutations were found the probands showed classical DC, with the mucocutaneous triad, bone marrow failure and telomere lengths below the first percentile of those found in healthy controls. Both sets of parents each carried one of the mutated alleles, were healthy and did not have short telomeres. Thus TCAB1 associated DC is inherited in both families in an autosomal recessive manner. The 4 mutations found in these 2 families were not found in a cohort of healthy controls, implying none of them are common polymorphisms, and may be sporadic mutations. If sporadic we might expect compound heterozygotes to crop up very rarely though the finding of 2 cases in one study seems to contradict that assumption. Data from a larger cohort would indicate the frequency of TCAB1 associated DC.
An intriguing aspect of the TCAB1 gene (also known as WRAP53(95)) is that it overlaps the p53 gene with the 2 genes in diverging, head to head orientation, making its transcript a natural anti-sense RNA to p53. Evidence was obtained that WRAP53, through interaction at the RNA level is an important regulator of p53. So far no mechanistic or functional connection has been made between these 2 roles ascribed to the same gene.
Is C16orf57 a DC gene?
Genetic mapping of consanguineous families from the Dyskeratosis congenita registry led Walne et al (97) to discover that homozygous mutations at the C16orf57 gene were responsible in 4 families. They went on to sequence the gene in 124 families whose mutation was unknown and found another 4 families where homozygous C16orf57 mutations were present. They also found homozygous C16orf57 mutations in 2 of 6 patients who had a diagnosis of Rothmund – Thomson syndrome (RTS, OMIM #268400). It is notable that in a large kindred with poikiloderma with neutrapenia (PN, OMIM #604173) homozygous mutations in C16orf57 were found to be responsible (98). Clinical features of DC, RTS and PN overlap to some extent, in particular all 3 show poikiloderma. DC and PN both have bone marrow manifestations, progressive bone marrow failure in the case of DC and variable neutropenia, often cyclical, in the case of PN. DC and RTS share predisposition to malignancies, MDS and epithelial cancers of the GI tract in DC and osteosarcoma in RTS. Most RTS patients have mutations in RECQL4 a DNA helicase that belongs to the same family as the proteins associated with Bloom’s syndrome (BLM, OMIM #210900) and Werner’s syndrome (WRN, OMIM #277700). The some overlapping clinical features of DC, RTS and PN make distinguishing them from one another at the diagnostic level sometimes somewhat difficult but it is not clear if there is any mechanistic similarity between them. Notably while DC is established as a disease caused by defective telomere maintenance, and usually presents with very short telomeres the individuals with homozygous mutations in C16orf57 have telomeres of normal length. However, as pointed out by Walne et al (97) telomere dysfunction does not necessarily go hand in hand with short telomeres (99). The resolution of this issue awaits the elucidation of the functional role of the C16orf57 protein in normal cells and in PN and RTS.
Dyskeratosis congenita and cancer
In common with other inherited bone marrow failure syndromes DC is associated with an increased susceptibility to cancer. Although many of the more severe cases of DC/HH do not live long enough for cancer to develop those that survive into their thirties and later are prone to develop malignant disease, most commonly MDS, AML and squamous cell carcinoma of the head and neck (100, 101). Cancer is more common in DC caused by TERT and TERC and least common in DC caused by TINF2, likely reflecting the fact that patients with TERT and TERC disease tend to live longer, and those with TINF2 much shorter (102). Disease caused by DKC1 is intermediate in age of presentation and cancer incidence. In these rare conditions the actual size of the increase in cancer incidence is difficult to estimate because it depends on the gene mutated and the age of the patient. Data from the NCI BMFS cohort suggests an overall increase in incidence of 11 fold(100).
The increase in cancer incidence in DC is somewhat puzzling at first glance since telomerase activity is usually an important feature of the malignant phenotype, with most cancers having substantially more telomerase activity than the cells from which they develop. There may be several explanations for this(103). Firstly short telomeres can lead to malignant transformation because when telomeres get critically short they trigger a cell cycle arrest via a signaling pathway involving p53, most likely because the short telomeres are recognized as DNA damage by the cellular DNA repair machinery. Rarely mutations in the signaling pathway or in genes mediating cell cycle arrest may occur in these cells resulting in further replication and cell division and giving rise to chromosome instability as telomeres from different chromosomes are fused together by DNA repair proteins. Circular chromosomes formed by this process will break during mitosis and cells will enter a phase of chromosome fusion/breakage that will produce translocations, amplifications and deletions, with the possibility of tumorigenic cells evolving. DC cells have all they need to increase telomerase activity to levels that would sustain tumorigenic growth since none of them are null for telomerase activity. Increased production of TERC or TERT may provide sufficient telomerase activity, or telomeres may be maintained by the ALT (alternative lengthening of telomeres) pathway which involves recombination between telomeres and does not require telomerase activity(104). It is not known which of these mechanisms are used to maintain the telomeres in cancers in DC patients because of the rarity of the disease but it would be interesting to determine the mechanism in DC or in appropriate animal models.
