In practicing Dermatology one soon realizes that the ‘severity’ of most skin diseases is not easily assessed and communicated. Almost none of the conditions we treat can be followed by laboratory values, and typically the patients we care for survive with (rather than die from) their diseases. Also, we quickly learn that the visible extent of disease often does not correlate with the degree to which patients are disturbed by it; patients with ‘minimal’ clinical involvement may be highly distressed, but others with extensive involvement may not be bothered. In fact, the severity of a skin disease is related to both its clinical extent (using ‘clinimetric’ measures) and its effects on patients’ quality of life (using ‘psychometric’ measures).
In this paper I will describe how we have designed and worked with a measure of the effects of skin disease on quality of life, called Skindex. I will review the development of the two versions of Skindex, discuss their measurement properties and interpretability, and give examples of how they have been used and adapted for dermatologic research internationally. I will specifically discuss our studies of quality of life in patients with nonmelanoma skin cancer, to illustrate how we have used Skindex to understand quality of life and to compare effectiveness of different treatments for this highly prevalent condition.
Development of Skindex
When we began to develop Skindex, we were greatly informed by Andrew Finlay’s previous work on measuring disability from skin disease 1,2. Our goal was to develop an instrument to measure comprehensively the effects of skin disease on health-related quality of life, and we specifically designed the instrument be able to discriminate between patients with different effects, as well as to detect changes in patients over time 3. We followed an incremental strategy that began with an hypothesis: based on our literature review of previous clinical and psychological studies as well as substantial input from patients and clinicians, we constructed a comprehensive conceptual framework for the ways in which we hypothesized skin diseases affected patients. We composed survey items to measure all domains in the framework, and then we tested our hypothesis by examining the validity of the items in a series of psychometric tests using the responses of a large sample of patients.
Original Conceptual Framework
We proposed that skin diseases affect patients in either psychosocial or physical ways. We suggested that psychosocial effects could be cognitive (beliefs about self or others), social, or emotional. Subdimensions of emotional effects include depression, fear, embarrassment, and anger. Physical effects are either discomfort or limitations in physical functioning. This original hypothesized framework is depicted in Figure 1.
Figure 1.

Original hypothesized conceptual framework for the effects of skin disease on the quality of life of affected patients.
Item composition and prototype Skindex
Our team consisted of two psychometricians, and, using conventional principles, we composed 65 items to assess the dimensions in the conceptual framework. We pilot-tested this draft survey and changed or deleted ambiguous and redundant items, which left a 61-item prototype version of Skindex. The measurement properties of this trial version were tested in a series of studies that demonstrated it to be reliable and to have substantial evidence of validity as a measure of the effects of skin disease on quality of life 4.
Refinement into Skindex-29
We wanted to improve the ability of the prototype Skindex to discriminate among patients with likely different degrees of quality-of-life effect, as well as to be more sensitive to even modest changes in patients’ experiences over time. Also, if possible, we wanted to shorten the instrument to make it more useful in research and clinical settings. To accomplish these goals, we assessed the performance of each item, using not only qualitative judgments but also based on a priori criteria for suboptimal item performance, including reproducibility, discriminant validity, complexity, ambiguity, response distribution, and item-total correlation. We analyzed the factors, or themes, that explained the variability in responses to the psychometrically most sound items, which permitted us to test and refine our theorized model for the effects of skin disease on quality of life. Finally, we composed new items that we judged would improve discriminative and evaluative capability of the instrument.
This sequential process generated a refined conceptual framework; we now propose that the effects of skin disease on quality of life can be understood in three domains: Symptoms, Emotions, and Functioning (Figure 2). The analyses yielded a 29-item version of Skindex that remained reliable and valid, but that had reduced respondent burden, and improved discriminative and evaluative capability 5.
Figure 2.
