Abstract
Models of hematopoiesis often depict lymphocyte production as a uniform process in which a homogenous population of hematopoietic stem cells (HSCs) generates progenitors from which all types of lymphocytes are derived. However, it is increasingly evident that these schemes are too simplistic and that the lymphoid potential of HSCs and precursors arising in the embryo, fetus, neonate, and adult is remarkably distinct. We review recent findings regarding the development of B lymphocytes, and the B-1 B cell lineage in particular, as a case in point. These studies show that B-1 and B-2 B cells involved in innate and adaptive immune responses, respectively, arise in staggered waves of development from distinct progenitors. We discuss the implications of this layered model of B cell development for understanding normal and dysregulated B lymphopoiesis.
Introduction
Two main populations of B lymphocytes, referred to as B-1 and B-2 B cells, exist (Figure 1). B-1 cells are part of the innate immune system and produce immunoglobulins (Ig) distinguished by their recognition of self-antigens and those with repetitive epitopes such as carbohydrates. The functional properties of B-1 cells, which include B-1a and B-1b subsets, and their role in providing immunity to specific pathogens have been reviewed extensively (Alugupalli and Gerstein, 2005; Alugupalli et al., 2004; Baumgarth, 2011; Haas et al., 2005; Hardy and Hayakawa, 2005). B-2 cells are present in secondary lymphoid organs and are generally considered to be mediators of adaptive immunity. They include a predominant population of follicular (FO) and a minor population of marginal zone (MZ) B cells, both of which can undergo Ig class switching and differentiate into memory cells (Martin and Kearney, 2002; Allman and Pillai, 2008; Hardy et al., 2007; Monroe and Dorshkind, 2007).
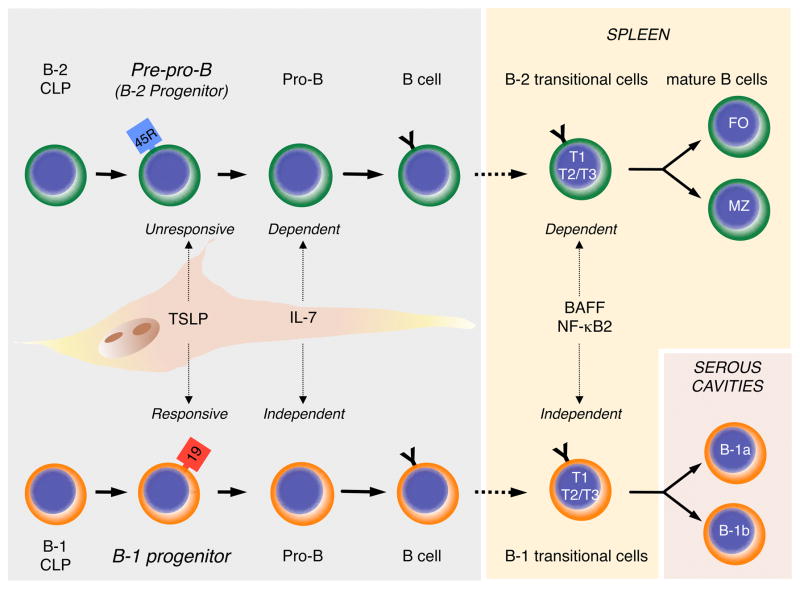
Figure 1.
B-1 and B-2 development. B-2 cells (Top) are produced in the bone marrow after birth. Common lymphoid progenitors (CLP) mature sequentially through pre-pro-B, pro-B, and pre-B (not shown) cell intermediates into immature sIgM+ B cells (shown as “B cell” in figure). Pre-pro-B cells are referred to as B-2 progenitors in the text. Immature sIgM+ cells migrate to the spleen where they mature through B-2 transitional 1 (T1) and Transitional 2 and 3 (T2 and T3) stages into Follicular (FO) or Marginal Zone (MZ) B cells. The stages of B-1 development are increasingly well defined, and it is now possible to propose a model of development based on the data summarized in this review (Bottom). Mature B-1 cells are generated from B-1 specified CLPs that sequentially differentiate through B-1 progenitor, pro-B, pre-B (not shown) and immature sIgM+ B-1 cell stages. The latter cells then mature in the spleen through the Transitional cell stages shown. Mature B-1 cells that migrate to serous cavities acquire the B-1a and B-1b phenotype. The figure also shows that B-1, but not B-2, progenitors are TSLP responsive, that B-2, but not B-1, development is dependent on IL-7, and that B-1 transitional cell survival and maturation is BAFF and NF-κB2 independent.
