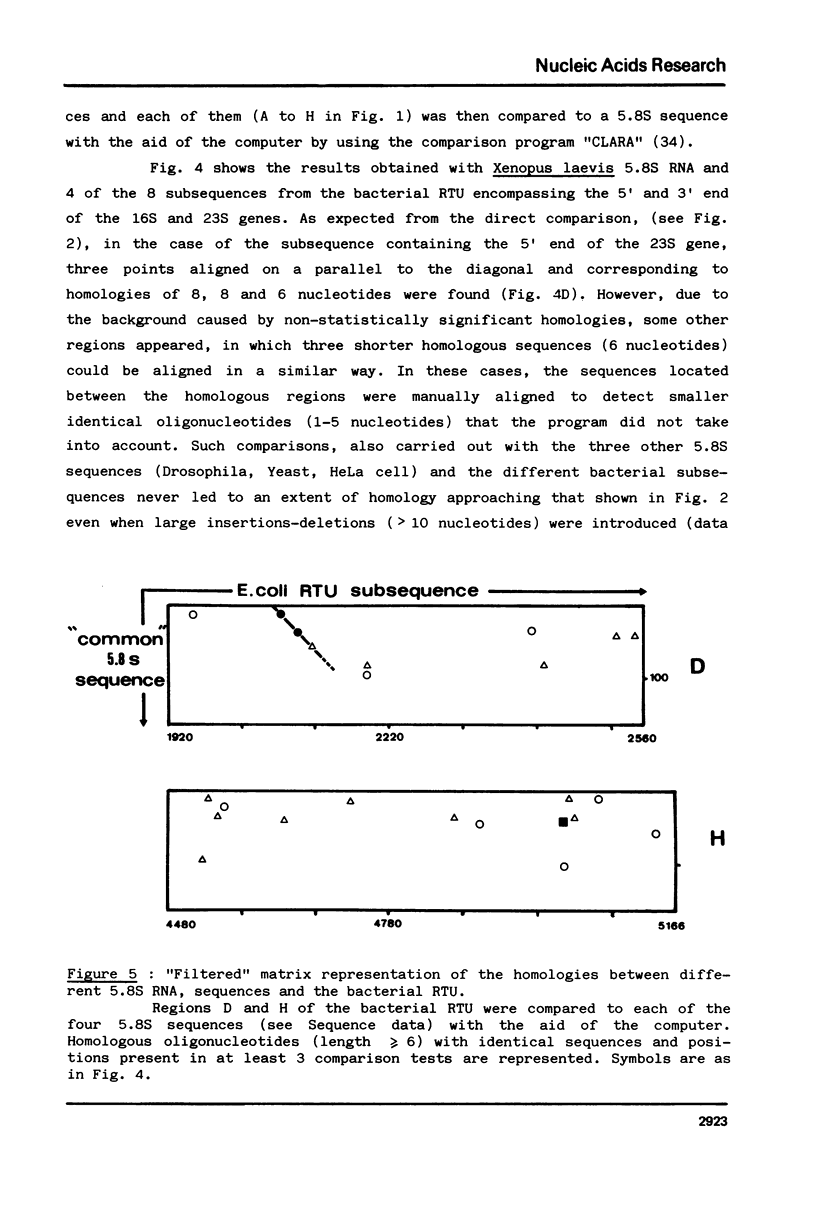
Abstract
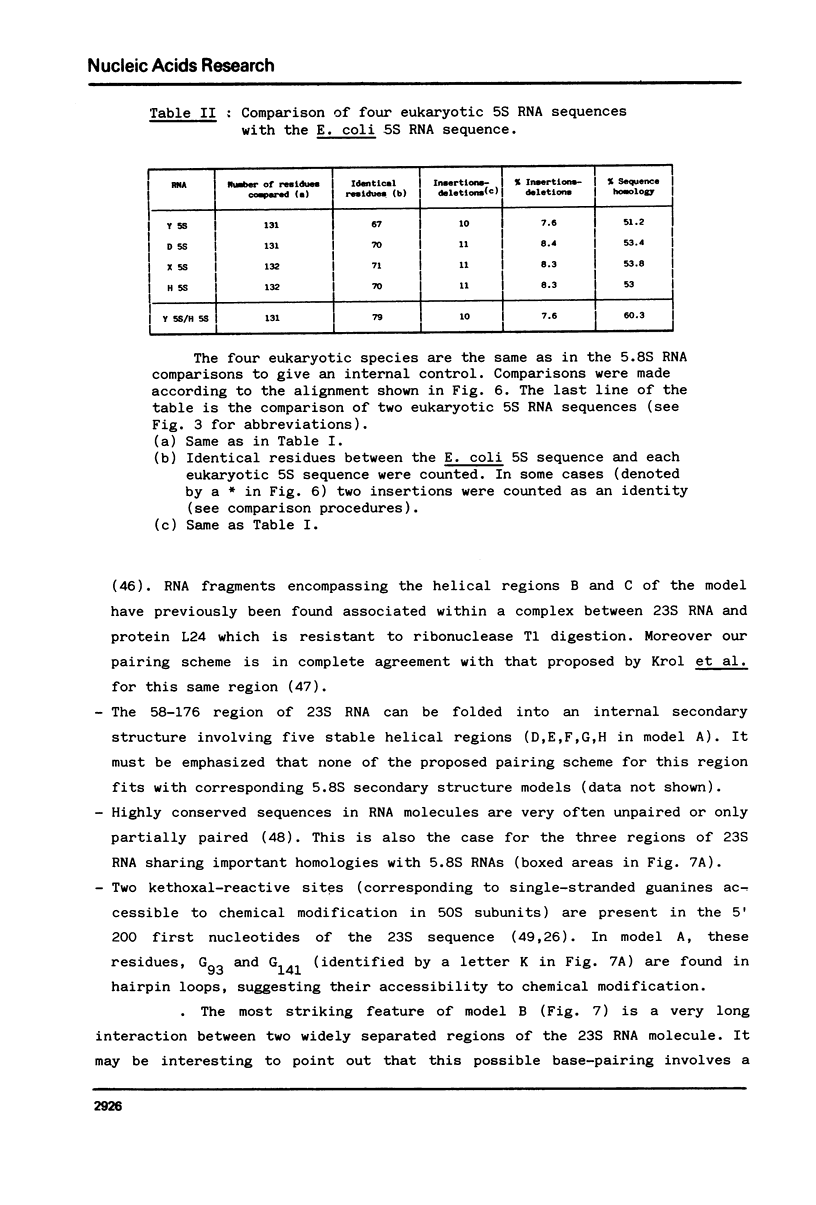
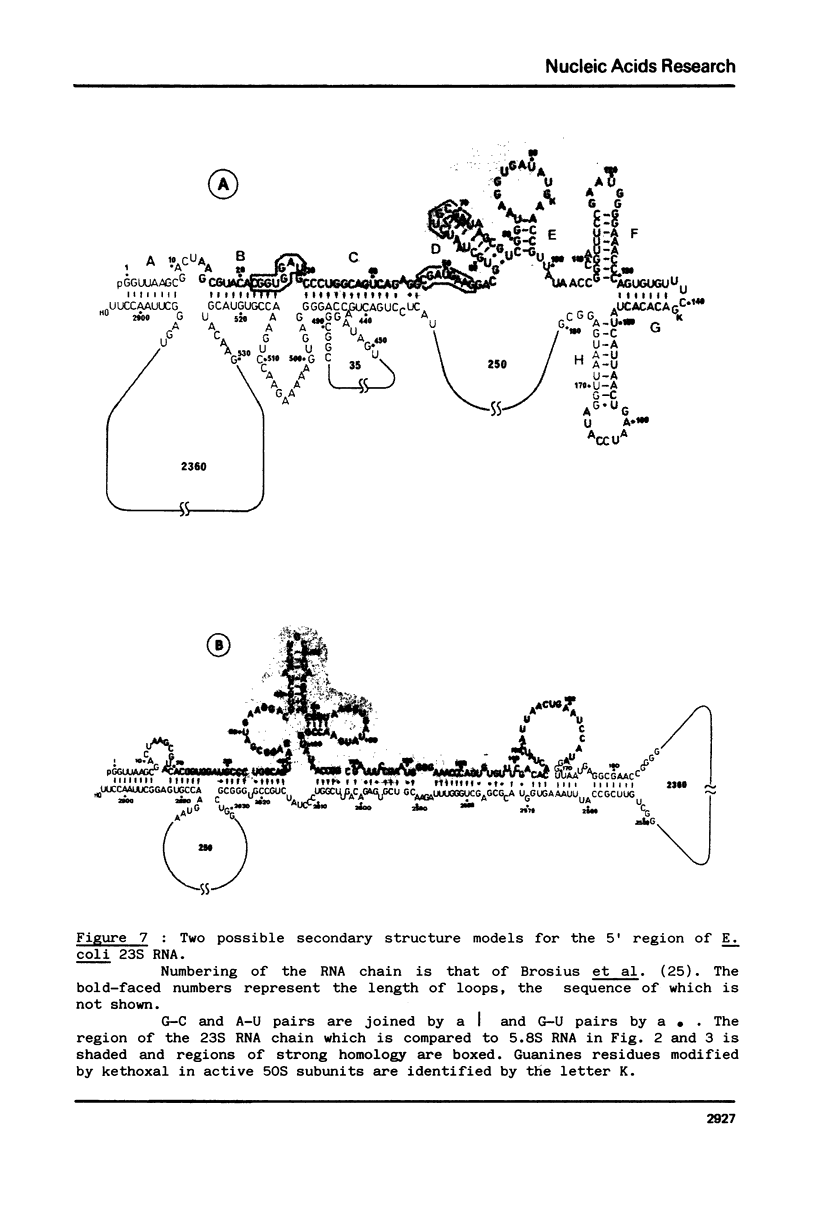
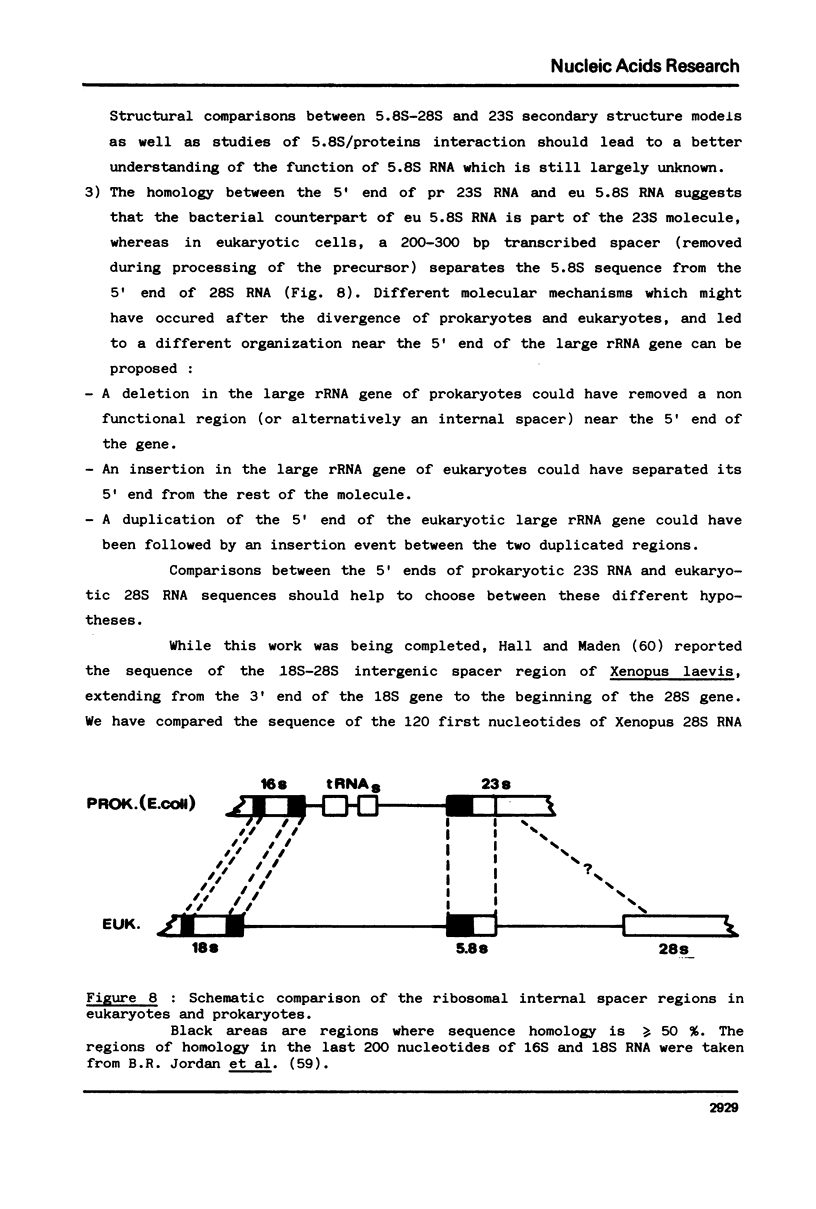
The question of the evolutionary origin of eukaryotic 5.8S rRNA was re-examined after the recent publication of the E. coli 23S rRNA sequence (26,40). A region of the 23S RNA located at its 5' end was found to be approximately 50% homologous to four different eukaryotic 5.8S rRNAs. A computer comparison analysis indicates that no other region of the E. coli ribosomal transcription unit (greater than 5 000 nucleotides in length) shares a comparable homology with 5.8S rRNA. Homology between the 5' end of e. coli 23S and four different eukaryotic 5.8S rRNAs falls within the same range as that between E. coli 5S RNA from the same four eukaryotic species. All these data strongly suggest that the 5' end of prokaryotic 23S rRNA and eukaryotic 5.8S RNA have a common evolutionary origin. Secondary structure models are proposed for the 5' region of E. coli 23S RNA.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B., Rochaix J. D. Structure analysis at the ends of the intervening DNA sequences in the chloroplast 23S ribosomal genes of C. reinhardii. Cell. 1979 Sep;18(1):55–60. doi: 10.1016/0092-8674(79)90353-2. [DOI] [PubMed] [Google Scholar]
- Azad A. A., Lane B. G. A possible role for 5 S rRNA as a bridge between ribosomal subunits. Can J Biochem. 1973 Dec;51(12):1669–1672. doi: 10.1139/o73-224. [DOI] [PubMed] [Google Scholar]
- Bellemare G., Vigne R., Jordan B. R. Interaction between Escherichia coli ribosomal proteins and 5S RNA molecules: recognition of prokaryotic 5S RNAs and rejection of eukaryotic 5S RNAs. Biochimie. 1973;55(1):29–35. doi: 10.1016/s0300-9084(73)80233-0. [DOI] [PubMed] [Google Scholar]
- Benhamou J., Jordan B. R. Nucleotide sequence of Drosophila melanogaster 5S RNA: evidence for a general 5S RNA model. FEBS Lett. 1976 Feb 15;62(2):146–149. doi: 10.1016/0014-5793(76)80039-7. [DOI] [PubMed] [Google Scholar]