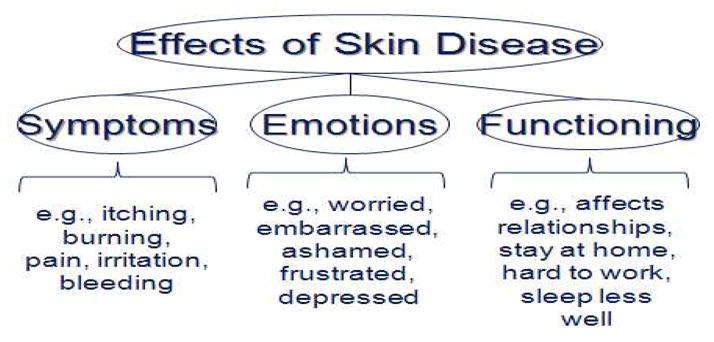
Refined conceptual framework for the effects of skin disease on the quality of life of affected patients.
Skindex-29 inquires about how often (Never, Rarely, Sometimes, Often, All the time) during the previous four weeks the patient experienced the effect described in each item. Seven items address the Symptoms domain, ten items the Emotional domain, and twelve items the Functioning domain. All responses are transformed to a linear scale of 100, varying from 0 (no effect) to 100 (effect experienced all the time). Skindex scores are reported as three scale scores, corresponding to the three domains; a scale score is the average of a patient’s responses to items in a given domain.
Construction of Skindex-16
Longitudinal research studies often require waves of data collection with lengthy survey instruments to assess multiple aspects of patients’ experience. We wanted to develop a version of Skindex that would remain accurate and responsive as a measure of skin-related quality of life, but that would be brief (contained on one page). Also, we wanted to assess not only how often patients have a particular experience, but how much they are bothered by it. Thus, we used Skindex-29 as the substrate for a series of studies that developed a different, single-page version, Skindex-16. We used item analyses similar to those described above, to select only those items that performed well according to our criteria, and eliminated items to which a majority of patients responded ‘Never’. We also composed new items to address aspects of quality of life that patients mentioned often in their qualitative responses but that had not been addressed in Skindex-29. This process generated a new instrument, which fits on a single page. The header inquires “During the past x weeks, how often have you been bothered by …” The response choices are on continuous bipolar scale with seven boxes anchored by the words “Never Bothered” and “Álways Bothered” at each end. As with the parent instrument, scores vary from 0 (no effect) to 100 (effect experienced all the time), and responses are aggregated in Symptoms (four items), Emotions (seven items), and Functioning scales (five items). We tested the performance of this new instrument, Skindex-16, in over 500 patients, and determined that it was reliable, retained substantial evidence of validity, and was responsive to clinical change 6.
Choosing between Skindex-29 and Skindex-16
The Skindex instruments are copyrighted to ensure standardization in their use and scoring; permission to use either version is obtained by contacting the authors at chrenm@derm.ucsf.edu. Investigators often inquire about which of the two versions they should use for their studies. This decision typically depends on the research question being addressed. Because it is longer, Skindex-29 is more comprehensive, and it might be more suitable if the goal of a project is to investigate and understand the effects of a given condition on quality of life. Also, because Skindex-29 is older and has been used more broadly in clinical research, typical scores of patients with different skin conditions are widely available and can be compared with those of patients with the disease in question. For example, we were interested in learning more about quality-of-life effects from vulvodynia, a highly painful vulvar condition that is poorly understood. We used Skindex-29 in a large sample of women and determined that those with vulvodynia were substantially more likely than those with other vulvar conditions to have feelings of depression, anger, and frustration, and to report that the vulvodynia affected broad aspects of their social and physical functioning 7.
