SUMMARY
Long-term potentiation (LTP) of excitatory synapses is a compelling synaptic correlate of learning and memory. LTP induction requires NMDA receptor (NMDAR) activation, which triggers SNARE-dependent exocytosis of AMPA receptors (AMPARs). However, the molecular mechanisms mediating AMPAR exocytosis induced by NMDAR activation remain largely unknown. Here, we show that complexin, a protein that regulates neurotransmitter release via binding to SNARE complexes, is essential for AMPAR exocytosis during LTP but not for the constitutive AMPAR exocytosis that maintains basal synaptic strength. The regulated postsynaptic AMPAR exocytosis during LTP requires binding of complexin to SNARE complexes. In hippocampal neurons, presynaptic complexin acts together with synaptotagmin-1 to mediate neurotransmitter release. However, postsynaptic synaptotagmin-1 is not required for complexin-dependent AMPAR exocytosis during LTP. These results suggest a new complexin-dependent molecular mechanism for regulating AMPAR delivery to synapses, a mechanism that is surprisingly similar to presynaptic exocytosis but controlled by regulators other than synpatotagmin-1.
INTRODUCTION
Constitutive and regulated exocytosis play critical roles in the delivery of proteins to the plasma membrane and the release of substances into the extracellular environment. These events are frequently targeted to specific subcellular domains that mediate distinct cellular functions. A prototypic example of such subcellular specialization occurs at mammalian excitatory synapses. Presynaptic nerve terminals contain an active zone composed of a web of scaffolding proteins, ion channels and neurotransmitter-containing vesicles, some of which sit docked at the plasma membrane waiting for the appropriate signal to trigger their fusion. Like all intracellular membrane fusion events except for mitochondrial fusion, the exocytosis of presynaptic vesicles requires assembly of SNARE complexes that are composed of the plasma membrane proteins, syntaxin-1 and SNAP 25, and of the vesicle protein synaptobrevin/VAMP (Sudhof, 2004; Sudhof and Rothman, 2009). SNARE complex formation is critical for bringing the vesicle and plasma membrane into tight apposition and thereby preparing the vesicle for fusion (Rizo and Rosenmund, 2008; Sudhof and Rothman, 2009; Weber et al., 1998). The actual exocytotic fusion event, however, is prevented until an action potential invades the terminal and elicits a rise in calcium at which point neurotransmitter release occurs within one millisecond. Calcium triggers fusion pore opening by binding to synaptotagmins, which are calcium-sensors for synaptic vesicle exocytosis (Fernandez-Chacon et al., 2001; Sudhof and Rothman, 2009). Specific synaptotagmin isoforms have been implicated in presynaptic exocytosis with synaptotagmin-1 accounting for nearly all neurotransmitter release in forebrain neurons (Geppert et al., 1994) However, synaptotagmins do not function alone but require the presence of complexins, which are small soluble proteins that bind to SNARE complexes (McMahon et al., 1995). Complexins perform several functions in presynaptic exocytosis: priming of synaptic vesicles probably by promoting SNARE-complex assembly (Yang et al., 2010), activation of SNARE complexes to allow subsequent calcium-triggering of fusion pore opening via synaptotagmin (Reim et al., 2001; Xue et al., 2008) and in some preparations clamping of SNARE complexes to prevent inappropriate fusion pore opening (Giraudo et al., 2006; Huntwork and Littleton, 2007: Maximov et al., 2009; Tang et al., 2006; Xue et al., 2009; Yang et al., 2010).
Opposing presynaptic nerve terminals, the postsynaptic compartment of excitatory synapses contains a postsynaptic density (PSD) that is precisely aligned with the presynaptic active zone. The PSD contains a different set of scaffolding proteins that function to position glutamate receptors and intracellular signaling proteins in the appropriate subsynaptic domains so that they can respond to the release of glutamate (Elias and Nicoll, 2007; Scannevin and Huganir, 2000; Sheng and Sala, 2001). The composition of the PSD is not static but is influenced by synaptic activity, such that the numbers and properties of glutamate receptors can be modified resulting in long-lasting changes in synaptic strength. Specifically, long-term depression (LTD) triggered by activation of either NMDA receptors (NMDARs) or metabotropic glutamate receptors (mGluRs) is due to the endocytosis of AMPA receptors (AMPARs), while long-term potentiation (LTP) triggered by NMDARs requires the exocytosis of AMPARs (Bredt and Nicoll, 2003; Collingridge et al., 2004; Malinow and Malenka, 2002; Shepherd and Huganir, 2007).
Maintaining a steady complement of AMPARs in the PSD and thereby maintaining basal synaptic strength while simultaneously allowing plasticity (i.e. LTP and LTD) requires complex regulation of the trafficking of these receptors. Immediately adjacent to the PSD are endocytic zones that contain clathrin and other endocytic proteins such as AP-2 and dynamin (Henley et al., 2011; Kennedy and Ehlers, 2006). A commonly held view is that during LTD AMPARs diffuse laterally out of the PSD where they are captured and endocytosed by clathrin-coated vesicles. The site of NMDAR-triggered AMPAR exocytosis during LTP is not completely clear with most results suggesting that AMPARs are inserted into the plasma membrane outside of the PSD and then laterally diffuse into the PSD where they are “captured” by scaffolding proteins (Henley et al., 2011; Kennedy and Ehlers, 2006). Compared to the wealth of knowledge about the molecular mechanisms underlying the exocytosis of presynaptic vesicles mediating neurotransmitter release, little is known about the mechanisms underlying the regulated exocytosis of AMPARs during LTP other than SNARE proteins are involved (Kennedy et al., 2010; Lledo et al., 1998; Lu et al., 2001; Patterson et al., 2010; Yang et al., 2008).
