Abstract
The nuclear receptor (NR) superfamily is composed of 48 members in humans and includes receptors for steroid hormones, thyroid hormone, various lipids and oxysterols. This superfamily has been a rich source of drug targets for myriad diseases including inflammation, cancer, and metabolic disorders. Approximately half of the superfamily have well characterized natural ligands while the remaining receptors are considered orphan receptors and remain a focus of a number of investigators assessing their ability to be regulated by ligands. Here, we review recent discoveries that yield important insight into the druggability of three orphan nuclear receptors: the retinoic acid receptor-like orphan receptors (RORs), peroxisome proliferator-activated receptor γ (PPARγ), and liver receptor homologue-1 (LRH-1).
The nuclear receptor (NR) superfamily is composed of 48 members in humans and includes receptors for steroid hormones, thyroid hormone, various lipids and oxysterols. NRs function as ligand-dependent transcription factors and are share a modular domain structure (Fig. 1) (Mangelsdorf, et al., 1995). A general characteristic of members of the superfamily is a highly conserved DNA binding domain (DBD) also called a C region. Amino-terminal to the C region is the A/B region and is highly variable among superfamily members. This domain, depending on the receptor, may contain ligand-independent transcriptional activation activity. The second most conserved region is carboxy-terminal to the DBD is the ligand binding domain (LBD) or E region. This domain is responsible for recognition and binding of the receptor’s ligand as well as ligand-dependent transcriptional activity. A relatively short region connects the C region to the E region (region D) and is known as the hinge domain. Some receptors also contain a region carboxy-terminal to the ligand binding domain known as the F region.
Figure 1.
Structural domain organization of nuclear receptors. Regions (A/B, C, D, E, and F) are indicated above the schematic and domains are indicated below the schematic. AF=Activation function, DBD=DNA binding domain, LBD=Ligand binding domain.
Approximately half of the superfamily have well characterized natural ligands while the remaining receptors are considered orphan receptors and remain a focus of a number of investigators assessing their ability to be regulated by ligands (Kliewer, et al., 1999; Mangelsdorf and Evans, 1995). The vast majority of receptors that have identified natural ligands are also validated targets for clinical drugs. This superfamily has been a rich source of drug targets for myriad diseases including inflammation, cancer, and metabolic disorders. Thus, there remains significant interest in identification of ligands that regulate orphan members of the NR superfamily due to their potential for utilization as potential drugs to treat human disease.
Over the past 2 years, there have been significant breakthroughs in the identification of novel ligands for several orphan NRs and in this review we examine the progress that has been made toward identification of ligands for retinoic acid receptor-related orphan receptors (RORs) and the liver receptor homologue 1 (LRH1) as well as a novel class of ligand for peroxisome proliferator-activated receptor γ (PPARγ) that holds promise for an improved pharmacological profile over older glitazone agonists.
Pharmacological Targeting of Retinoic Acid Receptor-Related Orphan Receptors (RORs) for Autoimmune Diseases
As the canonical domain structure and conserved sequence of members of the NR superfamily became apparent in the 1980s, several laboratories isolated additional members of this superfamily that had no identified ligands. The first member of the ROR subfamily of receptors (RORα) was based on sequence similarities to the retinoic acid receptor (RAR) and the retinoid X receptor (RAR) yielding the name “retinoic acid related-related orphan receptor” (Beckerandre, et al., 1993; Giguere, et al., 1994). Similar receptors, RORβ and RORγ, were identified shortly after RORα was identified (Carlberg, et al., 1994; Hirose, et al., 1994). RORα, RORβ and RORγ display significant sequence similarities and each ROR gene generates multiple isoforms based on alternative promoter usage and splicing. All of the isoforms vary only in the amino-terminal A/B regions of the receptors. RORα, RORβand RORγ display distinct patterns of expression. RORα is widely expressed and is found in the liver, skeletal muscle, skin, lungs, adipose tissue, kidney, thymus and brain (Hamilton, et al., 1996; Steinmayr, et al., 1998). RORβ displays a very restricted pattern of expression and is limited to the central nervous system (Andre, et al., 1998; Andre, et al., 1998). RORγ is most highly expressed in immune tissues including the thymus, but significant expression is also found in the liver, skeletal muscle, adipose tissue and kidney (Medvedev, et al., 1996). RORγt, is exclusively expressed in key cells within the immune system (Miller and Weinmann, 2009). When bound to their specific DNA response elements within the promoter of a target gene, all three RORs constitutively recruit coactivators resulting in activation of transcription of their target genes. Interestingly, another group of orphan nuclear receptors, REV-ERBs, recognize the same response elements as the RORs and are coexpressed in many tissues (Duez and Staels, 2009; Solt, et al., 2011; Yin, et al., 2010). REV-ERBs are ligand-dependent transcriptional repressors and in many cases, functionally antagonize the action of the RORs (Burris, 2008; Raghuram, et al., 2007; Yin, et al., 2007).
Distinct adaptive immune responses directed towards protection against various classes of pathogens are facilitated by differentiation of CD4+ T cells into specific types of effector T cells (TH1, TH2, and TH17 cells). RORα and RORγ have garnered significant attention over the past several years due to their essential role in development of TH17 cells. Prior to the discovery of TH17 cells, TH1 cells were considered the effector T cell type responsible for the pathology of autoimmune diseases including psoriasis, multiple sclerosis (MS), and rheumatoid arthritis among others. Key experiments in interferon γ receptor-deficient mice suggested that this may not be the case since since these mice, deficient in TH1-IFNγ signaling, were actually more susceptible to autoimmune disease (experimental autoimmune encephalomyelitis (EAE) – an animal model of MS) rather than less susceptible to disease (Ferber, et al., 1996; Willenborg, et al., 1996). These data suggested that there may be another, as of yet unidentified, cell type responsible for at least a portion of the autoimmune pathology associated with EAE. Later studies demonstrated that the IL23/IL17 cytokine axis was crucial for autoimmune disease progression in the EAE and collagen induced arthritis (CIA) models and progression was independent of the TH1 and TH2 cells (Cua, et al., 2003; Murphy, et al., 2003; Zhang, et al., 2003). Indeed, a distinct lineage of T effector cells that produce IL17 (TH17 cells) was soon identified (Harrington, et al., 2005; Park, et al., 2005). TH17 cells have since become recognized as critical mediators of autoimmune pathology (Bettelli, et al., 2008; Littman and Rudensky, 2010). Thus, it became apparent that one might be able to modulate autoimmune disease progression by regulation of TH17 cell development.