Skindex-16, on the other hand, consists of the items that had the best performance characteristics in the longer instrument, as well as additional items that are not in Skindex-29, but that address aspects of skin disease that many patients had mentioned in response to our qualitative research (for example, bother from the persistence or reoccurrence of the skin condition). Also, as noted above, Skindex-16 measures bother rather than frequency of experience, which we reasoned may more directly assess effects on patients’ quality of life. Finally, because it has been refined into a single page, Skindex-16 is useful for studies in which respondent burden is a concern. For example, we have used Skindex-16 in waves of data collection over ten years in a longitudinal study of over 1500 patients with nonmelanoma skin cancer, as part of research to document and compare outcomes after therapy, as described below.
Interpretation of Scores
To use quality-of-life measures to study disease and improve patient care, we want to know not only raw scores, but also what the scores mean with respect to severity of effect and comparison with other patients 8. Because the Skindex instruments are generic in the sense that they can be used in patients with skin disease of any sort, valuable information can be obtained by comparisons of mean scores of groups of patients with certain diseases. Table 1 contains mean Skindex scores in unselected groups of patients with a variety of skin conditions.
Table 1.
Mean Skindex scores in patients with different dermatological diagnoses
| SKINDEX-29 SCORES* | SKINDEX-16 SCORES* | |||||||
|---|---|---|---|---|---|---|---|---|
| DIAGNOSIS | # | SYMPTOMS | EMOTIONS | FUNCTIONING | # | SYMPTOMS | EMOTIONS | FUNCTIONING |
| Eczematous dermatitis | 102 | 48 ± 23 | 41 ± 27 | 26 ± 26 | 84 | 42 ± 31 | 52 ± 30 | 24 ± 29 |
| Psoriasis | 44 | 42 ± 21 | 39 ± 27 | 23 ± 27 | 27 | 49 ± 29 | 68 ± 25 | 39 ± 33 |
| Acne vulgaris | 63 | 30 ± 19 | 41 ± 25 | 16 ± 16 | 38 | 31 ± 24 | 75 ± 23 | 38 ± 30 |
| Warts | 24 | 23 ± 18 | 22 ± 16 | 6 ± 13 | 33 | 23 ± 23 | 48 ± 31 | 24 ± 31 |
| Other benign growths | 76 | 22 ± 20 | 21 ± 21 | 9 ± 17 | 56 | 15 ± 20 | 34 ± 29 | 12 ± 21 |
Mean ± SD
In addition to these comparisons, both distribution-based and anchor-based methods have been used to aid interpretation of Skindex-29 scores 8. Using mixture analyses to assess whether the distribution of responses could be clustered into statistically distinct categories based on degree of quality-of-life effect, Nijsten et al demonstrated five distinct categories for the Symptoms scale, and four for the Emotions and Functioning scales. For example, for the Symptoms scale, the cutoff value for “very little” effect was ≤ 3, “mild” effect 4–10, “moderate” effect 11–25; “severe” effect 26–49, and “extremely severe” effect ≥ 50 9. Using an anchor-based method, Prinsen et al determined these cut-off Skindex-29 scores for severe effect: Symptoms ≥ 52, Emotions ≥ 39, Functioning ≥ 37 10. Similar studies to aid interpretation of Skindex-16 scores have not yet been performed.
Using Skindex in Research Studies and in Clinic
Understanding Quality of Life in Dermatologic Conditions
Skindex has been used to study the effects of a wide variety of skin conditions on patients’ lives, and in addition to answering research questions these investigations can lead to new insights to inform patient care. For example, we found that, even controlling for clinical severity, the quality of life of older patients (≥ 40 years) with acne vulgaris is more affected than that of younger patients 11. Patients with cutaneous lymphoma experience many quality-of-life effects, including skin sensitivity as well as annoyance about the disease, worry that it could worsen, and effects on sexual life 12. Similar quality-of-life assessments have been made in patients with psoriasis 13, dermatitis 14, alopecia 15, and urticaria 16, among others.
Clinical Trials
Because it was developed to be responsive to changes in quality of life, Skindex can be used as an outcomes measure in clinical trials. Examples of this use include studies of therapies for psoriasis 17,18, acne vulgaris 19, and atopic dermatitis 20.