Here we focus on the role of postsynaptic complexin, which unlike core SNARE proteins is not generally involved in membrane fusion events but is specifically required for calcium-dependent synaptic vesicle exocytosis. Although knockout mice lacking complexin-2 have been reported to exhibit impaired LTP (Huang et al., 2000; Takahashi et al., 1999), the interpretation of this result is ambiguous since presynaptic effects on transmitter release during LTP induction cannot be ruled out. Using viral mediated expression of shRNAs to complexin-1 and -2 in vivo, we find that knockdown of complexin-1 and -2 in CA1 pyramidal cells of the hippocampus impairs LTP without detectably altering basal synaptic transmission. Rescue experiments reveal that the postsynaptic function of complexin in LTP requires binding to SNARE complexes and its N-terminal activation domain. Identical results were obtained in a culture model of LTP in which NMDAR-triggered trafficking of AMPARs to synapses was assayed. In forebrain neurons, complexins are known to function in presynaptic vesicle exocytosis with synaptotagmin-1, but we find that postsynaptic synaptotagmin-1 is not essential for LTP. Together, these results suggest that the mechanisms underlying regulated postsynaptic exocytosis of AMPARs during LTP are unexpectedly similar to those regulating presynaptic vesicle exocytosis in that both require complexins. However, the requirement for synaptotagmin-1 in calcium-triggered presynaptic vesicle exocytosis but not for AMPAR delivery during LTP indicates that complexins act in conjunction with distinct regulators on the pre- versus postsynaptic sides of excitatory synapses.
RESULTS
Postsynaptic Complexin Knockdown In Vivo Blocks LTP
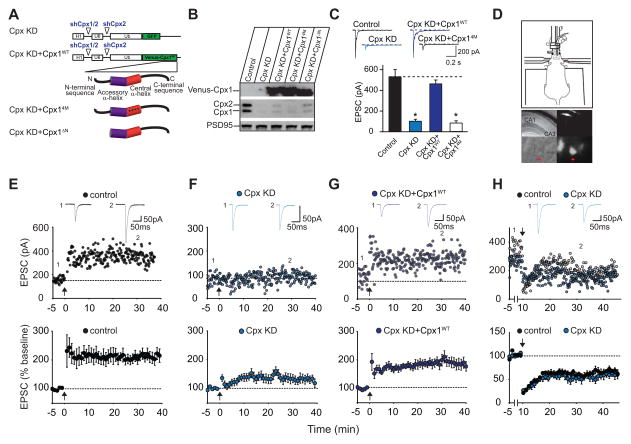
To selectively test the postsynaptic role of complexin in modulating excitatory synaptic transmission, we used a lentiviral molecular replacement strategy. Consistent with previous work (Maximov et al., 2009), simultaneous expression of two shRNAs targeted to complexin-1 and -2 (shCpx1/2) and complexin-2 alone (shCpx2) in a multipromoter lentivirus (Figure 1A) efficiently knocked down endogenous complexin-1 and -2 (Cpx KD) in dissociated cultured neurons (Figure 1B, Figure S1) resulting in a dramatic decrease in evoked EPSCs (Figure 1C). This presynaptic effect on evoked synaptic transmission in cultured neurons was rescued by simultaneous expression of an shRNA-resistant complexin-1 fused to the GFP variant, Venus, at its N-terminus (Figure 1A–C). In contrast, a mutant form of complexin-1 (Cpx14M) that is unable to bind SNARE complexes due to 4 amino acid substitutions in its central α-helix domain (R48A/R59A/K69A/Y70A) (Maximov et al., 2009) did not rescue evoked EPSCs (Figure 1A–C). These results demonstrate the effectiveness and specificity of the lenitiviral Cpx KD and confirm that the interaction of complexin with the SNARE complex is required for controlling presynaptic vesicle fusion.
Figure 1. Postsynaptic Knockdown of Complexin-1 and -2 (Cpx KD) In Vivo Blocks LTP.
(A) Diagram of shRNA-expressing lentiviruses. (B) Western blots from cultured neurons showing efficacy of Cpx KD and its replacement by Venus-complexins. (C) Effects of Cpx KD and its replacement by Cpx1WT and Cpx14M on evoked AMPAR EPSCs in cultured neurons. Sample traces are shown above the bar graph. For each group, data are from 15 different neurons from 3 different cultures (Bar graphs represent means ± SEM; *p < 0.001). (D) Diagram (upper panel) showing mouse on stereotaxic apparatus for in vivo injection of lentiviruses. Low magnification DIC image of an acute hippocampal slice (middle left panel) and CA1 pyramidal cell layer (at 40X magnification, lower left panel) from injected animal. Same images (middle and lower right panels) showing GFP expression. (E) Sample experiment (top panel) and summary graph (lower panel) of LTP in control CA1 pyramidal cells recorded from slices prepared from animals injected with Cpx KD lentiviruses. In this and all subsequent figures: arrow indicates the time of tetanic stimulation; sample averaged EPSCs during the baseline (1) and 30 min post-LTP induction (2) are shown above the sample experiment. (F) Sample experiment (top panel) and summary graph (lower panel) of impaired LTP in Cpx KD cells. (G) Sample experiment (top panel) and summary graph (bottom panel) of LTP in Cpx KD cells also expressing recombinant full-length complexin-1 (Cpx KD+Cpx1WT). (H) Sample experiment (top panel) and summary graph (bottom panel) of LTD in control cells and Cpx KD cells. Bottom graphs in E–H represent means ± SEM.
The Cpx KD lentiviruses provided tools to study the role of postsynaptic complexin in LTP at excitatory synapses on CA1 pyramidal cells in acute hippocampal slices, the synapses and preparations from which most mechanistic understanding of LTP has been generated (Malenka and Nicoll, 1999). We stereotactically injected the lentiviruses into the CA1 region of young adult mice in vivo (Figure 1D) and prepared acute hippocampal slices 10–15 days later. Whole-cell patch-clamp recordings were then made from infected CA1 pyramidal cells in slices in which the presynaptic release machinery was unaltered by the complexin shRNAs as evidenced by the absence of GFP in CA3 pyramidal cells (Figure 1D). Thus, Cpx KD only occurred in the postsynaptic compartment of the synapses that were studied. Control, uninfected cells recorded in slices prepared from injected animals exhibited robust LTP (Figure 1E; 217 ± 18% of baseline, n=10 cells, 9 mice). In contrast, Cpx KD cells exhibited a marked deficit in LTP (Figure 1F; 139 ± 15%, n=14 cells, 9 mice). The impairment in LTP caused by Cpx KD was almost completely rescued by simultaneous expression of shRNA-resistant full-length complexin-1 fused to Venus (Cpx KD+Cpx1WT) (Figure 1G; 190 ± 17%, n=9 cells, 7 mice). The rescue of LTP by Cpx1WT provides strong evidence that the impairment of LTP caused by Cpx KD was not due to off-target effects of the shRNAs. Importantly, in these experiments neurons providing presynaptic inputs to the cells from which we recorded were not infected. Thus the observed effect on LTP must be postsynaptic.