- Boseley P. G., Tuyns A., Birnstiel M. L. Mapping of the Xenopus laevis 5.8S rDNA by restriction and DNA sequencing. Nucleic Acids Res. 1978 Apr;5(4):1121–1137. doi: 10.1093/nar/5.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bram R. J., Young R. A., Steitz J. A. The ribonuclease III site flanking 23S sequences in the 30S ribosomal precursor RNA of E. coli. Cell. 1980 Feb;19(2):393–401. doi: 10.1016/0092-8674(80)90513-9. [DOI] [PubMed] [Google Scholar]
- Branlant C., Krol A., Machatt M. A., Ebel J. P. Structural study of ribosomal 23 S RNA from Escherichia coli. FEBS Lett. 1979 Nov 1;107(1):177–181. doi: 10.1016/0014-5793(79)80490-1. [DOI] [PubMed] [Google Scholar]
- Branlant C., Widada J. S., Krol A., Ebel J. P. Extensions of the known sequences at the 3' and 5' ends of 23S ribosomal RNA from Escherichia coli, possible base pairing between these 23S RNA regions and 16S ribosomal RNA. Nucleic Acids Res. 1976 Jul;3(7):1671–1687. doi: 10.1093/nar/3.7.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Noller H. F. Complete nucleotide sequence of a 23S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):201–204. doi: 10.1073/pnas.77.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee G. G., Cartwright E. M. The nucleotide sequence of the 5S RNA of chicken embryo fibroblasts. Nucleic Acids Res. 1975 Dec;2(12):2279–2288. doi: 10.1093/nar/2.12.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee G. G., Cartwright E., McShane T., Williamson R. The nucleotide sequence of somatic 5 S RNA from Xenopus laevis. FEBS Lett. 1972 Sep 1;25(1):8–12. doi: 10.1016/0014-5793(72)80442-3. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F., Barrell B. G. Nucleotide sequence of 5S-ribosomal RNA from Escherichia coli. Nature. 1967 Aug 12;215(5102):735–736. doi: 10.1038/215735a0. [DOI] [PubMed] [Google Scholar]