Cultural adaptation/Translations of Skindex
Skindex has been translated into several languages, typically using adaptations of conventional guidelines for ensuring cultural equivalence in quality-of-life instruments 21. A good example is the development of the Spanish version of Skindex-29 in which a step-wise process was used to forward-translate, back-translate, pilot test, refine, and evaluate the measurement properties of the adapted version 22. For certain expressions a simple translation was not sufficient; for example, further consideration was necessary to determine the best translations for ‘embarrassed’ and ‘ashamed,’ which is typical of linguistic issues in other translations as well (e.g. 23).
Quality-of-Life Instruments Based on Skindex
Skindex is a generic instrument in the sense that it is intended to be used by patients with any skin condition. It has been used as a basis for several more specific instruments or modules to measure quality of life in particular populations or in patients with certain diagnoses. For example, variations of Skindex have been developed for patients with leg ulcers 24, onychomycosis 25, scalp disorders 26, and for teenagers 27.
Outcomes Research and Comparative Effectiveness of Therapies
Because quality-of-life is a central outcome of nonfatal conditions such as most skin diseases, Skindex can be used to assess how patients progress over time or after therapies. For example, we have used Skindex-16 to document the effects of nonmelanoma skin cancer (NMSC) and its treatments on quality of life over time, as part of a prospective cohort study of a large sample of patients with this common tumor.
We determined the skin-related quality of life of 633 consecutive patients with NMSC diagnosed in 1999 and 2000 and treated with the three major treatments used in the US, electrodessication and curettage (ED&C), excision, or Mohs surgery 28. Scores for the Symptoms, Emotions, and Functioning subscales of Skindex-16 in the three treatment groups before therapy are contained in Table 2. Compared with Skindex-16 scores of patients with inflammatory dermatologic conditions (Table 1), the scores of these patients with NMSC were relatively low, indicating that overall the tumors have less effect on quality of life than the effects of psoriasis, eczematous dermatitis, or acne vulgaris. There was no significant difference in the treatment groups in mean Symptoms or Emotions scores, but the mean Emotions score of patients whose tumors were ultimately treated with Mohs surgery were higher than mean Emotions scores of those treated with the other two treatments (P<0.0001). After treatment, in analyses that adjusted for differences in treatment groups, the scores of patients treated with excision or Mohs surgery improved in all three Skindex domains, but patients treated with ED&C had no change in tumor-related quality of life (Table 3).
Table 2.
Skindex-16 scores (Mean ± SD) before treatment of 633 patients with nonmelanoma skin cancer
| Skindex Subscale | Treatment Groups | ||
|---|---|---|---|
| ED&C | Excision | Mohs surgery | |
| Symptoms | 20 (24) | 22 (23) | 22 (24) |
| Emotions | 33 (28) | 39 (30) | 46 (27) |
| Functioning | 12 (22) | 15 (25) | 14 (21) |
Table 3.
Improvement in Skindex-16 scores of 633 patients after treatment of nonmelanoma skin cancer
| Treatment Group | Adjusted Change Score, mean | ||
|---|---|---|---|
| Symptoms | Emotions | Functioning | |
| ED&C | −3 | −5 | 2 |
| Excision | −10* | −19* | −3* |
| Mohs surgery | −10* | −22* | −5* |
P<0.05 for difference before and after treatment
We were also interested in determining whether characteristics of the patients, tumors, or care were associated with better skin-related quality of life after treatment for NMSC 29. We found that the strongest independent predictor of quality of life after treatment was quality of life before treatment. Fewer comorbid illnesses and better mental health statu were also independent predictors. Tumor characteristics, however, did not predict quality of life. These results may improve clinical care by permitting clinicians to recognize patients at higher risk for poor quality-of-life outcomes.
