Abstract
In Drosophila, normal and transformed cells compete with each other for survival in a process called cell competition. However, it is not known whether comparable phenomena also occur in mammals. Scribble is a tumor suppressor protein in Drosophila and mammals. In this study we examine the interface between normal and Scribble-knockdown epithelial cells using Madin–Darby Canine Kidney (MDCK) cells expressing Scribble short hairpin RNA (shRNA) in a tetracycline-inducible manner. We observe that Scribble-knockdown cells undergo apoptosis and are apically extruded from the epithelium when surrounded by normal cells. Apoptosis does not occur when Scribble-knockdown cells are cultured alone, suggesting that the presence of surrounding normal cells induces the cell death. We also show that death of Scribble-knockdown cells occurs independently of apical extrusion. Finally, we demonstrate that apoptosis of Scribble-knockdown cells depends on activation of p38 mitogen-activated protein kinase (MAPK). This is the first demonstration that an oncogenic transformation within an epithelium induces cell competition in a mammalian cell culture system.
Key words: MDCK cells, Scribble, Cell competition, p38 MAPK
Introduction
Since the first oncogene Src was identified, a variety of oncogenes and tumor suppressor genes have been found, and cellular functions and downstream signaling pathways of the encoded proteins have been revealed (Hanahan and Weinberg, 2000; Hanahan and Weinberg, 2011). In most of these studies, however, the fact that transformation occurs in a single normal cell and that the transformed cell grows while being surrounded by neighboring normal cells has been largely overlooked. Thus, it is still not clearly understood what happens at the interface between normal and transformed cells at the initial stage of carcinogenesis. In Drosophila melanogaster, various phenomena have been reported to occur at the interface between normal and transformed epithelial cells. In particular, normal and transformed cells often compete with each other for survival. For example, when Drosophila Myc-overexpressing cells contact wild-type cells, wild-type cells undergo apoptosis and Myc-overexpressing cells proliferate and fill the vacant spaces (de la Cova et al., 2004; Moreno and Basler, 2004). By contrast, when Lethal giant larvae (Lgl)-knockout cells are surrounded by wild-type cells, Lgl-knockout cells die by apoptosis (Grzeschik et al., 2007; Tamori et al., 2010). These phenomena are called ‘cell competition’, which has been intensively studied in Drosophila (Baker and Li, 2008; Diaz and Moreno, 2005; Johnston, 2009). However, it remains unknown whether comparable phenomena also occur in vertebrates (Fujita, 2011; Hogan et al., 2011).
Scribble is a neoplastic tumor suppressor gene that was identified in Drosophila. In epithelia of Scribble homozygous mutant larvae, apicobasal cell polarity and proliferative control are lost, leading to multilayered amorphous tumor formation (Bilder and Perrimon, 2000). Scribble is a LAP (leucine-rich repeats and PDZ) protein that contains 16 leucine-rich repeat (LRR) and four PDZ [PSD95, Discs large and Zonula adherens-1 (ZO-1)] domains (Bilder and Perrimon, 2000) and is localized at the basolateral membrane in Drosophila and mammalian epithelial cells. Scribble has also been shown to function as a tumor suppressor protein in mice (Zhan et al., 2008), and decreased Scribble expression is observed in human colon and breast cancers (Gardiol et al., 2006; Navarro et al., 2005). In addition, Scribble has been reported to be involved in cell competition in Drosophila (Brumby and Richardson, 2003). When clones of homozygous scrib mutant cells are surrounded by wild-type cells in eye imaginal discs, scrib mutant cells are eliminated from the epithelium by Jun N-terminal kinase (JNK) pathway-mediated apoptosis. By contrast, when all epithelial cells are scrib mutant cells, they do not die, but overproliferate and form tumors. These data suggest that the presence of surrounding wild-type cells induces apoptosis of scrib mutant cells. The underlying molecular mechanism is not fully understood, although the involvement of endocytic activation of Eiger/TNF and induction of phagocytosis has been suggested (Igaki et al., 2009; Ohsawa et al., 2011). In this study, we show that loss of Scribble causes cell competition in mammalian cells and investigate the molecular mechanism whereby death of Scribble-knockdown cells is induced.
Results
Effect of Scribble knockdown on cell polarity and morphology in MDCK cells
To examine the interaction between normal and Scribble-knockdown epithelial cells, we established MDCK epithelial cells stably expressing Scribble shRNA in a tetracycline-inducible manner (MDCK-pTR Scribble shRNA cells). At 48 hours after tetracycline addition, the expression level of Scribble was knocked down by 90% (Fig. 1A). Expression of other intercellular junction proteins, including E-cadherin and β-catenin, was not affected (Fig. 1B). Genetic studies in Drosophila have revealed that three tumor suppressor proteins, Scribble, Discs large (Dlg), and Lethal giant larvae (Lgl), cooperatively regulate cell polarity (Bilder et al., 2000). However, expression of neither Lgl nor Dlg was affected by knockdown of Scribble (supplementary material Fig. S1). As previously reported (Qin et al., 2005), Scribble-knockdown MDCK cells lost epithelial morphology with a flattened appearance when cultured at low density (Fig. 1C). However, when cultured at high density, they maintained apicobasal polarity, at least to a certain extent, as shown by localization of gp135 at the apical domain and of ZO-1 at tight junctions (Fig. 1D; and data not shown). By contrast, the distribution of E-cadherin was significantly disrupted in Scribble-knockdown cells; there was some E-cadherin localized at cell–cell contact sites, but the majority of E-cadherin was localized at the basal membrane (Fig. 1D), which is comparable with observations in a previous report (Qin et al., 2005). In all subsequent experiments, we cultured cells at high density.