Conclusions – genetic and clinical heterogeneity in DC
Unraveling the genetic basis of dyskeratosis congenita over the last 13 years has led not only to the discovery that this rare but interesting condition is due to defective telomere maintainance but has helped to generate greater understanding of a variety of other diseases including aplastic anemia and pulmonary fibrosis. In terms of translational “bench to bedside” research this greater understanding will certainly lead to improvements in patient management, some of which, such as the development of milder conditioning regimens prior to bone marrow transplantation in DC (105), the use of telomere length measurement as an aid to diagnosis (106, 107) and genotyping of patients’ relatives for early diagnosis are already in practice. Awareness of the possibility that AA and PF cases that seem sporadic may be due to inherited mutations will also inform patient care and in the case of PF may be used to instigate preventative measures such as not smoking. In turn the clinical work has stimulated some excellent basis “bedside to bench” research into the roles of dyskerin, and recently TINF2, in telomere maintenance and into the biology of snoRNPs and telomerase biogenesis in humans.
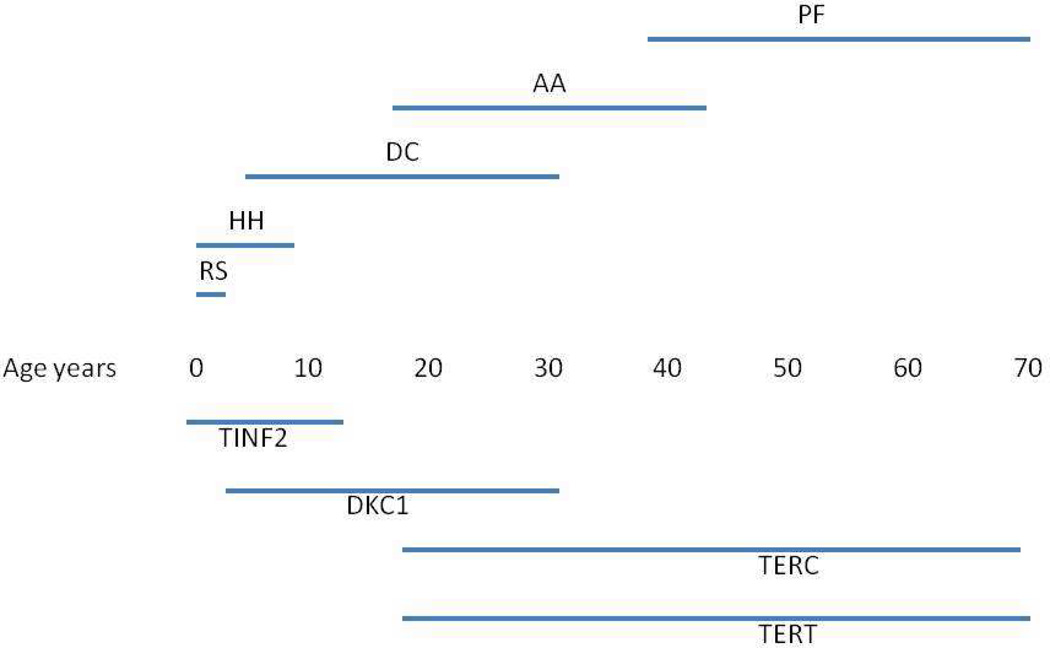
A unique and intriguing feature of DC is the relationship between the genetic heterogeneity, in terms of the different genes mutated, and the clinical heterogeneity, in terms of phenotypic abnormalities, age of onset and severity (Table 1 and Figure 3). DC presents with a wide range of disease severity, both in the degree and number of abnormalities. Not surprisingly there is a correlation between the age of onset and severity, with more severe disease presenting in younger patients. There is also some correlation between the causative gene and severity/age of onset and curiously the disease features at presentation are different at different ages. Thus mutations in TINF2 cause a very severe form of dyskeratosis congenita, usually with severe aplastic anemia, that can have features of Hoyeraal Hreidarrson syndrome (cerebellar hypoplasia, intrauterine growth retardation, immunodeficiency and progressive pancytopenia) or Revesz syndrome (retinopathy as well as pancytopenia, cerebellar hypoplasia and developmental delay). The age of onset is often below 5 years and 60% of TINF2 patients develop aplastic anemia before the age of 10 (84). Patients with DKC1 mutations also include some very severe Hoyeraal Hreidarrson cases but in general they are less severe than TINF2 patients. Typically a boy with a DKC1 mutation will develop the mucocutaneous triad in his first decade and bone marrow failure in his second decade, and will die from complications of bone marrow failure in his third decade. There is a wide range of disease courses however from HH to patients who live into their 30s and 40s (102). Patients with TERC and TERT mutations are, on the whole older than DKC1 patients and they present with features ranging from classical DC to aplastic anemia with no mucocutaneous features to pulmonary fibrosis. As discussed above, due to anticipation, some mutation carriers, often parents of DC patients, may be asymptomatic or have mild anemia. Patients with autosomal recessive disease caused by mutations in NOP10, NHP2 and TCAB1 are too scarce to make generalizations but those found so far in these 3 groups presented with features similar to those found in DKC1 patients, classical DC with bone marrow failure. Why does telomere shortening manifest differently at different ages? Looking at the telomere lengths of DC patients at the time of presentation they are all fairly similar – there is no downward trend with age as seen in healthy controls. This implies that in all cases a critically short telomere length is reached and then features of DC develop but why do the effects of critically short telomeres vary with age? It could be that tissue specific telomerase requirements vary with age. Thus initially telomerase is required to maintain telomere length throughout embryonic development and this is only not met in the most severe cases, causing the HH and RS type disease found in many cases of TINF2 and some cases of DKC1 disease. Maintaining telomere length throughout the constant renewal of epithelia and bone marrow in the first few decades of life requires more telomerase activity and this is not achieved without wild type dyskerin, or, when telomere lengths have been shortened by anticipation, without the full complement of TERT and TERC. Finally telomerase is required to maintain the renewable cells needed for maintaining healthy lung, liver and other adult tissues. If the function of the telomerase complex is compromised by mutation, and short telomeres were inherited, telomere maintenance may not be maintained in these tissues leading to susceptibility to pulmonary fibrosis or liver cirrhosis or other conditions. Thus with age in DC there is a trend of decreasing penetrance with environmental factors such as inflammation, alcohol, smoking and life stress (108) becoming more important. For these less penetrant effects of telomere maintenance diseases there is much scope for improvement and prevention by longitudinal studies and increased surveillance of families at risk.