Future Directions
The development of quality-of-life measures is a dynamic process. The interpretation of Skindex-29 and Skindex-16 scores will be enhanced as they are used and studied more widely. The instruments were developed using classical test theory methods, and the application of newer psychometric techniques such as item response theory and computerized adaptive testing may improve their measurement properties 30. As tools to measure complex aspects of health become more commonplace in dermatology, investigators, clinicians, and other stakeholders may develop a consensus about features that should be present in widely-accepted measures of disease severity 31; these features will likely include at least some measure of patients’ experience such as symptoms or quality of life. Features to be included in an ‘ideal’ quality-of-life measure could be defined using similar consensus techniques, and perhaps it will be possible to develop a core set of questions and metrics or repositories of items that perform well. Finally, because the patient report is a vital sign for dermatologic disease 32, an exciting development would be testing whether for selected subgroups of patients the inclusion in the clinic of quantitative measures of patient reports (such as Skindex) can improve care.
Summary
Skindex-29 and Skindex-16 are validated measures of the effects of skin diseases on quality of life that are suitable for use in research about patients’ experiences of illness and its treatment. This article reviews the development of Skindex and its use in a variety of clinical research studies.
Synopsis.
Skindex-29 and Skindex-16 are validated measures of the effects of skin diseases on patients’ quality of life. This paper reviews the development of both versions of Skindex, discusses their measurement properties and interpretability, and gives examples of how they have been used and adapted for dermatologic research internationally. Studies of quality of life in patients with nonmelanoma skin cancer are described to illustrate the use of Skindex to understand quality of life and to compare effectiveness of different treatments for this highly prevalent condition.
Acknowledgments
FUNDING SUPPORT: This work was supported by grant K24 AR052667 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health.
Footnotes
DISCLOSURE: The author has nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Finlay AY, Kelly SE. Psoriasis--an index of disability. Clin Exper Dermatol. 1987;12:8–11. doi: 10.1111/j.1365-2230.1987.tb01844.x. [DOI] [PubMed] [Google Scholar]
- 2.Finlay AY, Khan GK. Dermatology life quality index (DLQI)--a simple practical measure for routine clinical use. Clin Exper Dermatol. 1994;19:210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 3.Kirshner B, Guyatt G. A methodological framework for assessing health indices. J Chron Dis. 1985;38(1):27–36. doi: 10.1016/0021-9681(85)90005-0. [DOI] [PubMed] [Google Scholar]
- 4.Chren MM, Lasek RJ, Quinn LM, Mostow EN, Zyzanski SJ. Skindex, a quality-of-life measure for patients with skin disease: reliability, validity, and responsiveness. J Invest Dermatol. 1996;107:707–713. doi: 10.1111/1523-1747.ep12365600. [DOI] [PubMed] [Google Scholar]
- 5.Chren MM, Lasek RJ, Flocke SA, Zyzanski SJ. Improved discriminative and evaluative capability of a refined version of Skindex, a quality-of-life instrument for patients with skin diseases. Arch Dermatol. 1997;133(11):1433–1440. [PubMed] [Google Scholar]