Soon after the description of B-1 cells, controversy regarding their origin and developmental relationship with B-2 B cells arose and centered on two competing models. The selection model proposed that the decision of an Ig expressing B lymphocyte to become a B-1 or B-2 cell was driven by the response to particular antigens (Haughton et al., 1993; Berland and Wortis, 2002). In contrast, the “layered immune system hypothesis” formulated by the Herzenbergs (Herzenberg and Herzenberg, 1989) proposed that “conventional [B-2] and Ly-1 [B-1] cells belong to separate lineages deriving from distinct progenitors emerging at different times during development”.
The pros and cons of these two models have been reviewed extensively (Berland and Wortis, 2002; Herzenberg and Tung, 2006; Kantor and Herzenberg, 1993), and we revisit this debate only briefly. Instead, this review will focus on abundant evidence that has been accumulating over the last decade in support of the layered immune system model. In addition to synthesizing these observations and emphasizing that B-1 and B-2 development occurs in distinct, differentially regulated waves, we discuss the implications of these observations for modeling murine and human B cell differentiation as well as understanding dysregulated B lymphopoiesis.
Origin of the Lineage Model of B-1 B Cell Development
In the early 1980s, B cell tumors that expressed CD5 (Lanier et al., 1981), referred to as Ly-1 in these early reports, were described, and this prompted a search for normal B cells that also expressed that determinant. Such populations were identified at a low frequency in the spleen but were abundant in the peritoneal cavity (Hardy et al., 1984; Hayakawa et al., 1985; Hayakawa et al., 1983). These observations established the existence of B-1 B cells, and additional studies performed in ensuing years led to the characterization of two distinct B-1 B cell subpopulations: B-1a B cells are sIgM+ CD11b+ CD5+ cells whereas B-1b B cells are sIgM+ CD11b+ CD5− (Kantor and Herzenberg, 1993). It is now realized that these phenotypes distinguish B-1 cells present in serous cavities and that different combinations of cell surface determinants must be used to resolve B-1 B cells in other tissues such as the spleen (Allman et al., 2001; Hardy, 2003).
The definition of B-1 cells prompted studies to define their developmental origin, particularly in relation to B-2 cells, and this was done by comparing the potential of cells from various donor tissues to generate the two types of lymphocytes. The first evidence to support the conclusion that these two B cell populations might be developmentally distinct was shown in transplantation studies that compared the potential of neonatal liver and adult bone marrow to generate B-1 and B-2 cells. Both donor populations produced B-2 cells, which was not surprising given that each included hematopoietic stem cells (HSCs). However, the striking result was that neonatal liver cells efficiently reconstituted B-1 cells upon transfer into irradiated recipients while adult bone marrow cells did so poorly.
The efficient generation of B-1 cells from neonatal liver suggested that they were derived from fetal progenitors. Pioneering studies performed in the early 1970s had established that B lymphocyte production initiates in the fetus and identified the liver and bone marrow as sites in which this occurred (Owen et al., 1974; Owen et al., 1975). These initial observations were followed by several studies showing that B lineage cells were present in additional fetal tissues that included the spleen, blood, and placenta as recently reviewed (Dorshkind and Montecino-Rodriguez, 2007). However, whereas reports published prior to the mid-1980s did not distinguish between B-1 and B-2 cells because the existence of these two subsets was not appreciated, many that appeared subsequently specifically demonstrated that various fetal tissues were a rich source of B-1 precursors. For example, fetal liver was shown to reconstitute B-1 cells in irradiated recipients (Kantor et al., 1992;), and the fetal omentum proved to be a selective source of B-1 B cells (Solvason et al., 1991). The latter observation was particularly intriguing, because the potential to generate B-1 but not B-2 B cells strongly suggested that a separate B-1 progenitor must exist.