- Cedergren R. J., Sankoff D. Evolutionary origin of 5.8S ribosomal RNA. Nature. 1976 Mar 4;260(5546):74–75. doi: 10.1038/260074a0. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle W. F., Pace N. R. Transcriptional organization of the ribosomal RNA cistrons in Escherichia coli. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1786–1790. doi: 10.1073/pnas.68.8.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dujon B. Sequence of the intron and flanking exons of the mitochondrial 21S rRNA gene of yeast strains having different alleles at the omega and rib-1 loci. Cell. 1980 May;20(1):185–197. doi: 10.1016/0092-8674(80)90246-9. [DOI] [PubMed] [Google Scholar]
- Ehresmann C., Stiegler P., Carbon P., Ebel J. P. Recent progress in the determination of the primary sequence of the 16 S RNA of Escherichia coli. FEBS Lett. 1977 Dec 15;84(2):337–341. doi: 10.1016/0014-5793(77)80720-5. [DOI] [PubMed] [Google Scholar]
- Eperon I. C., Anderson S., Nierlich D. P. Distinctive sequence of human mitochondrial ribosomal RNA genes. Nature. 1980 Jul 31;286(5772):460–467. doi: 10.1038/286460a0. [DOI] [PubMed] [Google Scholar]
- Erdmann V. A. Collection of published 5S and 5.8S rRNA sequences and their precursors. Nucleic Acids Res. 1980 Jan 11;8(1):r31–r47. doi: 10.1093/nar/8.1.197-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann V. A. Structure and function of 5S and 5.8 S RNA. Prog Nucleic Acid Res Mol Biol. 1976;18:45–90. [PubMed] [Google Scholar]
- Ford P. J., Mathieson T. The nucleotide sequences of 5.8-S ribosomal RNA from Xenopus laevis and Xenopus borealis. Eur J Biochem. 1978 Jun 1;87(1):199–214. doi: 10.1111/j.1432-1033.1978.tb12367.x. [DOI] [PubMed] [Google Scholar]
- Ginsburg D., Steitz J. A. The 30 S ribosomal precursor RNA from Escherichia coli. A primary transcript containing 23 S, 16 S, and 5 S sequences. J Biol Chem. 1975 Jul 25;250(14):5647–5654. [PubMed] [Google Scholar]