One key factor in development of the TH17 cells has been shown to be expression of RORs. RORγt was demonstrated to play a critical role in development of TH17 cells since overexpression of RORγt in naïve CD4+ T cells resulted in induction of development of IL17 producing TH17 cells and RORγt-deficient mice display impaired TH17 cell differentiation (Ivanov, et al., 2006). The transcriptional cascade leading to TH17 differentiation is complex and additional transcription factors involved in metabolic regulation such as HIF-1 and RORα have also been demonstrated to play an important role (Dang, et al., 2011; Yang, et al., 2008). Mice deficient in either RORγ or RORα or both receptors displayed resistance to development of autoimmunity (Ivanov, et al., 2006; Yang, et al., 2008) suggesting that one mechanism to reduce autoimmune pathology may be to inhibit these receptors leading to decreased TH17 cell differentiation. At the time of these discoveries, ligands had not been identified for either of these receptors but developments in 2010 suggested that both RORα and RORγ were indeed “druggable”.
Crystallographic studies of RORα suggested that a sterol such as cholesterol or cholesterol sulfate may function as a natural ligand of this receptor (Kallen, et al., 2004; Kallen, et al., 2002). We later found that hydroxycholesterols were high affinity ligands for both RORα and RORγ and that some of these such as 7-oxygenated sterols (Fig. 2) functioned as inverse agonists suppressing the constitutive activity of both of these receptors (Wang, et al., 2010; Wang, et al., 2010). The structure of the RORγ LBD bound to several oxysterols provided a clear structural basis for these sterols as natural ROR ligands (Jin, et al., 2010). A key breakthrough with this discovery was the ability to develop a radioligand binding assay for RORα and RORγ using high affinity radiolabeled oxysterols and this allowed our group to identify the first synthetic ligands for these two “orphan” receptors. The characterized liver X receptor (LXR) agonist, T0901317 (T1317) was identified as an inverse agonist for both RORα and RORγ and shown to directly bind to the LBD’s of these receptors and inhibit coactivator binding leading to suppression of their constitutive transcriptional activity (Kumar, et al., 2010). T1317 displays promiscuous activity at several NRs including LXR, farnesoid X receptor, pregnane X receptor, and RORα and RORγ limiting its ability to be used as a chemical tool to probe the utility of a selective RORα/RORγ inverse agonist in models of TH17 cell differentiation; however, a focused medicinal chemistry effort was able to develop a analogue of T1317 (SR1001) that retained activity at both RORα and RORγ but lacked activity at any other NR (Solt, et al., 2011).
Figure 2.
Chemical structure of several ROR ligands including sterol derivatives (cholesterol, cholesterol sulfate, 7α-hydroxycholesterol, ursolic acid, and digoxin as well as its derivatives) and non-steroidal compounds such as T0901317 and SR1001.
Unlike the natural ligands that have been identified, SR1001 does not contain a sterol or steroid-like structure (Fig. 2). We were able to demonstrate that SR1001 directly binds to the LBD’s of RORα and RORγ (Ki=111 to 172 nM) leading to a conformational change resulting in loss of affinity for the receptors for coactivator (SRC-2) and increased affinity for corepressor (NCoR) (Solt, et al., 2011). SR1001 effectively suppressed IL17 expression and TH17 cell development without affecting other T cell lineages (Solt, et al., 2011). Most importantly, SR1001 was effective in delaying the onset and severity of EAE in mice demonstrating proof-of-principle that a small molecule inhibitor of ROR transcriptional activity is effective in suppressing autoimmunity (Solt, et al., 2011).
Littman’s group also identified a small molecule inhibitor of RORγ and this compound was the well-known cardiac glycoside, digoxin (Huh, et al., 2011) (Fig. 2). Digoxin is used clinically for treatment of atrial fibrillation where it directly alters electrical conduction in the heart targeting the Na+/K+-ATPase. Digoxin displayed a potency (IC50) of ~2 µM for suppression of RORγ-mediated activity (Huh, et al., 2011). Digoxin displayed RORγ specificity and did not affect RORα, DHR3, DAF-12, or the androgen receptor (Huh, et al., 2011). Similar to SR1001, digoxin inhibited TH17 cell differentiation and delayed the onset and severity of EAE in mice (Huh, et al., 2011). One significant issue of digoxin is the toxicity associated with this drug and the small therapeutic window. These authors examined analogues of digoxin and demonstrated that these compounds also demonstrate activity against RORγ and TH17 cell differentiation and that the anti-TH17 cell activity does not correlate with the cardiac glycoside activity suggesting that there may be opportunities for optimization of the RORγ activity with removal of the potential cardiac toxicity (Huh, et al., 2011). A crystal structure of the RORγ LBD bound to digoxin demonstrating the molecular mechanism of inhibition of coactivator binding was reported soon after the original study identifying dixogen as a RORγ inhibitor (Fujita-Sato, et al., 2011). Given the severity of the toxicity associated with and the limited intellectual property space associated with this chemical scaffold, it is unlikely that this would be pursued by pharmaceutical companies even though Huh et al indicate that some digoxin derivatives (Fig. 2) do display RORγ inhibitory activity at levels where they do not display cellular toxicity. However, of critical importance is the fact that these data demonstrate the feasibility of targeting RORγ with small molecule inhibitors for treatment of autoimmune disorders.