Table 1. Mutations and disease phenotype in DC.
Disease phenotypes are variable, the most common are shown in the table. DC (dyskeratosis congenita – abnormal skin pigmentation, nail dystrophy, leukoplakia, bone marrow failure and short telomeres) HH (Hoyeraal-Hreidarrson syndrome – intra-uterine growth retardation, cerebellar hypoplasia, immunodeficiency and bone marrow failure, AA(aplastic anemia without the mucocutaneous features of DC), RS (Revesz syndrome – bilateral retinopathy, developmental delay, the DC triad and cerebellar hypoplasia). For a discussion of diagnosis and terminology see (109–111).
| Gene (OMIM#) |
Mutation profile | Disease phenotype | Comments |
|---|---|---|---|
| TINF2 (604319) | Mainly point mutations causing single amino acid substitutions. Highly clustered. Nearly always de novo. | Severe to classical DC, HH or RS. Early age of onset. | High penetrance. R282H and R282C most common. Others mostly affect amino acids 280–298. |
| DKC1 (300126) | Mainly point mutations causing single amino acid substitutions. Mainly in 2 regions of protein that associate forming RNA binding domain. Sometimes de novo. | Classical DC. Occasionally HH. | Common recurrent mutation A353V accounts for about 40% of cases and is often de novo. High penetrance. |
| TERC (602322) | Point mutations, deletions, insertions. Many are in the pseudoknot essential for telomerase activity. | DC, AA, PF. | Anticipation with shorter telomeres in later generations. Homozygotes can have severe disease. |
| TERT (187270) | Mainly point mutations causing single amino acid substitutions. Spread throughout protein but more in reverse transcriptase domain. | DC, AA, PF. | Some polymorphisms that may contribute to disease. Anticipation with shorter telomeres in later generations. Homozygotes can have severe disease. |
| NOP10 (606471) NHP2 (606470) | Homozygous R34W mutation causes DC in 1 large family. | Classical DC | Aka NOLA3 |
| NHP2 (606470) | 1 homozygous missense mutation 1 heterozygous for a missense and a mutated STOP codon. | Classical DC | Aka NOLA2 |
| TCAB1 (612661) | 2 compound heterozygotes with 4 missense mutations. | Classical DC | Aka WDR79, WRAP53 |
| Gene | Mutation profile | Disease phenotype | Comments |
| TINF2 (604319) | Mainly point mutations causing single amino acid substitutions. Highly clustered. Nearly always de novo. | Severe to classical DC, HH or RS. Early age of onset. | High penetrance. R282H and R282C most common. Others mostly affect amino acids 280–298. |
| DKC1 (300126) | Mainly point mutations causing single amino acid substitutions. Mainly in 2 regions of protein that associate forming RNA binding domain. Sometimes de novo. | Classical DC. Occasionally HH. | Common recurrent mutation A353V accounts for about 40% of cases and is often de novo. High penetrance. |
| TERC (602322) | Point mutations, deletions, insertions. Many are in the pseudoknot essential for telomerase activity. | DC, AA, PF. | Anticipation with shorter telomeres in later generations. Homozygotes can have severe disease. |
| TERT (187270) | Mainly point mutations causing single amino acid substitutions. Spread throughout protein but more in reverse transcriptase domain. | DC, AA, PF. | Some polymorphisms that may contribute to disease. Anticipation with shorter telomeres in later generations. Homozygotes can have severe disease. |
| NOP10 (606471) NHP2 (606470) | Homozygous R34W mutation causes DC in 1 large family. | Classical DC | |
| NHP2 (606470) | 1 homozygous missense mutation 1 heterozygous for a missense and a mutated STOP codon. | Classical DC | |
| TCAB1 (612661) | 2 compound heterozygotes with 4 missense mutations. | Classical DC |
Fig.3. Schematic representation of age specific effects of mutations in different DC genes.
The primary manifestation of DC differs with the age of disease onset and is dependent on the underlying gene mutation(s). This is a schematic presentation of the age dependent presentation of disease and the most frequent gene mutation responsible for this phenotype. The mutated genes are shown below and the presentation at disease onset is indicated above.