Fig. 1.
Characterization of MDCK cells stably expressing Scribble shRNA in a tetracycline-inducible manner. (A,B) Effect of tetracycline addition on the expression of Scribble (A), E-cadherin and β-catenin (B) in MDCK-pTR Scribble shRNA cells. MDCK or MDCK-pTR Scribble shRNA cells were incubated with tetracycline for the indicated times, and cell lysates were examined by western blotting using anti-Scribble, anti-E-cadherin, anti-β-catenin or anti-GAPDH antibody. (C) Phase-contrast images of MDCK-pTR Scribble shRNA cells cultured in the presence of tetracycline for the indicated times. (D) Effect of Scribble knockdown on cell polarity. MDCK-pTR Scribble shRNA cells were incubated without or with tetracycline for 72 hours and were analyzed by immunofluorescence using anti-gp135 and anti-E-cadherin antibodies. Nuclei were stained with Hoechst 33342 dye. Scale bars: 15 μm (C), 10 μm (D).
Scribble-knockdown cells are removed from a monolayer of normal epithelial cells
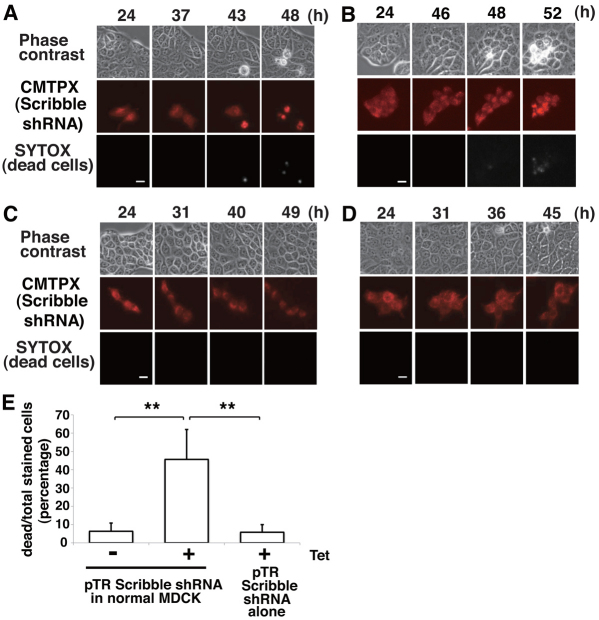
To examine the fate of Scribble-knockdown cells within a monolayer of normal cells, we labeled MDCK-pTR Scribble shRNA cells with a red fluorescent dye (CMTPX) and mixed them with normal MDCK cells at a ratio of 1:10. The mixture of cells was cultured in the absence of tetracycline until a monolayer was formed. We then added tetracycline to induce knockdown of Scribble and followed the fate of Scribble-knockdown cells using time-lapse microscopy. To identify dead cells, we used SYTOX Blue fluorescent dye, which only passes through damaged cell membranes, binds DNA and emits blue fluorescence under violet light. After 60 hours of incubation with tetracycline, approximately 45% of Scribble-knockdown cells died and left the epithelial monolayer (Fig. 2A,B,E; supplementary material Movies 1, 2). Death of Scribble-knockdown cells was observed when either a small (Fig. 2A) or a large cluster (Fig. 2B) of Scribble-knockdown cells was surrounded by normal cells. Expression of exogenous human Scribble in Scribble shRNA cells significantly reduced cell death (supplementary material Fig. S2A,B), indicating that cell death is indeed caused by Scribble knockdown. Scribble shRNA cells did not die in the absence of tetracycline (Fig. 2C,E; supplementary material Movie 3). Importantly, fluorescently labeled Scribble-knockdown cells did not die when surrounded by unlabeled Scribble-knockdown cells (Fig. 2D,E). Collectively, these data suggest that the presence of surrounding normal cells induces death of Scribble-knockdown cells. This is the first demonstration that knockdown of a tumor suppressor protein causes cell competition in mammalian epithelial cells.
Fig. 2.
Knockdown of Scribble expression induces cell competition in MDCK cells. (A–C) MDCK-pTR Scribble shRNA cells were fluorescently labeled with CMTPX dye (red) and mixed with normal MDCK cells at a ratio of 1:10, and cultured in the presence (A and B) or absence (C) of tetracycline for the indicated times. Cells were stained with SYTOX Blue to label dead cells. (D) MDCK-pTR Scribble shRNA cells were fluorescently labeled with CMTPX (red), mixed with MDCK-pTR Scribble shRNA cells at a ratio of 1:10, and cultured in the presence of tetracycline for the indicated times. All images were extracted from a representative time-lapse analysis. Scale bars: 10 μm. (E) Quantification of death of MDCK-pTR Scribble shRNA cells. Fluorescently labeled MDCK-pTR Scribble shRNA cells were mixed with normal MDCK or MDCK-pTR Scribble shRNA cells, and cultured in the absence or presence of tetracycline for 64 hours. Frequency of cell death that occurred in labeled MDCK-pTR Scribble shRNA cells was analyzed under each experimental condition, and the results represent the means ± s.d. **P<0.005; n=90, 118 and 106 from three independent experiments.