Ursolic acid has also been recently shown to inhibit TH17 cell differentiation via targeting RORγ (Xu, et al., 2011) (Fig. 2). Ursolic acid also delayed the onset and decreased the severity of EAE in mice (Xu, et al., 2011). Ursolic acid, like digoxin, displays a steroid-like chemical structure and, in fact, several have reported that ursolic acid modulates the activity of the glucocorticoid receptor (GR) (Cha, et al., 1998; Kassi, et al., 2009). Biochemical assays indicate that ursolic acid effectively bindings to the RORγ LBD and blocks coactivator binding, but was considerably less active on RORα (Xu, et al., 2011). The possibility that ursolic acid exhibits activity at the GR complicates the interpretation of the results since glucocorticoids are very effective in the EAE model.
Given the validated results in the development of small molecule ligands for RORα and RORγ by multiple teams it is clear that these orphan receptors are druggable. The data also clearly indicate that these compounds have efficacy in suppression of TH17 cell development and an in vivo model of autoimmunity (EAE model). Thus, targeting the RORs represents a very promising path forward for novel treatments for autoimmunity that offers potential significant advantages over current therapies that broadly target the immune system and/or are typically biologics that must be injected.
Revival of PPARγ Ligands for Type 2 Diabetes
The incidence of diabetes is increasing rapidly as the percentage of the population ages and becomes more obese. According to the National Center for Health Statistics diabetes is now the sixth leading cause of death in the US. Adipose tissue is at the center of the metabolic syndrome, which encompasses an array of medical disorders including insulin resistance and diabetes. Excessive body fat defines obesity and leads to insulin resistance, dyslipidemia, type 2 diabetes (T2D), and cardiovascular disease. Understanding the molecular pathways that link adipose tissue biology to these pathologies is of critical scientific and medical importance. Adipose tissue secretes a variety of cytokines and cytokine-like molecules, called adipokines, which have both positive and negative effects on peripheral insulin sensitivity (Kershaw and Flier, 2004). In obesity, TNFα, IL-1 and resistin are secreted from adipose tissues and suppress insulin action on peripheral tissues (Hotamisligil, et al., 1993; Lagathu, et al., 2006; Steppan, et al., 2001). Conversely, adipose tissues from lean individuals secrete higher levels of adiponectin, a circulating protein that has insulin-sensitizing effects on liver and other tissues (Berg, et al., 2001; Hu, et al., 1996; Yamauchi, et al., 2006).
The nuclear receptor peroxisome proliferator-activated receptor gamma (PPARγ) is viewed as a master regulator of fat cell biology and differentiation, being both necessary and sufficient to drive conversion of fibroblastic precursors into fat cells (Morrison and Farmer, 2000; Tontonoz, et al., 1994; Willson, et al., 2001). Transcription factors such as C/EBPs and EBF proteins regulate the expression of PPARγ (Jimenez, et al., 2007; Wu, et al., 1996). The glitazones or thiazolidinedione (TZDs) class of drugs which include rosiglitazone and pioglitazone are synthetic ligands that demonstrate high affinity binding to PPARγ (~100nM Kd) and these compounds function as full agonists of the receptor (Day, 1999; Reginato and Lazar, 1999) (Fig. 3). Treatment of animal models of diabetes and diabetic patients with TZDs results in potent insulin-sensitizing activity (Lehmann, et al., 1995) concomitant with decreased expression of insulin resistance-inducing adipokines including TNF-a, IL-1 and resistin, and increased production of the insulin-sensitizing hormone, adiponectin (Sharma and Staels, 2007; Trujillo and Scherer, 2006). Unfortunately, PPARγ agonists can have long-term adverse effects on the health of certain patients, such as increased body weight, fluid retention, and increased risk of heart failure (Lipscombe, et al., 2007). This is unfortunate, as TZDs have consistently shown robust efficacy for treatment of T2D. More recently concerns have increased on the association of TZD use with bone loss (Schwartz, et al., 2006; Wei, et al., 2010). The latter risk is troublesome as detection is typically only made when a patient suffers a fracture. The biguanide metformin is now the first-line medication used for treatment of T2DM as safety concerns over the use of TZDs has grown.
Figure 3.
A. Chemical structure of various PPARγ agonists, partial agonists, and “non-agonists”. B. The desired profile of a PPARγ modulator is high affinity binding with minimal to no classical agonism and potent blockage of S273 phosphorylation by CDK5. A compound with this profile will result in improved therapeutic index (maximal efficacy with minimal side effects).
Interestingly, studies in preclinical models and in clinical trials have shown that weight gain and plasma volume expansion can be minimized without loss of insulin sensitization by the use of modulators that are weak or partial agonists of PPARγ. Partial agonists have been referred to as selective PPARγ modulators or SPPARγMs and this class of ligand has been shown to have a different binding mode in the PPARγ ligand binding pocket (LBP) as compared to the full agonists (Berger, et al., 2005). Selective recruitment of transcriptional coactivators by partial agonists has also been demonstrated. A combination of different ligand binding mode and distinct coactivator recruitment profile may explain the change in gene expression patterns compared to that of full agonists (Berger, et al., 2003). These findings highlight that several important aspects of PPARγ action remain unclear. First, there is no general deficiency in PPARγ function in obesity or insulin-resistant states. Hence, it is not clear why synthetic activation of a receptor should afford anti-diabetic effects. Second, while anti-diabetic potency of the PPARγ ligand drugs correlates very well with their binding affinities (Willson, et al., 1996), some ligands with full agonist action, like rosiglitazone, have powerful insulin-sensitizing actions, while other compounds with poor agonist activities, such as the benzyl indole MRL24, retain very good anti-diabetic effects (Acton, et al., 2005).