Acknowledgements
The work has been supported by the Buck Family Endowed Chair in Hematology, and by NCI NIH grants 2R01CA106995 to PJM, and2R01 CA105312 to MB. We would like to thank all of the patients and their families for their continuing participation in our bone marrow failure studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zinsser F. Atrophia cutis reticularis pigmentione, dystrophia unguium et leukoplakia oris (poikilodermia atrophicans vascularis Jacobi) Ikonogr Derm (Kyoto. 1906;5:219–223. [Google Scholar]
- 2.Engman MA. A unique case of reticular pigmentation of the skin with atrophy. Arch Derm Syph Suppl. 1926;13:685–687. [Google Scholar]
- 3.Cole H, Rauschkolb J, Toomey J. Dyskeratosis congenita with pigmentation, dystrophia unguis and leukokeratosis oris. Arch Derm Syph Suppl. 1930;21:71–95. doi: 10.1001/archderm.1955.01540280027005. [DOI] [PubMed] [Google Scholar]
- 4.Drachtman RA, Alter BP. Dyskeratosis congenita. Dermatol Clin. 1995;13:33–39. [PubMed] [Google Scholar]
- 5.Dokal I. Dyskeratosis congenita in all its forms. Br J Haematol. 2000;110:768–779. doi: 10.1046/j.1365-2141.2000.02109.x. [DOI] [PubMed] [Google Scholar]
- 6.Vulliamy TJ, Marrone A, Knight SW, et al. Mutations in dyskeratosis congenita: their impact on telomere length and the diversity of clinical presentation. Blood. 2006;107:2680–2685. doi: 10.1182/blood-2005-07-2622. [DOI] [PubMed] [Google Scholar]
- 7.Savage SA, Alter BP. Dyskeratosis congenita. Hematol Oncol Clin North Am. 2009;23:215–231. doi: 10.1016/j.hoc.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knight SW, Vulliamy TJ, Heiss NS, et al. 1.4 Mb candidate gene region for X linked dyskeratosis congenita defined by combined haplotype and X chromosome inactivation analysis. J Med Genet. 1998;35:993–996. doi: 10.1136/jmg.35.12.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heiss NS, Knight SW, Vulliamy TJ, et al. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet. 1998;19:32–38. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- 10.Meier UT, Blobel G. NAP57, a mammalian nucleolar protein with a putative homolog in yeast and bacteria. J Cell Biol. 1994;127:1505–1514. doi: 10.1083/jcb.127.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meier UT. The many facets of H/ACA ribonucleoproteins. Chromosoma. 2005;114:1–14. doi: 10.1007/s00412-005-0333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiss T, Fayet-Lebaron E, Jady BE. Box H/ACA small ribonucleoproteins. Mol Cell. 2010;37:597–606. doi: 10.1016/j.molcel.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 13.Watkins NJ, Gottschalk A, Neubauer G, et al. Cbf5p, a potential pseudouridine synthase, and Nhp2p, a putative RNA-binding protein, are present together with Gar1p in all H BOX/ACA-motif snoRNPs and constitute a common bipartite structure. RNA. 1998;4:1549–1568. doi: 10.1017/s1355838298980761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lafontaine DL, Bousquet-Antonelli C, Henry Y, et al. The box H + ACA snoRNAs carry Cbf5p, the putative rRNA pseudouridine synthase. Genes Dev. 1998;12:527–537. doi: 10.1101/gad.12.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cadwell C, Yoon HJ, Zebarjadian Y, et al. The yeast nucleolar protein Cbf5p is involved in rRNA biosynthesis and interacts genetically with the RNA polymerase I transcription factor RRN3. Mol Cell Biol. 1997;17:6175–6183. doi: 10.1128/mcb.17.10.6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reichow SL, Hamma T, Ferre-D'Amare AR, et al. The structure and function of small nucleolar ribonucleoproteins. Nucleic Acids Res. 2007;35:1452–1464. doi: 10.1093/nar/gkl1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell JR, Wood E, Collins K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell JR, Cheng J, Collins K. A box H/ACA small nucleolar RNA-like domain at the human telomerase RNA 3' end. Mol Cell Biol. 1999;19:567–576. doi: 10.1128/mcb.19.1.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venteicher AS, Abreu EB, Meng Z, et al. A human telomerase holoenzyme protein required for Cajal body localization and telomere synthesis. Science. 2009;323:644–648. doi: 10.1126/science.1165357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu D, Collins K. Human telomerase and Cajal body ribonucleoproteins share a unique specificity of Sm protein association. Genes Dev. 2006;20:531–536. doi: 10.1101/gad.1390306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jady BE, Bertrand E, Kiss T. Human telomerase RNA and box H/ACA scaRNAs share a common Cajal body-specific localization signal. J Cell Biol. 2004;164:647–652. doi: 10.1083/jcb.200310138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theimer CA, Jady BE, Chim N, et al. Structural and functional characterization of human telomerase RNA processing and cajal body localization signals. Mol Cell. 2007;27:869–881. doi: 10.1016/j.molcel.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Darzacq X, Jady BE, Verheggen C, et al. Cajal body-specific small nuclear RNAs: a novel class of 2'-O-methylation and pseudouridylation guide RNAs. EMBO J. 2002;21:2746–2756. doi: 10.1093/emboj/21.11.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong JM, Collins K. Telomerase RNA level limits telomere maintenance in X-linked dyskeratosis congenita. Genes Dev. 2006;20:2848–2858. doi: 10.1101/gad.1476206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong JM, Kyasa MJ, Hutchins L, et al. Telomerase RNA deficiency in peripheral blood mononuclear cells in X-linked dyskeratosis congenita. Hum Genet. 2004;115:448–455. doi: 10.1007/s00439-004-1178-7. [DOI] [PubMed] [Google Scholar]