Scribble-knockdown cells undergo apoptosis independently of apical extrusion
Next, we examined the cell death of Scribble-knockdown cells by immunofluorescence. We found that apically extruded Scribble-knockdown cells were always positively immunostained with anti-active caspase-3 antibody and had fragmented nuclei, indicating that they had undergone apoptosis (Fig. 3A). By contrast, when cells expressing scramble shRNA were surrounded by normal cells, no significant active caspase-3 staining was observed (supplementary material Fig. S2C,D). 48 hours after tetracycline addition, active caspase-3-positive Scribble-knockdown cells often remained within the epithelial monolayer (supplementary material Fig. S3A), suggesting that caspase activation might occur before apical extrusion. Bcl-2 homologous antagonist/killer (Bak) and Bcl-2 associated X protein (Bax) are proapoptotic members of the Bcl-2 family and promote apoptosis through their effect on mitochondria (Youle and Strasser, 2008). We found that Bak and Bax were activated in apically extruded Scribble-knockdown cells (Fig. 3B,C), suggesting the involvement of the mitochondrial apoptosis pathway in their death. Addition of the poly-caspase inhibitor Z-VAD-FMK blocked activation of caspase-3 (Fig. 3D; supplementary material Fig. S3B,C), but did not substantially affect activation of Bak and Bax (Fig. 3D; supplementary material Fig. S3C; and data not shown) in Scribble-knockdown cells surrounded by normal cells, suggesting that Z-VAD-FMK-sensitive caspases are not required for activation of Bak and Bax. Surprisingly, addition of Z-VAD-FMK did not significantly block death of Scribble-knockdown cells surrounded by normal cells (supplementary material Fig. S3D). It is possible that Z-VAD-FMK-insensitive caspases (Chauvier et al., 2007) are involved in the cell death or that inhibition of caspases induces alternative cell death pathways.
Fig. 3.
Scribble-knockdown cells surrounded by normal cells undergo apoptosis independently of apical extrusion. (A-C) Immunofluorescence analyses of apoptosis markers in MDCK-pTR Scribble shRNA cells apically extruded from the monolayer of normal MDCK cells. MDCK-pTR Scribble shRNA cells were labeled with CMFDA dye (green), mixed with normal MDCK cells, and incubated with tetracycline for 64 hours. Immunofluorescence analyses were performed using anti-active caspase-3 antibody and Alexa-Fluor-647-conjugated phalloidin (A), anti-active Bak antibody (B) or anti-active Bax antibody (C). (D) Effect of the caspase inhibitor Z-VAD-FMK on immunofluorescence for active Bak. Cells were cultured as described above except with addition of 100 μM Z-VAD-FMK for the final 24 hours of incubation. Immunostaining was performed using anti-active caspase-3 and anti-active Bak antibodies. (E) Immunofluorescence analyses for an apoptosis marker in non-extruded MDCK-pTR Scribble shRNA cells. Cells were cultured as described above except with addition of 30 μM blebbistatin for the final 24 hours of incubation. Immunostaining was performed using anti-active caspase-3 antibody. Scale bars: 10 μm. (F) Quantification of fluorescently labeled MDCK-pTR Scribble shRNA cells that were positively immunostained with anti-active caspase-3 antibody. Fluorescently labeled Scribble shRNA cells were analyzed under each experimental condition, and the results represent the means ± s.d. It should be noted that the values shown in this figure might underscore the frequency of apoptotic events because apically extruded apoptotic cells were often lost during the washing steps in immunostaining. *P<0.05; n=302, 354 and 489 from three independent experiments.
To determine whether Scribble-knockdown cells surrounded by normal cells die before or after apical extrusion, we treated cells with the myosin-II inhibitor blebbistatin, which blocks apical extrusion of apoptotic cells from the epithelial monolayer (Rosenblatt et al., 2001). In the presence of blebbistatin, the extrusion of apoptotic Scribble-knockdown cells, as denoted by fragmented nuclei and positive staining for the active form of caspase-3, was inhibited, and cells remained within the monolayer (Fig. 3E). Under these conditions, 24% of Scribble-knockdown cells surrounded by normal cells were positive for active caspase-3 (Fig. 3E,F). By contrast, when Scribble shRNA cells were cultured with normal cells without tetracycline, or Scribble-knockdown cells were cultured alone, positive immunostaining for active caspase-3 was not frequently observed (Fig. 3F). Addition of tetracycline did not affect activation of caspase-3 in normal cells or scramble-knockdown cells (supplementary material Fig. S3E). These data suggest that activation of the apoptotic pathway of Scribble-knockdown cells surrounded by normal cells can occur independently of apical extrusion.