A recent study by Choi et al demonstrated that many PPARγ-based drugs can activate the receptor by recruitment of co-activators but these compounds have a separate biochemical activity involving blocking the obesity-linked phosphorylation of PPARγ by Cdk5 on S273 of the receptor (Choi, et al., 2010). In this study it was shown that the insulin sensitization efficacy of a rosiglitazone and the partial agonist MRL24 correlates with their ability to block S273 phosphorylation. While it is likely that other kinases can phosphorylate PPARγ on S273, this study demonstrates that activation of CDK5 by pro-inflammatory cytokines leads to an increase of phosphorylation of S273 in cellular models and increased S273-P was observed in obese diabetic mice (Choi, et al., 2010). The level of S273-P correlates with the repression of a subset of PPARγ target genes that are dysregulated in obesity and associated with insulin resistance (Choi, et al., 2010) and interestingly, a recent study indicates that deletion of NCoR in adipocytes facilitates S273 phosphorylation by CDK5 and mimics the action of the TZD treatment. These studies suggested the possibility that the mechanism driving efficacy of PPARγ drugs can be separated from classical activation of the receptor, which if not required for efficacy likely contributes to the adverse event profile of TZDs.
Efforts to discover high affinity PPARγ ligands that lacked classical activation of the receptor while retaining the ability to block S273 phosphorylation in cultured adipocytes and in diabetic mice led to the discovery of SR1664 (Fig. 2.). SR1664 has similar binding affinity as rosiglitazone for PPARγ in a lanthascreen ligand displacement assay (Ki~160nM). In a well established cell-based co-transfection promoter/reporter assay, rosiglitazone induces significant transcriptional activation of the reporter whereas SR1664 failed to transcriptionally activate the reporter even at the highest concentrations tested. On purified PPARγ, both rosiglitazone and SR1664 demonstrated dose-dependent inhibition of the CDK5-dependent phosphorylation of S273. The blockage of phosphorylation of S273 is a direct action of the ligands that requires binding to the LBD of the receptor to induce a conformational change that interferes with the ability of Cdk5 to phosphorylate serine 273. Adipogenesis was the first known biological function of PPARγ and PPARγ agonists have been shown to potently stimulate the differentiation of pre-adipose cell lines (e.g., 3T3-L1 cells). In this study, rosiglitazone potently stimulated fat cell differentiation, where in contrast, SR1664 did not stimulate increased lipid accumulation or changes in morphology characteristic of differentiating fat cells. More importantly, glucose tolerance tests were markedly improved with both rosiglitazone and SR1664. While SR1664 demonstrated potent anti-diabetic activity in diabetic mice there was no measurable increase in fluid retention or weight gain. Not surprising, significant plasma volume expansion and weight gain was observed in the rosiglitazone treated animals. Unlike rosiglitazone, SR1664 did not interfere with bone formation in cultured cells.
These data illustrate that it is possible to develop a new class of anti-diabetes drugs that allosterically block the phosphorylation of PPARγ at S273. Even though SR1664 is only a proof of concept compound that needs to be optimized for improved pharmaceutical properties, these studies represent a significant advancement towards developing insulin sensitizers with improved therapeutic index. Several questions need to be addressed to facilitate development of compounds like SR1664. First, what is the mechanism by which phosphorylation of S273 represses the transcriptional output of a subset of genes that are dysregulated in obesity? Second, what are the molecular and structural determinants that facilitate high affinity binding to PPARγ without inducing classical activation yet maintaining potent blockage of phosphorylation of S273? And third, will non-activating/S273 phosphorylation blockers like SR1664 completely dissociate efficacy from the major side effects associated with TZDs? While the answer to the last question will require testing compounds in man, it seems highly plausible that, as shown in Figure 3, that the therapeutic index will improve as agonism if removed while maintaining blockage of S273 phosphorylation.
Synthetic agonists for LRH-1 for Treatment of Diabetes and Metabolic Diseases
Liver receptor homolog-1 (LRH-1) is a member of the NR5A family of nuclear receptors that also includes the close homolog, steroidogenic factor-1 (SF-1). Although these receptors are considered orphan receptors, a number of reports have identified the presence of bacterial phospholipids in the binding pockets of both SF-1 and LRH-1, but it remains to be determined whether these represent merely structural or fortuitous ligands or if they can modulate receptor activity (Krylova, et al., 2005; Li, et al., 2005; Ortlund, et al., 2005). LRH-1 plays important roles in embryonic development and is highly expressed in the intestine liver, pancreas and ovary (Lee and Moore, 2008). In addition, it is well established that LRH-1 regulates critical enzymes involved in cholesterol homeostasis and bile-acid biosynthesis in the liver in addition to regulating the expression of aromatase in the breast and ovaries (Lee, et al., 2008; Santen, et al., 2009).