- Hagenbüchle O., Santer M., Steitz J. A., Mans R. J. Conservation of the primary structure at the 3' end of 18S rRNA from eucaryotic cells. Cell. 1978 Mar;13(3):551–563. doi: 10.1016/0092-8674(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Hall L. M., Maden B. E. Nucleotide sequence through the 18S-28S intergene region of a vertebrate ribosomal transcription unit. Nucleic Acids Res. 1980 Dec 20;8(24):5993–6005. doi: 10.1093/nar/8.24.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatlen L. E., Amaldi F., Attardi G. Oligonucleotide pattern after pancreatic ribonuclease digestion and the 3' and 5' termini of 5S ribonucleic acid from HeLa cells. Biochemistry. 1969 Dec;8(12):4989–5005. doi: 10.1021/bi00840a048. [DOI] [PubMed] [Google Scholar]
- Herr W., Noller H. F. A fragment of 23S RNA containing a nucleotide sequence complementary to a region of 5S RNA. FEBS Lett. 1975 May 1;53(2):248–252. doi: 10.1016/0014-5793(75)80030-5. [DOI] [PubMed] [Google Scholar]
- Hori H. Evolution of 5sRNA. J Mol Evol. 1975 Dec 31;7(1):75–86. doi: 10.1007/BF01732181. [DOI] [PubMed] [Google Scholar]
- Jarry B., Rosset R. Heterogeneity of 5S RNA in Escherichia coli. Mol Gen Genet. 1971;113(1):43–50. doi: 10.1007/BF00335006. [DOI] [PubMed] [Google Scholar]
- Jordan B. R., Jourdan R., Jacq B. Late steps in the maturation of Drosophila 26 S ribosomal RNA: generation of 5-8 S and 2 S RNAs by cleavages occurring in the cytoplasm. J Mol Biol. 1976 Feb 15;101(1):85–105. doi: 10.1016/0022-2836(76)90067-x. [DOI] [PubMed] [Google Scholar]
- Jordan B. R., Latil-Damotte M., Jourdan R. Coding and spacer sequences in the 5.8S-2S region of Sciara coprophila ribosomal DNA. Nucleic Acids Res. 1980 Aug 25;8(16):3565–3573. doi: 10.1093/nar/8.16.3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan B. R., Latil-Damotte M., Jourdan R. Sequence of the 3'-terminal portion of Drosophila melanogaster 18 S rRNA and of the adjoining spacer: comparison with corresponding prokaryotic and eukaryotic sequences. FEBS Lett. 1980 Aug 11;117(1):227–231. doi: 10.1016/0014-5793(80)80951-3. [DOI] [PubMed] [Google Scholar]