- 26.Mochizuki Y, He J, Kulkarni S, et al. Mouse dyskerin mutations affect accumulation of telomerase RNA and small nucleolar RNA, telomerase activity, and ribosomal RNA processing. Proc Natl Acad Sci U S A. 2004;101:10756–10761. doi: 10.1073/pnas.0402560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gu BW, Bessler M, Mason PJ. A pathogenic dyskerin mutation impairs proliferation and activates a DNA damage response independent of telomere length in mice. Proc Natl Acad Sci U S A. 2008;105:10173–10178. doi: 10.1073/pnas.0803559105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen SB, Graham ME, Lovrecz GO, et al. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007;315:1850–1853. doi: 10.1126/science.1138596. [DOI] [PubMed] [Google Scholar]
- 29.Bellodi C, Kopmar N, Ruggero D. Deregulation of oncogene-induced senescence and p53 translational control in X-linked dyskeratosis congenita. EMBO J. 2010;29:1865–1876. doi: 10.1038/emboj.2010.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoon A, Peng G, Brandenburger Y, et al. Impaired control of IRES-mediated translation in X-linked dyskeratosis congenita. Science. 2006;312:902–906. doi: 10.1126/science.1123835. [DOI] [PubMed] [Google Scholar]
- 31.Ruggero D, Grisendi S, Piazza F, et al. Dyskeratosis congenita and cancer in mice deficient in ribosomal RNA modification. Science. 2003;299:259–262. doi: 10.1126/science.1079447. [DOI] [PubMed] [Google Scholar]
- 32.Bessler M, Mason P, Link D, et al. Inherited bone marrow failure syndromes. In: Orkin S, Nathan D, Ginsburg D, Look A, Fisher D, Lux S, editors. Nathan and Oski's Hematology of Infancy and Childhood. Philadelphia: Saunders; 2008. pp. 307–395. [Google Scholar]
- 33.Hoyeraal HM, Lamvik J, Moe PJ. Congenital hypoplastic thrombocytopenia and cerebral malformations in two brothers. Acta Paediatr Scand. 1970;59:185–191. doi: 10.1111/j.1651-2227.1970.tb08986.x. [DOI] [PubMed] [Google Scholar]
- 34.Hreidarsson S, Kristjansson K, Johannesson G, et al. A syndrome of progressive pancytopenia with microcephaly, cerebellar hypoplasia and growth failure. Acta Paediatr Scand. 1988;77:773–775. doi: 10.1111/j.1651-2227.1988.tb10751.x. [DOI] [PubMed] [Google Scholar]
- 35.Knight SW, Heiss NS, Vulliamy TJ, et al. Unexplained aplastic anaemia, immunodeficiency, and cerebellar hypoplasia (Hoyeraal-Hreidarsson syndrome) due to mutations in the dyskeratosis congenita gene, DKC1. Br J Haematol. 1999;107:335–339. doi: 10.1046/j.1365-2141.1999.01690.x. [DOI] [PubMed] [Google Scholar]
- 36.Knight SW, Heiss NS, Vulliamy TJ, et al. X-linked dyskeratosis congenita is predominantly caused by missense mutations in the DKC1 gene. Am J Hum Genet. 1999;65:50–58. doi: 10.1086/302446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rashid R, Liang B, Baker DL, et al. Crystal structure of a Cbf5-Nop10-Gar1 complex and implications in RNA-guided pseudouridylation and dyskeratosis congenita. Mol Cell. 2006;21:249–260. doi: 10.1016/j.molcel.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 38.Li L, Ye K. Crystal structure of an H/ACA box ribonucleoprotein particle. Nature. 2006;443:302–307. doi: 10.1038/nature05151. [DOI] [PubMed] [Google Scholar]
- 39.Hamma T, Reichow SL, Varani G, et al. The Cbf5-Nop10 complex is a molecular bracket that organizes box H/ACA RNPs. Nat Struct Mol Biol. 2005;12:1101–1107. doi: 10.1038/nsmb1036. [DOI] [PubMed] [Google Scholar]
- 40.Vulliamy TJ, Knight SW, Heiss NS, et al. Dyskeratosis congenita caused by a 3' deletion: germline and somatic mosaicism in a female carrier. Blood. 1999;94:1254–1260. [PubMed] [Google Scholar]
- 41.Knight SW, Vulliamy TJ, Morgan B, et al. Identification of novel DKC1 mutations in patients with dyskeratosis congenita: implications for pathophysiology and diagnosis. Hum Genet. 2001;108:299–303. doi: 10.1007/s004390100494. [DOI] [PubMed] [Google Scholar]
- 42.Vulliamy T, Marrone A, Goldman F, et al. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–435. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- 43.Blackburn EH, Collins K. Telomerase: an RNP enzyme synthesizes DNA. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Autexier C, Lue NF. The structure and function of telomerase reverse transcriptase. Annu Rev Biochem. 2006;75:493–517. doi: 10.1146/annurev.biochem.75.103004.142412. [DOI] [PubMed] [Google Scholar]