To determine if postsynaptic complexins are specifically required for LTP and not more globally involved in multiple forms of plasticity, we examined NMDAR-dependent long-term depression (LTD), which involves internalization of synaptic AMPARs, not their delivery to synapses (Bredt and Nicoll, 2003; Collingridge et al., 2004; Malinow and Malenka, 2002; Shepherd and Huganir, 2007). There was no difference in the generation of LTD between control, uninfected cells and Cpx KD cells (Figure 1H; control 61 ± 7%, n=5 cells, 5 mice; Cpx KD 59 ± 8%, n=5 cells, 5 mice). This result is consistent with the hypothesis that complexin plays a specific role in the membrane fusion events underlying the exocytosis of AMPARs during LTP and that its knockdown is not generally impairing plasticity mechanisms.
Postsynaptic Complexin Knockdown Does Not Affect Basal Synaptic Transmission
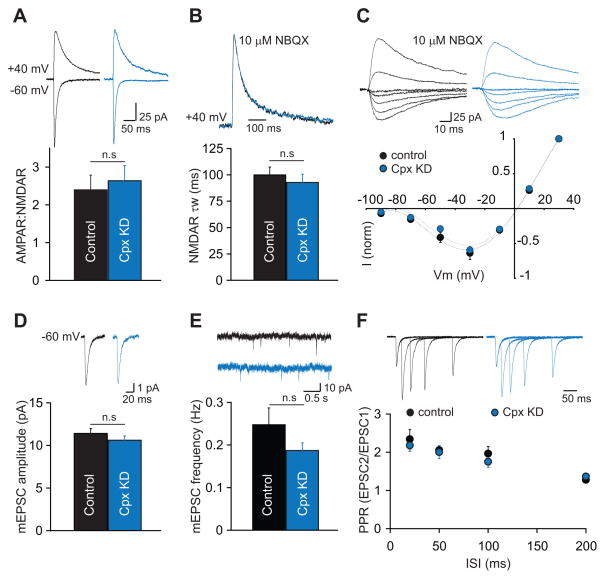
A critical question for interpreting the effects of Cpx KD on LTP and for understanding complexin’s postsynaptic role in regulating excitatory synaptic transmission is whether the Cpx KD affects basal AMPAR-mediated and/or NMDAR-mediated synaptic responses. Complexin might be involved in the constitutive exocytosis that maintains basal levels of AMPARs and NMDARs at synapses. As an assay for effects of postsynaptic Cpx KD on basal synaptic responses we measured the ratio of AMPAR-mediated EPSCs (AMPAR EPSCs) to NMDAR-mediated EPSCs (NMDAR ESPCs), a standard measure for detecting changes in synaptic strength (Kauer and Malenka, 2007). This ratio was not affected by Cpx KD (Figure 2a; control 2.40 ± 0.39, n=9 cells, 6 mice; Cpx KD 2.63 ± 0.40, n=11 cells, 8 mice), indicating that if postsynaptic Cpx KD altered basal synaptic responses, it affected both AMPARs and NMDARs to equivalent degrees. To determine if the lack of LTP due to the Cpx KD was caused by a change in the composition of synaptic NMDARs, we compared the weighted decay time constants of isolated NMDAR EPSCs at +40 mV. The time course of NMDAR EPSCs was the same in both Cpx KD and control cells demonstrating that the subunit composition of synaptic NMDARs was unaffected (Figure 2B; control 100 ± 8 ms, n=8 cells, 4 mice; Cpx KD 93 ± 8 ms, n=8 cells, 4 mice). In addition, the current-voltage relationship of NMDAR EPSCs was normal in Cpx KD cells (Figure 2C). These findings, taken together with the normal NMDAR-dependent LTD in Cpx KD cells (Figure 1H), provides strong evidence that an impairment in NMDAR function does not account for the impairment in LTP caused by Cpx KD.
Figure 2. Postsynaptic Knockdown of Complexin-1 and -2 (Cpx KD) In Vivo Does Not Alter Basal Synaptic Transmission.
(A) The ratio of AMPAR- to NMDAR-mediated EPSCs is unchanged in Cpx KD cells. Representative EPSCs recorded at −60 mV and +40 mV are shown above the bar graph. (B) The weighted decay time constant of isolated NMDAR EPSCs at +40 mV is unchanged by postsynaptic Cpx KD. Scaled NMDAR EPSCs from two representative cells are shown above the bar graph. (C) Postsynaptic Cpx KD does not affect the I/V relationship of isolated NMDAR EPSCs. Representative traces from the two cells are shown above the graph. (D) The mean amplitude of mEPSCs is unaltered by postsynaptic Cpx KD. Averaged mEPSCs from representative cells are shown above the graph. (E) The mean frequency of mEPSCs is also unchanged by Cpx KD. Short traces of recordings from representative cells are shown above the graph. (F) Paired-pulse ratios of AMPAR EPSCs are unchanged in Cpx KD cells. EPSCs from two representative cells are shown above the graph. In all panels, bar graphs and individual points represent means ± SEM.
To further investigate possible effects of the Cpx KD on AMPAR-mediated transmission, we recorded miniature EPSCs (mEPSCs; in 1 μM TTX). Average mEPSC amplitude was not affected by the Cpx KD (Figure 2D; control 11.4 ± 0.6 pA, n=11 cells, 6 mice; Cpx KD 10.6 ± 0.5 pA, n=13 cells, 6 mice) nor was average mEPSC frequency (Figure 2E; control 0.25 ± 0.04 Hz, Cpx KD 0.19 ± 0.02 Hz). Together with the normal AMPAR/NMDAR EPSC ratios, these results suggest that postsynaptic Cpx KD does not affect basal AMPAR- nor NMDAR-mediated synaptic transmission. As a final test for effects on basal synaptic transmission, we calculated paired-pulse ratios and found that postsynaptic Cpx KD had no effects suggesting that, as expected, presynaptic function at synapses on Cpx KD cells was also unaffected (Figure 2F; PP20 control 2.34 ± 0.26, Cpx KD 2.18 ± 0.15; PP50 control 2.05 ± 0.12, Cpx KD 2.00 ± 0.17; PP100 control 1.96 ± 0.18, Cpx KD 1.75 ± 0.14; PP200 control 1.28 ± 0.08, Cpx KD 1.36 ± 0.04; control n=6 cells, 4 mice; Cpx KD n=7 cells, 5 mice).