Although these results provided evidence for the lineage hypothesis, that model nevertheless generated controversy for several reasons. The first was the formulation of the competing selection model. Studies of transgenic mice engineered to express Ig heavy and light chains frequently utilized by B-1 cells demonstrated that B-1 B cells were preferentially generated (Arnold et al., 1994; Haughton et al., 1993), which led to the conclusion that antigen selection at the surface Ig stage determines whether a B cell will adopt a B-1 fate. In addition, reports that mature B-2 cells could acquire the characteristics of B-1 cells began to appear. For example, B-2 cells could be induced to express CD5 (Berland and Wortis, 2002). Another reason for challenging the lineage model was that a distinct B-1 progenitor had not been indentified. Thus, the controversy of whether B-1 B cells were “made or born” (Haughton et al., 1993) “simmered and bubbled” (Herzenberg and Tung, 2006).
Identification of B-1 Specified Progenitors
The 1980s and 1990s were a period during which tremendous advances in flow cytometry were made, and it became possible to phenotypically identify and isolate cells at various stages of hematopoietic development. In particular, several laboratories formulated increasingly refined schemes of B cell development in which the differential expression of distinct cell surface determinants was correlated with the status of Ig heavy and light chain rearrangements and expression of various intracellular signaling molecules and transcription factors (Hardy et al., 1991; Rolink and Melchers, 1996). As a result, we now take for granted schemes of bone marrow B cell development in which HSCs differentiate into early lymphoid progenitors (ELPs) and then common lymphoid progenitors (CLPs) from which pro-B, pre-B, and finally immature B cells are produced (Ikuta et al., 1992; Kondo et al., 1997; Pelayo et al., 2006). The scheme defined by Hardy and collaborators (Hardy et al., 1991) is widely used by many laboratories, including our own, and indicates that the transition from CLPs into pre-pro-B cells is accompanied by up-regulation of CD45R(B220) and that CD19 expression does not occur until these cells have matured into pro-B cells (Figure 1).
During the course of studies aimed at comparing the properties of fetal and adult B cell progenitors, we unexpectedly identified a population of lineage negative (Lin−) CD93(AA4.1)+ CD45R− or lo CD19+ cells whose existence was not predicted by the Hardy scheme (Montecino-Rodriguez et al., 2006). These cells were present in high numbers in fetal liver, peaked in number at around day 17 of gestation, and then declined in both frequency and total number in the weeks after birth. We had previously reported that cells with a similar phenotype were present at a low frequency in the bone marrow of young adults (Montecino-Rodriguez et al., 2001), and de Andres et al. had also identified low numbers of CD45R− or lo CD19+ B cell progenitors in the fetal liver and aorta-gonad-mesonephric (AGM) region of embryos at day eleven of gestation (de Andres et al., 2002).
When the Lin− CD93(AA4.1)+ CD45R− or lo CD19+ cells were cultured in vitro, they upregulated expression of CD45R(B220) and matured into sIgM+ B cells. A proportion of the latter cells co-expressed CD11b (Montecino-Rodriguez et al., 2006). We further confirmed that the Lin− CD93(AA4.1)+ CD45R− or lo CD19+ cells transplanted in vivo differentiated into both B-1a and B-1b B cells in the peritoneal cavity of recipients. The latter cells expressed Ig heavy chain genes known to be utilized by B-1 cells and secreted Igs with reactivity towards the B-1 antigen phosphorylcholine. Furthermore, Lin− CD93(AA4.1)+ CD45R− or lo CD19+ cells did not generate B-2 cells or cells from any other hematopoietic lineage (Montecino-Rodriguez et al., 2006). Taken together, these data demonstrated the existence of distinct B-1 progenitors, thus providing support for the lineage model. The existence of the Lin− CD45R− or lo CD19+ B-1 progenitors has subsequently been independently confirmed by several laboratories (Esplin et al., 2009; Ghosn et al., 2011; Tung et al., 2006; Yoshimoto et al., 2011).