- 45.Wright WE, Piatyszek MA, Rainey WE, et al. Telomerase activity in human germline and embryonic tissues and cells. Dev Genet. 1996;18:173–179. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 46.Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 47.Artandi SE, DePinho RA. A critical role for telomeres in suppressing and facilitating carcinogenesis. Curr Opin Genet Dev. 2000;10:39–46. doi: 10.1016/s0959-437x(99)00047-7. [DOI] [PubMed] [Google Scholar]
- 48.Field JJ, Mason PJ, An P, et al. Low frequency of telomerase RNA mutations among children with aplastic anemia or myelodysplastic syndrome. J Pediatr Hematol Oncol. 2006;28:450–453. doi: 10.1097/01.mph.0000212952.58597.84. [DOI] [PubMed] [Google Scholar]
- 49.Vulliamy T, Marrone A, Szydlo R, et al. Disease anticipation is associated with progressive telomere shortening in families with dyskeratosis congenita due to mutations in TERC. Nat Genet. 2004;36:447–449. doi: 10.1038/ng1346. [DOI] [PubMed] [Google Scholar]
- 50.Ball SE, Gibson FM, Rizzo S, et al. Progressive telomere shortening in aplastic anemia. Blood. 1998;91:3582–3592. [PubMed] [Google Scholar]
- 51.Vulliamy T, Marrone A, Dokal I, et al. Association between aplastic anaemia and mutations in telomerase RNA. Lancet. 2002;359:2168–2170. doi: 10.1016/S0140-6736(02)09087-6. [DOI] [PubMed] [Google Scholar]
- 52.Fogarty PF, Yamaguchi H, Wiestner A, et al. Late presentation of dyskeratosis congenita as apparently acquired aplastic anaemia due to mutations in telomerase RNA. Lancet. 2003;362:1628–1630. doi: 10.1016/S0140-6736(03)14797-6. [DOI] [PubMed] [Google Scholar]
- 53.Yamaguchi H, Baerlocher GM, Lansdorp PM, et al. Mutations of the human telomerase RNA gene (TERC) in aplastic anemia and myelodysplastic syndrome. Blood. 2003;102:916–918. doi: 10.1182/blood-2003-01-0335. [DOI] [PubMed] [Google Scholar]
- 54.Goldman F, Bouarich R, Kulkarni S, et al. The effect of TERC haploinsufficiency on the inheritance of telomere length. Proc Natl Acad Sci U S A. 2005;102:17119–17124. doi: 10.1073/pnas.0505318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Armanios M, Chen JL, Chang YP, et al. Haploinsufficiency of telomerase reverse transcriptase leads to anticipation in autosomal dominant dyskeratosis congenita. Proc Natl Acad Sci U S A. 2005;102:15960–15964. doi: 10.1073/pnas.0508124102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blasco MA, Lee HW, Hande MP, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 57.Hao LY, Armanios M, Strong MA, et al. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell. 2005;123:1121–1131. doi: 10.1016/j.cell.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 58.Armanios M, Alder JK, Parry EM, et al. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am J Hum Genet. 2009;85:823–832. doi: 10.1016/j.ajhg.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen JL, Greider CW. Telomerase RNA structure and function: implications for dyskeratosis congenita. Trends Biochem Sci. 2004;29:183–192. doi: 10.1016/j.tibs.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 60.Fu D, Collins K. Distinct biogenesis pathways for human telomerase RNA and H/ACA small nucleolar RNAs. Mol Cell. 2003;11:1361–1372. doi: 10.1016/s1097-2765(03)00196-5. [DOI] [PubMed] [Google Scholar]
- 61.Ly H, Calado RT, Allard P, et al. Functional characterization of telomerase RNA variants found in patients with hematological disorders. Blood. 2004 doi: 10.1182/blood-2004-09-3659. [DOI] [PubMed] [Google Scholar]
- 62.Marrone A, Stevens D, Vulliamy T, et al. Heterozygous telomerase RNA mutations found in dyskeratosis congenita and aplastic anemia reduce telomerase activity via haploinsufficiency. Blood. 2004;104:3936–3942. doi: 10.1182/blood-2004-05-1829. [DOI] [PubMed] [Google Scholar]
- 63.Beattie TL, Zhou W, Robinson MO, et al. Functional multimerization of the human telomerase reverse transcriptase. Mol Cell Biol. 2001;21:6151–6160. doi: 10.1128/MCB.21.18.6151-6160.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wenz C, Enenkel B, Amacker M, et al. Human telomerase contains two cooperating telomerase RNA molecules. EMBO J. 2001;20:3526–3534. doi: 10.1093/emboj/20.13.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marrone A, Sokhal P, Walne A, et al. Functional characterization of novel telomerase RNA (TERC) mutations in patients with diverse clinical and pathological presentations. Haematologica. 2007;92:1013–1020. doi: 10.3324/haematol.11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Comolli LR, Smirnov I, Xu L, et al. A molecular switch underlies a human telomerase disease. Proc Natl Acad Sci U S A. 2002;99:16998–17003. doi: 10.1073/pnas.262663599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ly H, Xu L, Rivera MA, et al. A role for a novel 'trans-pseudoknot' RNA-RNA interaction in the functional dimerization of human telomerase. Genes Dev. 2003;17:1078–1083. doi: 10.1101/gad.1060803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vulliamy T, Walne A, Baskaradas A, et al. Mutations in the reverse transcriptase component of telomerase (TERT) in patients with bone marrow failure. Blood cells molecules and disease. 2005 doi: 10.1016/j.bcmd.2004.12.008. In Press. [DOI] [PubMed] [Google Scholar]