SNARE Complex Interaction and Activation Domain of Postsynaptic Complexin Are Required for LTP
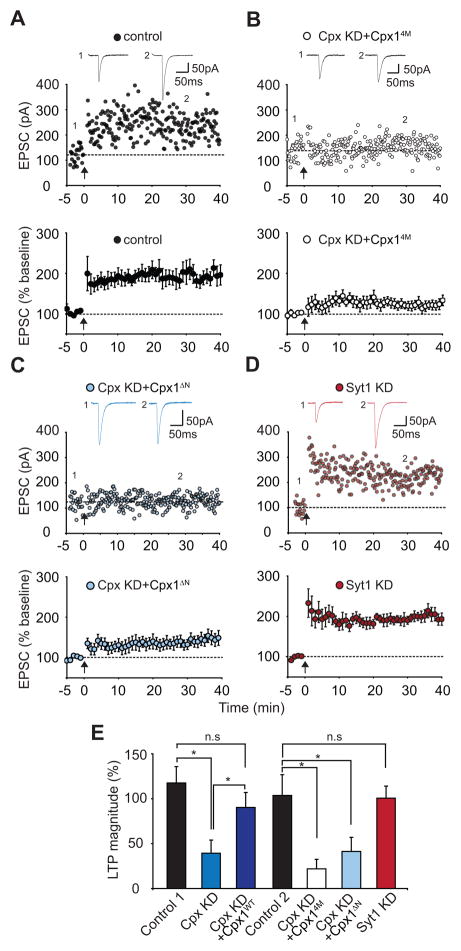
Our results thus far suggest that postsynaptic complexin plays a critical role in the NMDAR-dependent delivery of AMPARs during LTP, yet is not required for the mechanisms underlying the constitutive delivery of AMPARs and NMDARs to synapses. To further explore the molecular mechanisms by which complexin functions in LTP, we replaced endogenous complexin-1 and -2 with mutant forms of complexin-1 with known effects on presynaptic function. We first tested the SNARE-dependence of postsynaptic complexin function by expressing the shRNA-resistant 4M-mutant of complexin-1 (Cpx14M) along with the shRNAs (Cpx KD+Cpx14M). Cells expressing Cpx KD+Cpx14M showed greatly reduced LTP compared to interleaved control cells (Figure 3A and B; control, 197 ± 13%, n=9 cells, 9 mice; Cpx KD+Cpx14M, 122 ± 11%, n=8 cells, 6 mice). We next examined an N-terminal mutant form of complexin (Cpx1ΔN) in which its first 26 amino acid residues were deleted. These amino acids are critical for calcium-triggered synaptic vesicle exocytosis (Maximov et al., 2009; Xue et al., 2008). Cells expressing the replacement Cpx1ΔN construct (Cpx KD+Cpx1ΔN) also exhibited impaired LTP (Figure 3C; 141 ± 15%, n=9 cells, 7 mice).
Figure 3. Postsynaptic Complexin Function in LTP Requires Its Interaction With the SNARE Complex and Its N-terminal Activation Domain.
(A) Sample experiment (top panel) and summary graph (bottom panel) of LTP in control cells that were interleaved with the complexin replacement experiments. (B) Sample experiment (top panel) and summary graph (bottom panel) of impaired LTP in Cpx KD cells expressing the Cpx14M mutant (Cpx KD+Cpx14M). (C) Sample experiment (top panel) and summary graph (bottom panel) of impaired LTP in Cpx KD cells expressing Cpx1ΔN mutant (Cpx KD+Cpx1ΔN). (D) Sample experiment (top panel) and summary graph (bottom panel) of LTP in synaptotagmin-1 knockdown (Syt1 KD) cells. (E) Summary graph of the effects of the molecular manipulations of Cpx and Syt1 on LTP. *p < 0.05. Bottom graphs in A–D and bar graphs in E represent means ± SEM.
During neurotransmitter release at synapses on hippocampal pyramidal neurons, complexin functions as an obligatory component of the calcium triggering of vesicle fusion by synaptotagmin-1 (Sudhof and Rothman, 2009; Tang et al., 2006). Therefore, a critical question is whether complexin postsynaptically also acts in conjunction with synaptotagmin-1 to trigger AMPAR exocytosis. To address this question, we injected a lentivirus expressing a highly effective shRNA to synaptotagmin-1 (Yang et al., 2010). Postsynaptic expression of the synaptotagmin-1 shRNA in CA1 pyramidal cells in vivo had no detectable effect on LTP (Figure 3D; 200 ± 13%, n=6 cells, 5 mice). Together these results (Figure 3E) suggest that complexin functions by a similar SNARE-dependent mechanism in presynaptic vesicle exocytosis and postsynaptic AMPAR exocytosis during LTP but utilizes different regulators.