- 6.Chren MM, Lasek RJ, Sahay AP, Sands LP. Measurement properties of Skindex-16, A brief quality-of-life measure for patients with skin diseases. Journal of Cutaneous Medicine and Surgery. 2001;5(2):105–110. doi: 10.1007/BF02737863. [DOI] [PubMed] [Google Scholar]
- 7.Ponte M, Klemperer E, Sahay A, Chren MM. Effects of vulvodynia on quality of life. J Am Acad Dermatol. 2009 Jan;60(1):70–76. doi: 10.1016/j.jaad.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chren M. Interpretation of quality-of-life scores. J Invest Dermatol. 2010 doi: 10.1038/jid.2010.51. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nijsten T, Sampogna F, Abeni D. Categorization of Skindex-29 scores using mixture analysis. Dermatology. 2009;218(2):151–154. doi: 10.1159/000182253. [DOI] [PubMed] [Google Scholar]
- 10.Prinsen CA, Lindeboom R, Sprangers MA, Legierse CM, de Korte J. Health-Related Quality of Life Assessment in Dermatology: Interpretation of Skindex-29 Scores Using Patient-Based Anchors. J Invest Dermatol. 2010;130:1318–1322. doi: 10.1038/jid.2009.404. [DOI] [PubMed] [Google Scholar]
- 11.Lasek RJ, Chren MM. Acne vulgaris and the quality of life of adult dermatology patients. Arch Dermatol. 1998;134(4):454–458. doi: 10.1001/archderm.134.4.454. [DOI] [PubMed] [Google Scholar]
- 12.Sampogna F, Frontani M, Baliva G, et al. Quality of life and psychological distress in patients with cutaneous lymphoma. Br J Dermatol. 2009 Apr;160(4):815–822. doi: 10.1111/j.1365-2133.2008.08992.x. [DOI] [PubMed] [Google Scholar]
- 13.De Korte J, Mombers FM, Sprangers MA, Bos JD. The suitability of quality-of-life questionnaires for psoriasis research: a systematic literature review. Arch Dermatol. 2002 Sep;138(9):1221–1227. doi: 10.1001/archderm.138.9.1221. discussion 1227. [DOI] [PubMed] [Google Scholar]
- 14.Zug KA, Aaron DM, Mackenzie T. Baseline quality of life as measured by Skindex-16+5 in patients presenting to a referral center for patch testing. Dermatitis. 2009 Jan-Feb;20(1):21–28. [PubMed] [Google Scholar]
- 15.Reid EE, Haley AC, Borovicka JH, et al. Clinical severity does not reliably predict quality of life in women with alopecia areata, telogen effluvium, or androgenic alopecia. J Am Acad Dermatol. 2011 May 21; doi: 10.1016/j.jaad.2010.11.042. [DOI] [PubMed] [Google Scholar]
- 16.Maurer M, Ortonne JP, Zuberbier T. Chronic urticaria: a patient survey on quality-of-life, treatment usage and doctor-patient relation. Allergy. 2009 Apr;64(4):581–588. doi: 10.1111/j.1398-9995.2008.01853.x. [DOI] [PubMed] [Google Scholar]
- 17.Ortonne JP, Ganslandt C, Tan J, Nordin P, Kragballe K, Segaert S. Quality of life in patients with scalp psoriasis treated with calcipotriol/betamethasone dipropionate scalp formulation: a randomized controlled trial. Eur Acad Dermatol Venereol. 2009 Aug;23(8):919–926. doi: 10.1111/j.1468-3083.2009.03221.x. [DOI] [PubMed] [Google Scholar]
- 18.de Korte J, van der Valk PG, Sprangers MA, et al. A comparison of twice-daily calcipotriol ointment with once-daily short-contact dithranol cream therapy: quality-of-life outcomes of a randomized controlled trial of supervised treatment of psoriasis in a day-care setting. Br J Dermatol. 2008 Feb;158(2):375–381. doi: 10.1111/j.1365-2133.2007.08337.x. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi N, Kawashima M. Efficacy of oral antibiotics on acne vulgaris and their effects on quality of life: a multicenter randomized controlled trial using minocycline, roxithromycin and faropenem. Dermatol. 2011 Feb;38(2):111–119. doi: 10.1111/j.1346-8138.2010.00969.x. [DOI] [PubMed] [Google Scholar]