- 69.Yamaguchi H, Calado RT, Ly H, et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005;352:1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 70.Du HY, Idol R, Robledo S, et al. Telomerase reverse transcriptase haploinsufficiency and telomere length in individuals with 5p- syndrome. Aging Cell. 2007;6:689–697. doi: 10.1111/j.1474-9726.2007.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Armanios MY, Chen JJ, Cogan JD, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 72.Tsakiri KD, Cronkhite JT, Kuan PJ, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci U S A. 2007;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Calado RT, Regal JA, Kleiner DE, et al. A spectrum of severe familial liver disorders associate with telomerase mutations. PLoS One. 2009;4:e7926. doi: 10.1371/journal.pone.0007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Calado RT, Regal JA, Hills M, et al. Constitutional hypomorphic telomerase mutations in patients with acute myeloid leukemia. Proc Natl Acad Sci U S A. 2009;106:1187–1192. doi: 10.1073/pnas.0807057106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Calado RT, Brudno J, Mehta P, et al. Constitutional telomerase mutations are genetic risk factors for cirrhosis. Hepatology. 2011;53:1600–1607. doi: 10.1002/hep.24173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Walne AJ, Vulliamy T, Marrone A, et al. Genetic heterogeneity in autosomal recessive dyskeratosis congenita with one subtype due to mutations in the telomerase-associated protein NOP10. Hum Mol Genet. 2007;16:1619–1629. doi: 10.1093/hmg/ddm111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vulliamy T, Beswick R, Kirwan M, et al. Mutations in the telomerase component NHP2 cause the premature ageing syndrome dyskeratosis congenita. Proc Natl Acad Sci U S A. 2008;105:8073–8078. doi: 10.1073/pnas.0800042105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trahan C, Martel C, Dragon F. Effects of dyskeratosis congenita mutations in dyskerin, NHP2 and NOP10 on assembly of H/ACA pre-RNPs. Hum Mol Genet. 2010;19:825–836. doi: 10.1093/hmg/ddp551. [DOI] [PubMed] [Google Scholar]
- 79.Grozdanov PN, Roy S, Kittur N, et al. SHQ1 is required prior to NAF1 for assembly of H/ACA small nucleolar and telomerase RNPs. RNA. 2009;15:1188–1197. doi: 10.1261/rna.1532109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leulliot N, Godin KS, Hoareau-Aveilla C, et al. The box H/ACA RNP assembly factor Naf1p contains a domain homologous to Gar1p mediating its interaction with Cbf5p. J Mol Biol. 2007;371:1338–1353. doi: 10.1016/j.jmb.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 81.Fatica A, Dlakic M, Tollervey D. Naf1 p is a box H/ACA snoRNP assembly factor. RNA. 2002;8:1502–1514. [PMC free article] [PubMed] [Google Scholar]
- 82.Savage SA, Giri N, Baerlocher GM, et al. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. Am J Hum Genet. 2008;82:501–509. doi: 10.1016/j.ajhg.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Riyaz A, Riyaz N, Jayakrishnan MP, et al. Revesz syndrome. Indian J Pediatr. 2007;74:862–863. doi: 10.1007/s12098-007-0155-2. [DOI] [PubMed] [Google Scholar]
- 84.Walne AJ, Vulliamy T, Beswick R, et al. TINF2 mutations result in very short telomeres: analysis of a large cohort of patients with dyskeratosis congenita and related bone marrow failure syndromes. Blood. 2008;112:3594–3600. doi: 10.1182/blood-2008-05-153445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Du HY, Mason PJ, Bessler M, et al. TINF2 mutations in children with severe aplastic anemia. Pediatr Blood Cancer. 2009;52:687. doi: 10.1002/pbc.21903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sasa G, Ribes-Zamora A, Nelson N, et al. Three novel truncating TINF2 mutations causing severe dyskeratosis congenita in early childhood. Clin Genet. 2011 doi: 10.1111/j.1399-0004.2011.01658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Palm W, de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 88.Abreu E, Aritonovska E, Reichenbach P, et al. TIN2-tethered TPP1 recruits human telomerase to telomeres in vivo. Mol Cell Biol. 2010;30:2971–2982. doi: 10.1128/MCB.00240-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang D, He Q, Kim H, et al. TIN2 protein dyskeratosis congenita missense mutants are defective in association with telomerase. J Biol Chem. 2011;286:23022–23030. doi: 10.1074/jbc.M111.225870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Canudas S, Houghtaling BR, Bhanot M, et al. A role for heterochromatin protein 1{gamma} at human telomeres. Genes Dev. 2011;25:1807–1819. doi: 10.1101/gad.17325211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu L, Bailey SM, Okuka M, et al. Telomere lengthening early in development. Nat Cell Biol. 2007;9:1436–1441. doi: 10.1038/ncb1664. [DOI] [PubMed] [Google Scholar]