Postsynaptic Complexin Is Required for NMDA Receptor-Triggered Delivery of Endogenous AMPA Receptors in Cultured Neurons
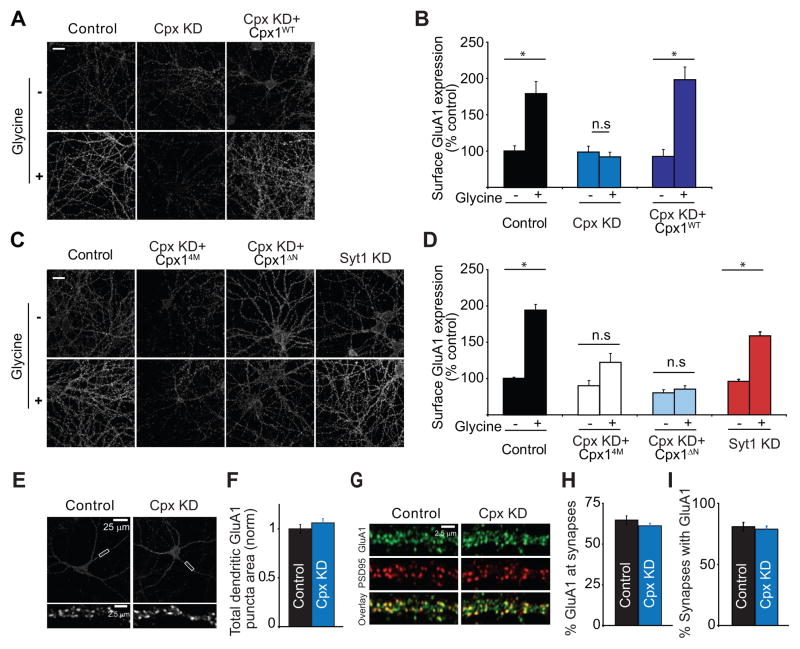
A major advantage of the approach we have taken is that the molecular manipulations of complexin were performed in vivo solely in the postsynaptic compartment of synapses, allowing LTP to be measured electrophysiologically in acute hippocampal slices. However, electrophysiological assays do not allow direct, unequivocal measurement of changes in the number of synaptic AMPARs. To directly test the role of complexin in the NMDAR-triggered delivery of endogenous AMPARs to the plasma membrane, we turned to a neuronal culture model of LTP in which pharmacological activation of NMDARs leads to an increase in the surface expression of synaptic AMPARs (Kennedy et al., 2010; Lu et al., 2001; Park et al., 2004; Passafaro et al., 2001). Consistent with prior results, application of the NMDAR co-agonist glycine in the presence of a GABAA receptor antagonist, a glycine receptor antagonist, and tetrodotoxin caused a significant increase in the surface expression of endogenous GluA1-containing AMPARs compared to unstimulated control cells (Figure 4A and B: control 100.3 ± 7.2%, n=30; glycine 179.5 ± 16.6%, n=30). This increase was blocked by D-APV (data not shown) and was associated with an increase in the amplitudes of miniature EPSCs (Figure S2) indicating that the increase in surface AMPARs increased synaptic strength.
Figure 4. Complexin-1 and -2 Knockdown (Cpx KD) Prevents Glycine-Induced Increase in Surface AMPAR Expression in Hippocampal Neuronal Cultures.
(A and B) Representative images (A) and summary graphs (B) showing increase in dendritic surface GluA1 following glycine-induced “LTP” in control cells but not in Cpx KD cells. The “LTP” was rescued by expression of wildtype complexin-1. Scale bar = 10 μm. (C and D) Representative images (C) and summary graphs (D) showing that the Cpx14M mutant and Cpx1ΔN mutant do not rescue the glycine-induced “LTP” that was generated in cells from the same culture preparations (Control) and that Syt1 KD does not block this cell culture model of LTP. (E and F) Representative images (E) and summary graphs (F) showing that the total pools of GluA1-containing AMPARs in dendrites are not affected by Cpx KD. (G–I) Representative images (G) and summary graphs (H and I) showing that the percentage of GluA1 puncta at synapses as defined by co-localization with PSD-95 (H) does not change after Cpx KD nor does the percentage of synapses containing GluA1 (I). In all panels, each bar represents mean ± SEM (*p < 0.05).
Consistent with the lack of effect of Cpx KD on basal synaptic responses in hippocampal slices, the Cpx KD in cultured neurons had no significant effect on the basal surface expression of AMPARs but blocked the NMDAR-triggered increase in AMPAR surface expression (Figure 4A and B: control Cpx KD 98.4 ± 6.9%, n=18; glycine Cpx KD 91.8 ± 6.5%, n=30). This effect of the Cpx KD was rescued by expression of the shRNA-resistant wildtype complexin-1 (Figure 4A and B: control Cpx KD+Cpx1WT 92.4 ± 9.5%, n=17; glycine Cpx KD+Cpx1WT 197.1 ± 17.3%, n=29) demonstrating again that off-target effects of the shRNAs are unlikely to account for their actions. Importantly, decreases in neurotransmitter release cannot account for the block of AMPAR insertion following glycine application since Cpx KD causes a significant increase in mESPC frequency in cultured neurons (Maximov et al., 2009; Yang et al., 2010), and action potentials were blocked by tetrodotoxin prior to glycine application.
We next examined the ability of the Cpx4M and Cpx1ΔN mutants to rescue the glycine-induced increase in AMPAR surface expression, while always confirming the efficacy of the glycine treatment in neurons from the same culture preparations (Figure 4C and D). Similar to their lack of effects on LTP in acute slices, these mutant forms of complexin did not rescue the block of the NMDAR-triggered increase in AMPAR surface expression caused by Cpx KD (control 100.0 ± 1.6%, n=30; glycine 193.8 ± 7.9%, n=30; control Cpx KD+Cpx14M 89.9 ± 7.2%, n=18; glycine Cpx KD+ Cpx14M 122.8 ± 12.3%, n=30; control Cpx KD+Cpx1ΔN 80.4 ± 4.2%, n=30; glycine Cpx KD+ Cpx1ΔN 85.3 ± 4.9%, n=30). Finally, we tested the effects of knocking down Syt1 in this culture model of LTP and found that this manipulation did not prevent the increase in AMPAR surface expression elicited by glycine application (Fig. 4C and D: control Syt1 KD 96.2 ± 2.7%, n=30; glycine Syt1 KD 159.8 ± 5.5%, n=30). These results provide an independent confirmation of the critical role of postsynaptic complexin, its interaction with SNARE complexes and its N-terminal sequence in the NMDAR-triggered exocytosis of AMPARs that is required for the normal expression of LTP.