- Khan M. S., Maden B. E. Nucleotide sequence relationships between vertebrate 5.8 S ribosomal RNAs. Nucleic Acids Res. 1977 Jul;4(7):2495–2505. doi: 10.1093/nar/4.7.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss A., Sain B., Venetianer P. The number of rRNA genes in Escherichia coli. FEBS Lett. 1977 Jul 1;79(1):77–79. doi: 10.1016/0014-5793(77)80354-2. [DOI] [PubMed] [Google Scholar]
- Krol A., Machatt M. A., Branlant C., Ebel J. P. RNA-RNA interactions in the binding site of protein L24 on 23S ribosomal RNA of E. coli. II. Sequence analysis of the interacting fragments. Nucleic Acids Res. 1978 Dec;5(12):4933–4947. doi: 10.1093/nar/5.12.4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoma G. A., Marshall A. G. Laser Raman evidence for new cloverleaf secondary structures for eukaryotic 5.8S RNA and prokaryotic 5S RNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4901–4905. doi: 10.1073/pnas.75.10.4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden B. E., Robertson J. S. Demonstration of the "5-8 S" ribosomal sequence in HeLa cell ribosomal precursor RNA. J Mol Biol. 1974 Aug 5;87(2):227–235. doi: 10.1016/0022-2836(74)90145-4. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki M. Studies on the nucleotide sequence of pseudouridine-containing 5S RNA from Saccharomyces cerevisiae. J Biochem. 1974 Jun;75(6):1407–1410. doi: 10.1093/oxfordjournals.jbchem.a130532. [DOI] [PubMed] [Google Scholar]
- Nazar R. N. A 5.8 S rRNA-like sequence in prokaryotic 23 S rRNA. FEBS Lett. 1980 Oct 6;119(2):212–214. doi: 10.1016/0014-5793(80)80254-7. [DOI] [PubMed] [Google Scholar]
- Nazar R. N., Roy K. L. Nucleotide sequence of rainbow trout (Salmo gairdneri) ribosomal 5.8 S ribonucleic acid. J Biol Chem. 1978 Jan 25;253(2):395–399. [PubMed] [Google Scholar]
- Nazar R. N., Sitz T. O., Busch H. Structural analyses of mammalian ribosomal ribonucleic acid and its precursors. Nucleotide sequence of ribosomal 5.8 S ribonucleic acid. J Biol Chem. 1975 Nov 25;250(22):8591–8597. [PubMed] [Google Scholar]