Molecular mechanisms of death of Scribble-knockdown cells
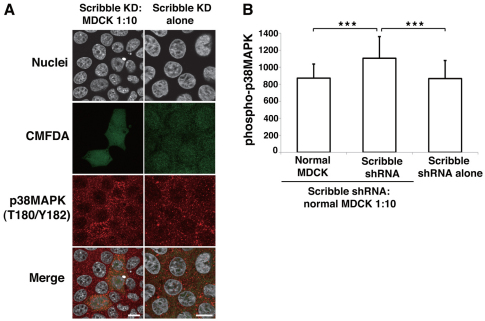
It has been reported in Drosophila that JNK plays an important role in death of outcompeted cells in cell competition (Brumby and Richardson, 2003; Igaki et al., 2009; Moreno and Basler, 2004; Tamori et al., 2010). Therefore, we next examined the role of JNK in the death of Scribble-knockdown MDCK cells. We did not observe phosphorylation of JNK in Scribble-knockdown cells surrounded by normal cells (data not shown). In addition, the JNK-specific inhibitor SP600125 did not significantly inhibit death of Scribble-knockdown cells surrounded by normal cells (supplementary material Fig. S4A). p38 MAPK (MAPK14) is another MAPK that is activated by various types of cellular stresses and is involved in apoptosis (Zarubin and Han, 2005). Immunofluorescence analyses with an antibody against phosphorylated p38 MAPK demonstrated that protein activity was significantly enhanced in Scribble-knockdown cells surrounded by normal cells, but not in normal cells or Scribble-knockdown cells cultured alone (Fig. 4A,B; supplementary material Fig. S5). Addition of the p38 MAPK inhibitor SB202190 significantly reduced activation of caspase-3 and death of Scribble-knockdown cells surrounded by normal cells (Fig. 5A; supplementary material Fig. S4B). Furthermore, expression of a dominant-negative form of p38 MAPK strongly suppressed death of Scribble-knockdown cells surrounded by normal cells (Fig. 5B,C). Collectively, these data indicate that p38 MAPK, not JNK, plays a crucial role in cell competition between normal and Scribble-knockdown MDCK cells. Finally, inhibition of p38 MAPK significantly suppressed activation of Bak in Scribble-knockdown cells surrounded by normal cells (Fig. 5D), suggesting that the mitochondrial apoptosis pathway is one of the downstream targets of p38 MAPK.
Fig. 4.
p38 MAPK is activated in Scribble-knockdown cells surrounded by normal cells. (A) Immunofluorescence analyses of active p38 MAPK in MDCK-pTR Scribble shRNA cells within a monolayer of normal MDCK cells. CMFDA-labeled MDCK-pTR Scribble shRNA cells (green) were mixed with normal MDCK cells (left panels) or MDCK-pTR Scribble shRNA cells (right panels) and incubated with tetracycline for 64 hours. Immunostaining was performed using antibody against phosphorylated p38 MAPK. Scale bars: 10 μm. Note that the MDCK-pTR Scribble shRNA cell line expresses a low level of GFP, which masked the presence of CMFDA-stained cells. (B) Quantification of immunofluorescence intensity of phosphorylated p38 MAPK staining. Data represent the means ± s.d. ***P<0.0001; n=42, 41 and 41 from two independent experiments.
Fig. 5.
p38MAPK is involved in Scribble-knockdown-mediated cell competition. (A) Effect of the p38 MAPK inhibitor SB202190 on death of MDCK-pTR Scribble shRNA cells surrounded by normal MDCK cells. MDCK-pTR Scribble shRNA cells were fluorescently labeled with CMFDA dye and mixed with normal MDCK cells at a ratio of 1:10, and cultured with tetracycline for 64 hours in the absence or presence of SB202190. SB202190 was added during the final 40 hours. Cells were incubated with SYTOX Blue to label dead cells. Results represent the means ± s.d. *P<0.05; n=172 and 170 from four independent experiments. (B) Establishment of MDCK-pTR Scribble shRNA cells stably expressing dominant-negative GFP–p38-MAPKα (p38DN) in a tetracycline-inducible manner. MDCK cells or MDCK-pTR Scribble shRNA + GFP–p38DN cells were incubated in the presence or absence of tetracycline for 64 hours. Cell lysates were examined by western blotting using anti-Scribble, anti-GFP, anti-p38-MAPKα or anti-GAPDH antibody. Arrows and arrowhead indicate the position of GFP–p38DN and endogenous p38MAPK proteins, respectively. It should be noted that expression of endogenous p38 MAPK is downregulated upon expression of exogenous GFP-p38DN. (C) Expression of dominant-negative p38MAPKα (p38DN) in Scribble shRNA cells rescues the cell competition phenotype. MDCK-pTR Scribble shRNA cells or MDCK-pTR Scribble shRNA + GFP–p38DN cells were fluorescently labeled with CMFDA dye and mixed with normal MDCK cells at a ratio of 1:10, and incubated in the presence of tetracycline for 64 hours. Results are expressed relative to the value for MDCK-pTR Scribble shRNA cells, and represent the mean ± s.d. **P<0.05, n=380 and 312 from three independent experiments. (D) Effect of the p38 MAPK inhibitor SB202190 on active Bak immunostaining in MDCK-pTR Scribble shRNA cells surrounded by normal MDCK cells. MDCK-pTR Scribble shRNA cells were fluorescently labeled with CMFDA dye and mixed with normal MDCK cells at a ratio of 1:10. Cells were incubated in the presence or absence of tetracycline for 64 hours with the addition of 30 μM blebbistatin for the final 24 hours to inhibit extrusion of apoptotic cells. SB202190 was added during the final 24 hours. Results represent the means ± s.d. *P<0.05; n=292, 265 and 267 from three independent experiments.