- 92.Zalzman M, Falco G, Sharova LV, et al. Zscan4 regulates telomere elongation and genomic stability in ES cells. Nature. 2010;464:858–863. doi: 10.1038/nature08882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhong F, Savage SA, Shkreli M, et al. Disruption of telomerase trafficking by TCAB1 mutation causes dyskeratosis congenita. Genes Dev. 2011;25:11–16. doi: 10.1101/gad.2006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tycowski KT, Shu MD, Kukoyi A, et al. A conserved WD40 protein binds the Cajal body localization signal of scaRNP particles. Mol Cell. 2009;34:47–57. doi: 10.1016/j.molcel.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mahmoudi S, Henriksson S, Corcoran M, et al. Wrap53, a natural p53 antisense transcript required for p53 induction upon DNA damage. Mol Cell. 2009;33:462–471. doi: 10.1016/j.molcel.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 96.Jady BE, Richard P, Bertrand E, et al. Cell cycle-dependent recruitment of telomerase RNA and Cajal bodies to human telomeres. Mol Biol Cell. 2006;17:944–954. doi: 10.1091/mbc.E05-09-0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Walne AJ, Vulliamy T, Beswick R, et al. Mutations in C16orf57 and normal-length telomeres unify a subset of patients with dyskeratosis congenita, poikiloderma with neutropenia and Rothmund-Thomson syndrome. Hum Mol Genet. 2010;19:4453–4461. doi: 10.1093/hmg/ddq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Volpi L, Roversi G, Colombo EA, et al. Targeted next-generation sequencing appoints c16orf57 as clericuzio-type poikiloderma with neutropenia gene. Am J Hum Genet. 2010;86:72–76. doi: 10.1016/j.ajhg.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lamm N, Ordan E, Shponkin R, et al. Diminished telomeric 3' overhangs are associated with telomere dysfunction in Hoyeraal-Hreidarsson syndrome. PLoS One. 2009;4:e5666. doi: 10.1371/journal.pone.0005666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alter BP, Giri N, Savage SA, et al. Malignancies and survival patterns in the National Cancer Institute inherited bone marrow failure syndromes cohort study. Br J Haematol. 2010;150:179–788. doi: 10.1111/j.1365-2141.2010.08212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alter BP, Giri N, Savage SA, et al. Cancer in dyskeratosis congenita. Blood. 2009;113:6549–6557. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vulliamy TJ, Kirwan MJ, Beswick R, et al. Differences in disease severity but similar telomere lengths in genetic subgroups of patients with telomerase and shelterin mutations. PLoS One. 2011;6:e24383. doi: 10.1371/journal.pone.0024383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Artandi SE, DePinho RA. Telomeres and telomerase in cancer. Carcinogenesis. 2010;31:9–18. doi: 10.1093/carcin/bgp268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cesare AJ, Reddel RR. Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet. 2010;11:319–330. doi: 10.1038/nrg2763. [DOI] [PubMed] [Google Scholar]
- 105.Dietz AC, Orchard PJ, Baker KS, et al. Disease-specific hematopoietic cell transplantation: nonmyeloablative conditioning regimen for dyskeratosis congenita. Bone Marrow Transplant. 2011;46:98–104. doi: 10.1038/bmt.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alter BP, Baerlocher GM, Savage SA, et al. Very short telomere length by flow fluorescence in situ hybridization identifies patients with dyskeratosis congenita. Blood. 2007;110:1439–1447. doi: 10.1182/blood-2007-02-075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Du HY, Pumbo E, Ivanovich J, et al. TERC and TERT gene mutations in patients with bone marrow failure and the significance of telomere length measurements. Blood. 2009;113:309–316. doi: 10.1182/blood-2008-07-166421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nelson ND, Bertuch AA. Dyskeratosis congenita as a disorder of telomere maintenance. Mutat Res. 2011 doi: 10.1016/j.mrfmmm.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Savage SA, Dokal I, Armanios M, et al. Dyskeratosis congenita: the first NIH clinical research workshop. Pediatr Blood Cancer. 2009;53:520–523. doi: 10.1002/pbc.22061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dokal I, Vulliamy T, Mason P, et al. Clinical utility gene card for: Dyskeratosis congenita. Eur J Hum Genet. 2011 doi: 10.1038/ejhg.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Egan ED, Collins K. Specificity and stoichiometry of subunit interactions in the human telomerase holoenzyme assembled in vivo. Mol Cell Biol. 2010;30:2775–2786. doi: 10.1128/MCB.00151-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang Q, Kim NK, Feigon J. Architecture of human telomerase RNA. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1100279108. [DOI] [PMC free article] [PubMed] [Google Scholar]