The intracellular pool of AMPARs that undergo exocytosis in response to NMDAR activation during LTP induction has been suggested to reside in recycling endosomes (RE’s) that also contain transferrin receptors (TfRs) (Park et al., 2004; Petrini et al., 2009). It is conceivable that the impairment of LTP caused by Cpx KD was due to a depletion of this pool or its mis-localization rather than a block of AMPAR exocytosis itself. Such an explanation for our results requires that the maintenance of AMPARs at synapses be independent of this pool since basal synaptic transmission in slices and AMPAR surface expression in cell culture were not affected by the Cpx KD. Nevertheless, to address this possibility we first measured the entire pool of GluA1-containing AMPARs in dendrites by permeablizing cells and staining with the GluA1 antibody. These experiments demonstrated that Cpx KD had no effect on the total levels of GluA1 in dendrites (Figure 4E and F: control 1.0 ± 0.04, n=20; Cpx KD 1.06 ± 0.04, n = 20). We next examined the percentage of GluA1 puncta that localized to synapses as defined by co-localization with PSD95 and again Cpx KD had no detectable effects (Figure 4G and H: control 64.8 ± 2.6%, n=14; Cpx KD 61.2 ± 1.6%, n = 14). Furthermore, the proportion of synapses that contained GluA1 was unaffected by the Cpx KD (Figure 4I: control 80.8 ± 3.5%, n=14; Cpx KD 78.9 ± 2.5%, n = 14). We also examined whether Cpx KD might affect the proportion of RE’s containing AMPARs. However, Cpx KD did not affect the percentage of RE’s containing GluA1 (Figure S3) nor the percentage of dendritic GluA1 puncta that co-localized with RE’s (Figure S3). Cpx KD also did not affect the subcellular localization of RE’s relative to dendritic spines as defined by simultaneous expression of recombinant TfR fused to mCherry and soluble GFP (Figure S3). Thus, consistent with the lack of effects of Cpx KD on basal synaptic transmission, these results demonstrate that Cpx KD had no detectable effects on the pool of intracellular AMPARs that are thought to be the source of the AMPARs that are exocytosed during LTP.
A final possibility is that the Cpx KD did in fact affect the constitutive delivery of AMPARs to synapses but that the basal surface expression of AMPARs and thus basal AMPAR EPSCs were not affected because of a compensatory decrease in the rate of steady state AMPAR endocytosis. To address this possibility, we measured the effect of Cpx KD on constitutive AMPAR endocytosis (Bhattacharyya et al., 2009). There was no detectable effect of Cpx KD on the constitutive endocytosis of endogenous surface AMPARs (Figure S3), thus ruling out this hypothesis.
DISCUSSION
Previous work showed that postsynaptic SNARE-mediated membrane fusion is required for LTP (Kennedy et al., 2010; Lledo et al., 1998; Lu et al., 2001). However, these experiments focused on SNARE proteins that are ubiquitously involved in both regulated and constitutive membrane fusion events. Thus, the molecular mechanisms underlying the regulated, calcium-dependent triggering of AMPAR exocytosis during LTP remained unknown. Using in vivo stereotaxic injection of lentiviruses, we have molecularly manipulated complexin only in CA1 pyramidal cells and thus only in the postsynaptic compartment of the excitatory synapses being studied in acute hippocampal slices. The results, which were confirmed in a neuronal culture model of LTP, provide strong evidence that complexin is a key component of the molecular mechanism by which NMDAR-mediated increases in calcium during LTP induction leads to the exocytosis of AMPARs at the postsynaptic membrane. The importance of postsynaptic complexin in LTP is consistent with immunohistochemical and electron microscopic studies that confirm the presence of complexin in dendritic spines and shafts (McMahon et al., 1995; Yamada et al., 1999). Our results also suggest that postsynaptic complexin is not required for the constitutive delivery of AMPARs and NMDARs into the synaptic plasma membrane, a process that likely occurs at a much slower timescale than the delivery of AMPARs during LTP (Adesnik et al., 2005; Washbourne et al., 2002). Consistent with this conclusion, Cpx KD had no effects on the intracellular pools of AMPARs found in dendrites nor on the dendritic RE’s that have been suggested to be the source of the AMPARs that are exocytosed during LTP (Park et al., 2004).
Further support for the idea that separate constitutive and regulated pathways for the exocytosis of AMPARs exist comes from the findings that botulinum toxins targeting synaptobrevin-2 block LTP (Lledo et al., 1998) yet deletion of synpatobrevin-2 has no effect on recruitment of AMPARs to synapses as assayed by mEPSC amplitudes (Schoch et al., 2001). A small (~25%) decrease in mEPSC amplitudes was previously observed in complexin double and triple knockout mice (Xue et al., 2008) but not in Cpx KD neurons (Maximov et al., 2009). Thus, it is possible that chronic deletion of complexins from a neuron also alters constitutive trafficking of AMPARs. However, because complexins were absent from both pre- and postsynaptic compartments in the knockout mice, the decrease in mEPSC amplitudes may reflect presynaptic changes in transmitter release kinetics, decreases in the transmitter content of vesicles or some modest effect on AMPAR content at synapses either due to the lack of LTP throughout development or some contributory but non-mandatory role for complexins in the delivery of synaptic AMPARs.
Examination of mutant forms of complexin revealed that complexin’s function during LTP requires binding to SNARE complexes and its N-terminal sequence, both of which are required for calcium-dependent neurotransmitter release. However, there are important differences in the properties of the calcium-triggered exocytosis underlying neurotransmitter release and postsynaptic insertion of AMPARs during LTP. In presynaptic terminals, neurotransmitter-containing vesicles are docked at the plasma membrane and primed such that fusion occurs rapidly, within 1 msec of the action-potential dependent rise in calcium. In contrast, in postsynaptic dendritic spines, intracellular organelles containing AMPARs have not been shown to sit “docked” closely adjacent to the plasma membrane and the exocytosis of AMPARs following NMDAR activation takes time to develop and lasts tens of seconds or minutes (Patterson et al., 2010; Petrini et al., 2009; Yang et al., 2008; Yudowski et al., 2007). Differences in the molecular machinery mediating pre- versus postsynaptic exocytosis likely contribute to these important functional differences.