Discussion
In this study, we have shown that Scribble-knockdown MDCK cells undergo apoptosis when surrounded by normal MDCK cells. It has been reported that apical extrusion of apoptotic epithelial cells occurs in a myosin-II-dependent manner at an early stage of apoptosis (Rosenblatt et al., 2001). Indeed, we have observed that caspase-3-positive Scribble-knockdown cells are apically extruded from the monolayer of normal cells. Inhibition of myosin-II with blebbistatin inhibits apical extrusion, but not cell death of Scribble-knockdown cells. These results indicate that apoptosis of Scribble-knockdown cells occurs prior to extrusion and is not caused by anoikis, which is compatible with observations for cell competition in Drosophila (Moreno and Basler, 2004; Tamori et al., 2010). In Drosophila, apoptotic cells are basally extruded and easily detected by immunofluorescence. By contrast, extruded scribble-knockdown MDCK cells loosely attach to the apical surface of the epithelium and are frequently lost during the washing steps of immunofluorescence. Thus time-lapse analyses are indispensible to properly quantify apical extrusion of apoptotic transformed cells in mammalian epithelial cell culture systems.
The molecular mechanism whereby outcompeted cells undergo apoptosis in cell competition is largely unknown in Drosophila or mammals. It was previously believed that fast-proliferating cells outcompete slow-proliferating cells. However, several reports have revealed that differences in proliferation rates are not always involved in cell competition (Tamori et al., 2010). Indeed, there were no significant differences in proliferation rates between normal and Scribble-knockdown cells (supplementary material Fig. S6), suggesting that differences in proliferation rates may not play a vital role in the cell competition. It has been also proposed that different types of cells compete for soluble survival factors; this is called the ligand capture hypothesis. However, it is unlikely that death of Scribble-knockdown MDCK cells is caused by this mechanism because the frequency of cell death was not substantially affected by repeated replacement of culture medium. In Drosophila, the JNK pathway has been shown to be involved in cell competition (Brumby and Richardson, 2003; Moreno and Basler, 2004; Moreno et al., 2002; Tamori et al., 2010). Brumby and colleagues demonstrated that expression of dominant-negative JNK suppresses apoptosis of Scribble mutant cells surrounded by wild-type cells in the eye disc epithelium (Brumby and Richardson, 2003). However, our data indicate the involvement of p38 MAPK, not JNK, in cell competition between normal and scribble-knockdown MDCK cells. In addition, unlike studies in Drosophila (Igaki et al., 2009), endocytosis was not significantly increased in Scribble-knockdown cells, compared with surrounding normal MDCK cells (supplementary material Fig. S7). Thus, cell competition caused by loss of Scribble is, at least partially, mediated by different molecular mechanisms in Drosophila and mammals. Previously we demonstrated that JNK is involved in apoptosis of Mahjong-knockdown MDCK cells surrounded by normal MDCK cells (Tamori et al., 2010), suggesting the involvement of distinct signaling pathways in these two types of mammalian cell competition. ASK1 is one of the upstream activators of p38 MAPK, but we did not observe increased activation of ASK1 in Scribble-knockdown cells surrounded by normal cells (data not shown). How the presence of surrounding normal cells influences signaling pathways including p38 MAPK in Scribble-knockdown cells remains to be resolved. Recent studies revealed the involvement of two molecules, Flower and Sparc, in cell competition in Drosophila (Portela et al., 2010; Rhiner et al., 2010). It also remains to be clarified whether homologous proteins also play a role in cell competition in mammals.
Another important question is whether a comparable cell competition phenotype can be observed in vivo in mammals. In most of the conventional mouse carcinogenesis model systems, epithelial-tissue specific promoters have been used where genetic changes are induced within the entire epithelium. These are useful systems to study cell-autonomous signaling pathways, but are not suitable to examine the interface between normal and transformed epithelial cells. Therefore, a novel murine model system needs to be established where oncogenic alterations can be induced in a mosaic manner within epithelial tissues in combination with in vivo live cell imaging.
Cell competition is a newly emerging field in cancer biology, which sheds light on the interaction between normal and transformed epithelial cells at the early stage of carcinogenesis. It is hoped that future studies will lead to a novel type of cancer prevention and treatment.
Materials and Methods
Plasmids, antibodies and materials
To construct pSUPERIOR Scribble shRNA, Scribble shRNA oligonucleotides (5′-GATCCCCCAGATGGTCCTCAGCAAGTTTCAAGAGAACTTGCTGAGGACCATCTGTTTTTC-3′ and 5′-TCGAGAAAAACAGATGGTCCTCAGCAAGTTCTCTTGAAACTTGCTGAGGACCATCTGGGG-3′) (Qin et al., 2005) were cloned into BglII and XhoI sites of pSUPERIOR.-neo+gfp (Oligoengine). To construct pSUPERIOR scramble shRNA, scramble shRNA oligonucleotides (5′-GATCCCCGGAGCGCTATCGGTCAAGATTCAAGAGATCTTGACCGATAGCGCTCCTTTTTC-3′ and 5′-TCGAGAAAAAGGAGCGCTATCGGTCAAGATCTCTTGAATCTTGACCGATAGCGCTCCGGG-3′) were cloned into BglII and XhoI sites of pSUPERIOR.-neo+gfp. pcDNA4/TO/human Scribble was constructed by digestion of pEGFP-C1 human Scribble (kindly provided by Jean-Paul Borg, The INSERM Cancer Research Centre, Marseille, France) with BamHI and XbaI and ligation into BamHI and XbaI sites of pcDNA4/TO (Dupre-Crochet et al., 2007). pcDNA4/TO/GFP-p38αDN was constructed by amplification of p38αK53N from pcDNA3-HA-p38k/n and ligation into XhoI and PstI sites of pcDNA4/TO/GFP. pcDNA3-HA-p38k/n was a kind gift from Mutsuhiro Takegawa, Nagoya University, Nagoya, Japan).