- Nazar R. N., Sitz T. O. Role of the 5'-terminal sequence in the RNA binding site of yeast 5.8 S rRNA. FEBS Lett. 1980 Jun 16;115(1):71–76. doi: 10.1016/0014-5793(80)80729-0. [DOI] [PubMed] [Google Scholar]
- Pace N. R., Walker T. A., Schroeder E. Structure of the 5.8S RNA component of the 5.8S-28S ribosomal RNA junction complex. Biochemistry. 1977 Nov 29;16(24):5321–5328. doi: 10.1021/bi00643a025. [DOI] [PubMed] [Google Scholar]
- Pavlakis G. N., Jordan B. R., Wurst R. M., Vournakis J. N. Sequence and secondary structure of Drosophila melanogaster 5.8S and 2S rRNAs and of the processing site between them. Nucleic Acids Res. 1979 Dec 20;7(8):2213–2238. doi: 10.1093/nar/7.8.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pene J. J., Knight E., Jr, Darnell J. E., Jr Characterization of a new low molecular weight RNA in HeLa cell ribosomes. J Mol Biol. 1968 May 14;33(3):609–623. doi: 10.1016/0022-2836(68)90309-4. [DOI] [PubMed] [Google Scholar]
- Price R., Penman S. A distinct RNA polymerase activity, synthesizing 5-5 s, 5 s and 4 s RNA in nuclei from adenovirus 2-infected HeLa cells. J Mol Biol. 1972 Oct 14;70(3):435–450. doi: 10.1016/0022-2836(72)90551-7. [DOI] [PubMed] [Google Scholar]
- Renkawitz R., Gerbi S. A., Glätzer K. H. Ribosomal DNA of fly Sciara coprophila has a very small and homogeneous repeat unit. Mol Gen Genet. 1979 May 23;173(1):1–13. doi: 10.1007/BF00267685. [DOI] [PubMed] [Google Scholar]
- Rubin G. M. The nucleotide sequence of Saccharomyces cerevisiae 5.8 S ribosomal ribonucleic acid. J Biol Chem. 1973 Jun 10;248(11):3860–3875. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankoff D., Cedergren R. J., Lapalme G. Frequency of insertion-deletion, transversion, and transition in the evolution of 5S ribosomal RNA. J Mol Evol. 1976 Mar 29;7(2):133–149. doi: 10.1007/BF01732471. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Identical 3'-terminal octanucleotide sequence in 18S ribosomal ribonucleic acid from different eukaryotes. A proposed role for this sequence in the recognition of terminator codons. Biochem J. 1974 Sep;141(3):609–615. doi: 10.1042/bj1410609a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speirs J., Birnstiel M. Arrangement of the 5-8 S RNA cistrons in the genome of Xenopus laevis. J Mol Biol. 1974 Aug 5;87(2):237–256. doi: 10.1016/0022-2836(74)90146-6. [DOI] [PubMed] [Google Scholar]