- 20.Gambichler T, Othlinghaus N, Tomi NS, et al. Medium-dose ultraviolet (UV) A1 vs. narrowband UVB phototherapy in atopic eczema: a randomized crossover study. Br J Dermatol. 2009 Mar;160(3):652–658. doi: 10.1111/j.1365-2133.2008.08984.x. [DOI] [PubMed] [Google Scholar]
- 21.Guillemin F, Bombardier C, Beaton D. Cross-cultural adaptation of health-related quality of life measures: literature review and proposed guidelines. Clin Epidemiol. 1993 Dec;46(12):1417–1432. doi: 10.1016/0895-4356(93)90142-n. [DOI] [PubMed] [Google Scholar]
- 22.Jones-Caballero M, Penas PF, Garcia-Diez A, Badia X, Chren MM. The Spanish version of Skindex-29. Cultural adaptation and preliminary evidence of validity and equivalence with the original American version. Int J Dermatol. 2000;39:907–912. doi: 10.1046/j.1365-4362.2000.00944.x. [DOI] [PubMed] [Google Scholar]
- 23.Higaki Y, Kawamoto K, Kamo T, Horikawa N, Kawashima M, Chren MM. The Japanese version of Skindex-16: a brief quality-of-life measure for patients with skin diseases. Dermatol. 2002 Nov;29(11):693–698. doi: 10.1111/j.1346-8138.2002.tb00205.x. [DOI] [PubMed] [Google Scholar]
- 24.Hareendran A, Doll H, Wild DJ, et al. The venous leg ulcer quality of life (VLU-QoL) questionnaire: development and psychometric validation. Wound Repair Regen. 2007 Jul-Aug;15(4):465–473. doi: 10.1111/j.1524-475X.2007.00253.x. [DOI] [PubMed] [Google Scholar]
- 25.Warshaw EM, Foster JK, Cham PM, Grill JP, Chen SC. NailQoL: a quality-of-life instrument for onychomycosis. Int J Dermatol. 2007 Dec;46(12):1279–1286. doi: 10.1111/j.1365-4632.2007.03362.x. [DOI] [PubMed] [Google Scholar]
- 26.Chen SC, Yeung J, Chren MM. Scalpdex: a quality-of-life instrument for scalp dermatitis. Arch Dermatol. 2002 Jun;138(6):803–807. doi: 10.1001/archderm.138.6.803. [DOI] [PubMed] [Google Scholar]
- 27.Smidt AC, Lai JS, Cella D, Patel S, Mancini AJ, Chamlin SL. Development and validation of Skindex-Teen, a quality-of-life instrument for adolescents with skin disease. Arch Dermatol. 2010 Aug;146(8):865–869. doi: 10.1001/archdermatol.2010.161. [DOI] [PubMed] [Google Scholar]
- 28.Chren MM, Sahay AP, Bertenthal DS, Sen S, Landefeld CS. Quality-of-life outcomes of treatments for cutaneous basal cell carcinoma and squamous cell carcinoma. Invest Dermatol. 2007 Jun;127(6):1351–1357. doi: 10.1038/sj.jid.5700740. [DOI] [PubMed] [Google Scholar]
- 29.Chen T, Bertenthal D, Sahay A, Sen S, Chren MM. Predictors of skin-related quality of life after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. Arch Dermatol. 2007 Nov;143(11):1386–1392. doi: 10.1001/archderm.143.11.1386. [DOI] [PubMed] [Google Scholar]
- 30.Nijsten TE, Sampogna F, Chren MM, Abeni DD. Testing and reducing skindex-29 using Rasch analysis: Skindex-17. Invest Dermatol. 2006 Jun;126(6):1244–1250. doi: 10.1038/sj.jid.5700212. [DOI] [PubMed] [Google Scholar]
- 31.Schmitt J, Langan S, Stamm T, Williams HC. Core outcome domains for controlled trials and clinical recordkeeping in eczema: international multiperspective Delphi consensus process. Invest Dermatol. 2011 Mar;131(3):623–630. doi: 10.1038/jid.2010.303. [DOI] [PubMed] [Google Scholar]
- 32.Chren MM. Measurement of vital signs for skin diseases. J Invest Dermatol. 2005 Oct;125(4):viii–ix. doi: 10.1111/j.0022-202X.2005.23796.x. [DOI] [PubMed] [Google Scholar]