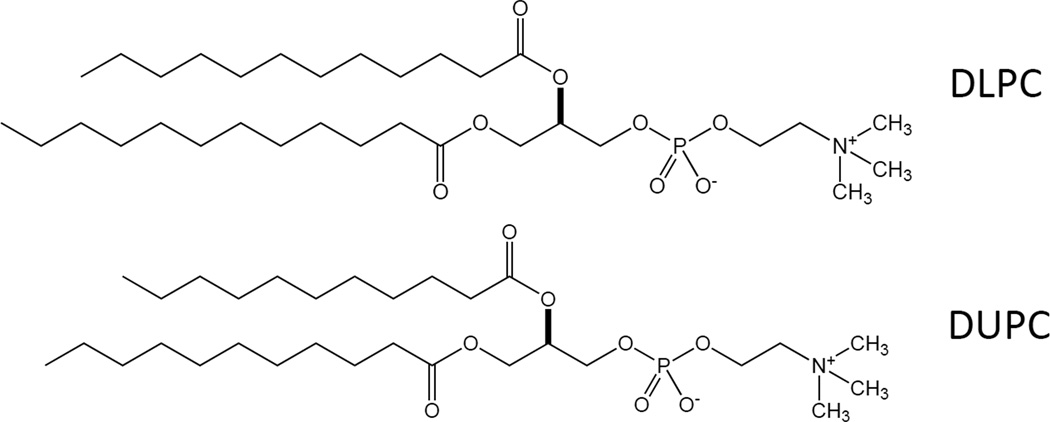
A report published recently in Nature by Lee and coworkers identified two unusual phosphatidylcholine (PC) species as LRH-1 agonists that also uncovered a previously unknown role for LRH-1 in the control of glucose homeostasis and insulin sensitivity (Lee, et al., 2011). Dilauroyl PC (DLPC; C12:0/C12:0) and diundecanoyl PC (DUPC; C11:0/C11:0) were shown to function as direct ligands of LRH-1 and modulate the receptor’s activity (Fig. 4). Both phospholipids were able to induce activation of LRH-1 specific promoters such as Shp and Oct4 while closely related phospholipids such as dipalmitoyl PC (DPPC; C16:0/C16:0) did not. In addition, the authors demonstrate that these phospholipids were specific to NR5A family members and did not cross react with other families of nuclear receptors such as PPARα. Mammalian two-hybrid assays confirmed the ability of these two phospholipids to bind to LRH-1 and selectively recruit important coactivators such as SRC-3. Moreover, coactivator peptide association assays with purified LRH-1 suggested that the binding of DLPC to LRH-1 is subnanomolar. The effects of these LRH-1 agonists were then explored in vivo where they were administered to normal mice and it was shown that both ligands produced significantly reduced levels of non-esterified fatty acids and glucose levels in serum as well as a reduction of hepatic triglycerides. These effects were further explored in two mouse models of insulin resistance, db/db and diet induced obese (DIO) mice and the results were equally striking. DLPC treatment of insulin-resistant leptin-receptor deficient db/db mice for two weeks saw improvements in glucose homeostasis as well as lower fasting serum insulin levels and significantly lowered hepatic triglyceride levels in these animals compared to vehicle control.
Figure 4.
Chemical structure of DLPC and DUPC (Dilauroyl phosphatidylcholine and diundecanoyl phosphatidylcholine), two LRH-1 ligands.
To further confirm the role of LRH-1 in this pathway, DIO mice, that either expressed wild-type hepatic LRH-1 or were knocked out for hepatic LRH-1, were fed a high fat diet to induce obesity and insulin resistance. These animals were then continued on the diet with the addition of either DLPC or vehicle control for an additional three weeks and then the same parameters of glucose homeostasis were measured. It was determined that consistent with the db/db mice, the DIO mice containing wild-type hepatic LRH-1 and treated with DLPC showed marked improvement of glucose homeostasis that was absent from the mice containing the LRH-1 knockout. Moreover, these animals displayed increased insulin sensitivity, decreased hepatic triglyceride levels, decreased non-esterified fatty acids and their livers appeared less pale and fatty than the animals with the LRH-1 knockout given DLPC. These data illustrate the utility of these two novel LRH-1 agonists in further elucidating the mechanisms that control this pathway and identifies a potential new target for the intervention into metabolic disease. Furthermore, the results suggest that a targeted synthetic chemistry approach to targeting LRH-1 is warranted.
Summary
Defining both natural and synthetic ligands for orphan NRs continues to be a rich area and discoveries in the areas of RORs and LRH-1 over the past 2 years suggests that this group of NRs as well as additional orphan NRs may develop into validated drug targets. Beyond autoimmunity, the RORs offer the potential for development of drugs targeting a range of disorders. Both RORα and RORγ play important roles in glucose and lipid metabolism, which is exemplified by the phenotypes of mice with mutations in RORα or RORγ {Mamontova, 1998 #772}{Lau, 2008 #776}{Kang, 2007 #748}. During the investigation of the effects of SR1001, we reported that metabolic genes known to be regulated by ROR were also affected {Solt, 2011 #1238}. Furthermore, we also demonstrated that a RORα-selective agonist, SR3335, suppresses hepatic glucose output in vivo {Kumar, 2011 #1240}. Thus, there are clear opportunities for assessing the ability of synthetic RORα/γ ligands for treatment of metabolic disorders such as type 2 diabetes. Currently, much less is known about targeting RORβ with small molecule synthetic ligands. RORβ expression is limited to the CNS and plays a critical role in regulation of the central circadian rhythm, but RORβ null mice also display reduced anxiety {Masana, 2007 #888}. Although RORα also plays a critical role in regulation of the circadian rhythm, the restricted pattern of expression of RORβ suggests that one may be able to develop RORβ-selective compounds that would modulate the central circadian rhythm without affecting the periphery.
The utilization of the TZD class of PPARγ agonists in the clinic has been declining significantly due to the association of these compounds with a range of side effects that has even led to the removal of drugs from the market. The observation that a new class of “modulator” compounds that selectively alter the post-translational modification of this receptor leading to “selective” pharmacology offers potential for a new class of PPARγ ligands that would offer the beneficial insulin sensitization effects without the detrimental side effects that are associated with typical agonists. This approach, while clearly promising, is in early stages and it is unclear if this will yield a superior drug in the clinic. With the array of selective modulators being developed for various nuclear receptors, it is interesting to speculate whether this method of selective modulation of post-transcriptional modification of the receptor leading to selective pharmacology will be applicable to a wider array of receptors.