- Van Etten R. A., Walberg M. W., Clayton D. A. Precise localization and nucleotide sequence of the two mouse mitochondrial rRNA genes and three immediately adjacent novel tRNA genes. Cell. 1980 Nov;22(1 Pt 1):157–170. doi: 10.1016/0092-8674(80)90164-6. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Magrum L. J., Gupta R., Siegel R. B., Stahl D. A., Kop J., Crawford N., Brosius J., Gutell R., Hogan J. J. Secondary structure model for bacterial 16S ribosomal RNA: phylogenetic, enzymatic and chemical evidence. Nucleic Acids Res. 1980 May 24;8(10):2275–2293. doi: 10.1093/nar/8.10.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrede P., Erdmann V. A. Activities of B. stearothermophilus 50 S ribosomes reconstituted with prokaryotic and eukaryotic 5 S RNA. FEBS Lett. 1973 Jul 15;33(3):315–319. doi: 10.1016/0014-5793(73)80219-4. [DOI] [PubMed] [Google Scholar]
- Wrede P., Erdmann V. A. Escherichia coli 5S RNA binding proteins L18 and L25 interact with 5.8S RNA but not with 5S RNA from yeast ribosomes. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2706–2709. doi: 10.1073/pnas.74.7.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Macklis R., Steitz J. A. Sequence of the 16 S-23 s spacer region in two ribosomal RNA operons of Escherichia coli. J Biol Chem. 1979 May 10;254(9):3264–3271. [PubMed] [Google Scholar]
- Young R. A., Steitz J. A. Tandem promoters direct E. coli ribosomal RNA synthesis. Cell. 1979 May;17(1):225–234. doi: 10.1016/0092-8674(79)90310-6. [DOI] [PubMed] [Google Scholar]
- de Boer H. A., Gilbert S. F., Nomura M. DNA sequences of promoter regions for rRNA operons rrnE and rrnA in E. coli. Cell. 1979 May;17(1):201–209. doi: 10.1016/0092-8674(79)90308-8. [DOI] [PubMed] [Google Scholar]