Rabbit antibodies against phosphorylated p38 MAPK (T180/Y182), mouse anti-p38-MAPKα and rabbit anti-cleaved caspase-3 were purchased from Cell Signaling Technology. Goat anti-Scribble and mouse anti-Dlg1 (2D11) antibodies were obtained from Santa-Cruz Biotechnology. Rat anti-E-cadherin antibody (Zymed) and mouse anti-E-cadherin antibody (BD Biosciences) were used for immunofluorescence and western blotting, respectively. Mouse anti-β-catenin antibody was from BD Biosciences. Mouse anti-Llgl2 antibody was from Abnova. Mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody was from Millipore. Mouse anti-active-Bax and mouse anti-tubulin antibodies were from Sigma-Aldrich. Mouse anti-active-Bak antibody was from Calbiochem. Mouse anti-gp135 antibody was kindly provided by George Ojakian (SUNY Downstate Medical Center, New York, NY). Rat anti-phosphorylated ASK antibody was a kind gift from Hidenori Ichijo (Tokyo University, Tokyo, Japan). Rabbit anti-GFP antibody was kindly provided by Mark Marsh (MRC, LMCB, London, UK). For immunofluorescence, anti-Scribble antibody was used at a dilution of 1:50, and all other primary antibodies were used at a dilution of 1:100. Alexa Fluor secondary antibodies (Invitrogen) were used at a dilution of 1:200. Alexa-Fluor-647-conjugated phalloidin (Invitrogen) was used at 1.5 μg/ml. For western blotting, anti-GAPDH antibody was used at 1:5000, and all other antibodies at 1:1000. Hoechst 33342 (Invitrogen) was used to visualize nuclei.
Cells were fluorescently labeled with a green CMFDA or red CMTPX dye (Invitrogen) according to manufacturer's instructions. Inhibitors were used at the following concentrations: JNK1,2,3 inhibitor SP600125 (Sigma-Aldrich), 5 μM; p38MAPK inhibitor SB202190 (Calbiochem), 10 μM; myosin II inhibitor (S)-(−)-blebbistatin (Toronto Research Chemicals), 30 μM; caspase inhibitor Z-VAD-FMK (Calbiochem), 100 μM. For immunofluorescence analyses, inhibitors were added 24 hours before fixation. For live-image analyses, inhibitors were added immediately before analyses and incubated for 40 hours.
Cell culture
MDCK cells were cultured as previously described (Dupre-Crochet et al., 2007). To establish MDCK cell lines that stably express Scribble shRNA in a tetracycline-inducible manner (MDCK-pTR Scribble shRNA cells), MDCK-pTR cells (Bialucha et al., 2007) were transfected with pSUPERIOR.neo+gfp Scribble shRNA using Metafectene Pro (Biontex), followed by selection in medium containing 5 μg/ml. blasticidin (Invitrogen) and 800 μg/ml. G418 (Calbiochem). To establish MDCK cell lines that stably express scramble shRNA in a tetracycline-inducible manner (MDCK-pTR scramble shRNA cells), MDCK-pTR cells were transfected with pSUPERIOR.neo+gfp scramble shRNA, followed by the same procedures as described above. To establish MDCK cell lines that stably expresses Scribble shRNA with human Scribble (human Scrib) or with human p38MAPKα dominant-negative (K53N) (p38DN) in a tetracycline-inducible manner (MDCK-pTR Scribble shRNA + human Scrib cells or + p38DN-GFP cells), MDCK-pTR Scribble shRNA cells were transfected with pcDNA4/TO/humanScrib or pcDNA4/TO/GFP-p38αK53N, followed by selection in medium containing 5 μg ml−1 blasticidin, 400 μg ml−1 zeocin (Invitrogen) and 800 μg ml−1 G418. MDCK-pTR scribble shRNA cells, MDCK-pTR scramble shRNA cells, MDCK-pTR Scribble shRNA + human Scrib cells and MDCK-pTR Scribble shRNA + p38DN-GFP cells were cultured in DMEM supplemented with 10% tetracycline-free fetal calf serum (PAA) and antibiotics. Where indicated, tetracycline was added at a final concentration of 10 μg ml−1. Western blotting was performed as described (Hogan et al., 2004). For supplementary material Fig. S3B, 1 μM staurosporine was added for 2–3 hours where indicated. To retain all dead cells, cells were scraped in culture medium and centrifuged.
Time-lapse analyses and immunofluorescence
Cells were seeded in a six-well glass-bottomed dish (MatTek Corporation) at a density of 3.85×105 cells/well. For mixed culture experiments, 3.5×105 non-labeled cells were mixed with 3.5×104 labeled cells. Where indicated, tetracycline was added 6 hours after plating. Before live-image analyses, medium was changed to Leibovitz's L-15 medium (Gibco) supplemented with 10% tetracycline-free fetal calf serum, 1% Glutamax and 1% penicillin-streptomycin. Live-image analyses were started 24 hours after the addition of tetracycline and continued for 40 hours. Cells were incubated with SYTOX Blue (Molecular Probes) to label dead cells according to the manufacturer's instructions. For quantification of the frequency of cell death, we followed the fate of CMTPX-labeled cells. For total stained cells, we counted the number of dead and remaining labeled cells including those that had undergone cell division. Images were acquired at 37°C using a Zeiss Axiovert 135TV microscope with a 20× 0.50 NA air objective lens (Zeiss) and a Hamamatsu Retiga Exi camera. Images were captured every 10 minutes and analyzed using Volocity 5.3 software (PerkinElmer). Immunofluorescence was performed as previously described (Hogan et al., 2009). For quantification of caspase or Bak activation, cells were treated with blebbistatin for 24 hours before fixation to prevent extrusion of apoptotic cells. It should be noted that apically extruded cells were often lost during the washing steps in immunostaining. Because culture of cells on collagen reduced the loss of cells during this procedure, cells were grown on collagen gels for immunofluorescence studies of apoptotic Scribble-knockdown cells. Type-1 collagen was obtained from Nitta Gelatin (Osaka, Japan) and was prepared according to the manufacturer's instructions.