The observation that DLPC regulates LRH-1 activity in vivo and is therapeutic for type 2 diabetes begs for a classical medicinal chemistry approach to be applied to this orphan receptor. With this recent publication from the Moore lab {Lee, 2011 #1776}, it is clear that there will be several groups investigating the potential for development of synthetic ligands that target LRH-1. This class of nuclear receptor ligands may also offer a unique method for treatment of metabolic diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acton JJ, Black RM, Jones AB, Moller DE, Colwell L, Doebber TW, MacNaul KL, Berger J, Wood HB. Benzoyl 2-methyl indoles as selective PPAR gamma modulators. Bioorganic & Medicinal Chemistry Letters. 2005;15:357–362. doi: 10.1016/j.bmcl.2004.10.068. [DOI] [PubMed] [Google Scholar]
- Andre E, Conquet F, Steinmayr M, Stratton SC, Porciatti V, Becker-Andre M. Disruption of retinoid-related orphan receptor beta changes circadian behavior, causes retinal degeneration and leads to vacillans phenotype in mice. Embo Journal. 1998;17:3867–3877. doi: 10.1093/emboj/17.14.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre E, Gawlas K, Steinmayr M, Becker-Andre M. A novel isoform of the orphan nuclear receptor ROR beta is specifically expressed in pineal gland and retina. Gene. 1998;216:277–283. doi: 10.1016/s0378-1119(98)00348-5. [DOI] [PubMed] [Google Scholar]
- Beckerandre M, Andre E, Delamarter JF. Identification of nuclear receptor messenger RNAs by RT-PCR amplification of conserved zinc finger motif sequences. Biochem Biophys Res Commun. 1993;194:1371–1379. doi: 10.1006/bbrc.1993.1976. [DOI] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Du XL, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nature Medicine. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic targets for metabolic disease. Trends in Pharmacological Sciences. 2005;26:244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Berger JP, Petro AE, Macnaul KL, Kelly LJ, Zhang BB, Richards K, Elbrecht A, Johnson BA, Zhou GC, Doebber TW, et al. Distinct properties and advantages of a novel peroxisome proliferator-activated protein gamma selective modulator. Molecular Endocrinology. 2003;17:662–676. doi: 10.1210/me.2002-0217. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris TP. Nuclear hormone receptors for heme: REV-ERBalpha and REV-ERBbeta are ligand-regulated components of the mammalian clock. Mol Endocrinol. 2008;22:1509–1520. doi: 10.1210/me.2007-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg C, Vanhuijsduijnen RH, Staple JK, Delamarter JF, Beckerandre M. RZRs, A new family of retinoid-related orphan receptors that function as both monomers heterodimers. Molecular Endocrinology. 1994;8:757–770. doi: 10.1210/mend.8.6.7935491. [DOI] [PubMed] [Google Scholar]
- Cha HJ, Park MT, Chung HY, Kim ND, Sato H, Seiki M, Kim KW. Ursolic acid-induced down-regulation of MMP-9 gene is mediated through the nuclear translocation of glucocorticoid receptor in HT1080 human fibrosarcoma cells. Oncogene. 1998;16:771–778. doi: 10.1038/sj.onc.1201587. [DOI] [PubMed] [Google Scholar]
- Choi JH, Banks AS, Estall JL, Kajimura S, Bostrom P, Laznik D, Ruas JL, Chalmers MJ, Kamenecka TM, Bluher M, et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPAR gamma by Cdk5. Nature. 2010;466 doi: 10.1038/nature09291. 451-U451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Dang EV, Barbi J, Yang H-Y, Jinasena D, Yu H, Zheng Y, Bordman Z, Fu J, Kim Y, Yen H-R, et al. Control of Th17/Treg Balance by Hypoxia-Inducible Factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day C. Thiazolidinediones: a new class of antidiabetic drugs. Diabetic Medicine. 1999;16:179–192. doi: 10.1046/j.1464-5491.1999.00023.x. [DOI] [PubMed] [Google Scholar]
- Duez H, Staels B. Rev-erb-alpha: an integrator of circadian rhythms and metabolism. Journal of Applied Physiology. 2009;107:1972–1980. doi: 10.1152/japplphysiol.00570.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber IA, Brocke S, TaylorEdwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalolmyelitis (EAE) Journal of Immunology. 1996;156:5–7. [PubMed] [Google Scholar]
- Fujita-Sato S, Ito S, Isobe T, Ohyama T, Wakabayashi K, Morishita K, Ando O, Isono F. Structural Basis of Digoxin That Antagonizes ROR gamma t Receptor Activity and Suppresses Th17 Cell Differentiation and Interleukin (IL)-17 Production. Journal of Biological Chemistry. 2011;286:31409–31417. doi: 10.1074/jbc.M111.254003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguere V, Tini M, Flock G, Ong E, Evans RM, Otulakowski G. Isoform specific amino-terminal domains dictate DNA binding properties of RORalpha, a novel family of orphan nuclear receptors. Genes & Development. 1994;8:538–553. doi: 10.1101/gad.8.5.538. [DOI] [PubMed] [Google Scholar]
- Hamilton BA, Frankel WN, Kerrebrock AW, Hawkins TL, FitzHugh W, Kusumi K, Russell LB, Mueller KL, vanBerkel V, Birren BW, et al. Disruption of the nuclear hormone receptor ROR alpha in staggerer mice. Nature. 1996;379:736–739. doi: 10.1038/379736a0. [DOI] [PubMed] [Google Scholar]
- Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4(+) effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nature Immunology. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- Hirose T, Smith RJ, Jetten AM. RORgamma - The 3rd member of ROR-RZR orphan receptor subfamily that is highly expressed in skeletal muscle. Biochem Biophys Res Commun. 1994;205:1976–1983. doi: 10.1006/bbrc.1994.2902. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor alpha - Direct role in aobesity linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Hu ED, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPAR gamma. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- Huh JR, Leung MWL, Huang PX, Ryan DA, Krout MR, Malapaka RRV, Chow J, Manel N, Ciofani M, Kim SV, et al. Digoxin and its derivatives suppress T(H)17 cell differentiation by antagonizing ROR gamma t activity. Nature. 2011;472:486–490. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor ROR gamma t directs the differentiation program of proinflammatory IL-17(+) T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Jimenez MA, Akerblad P, Sigvardsson M, Rosen ED. Critical role for Ebf1 and Ebf2 in the adipogenic transcriptional cascade. Molecular and Cellular Biology. 2007;27:743–757. doi: 10.1128/MCB.01557-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin LH, Martynowski D, Zheng SY, Wada T, Xie W, Li Y. Structural Basis for Hydroxycholesterols as Natural Ligands of Orphan Nuclear Receptor ROR gamma. Molecular Endocrinology. 2010;24:923–929. doi: 10.1210/me.2009-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallen J, Schlaeppi JM, Bitsch F, Delhon I, Fournier B. Crystal structure of the human ROR alpha ligand binding domain in complex with cholesterol sulfate at 2.2 angstrom. Journal of Biological Chemistry. 2004;279:14033–14038. doi: 10.1074/jbc.M400302200. [DOI] [PubMed] [Google Scholar]
- Kallen JA, Schlaeppi JM, Bitsch F, Geisse S, Geiser M, Delhon I, Fournier B. X-ray structure of the hROR alpha LBD at 1.63 angstrom: Structural and functional data that cholesterol or a cholesterol derivative is the natural ligand of ROR alpha. Structure. 2002;10:1697–1707. doi: 10.1016/s0969-2126(02)00912-7. [DOI] [PubMed] [Google Scholar]
- Kassi E, Sourlingas TG, Spiliotaki M, Papoutsi Z, Pratsinis H, Aligiannis N, Moutsatsou P. Ursolic Acid Triggers Apoptosis and Bcl-2 Downregulation in MCF-7 Breast Cancer Cells. Cancer Investigation. 2009;27:723–733. doi: 10.1080/07357900802672712. [DOI] [PubMed] [Google Scholar]
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. Journal of Clinical Endocrinology & Metabolism. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Lehmann JM, Willson TM. Orphan nuclear receptors: Shifting endocrinology into reverse. Science. 1999;284:757–760. doi: 10.1126/science.284.5415.757. [DOI] [PubMed] [Google Scholar]
- Krylova IN, Sablin EP, Moore J, Xu RX, Waitt GM, MacKay JA, Juzumiene D, Bynum JM, Madauss K, Montana V, et al. Structural analyses reveal phosphatidyl inositols as ligands for the NR5 orphan receptors SF-1 and LRH-1. Cell. 2005;120:343–355. doi: 10.1016/j.cell.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Kumar N, Solt LA, Conkright JJ, Wang Y, Istrate MA, Busby SA, Garcia-Ordonez R, Burris TP, Griffin PR. The benzenesulfonamide T0901317 is a novel ROR{alpha}/{gamma} Inverse Agonist. Molecular Pharmacology. 2010;77:228–236. doi: 10.1124/mol.109.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagathu C, Yvan-Charvet L, Bastard JP, Maachi M, Quignard-Boulange A, Capeau J, Caron M. Long-term treatment with interleukin-1 beta induces insulin resistance in murine and human adipocytes. Diabetologia. 2006;49:2162–2173. doi: 10.1007/s00125-006-0335-z. [DOI] [PubMed] [Google Scholar]
- Lee JM, Lee YK, Mamrosh JL, Busby SA, Griffin PR, Pathak MC, Ortlund EA, Moore DD. A nuclear-receptor-dependent phosphatidylcholine pathway with antidiabetic effects. Nature. 2011;474 doi: 10.1038/nature10111. 506-U135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y-K, Schmidt DR, Cummins CL, Choi M, Peng L, Zhang Y, Goodwin B, Hammer RE, Mangelsdorf DJ, Kliewer SA. Liver receptor homolog-1 regulates bile acid homeostasis but is not essential for feedback regulation of bile acid synthesis. Molecular Endocrinology. 2008;22:1345–1356. doi: 10.1210/me.2007-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Moore DD. Liver receptor homolog-1, an emerging metabolic modulator. Frontiers in Bioscience. 2008;13:5950–5958. doi: 10.2741/3128. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An Antidiabetic Thiazolidinedione Is a High Affinity Ligand for Peroxisome Proliferator-activated Receptor gamma. J. Biol. Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- Li Y, Choi M, Cavey G, Daugherty J, Suino K, Kovach A, Bingham NC, Kliewer SA, Xu HE. Crystallographic identification and functional characterization of phospholipids as ligands for the orphan nuclear receptor steroidogenic factor-1. Molecular Cell. 2005;17:491–502. doi: 10.1016/j.molcel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Lipscombe LL, Gomes T, Levesque LE, Hux JE, Juurlink DN, Alter DA. Thiazolidinediones and cardiovascular outcomes in older patients with diabetes. Jama-Journal of the American Medical Association. 2007;298:2634–2643. doi: 10.1001/jama.298.22.2634. [DOI] [PubMed] [Google Scholar]
- Littman DR, Rudensky AY. Th17 and Regulatory T Cells in Mediating and Restraining Inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Evans RM. The Rxr Heterodimers and Orphan Receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. The Nuclear Receptor Superfamily - the 2nd Decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev A, Yan ZH, Hirose T, Giguere V, Jetten AM. Cloning of a cDNA encoding the murine orphan receptor RZR/ROR gamma and characterization of its response element. Gene. 1996;181:199–206. doi: 10.1016/s0378-1119(96)00504-5. [DOI] [PubMed] [Google Scholar]
- Miller SA, Weinmann AS. Common themes emerge in the transcriptional control of T helper and developmental cell fate decisions regulated by the T-box, GATA and ROR families. Immunology. 2009;126:306–315. doi: 10.1111/j.1365-2567.2008.03040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison RF, Farmer SR. Hormonal signaling and transcriptional control of adipocyte differentiation. Journal of Nutrition. 2000;130:3116S–3121S. doi: 10.1093/jn/130.12.3116S. [DOI] [PubMed] [Google Scholar]
- Murphy CA, Langrish CL, Chen Y, Blumenschein C, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and Antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. Journal of Experimental Medicine. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortlund EA, Lee Y, Solomon IH, Hager JM, Safi R, Choi Y, Guan ZQ, Tripathy A, Raetz CRH, McDonnell DP, et al. Modulation of human nuclear receptor LRH-1 activity by phospholipids and SHP. Nature Structural & Molecular Biology. 2005;12:357–363. doi: 10.1038/nsmb910. [DOI] [PubMed] [Google Scholar]