We also demonstrate that the major calcium-trigger for neurotransmitter release in rostral brain regions, synaptotagmin-1, is not required for the postsynaptic expression of LTP. While formally it is possible that complexin may function independently of a synaptotagmin in the exocytosis of AMPARs, in all preparations that have been examined thus far, the membrane fusion reactions that require complexin also require a synaptotagmin isoform that is known to trigger synaptic or neuroendocrine exocytosis. (synaptotagmin-1,-2, -7, or -9: see Cai et al., 2008; Schonn et al., 2008; Xu et al., 2007). Since of these synaptotagmins only synaptotagmin-1 and -7 are known to be present in the cells we analyzed, it is possible that synaptotagmin-7 is involved. Alternatively, a different synaptotagmin isoform or related protein may mediate the calcium triggering of exocytosis during LTP induction. Given that that there are a total of 16 synaptotagmin isoforms, a major effort will be required to find the specific isoform(s) that are required for LTP. Importantly, this hypothesis does not require that the additional proteins required for the complexin-dependent exocytosis of AMPARs directly bind calcium. For example, the critical postsynaptic trigger for this exocytosis could be the target of any of the protein kinases implicated in the induction of LTP.
The specific SNARE proteins involved in the postsynaptic exocytosis of AMPARs are also likely to be different than those involved in transmitter release since results to date suggest that synaptobrevin-2 is selectively essential for regulated but not constitutive AMPAR exocytosis. It has been suggested that syntaxin-4 defines a postsynaptic microdomain for the exocytosis of RE’s that contain AMPARs (Kennedy et al., 2010). However, complexins do not bind to SNARE complexes containing syntaxin-4 but rather exhibit strong binding to SNARE complexes containing syntaxin-1 or -3 with reduced binding to SNARE complexes containing syntaxin 2 (Pabst et al., 2000). Further work will be needed to clarify this apparent discrepancy. The specific SNAP-25 homolog involved in AMPAR exocytosis during LTP is also not known although both SNAP-23 and SNAP-25 have been suggested to be important for the trafficking of synaptic NMDARs (Lau et al., 2010; Suh et al., 2010). Furthermore, the roles in postsynaptic AMPAR trafficking of Sec1-Munc18 proteins such as Munc18-1, which are required for all intracellular fusion reactions in conjunction with SNARE proteins (Sudhof and Rothman, 2009), will need to be defined for a molecular understanding of the mechanisms underlying LTP comparable to the current understanding of the molecular mechanisms responsible for neurotransmitter release.
EXPERIMENTAL PROCEDURES
Stereotaxic injections of lentiviruses were made into the CA1 region of P18-22 C57BL/6 mice and whole-cell patch clamp recordings were performed from CA1 pyramidal cells in acute hippocampal slices that were prepared 10–14 days later. Immunocytochemical assays were performed in 18–21 DIV dissociated hippocampal cultures 9–11 days after infection with lentiviruses. All experimental procedures are described in detail in Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Daniela Iona Ion and Scarlett Fang for technical assistance, Sandra Jurado for contributing data, the Chen lab for providing neuronal cultures, and members of the Malenka and Südhof labs for constructive comments and help during the course of the experiments. M.A. and J.S.P. performed electrophysiological recordings from acute slices and stereotaxic injections. D.G. performed AMPAR surface expression assays in hippocampal cultures. M.A. constructed plasmids, generated lentivirus, performed western blot analyses and colocalization imaging assays. X.Y. performed electrophysiological assays in hippocampal cultures. Y.J. K.-W. designed shCpx2 and Venus-Cpx1. M.A., J.S.P., TC.S. and R.C.M. wrote the manuscript. All authors reviewed the paper and edited it. R.C.M. and T.C.S. directed and coordinated the project. Supported by NIH grants MH06334 (to R.C.M.) and P50 MH0864 (R.C.M. and T.C.S.).
Footnotes
Supplemental information includes three figures and Supplemental Experimental Procedures and can be found with this article online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adesnik H, Nicoll RA, England PM. Photoinactivation of native AMPA receptors reveals their real-time trafficking. Neuron. 2005;48:977–985. doi: 10.1016/j.neuron.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Biou V, Xu W, Schluter O, Malenka RC. A critical role for PSD-95/AKAP interactions in endocytosis of synaptic AMPA receptors. Nat Neurosci. 2009;12:172–181. doi: 10.1038/nn.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Cai H, Reim K, Varoqueaux F, Tapechum S, Hill K, Sorensen JB, Brose N, Chow RH. Complexin II plays a positive role in Ca2+-triggered exocytosis by facilitating vesicle priming. Proc Natl Acad Sci USA. 2008;105:19538–19543. doi: 10.1073/pnas.0810232105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- Elias GM, Nicoll RA. Synaptic trafficking of glutamate receptors by MAGUK scaffolding proteins. Trends Cell Biol. 2007;17:343–352. doi: 10.1016/j.tcb.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Fernandez-Chacon R, Konigstorfer A, Gerber SH, Garcia J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Sudhof TC. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Sudhof TC. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Giraudo CG, Eng WS, Melia TJ, Rothman JE. A clamping mechanism involved in SNARE-dependent exocytosis. Science. 2006;313:676–689. doi: 10.1126/science.1129450. [DOI] [PubMed] [Google Scholar]
- Henley JM, Barker EA, Glebov OO. Routes, destinations and delays: recent advances in AMPA receptor trafficking. Trends Neurosci. 2011;34:258–268. doi: 10.1016/j.tins.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GZ, Ujihara H, Takahashi S, Kaba H, Yagi T, Inoue S. Involvement of complexin II in synaptic plasticity in the CA1 region of the hippocampus: the use of complexin II-lacking mice. Japan J Pharmacol. 2000;84:179–187. doi: 10.1254/jjp.84.179. [DOI] [PubMed] [Google Scholar]