BrdU labeling and quantification
Cells were seeded onto glass coverslips at a density of 2.2×105 cells per well in a six-well plate. Where appropriate, cells were incubated with tetracycline up to 72 hours. For the final 6 hours of incubation, culture medium was changed to FCS-free DMEM containing 10 μM bromodeoxyuridine (BrdU) according to the manufacturer's instructions (Calbiochem). Cells were fixed and labeled with anti-BrdU antibody (mouse, Calbiochem). BrdU-labeled cells were analyzed using a Leica Axiskop microscope. At least 200 cells were analyzed for each condition in three independent experiments.
Endocytosis assay
Cells were seeded onto glass coverslips in a 12-well plate at a density of 8×104 cells per well. For mixed cultures, 7.2×104 unstained cells were mixed with 0.8×104 stained cells. Tetracycline was added 6 hours after seeding. 64 hours after addition of tetracycline, Rhodamine-conjugated dextran (10,000 kDa) (Invitrogen) was added at a final concentration of 400 μg ml−1, and cells were further incubated for 30 minutes at 37°C. Cells on coverslips were washed once with ice-cold PBS, fixed in ice-cold 4% paraformaldehyde (PFA) for 15 minutes, and mounted with Mowiol.
Imaging
Phase-contrast images were acquired at ambient temperature using a Leica DMIRB microscope with a 20× 0.40 NA air objective lens (Leica) and a Hamamatsu C4742-95 Orca camera. Images were acquired using Openlab software (PerkinElmer). Confocal images were acquired using a Leica TCS SPE confocal microscope with an 63× 1.30 oil-immersion objective lens and Leica Application Suite (LAS) (Leica). Images were colored, and contrast and brightness were enhanced linearly using Photoshop CS4 (Adobe).
Data analyses
Student's t-tests were used to determine P values because this test requires variables with no fixed limits. For Fig. 4B, using Metamorph 6.0 digital analysis software (Universal Imaging), p38 MAPK fluorescence intensity was measured at eight random points (50 pixels each) in each cell, and the mean of the values was used for statistical analyses. For supplementary material Fig. S7, confocal images of 7–9 z sections at 0.42 μm intervals were acquired and processed using a maximum projection tool with Las AF software (Leica). The total number of fluorescent red puncta within each cell was counted, and the mean of the values was used for statistical analyses.
Supplementary Material
Acknowledgements
We thank George Ojakian for anti-gp135 antibody, Jean-Paul Borg for pEGFP-C1 human Scribble, Mutsuhiro Takegawa for pcDNA3-HA-p38k/n, and Hidenori Ichijo for anti-phosphorylated ASK antibody.
Footnotes
Funding
This work is supported by Medical Research Council funding to the Cell Biology Unit and by Funding Program for Next Generation World-Leading Researchers (NEXT Program). Y.F. is also supported by Takeda Science Foundation, the Naito Foundation, the Sagawa Foundation for promotion of Cancer Research, the Yasuda Medical Foundation, Ono Cancer Research Fund, the NOVARTIS Foundation (Japan) for the Promotion of Science and the Ichiro Kanehara Foundation. Deposited in PMC for release after 6 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.085803/-/DC1
References
- Baker N. E., Li W. (2008). Cell competition and its possible relation to cancer. Cancer Res. 68, 5505-5507 [DOI] [PubMed] [Google Scholar]
- Bialucha C. U., Ferber E. C., Pichaud F., Peak-Chew S. Y., Fujita Y. (2007). p32 is a novel mammalian Lgl binding protein that enhances the activity of protein kinase Czeta and regulates cell polarity. J. Cell Biol. 178, 575-581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder D., Perrimon N. (2000). Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 403, 676-680 [DOI] [PubMed] [Google Scholar]
- Bilder D., Li M., Perrimon N. (2000). Cooperative regulation of cell polarity and growth by Drosophila tumor suppressors. Science 289, 113-116 [DOI] [PubMed] [Google Scholar]
- Brumby A. M., Richardson H. E. (2003). scribble mutants cooperate with oncogenic Ras or Notch to cause neoplastic overgrowth in Drosophila. EMBO J. 22, 5769-5779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvier D., Ankri S., Charriaut-Marlangue C., Casimir R., Jacotot E. (2007). Broad-spectrum caspase inhibitors: from myth to reality? Cell Death Differ. 14, 387-391 [DOI] [PubMed] [Google Scholar]
- de la Cova C., Abril M., Bellosta P., Gallant P., Johnston L. A. (2004). Drosophila myc regulates organ size by inducing cell competition. Cell 117, 107-116 [DOI] [PubMed] [Google Scholar]
- Diaz B., Moreno E. (2005). The competitive nature of cells. Exp. Cell Res. 306, 317-322 [DOI] [PubMed] [Google Scholar]