- Park H, Li ZX, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nature Immunology. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB, Burris LL, Khorasanizadeh S, Burris TP, Rastinejad F. Identification of heme as the ligand for the orphan nuclear receptors REV-ERB[alpha] and REV-ERB[beta] Nat Struct Mol Biol. 2007;14:1207–1213. doi: 10.1038/nsmb1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginato MJ, Lazar MA. Mechanisms by which thiazolidinediones enhance insulin action. Trends in Endocrinology and Metabolism. 1999;10:9–13. doi: 10.1016/s1043-2760(98)00110-6. [DOI] [PubMed] [Google Scholar]
- Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A. History of Aromatase: Saga of an Important Biological Mediator and Therapeutic Target. Endocrine Reviews. 2009;30:343–375. doi: 10.1210/er.2008-0016. [DOI] [PubMed] [Google Scholar]
- Schwartz AV, Sellmeyer DE, Vittinghoff E, Palermo L, Lecka-Czernik B, Feingold KR, Strotmeyer ES, Resnick HE, Carbone L, Beamer BA, et al. Thiazolidinedione use and bone loss in older diabetic adults. Journal of Clinical Endocrinology & Metabolism. 2006;91:3349–3354. doi: 10.1210/jc.2005-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AM, Staels B. Review: Peroxisome proliferator-activated receptor gamma and adipose tissue - Understanding obesity-related changes in regulation of lipid and glucose metabolism. Journal of Clinical Endocrinology & Metabolism. 2007;92:386–395. doi: 10.1210/jc.2006-1268. [DOI] [PubMed] [Google Scholar]
- Solt LA, Kojetin DJ, Burris TP. The REV-ERBs and RORs: molecular links between circadian rhythms and lipid homeostasis. Future Medicinal Chemistry. 2011;3:623–638. doi: 10.4155/fmc.11.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solt LA, Kumar N, Nuhant P, Wang YJ, Lauer JL, Liu J, Istrate MA, Kamenecka TM, Roush WR, Vidovic D, et al. Suppression of T(H)17 differentiation and autoimmunity by a synthetic ROR ligand. Nature. 2011;472:491–494. doi: 10.1038/nature10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmayr M, Andre E, Conquet F, Rondi-Reig L, Delhaye-Bouchaud N, Auclair N, Daniel H, Crepel F, Mariani J, Sotelo C, et al. staggerer phenotype in retinoid-related orphan receptor alpha-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3960–3965. doi: 10.1073/pnas.95.7.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu ED, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPARgamma2, a lipid activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Trujillo ME, Scherer PE. Adipose tissue-derived factors: Impact on health and disease. Endocrine Reviews. 2006;27:762–778. doi: 10.1210/er.2006-0033. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kumar N, Crumbley C, Griffin PR, Burris TP. A second class of nuclear receptors for oxysterols: Regulation of RORalpha and RORgamma activity by 24S-hydroxycholesterol (cerebrosterol) Biochim Biophys Acta. 2010;1801:917–923. doi: 10.1016/j.bbalip.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kumar N, Solt LA, Richardson TI, Helvering LM, Crumbley C, Garcia-Ordonez RA, Stayrook KR, Zhang X, Novick S, et al. Modulation of RORalpha and RORgamma activity by 7-oxygenated sterol ligands. Journal of Biological Chemistry. 2010;285:5013–5025. doi: 10.1074/jbc.M109.080614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Wang XD, Yang M, Smith LC, Dechow PC, Wian YH. PGC1 beta Mediates PPAR gamma Activation of Osteoclastogenesis and Rosiglitazone-Induced Bone Loss. Cell Metabolism. 2010;11:503–516. doi: 10.1016/j.cmet.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenborg DO, Fordham S, Bernard CCA, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. Journal of Immunology. 1996;157:3223–3227. [PubMed] [Google Scholar]
- Willson TM, Cobb JE, Cowan DJ, Wiethe RW, Correa ID, Prakash SR, Beck KD, Moore LB, Kliewer SA, Lehmann JM. The structure-activity relationship between peroxisome proliferator-activated receptor gamma agonism and the antihyperglycemic activity of thiazolidinediones. Journal of Medicinal Chemistry. 1996;39:665–668. doi: 10.1021/jm950395a. [DOI] [PubMed] [Google Scholar]
- Willson TM, Lambert MH, Kliewer SA. Peroxisome proliferator-activated receptor gamma and metabolic disease. Annual Review of Biochemistry. 2001;70:341–367. doi: 10.1146/annurev.biochem.70.1.341. [DOI] [PubMed] [Google Scholar]
- Wu ZD, Bucher NLR, Farmer SR. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBP beta, C/EBP delta, and glucocorticoids. Molecular and Cellular Biology. 1996;16:4128–4136. doi: 10.1128/mcb.16.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Wang X, Zhong B, Nurieva RI, Ding S, Dong C. Ursolic Acid Suppresses Interleukin-17 (IL-17) Production by Selectively Antagonizing the Function of ROR lambda t Protein. Journal of Biological Chemistry. 2011;286:22707–22710. doi: 10.1074/jbc.C111.250407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kubota N, Hada Y, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and R2 abrogated adiponectin binding and impaired AMP kinase and PPARalpha activation, leading to diabetes. Diabetes. 2006;55:A13–A13. [Google Scholar]
- Yang XXO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, et al. Rev-erb{alpha}, a Heme Sensor That Coordinates Metabolic and Circadian Pathways. Science. 2007;318:1786–1789. doi: 10.1126/science.1150179. [DOI] [PubMed] [Google Scholar]
- Yin L, Wu N, Lazar MA. Nuclear receptor Rev-erbalpha: a heme receptor that coordinates circadian rhythm and metabolism. Nucl Recept Signal. 2010;8:e001. doi: 10.1621/nrs.08001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GX, Gran B, Yu S, Li JF, Siglienti I, Chen XH, Kamoun M, Rostami A. Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-beta 2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. Journal of Immunology. 2003;170:2153–2160. doi: 10.4049/jimmunol.170.4.2153. [DOI] [PubMed] [Google Scholar]