- Huntwork S, Littleton JT. A complexin fusion clamp regulates neurotransmitter release and synaptic growth. Nat Neurosci. 2007;10:1235–1237. doi: 10.1038/nn1980. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kennedy MJ, Davison IG, Robinson CG, Ehlers MD. Syntaxin-4 defines a domain for activity-dependent exocytosis in dendritic spines. Cell. 2010;141:524–535. doi: 10.1016/j.cell.2010.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ, Ehlers MD. Organelles and trafficking machinery for postsynaptic plasticity. Annu Rev Neurosci. 2006;29:325–362. doi: 10.1146/annurev.neuro.29.051605.112808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CG, Takayasu Y, Rodenas-Ruano A, Paternain AV, Lerma J, Bennett MV, Zukin RS. SNAP-25 is a target of protein kinase C phosphorylation critical to NMDA receptor trafficking. J Neurosci. 2010;30:242–254. doi: 10.1523/JNEUROSCI.4933-08.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo PM, Zhang X, Sudhof TC, Malenka RC, Nicoll RA. Postsynaptic membrane fusion and long-term potentiation. Science. 1998;279:399–403. doi: 10.1126/science.279.5349.399. [DOI] [PubMed] [Google Scholar]
- Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Ann Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Maximov A, Tang J, Yang X, Pang ZP, Sudhof TC. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science. 2009;323:516–521. doi: 10.1126/science.1166505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Missler M, Li C, Sudhof TC. Complexins: cytosolic proteins that regulate SNAP receptor function. Cell. 1995;83:111–119. doi: 10.1016/0092-8674(95)90239-2. [DOI] [PubMed] [Google Scholar]
- Pabst S, Hazzard JW, Antonin W, Sudhof TC, Jahn R, Rizo J, Fasshauer D. Selective interaction of complexin with the neuronal SNARE complex. Determination of the binding regions. J Biol Chem. 2000;275:19808–19818. doi: 10.1074/jbc.M002571200. [DOI] [PubMed] [Google Scholar]
- Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- Patterson MA, Szatmari EM, Yasuda R. AMPA receptors are exocytosed in stimulated spines and adjacent dendrites in a Ras-ERK-dependent manner during long-term potentiation. Proc Natl Acad Sci USA. 2010;107:15951–15956. doi: 10.1073/pnas.0913875107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini EM, Lu J, Cognet L, Lounis B, Ehlers MD, Choquet D. Endocytic trafficking and recycling maintain a pool of mobile surface AMPA receptors required for synaptic potentiation. Neuron. 2009;63:92–105. doi: 10.1016/j.neuron.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reim K, Mansour M, Varoqueaux F, McMahon HT, Sudhof TC, Brose N, Rosenmund C. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell. 2001;104:71–81. doi: 10.1016/s0092-8674(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Rizo J, Rosenmund C. Synaptic vesicle fusion. Nat Struct Mol Biol. 2008;15:665–674. doi: 10.1038/nsmb.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannevin RH, Huganir RL. Postsynaptic organization and regulation of excitatory synapses. Nat Rev Neurosci. 2000;1:133–141. doi: 10.1038/35039075. [DOI] [PubMed] [Google Scholar]
- Schoch S, Deak F, Konigstorfer A, Mozhayeva M, Sara Y, Sudhof TC, Kavalali ET. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- Schonn JS, Maximov A, Lao Y, Sudhof TC, Sorensen JB. Synaptotagmin-1 and -7 are functionally overlapping Ca2+ sensors for exocytosis in adrenal chromaffin cells. Proc Natl Acad Sci USA. 2008;105:3998–4003. doi: 10.1073/pnas.0712373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Sala C. PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci. 2001;24:1–29. doi: 10.1146/annurev.neuro.24.1.1. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh YH, Terashima A, Petralia RS, Wenthold RJ, Isaac JT, Roche KW, Roche PA. A neuronal role for SNAP-23 in postsynaptic glutamate receptor trafficking. Nat Neurosci. 2010;13:338–343. doi: 10.1038/nn.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Ujihara H, Huang GZ, Yagyu K, Sanbo M, Kaba H, Yagi T. Reduced hippocampal LTP in mice lacking a presynaptic protein: complexin II. Eur J Neurosci. 1999;11:2359–2366. doi: 10.1046/j.1460-9568.1999.00652.x. [DOI] [PubMed] [Google Scholar]
- Tang J, Maximov A, Shin OH, Dai H, Rizo J, Sudhof TC. A complexin/synaptotagmin 1 switch controls fast synaptic vesicle exocytosis. Cell. 2006;126:1175–1187. doi: 10.1016/j.cell.2006.08.030. [DOI] [PubMed] [Google Scholar]
- Washbourne P, Bennett JE, McAllister AK. Rapid recruitment of NMDA receptor transport packets to nascent synapses. Nat Neurosci. 2002;5:751–759. doi: 10.1038/nn883. [DOI] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Xu J, Mashimo T, Sudhof TC. Synaptotagmin-1, -2, and -9: Ca(2+) sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron. 2007;54:567–581. doi: 10.1016/j.neuron.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Xue M, Lin YQ, Pan H, Reim K, Deng H, Bellen HJ, Rosenmund C. Tilting the balance between facilitatory and inhibitory functions of mammalian and Drosophila Complexins orchestrates synaptic vesicle exocytosis. Neuron. 2009;64:367–380. doi: 10.1016/j.neuron.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Stradomska A, Chen H, Brose N, Zhang W, Rosenmund C, Reim K. Complexins facilitate neurotransmitter release at excitatory and inhibitory synapses in mammalian central nervous system. Proc Natl Acad Sci USA. 2008;105:7875–7880. doi: 10.1073/pnas.0803012105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Saisu H, Ishizuka T, Takahashi H, Abe T. Immunohistochemical distribution of the two isoforms of synaphin/complexin involved in neurotransmitter release: localization at the distinct central nervous system regions and synaptic types. Neuroscience. 1999;93:7–18. doi: 10.1016/s0306-4522(99)00104-9. [DOI] [PubMed] [Google Scholar]
- Yang X, Kaeser-Woo YJ, Pang ZP, Xu W, Sudhof TC. Complexin clamps asynchronous release by blocking a secondary Ca(2+) sensor via its accessory alpha helix. Neuron. 2010;68:907–920. doi: 10.1016/j.neuron.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang XB, Frerking M, Zhou Q. Delivery of AMPA receptors to perisynaptic sites precedes the full expression of long-term potentiation. Proc Natl Acad Sci USA. 2008;105:11388–11393. doi: 10.1073/pnas.0802978105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudowski GA, Puthenveedu MA, Leonoudakis D, Panicker S, Thorn KS, Beattie EC, von Zastrow M. Real-time imaging of discrete exocytic events mediating surface delivery of AMPA receptors. J Neurosci. 2007;27:11112–11121. doi: 10.1523/JNEUROSCI.2465-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.