- Dupre-Crochet S., Figueroa A., Hogan C., Ferber E. C., Bialucha U., Adams J., Richardson E. C., Fujita Y. (2007). Casein kinase 1 is a novel negative regulator of e-cadherin-based cell-cell contacts. Mol. Cell Biol. 27, 3804-3816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y. (2011). Interface between normal and transformed epithelial cells-A road to a novel type of cancer prevention and treatment. Cancer Sci. 102, 1749-1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiol D., Zacchi A., Petrera F., Stanta G., Banks L. (2006). Human discs large and scrib are localized at the same regions in colon mucosa and changes in their expression patterns are correlated with loss of tissue architecture during malignant progression. Int. J. Cancer 119, 1285-1290 [DOI] [PubMed] [Google Scholar]
- Grzeschik N. A., Amin N., Secombe J., Brumby A. M., Richardson H. E. (2007). Abnormalities in cell proliferation and apico-basal cell polarity are separable in Drosophila lgl mutant clones in the developing eye. Dev. Biol. 311, 106-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A. (2000). The hallmarks of cancer. Cell 100, 57-70 [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A. (2011). Hallmarks of cancer: the next generation. Cell 144, 646-674 [DOI] [PubMed] [Google Scholar]
- Hogan C., Serpente N., Cogram P., Hosking C. R., Bialucha C. U., Feller S. M., Braga V. M., Birchmeier W., Fujita Y. (2004). Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Mol. Cell. Biol. 24, 6690-6700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan C., Dupre-Crochet S., Norman M., Kajita M., Zimmermann C., Pelling A. E., Piddini E., Baena-Lopez L. A., Vincent J. P., Itoh Y., et al. (2009). Characterization of the interface between normal and transformed epithelial cells. Nat. Cell Biol. 11, 460-467 [DOI] [PubMed] [Google Scholar]
- Hogan C., Kajita M., Lawrenson K., Fujita Y. (2011). Interactions between normal and transformed epithelial cells: Their contributions to tumourigenesis. Int. J. Biochem. Cell Biol. 43, 496-503 [DOI] [PubMed] [Google Scholar]
- Igaki T., Pastor-Pareja J. C., Aonuma H., Miura M., Xu T. (2009). Intrinsic tumor suppression and epithelial maintenance by endocytic activation of Eiger/TNF signaling in Drosophila. Dev. Cell 16, 458-465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L. A. (2009). Competitive interactions between cells: death, growth, and geography. Science 324, 1679-1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Basler K. (2004). dMyc transforms cells into super-competitors. Cell 117, 117-129 [DOI] [PubMed] [Google Scholar]
- Moreno E., Basler K., Morata G. (2002). Cells compete for decapentaplegic survival factor to prevent apoptosis in Drosophila wing development. Nature 416, 755-759 [DOI] [PubMed] [Google Scholar]
- Navarro C., Nola S., Audebert S., Santoni M. J., Arsanto J. P., Ginestier C., Marchetto S., Jacquemier J., Isnardon D., Le Bivic A., et al. (2005). Junctional recruitment of mammalian Scribble relies on E-cadherin engagement. Oncogene 24, 4330-4339 [DOI] [PubMed] [Google Scholar]
- Ohsawa S., Sugimura K., Takino K., Xu T., Miyawaki A., Igaki T. (2011). Elimination of oncogenic neighbors by JNK-mediated engulfment in Drosophila. Dev. Cell 20, 315-328 [DOI] [PubMed] [Google Scholar]
- Portela M., Casas-Tinto S., Rhiner C., Lopez-Gay J. M., Dominguez O., Soldini D., Moreno E. (2010). Drosophila SPARC is a self-protective signal expressed by loser cells during cell competition. Dev. Cell 19, 562-573 [DOI] [PubMed] [Google Scholar]
- Qin Y., Capaldo C., Gumbiner B. M., Macara I. G. (2005). The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J. Cell Biol. 171, 1061-1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhiner C., Lopez-Gay J. M., Soldini D., Casas-Tinto S., Martin F. A., Lombardia L., Moreno E. (2010). Flower forms an extracellular code that reveals the fitness of a cell to its neighbors in Drosophila. Dev. Cell 18, 985-998 [DOI] [PubMed] [Google Scholar]
- Rosenblatt J., Raff M. C., Cramer L. P. (2001). An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr. Biol. 11, 1847-1857 [DOI] [PubMed] [Google Scholar]
- Tamori Y., Bialucha C. U., Tian A. G., Kajita M., Huang Y. C., Norman M., Harrison N., Poulton J., Ivanovitch K., Disch L., et al. (2010). Involvement of Lgl and Mahjong/VprBP in cell competition. PLoS Biol. 8, e1000422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle R. J., Strasser A. (2008). The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 9, 47-59 [DOI] [PubMed] [Google Scholar]
- Zarubin T., Han J. (2005). Activation and signaling of the p38 MAP kinase pathway. Cell Res. 15, 11-18 [DOI] [PubMed] [Google Scholar]
- Zhan L., Rosenberg A., Bergami K. C., Yu M., Xuan Z., Jaffe A. B., Allred C., Muthuswamy S. K. (2008). Deregulation of scribble promotes mammary tumorigenesis and reveals a role for cell polarity in carcinoma. Cell 135, 865-878 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.