The Emergence of B-1 Potential During Embryogenesis
The initial description of B-1 progenitors focused on their emergence in fetal liver and fetal bone marrow, but how early in gestation and in which tissues they arise remained unknown. Yoshimoto and colleagues recently completed a detailed study to address these questions (Yoshimoto et al., 2011). Particular focus was placed on the yolk sac and intraembryonic para-aortic splanchnopleura (PSp) because these tissues are the earliest sites of fetal hematopoiesis (Dzierzak and Speck, 2008; Medvinsky et al., 2011) and previous studies had implicated them as a source of B-1 precursors (Cumano et al., 1993; Godin et al., 1993). In particular, cells harvested from the PSp at embryonic day 8.5–9.0 of gestation reconstituted CD5 expressing B cells that localized to the peritoneal cavity and secreted IgM, suggesting that they were B-1a cells (Godin et al., 1993). However, the precursors from which these B cells were derived were not identified.
In their initial analysis, Yoshimoto et al. examined yolk sac and PSp for the presence of Lin− CD45R− or lo CD19+ B-1 progenitors at day 9.0–9.5 of gestation, but few cells with this phenotype were found. However, when hematopoietic cells from yolk sac or PSp were cultured on stromal cells under B lymphopoietic conditions (Montecino-Rodriquez and Dorshkind, 2006) for eight to ten days, foci that included CD93(AA4.1)+ CD19+ CD45R− or lo cells developed. No cells with a CD19− CD45R+ B-2 progenitor phenotype emerged. With extended time in culture, these CD93(AA4.1)+ CD19+ CD45R− or lo cells matured into CD19+ CD45R+ cells that only generated sIgM+ CD11b+ CD5+ B-1a and sIgM+ CD11b+ CD5− B-1b cells following in vivo transfer. That these cells were bona fide B-1 cells was shown by the fact that they used VH11 Ig genes, which is a characteristic of B-1 cells (Carmack et al., 1990), and secreted antibodies reactive against B-1 antigens (Yoshimoto et al., 2011).
Because B cell potential arose simultaneously in the yolk sac and PSp, it had been suggested that translocation of precursors from one tissue to the other did not occur and that each was an autonomous site of B-1 B cell development (Godin et al., 1995). Recent studies showing that yolk sac or PSp cells from Ncx1−/− embryos efficiently generated B-1 cells demonstrated that this is the case. Because the latter strain fails to initiate a heartbeat, and thus provides a circulation free environment, it was possible to conclude that CD19+ B220− or lo B-1 progenitors were generated autonomously in the yolk sac and PSp and did not derive from cells that had seeded those tissues from other sites through the circulation (Yoshimoto et al., 2011).
Taken together, the above observations provide evidence for the existence of B-1 specified progenitors that arise before B-2 progenitors in the embryo. The identity of the precursors in embryonic day 9.0 to 9.5 yolk sac and PSp that give rise to the B-1 progenitors is not known. However, because definitive HSCs do not arise until day 10.5 of gestation, it is intriguing to postulate the existence of a pre-HSC wave of B-1 development. In this regard, it is known that the fetal erythroid cells that develop in the yolk sac do so from restricted progenitors (Dzierzak and Speck, 2008; Medvinsky et al., 2011), and this could also apply to a fraction of B-1 B cells.
B-1 progenitors that arise at later times during gestation are almost certainly derived from HSCs. This view is based on the fact the number of Lin− CD93(AA4.1)+ CD45R− or lo CD19+ B-1 progenitors peaks at day sixteen to seventeen of gestation in fetal liver and bone marrow, which is several days following the emergence of definitive HSCs. In addition, Lin− CD117hi Sca-1+ enriched HSCs from fetal liver have been shown to efficiently generate B-1a and B-1b cells upon in vitro transfer (Kikuchi and Kondo, 2006).
Divergence of B-1 and B-2 potential
The fact that HSCs isolated from fetal liver or neonatal bone marrow can differentiate into B-1 and B-2 cells (Barber et al., 2011; Kikuchi and Kondo, 2006) raises the question of when commitment to the B-1 versus B-2 lineage occurs. It was recently reported that ELPs and CLPs purified from adult bone marrow are able to generate B-1 and B-2 progenitors but that pro-B cells primarily produce B-2 cells (Esplin et al., 2009). This result indicates that the divergence of B-1 and B-2 potential occurs at the CLP to pro-B cell transition. However, these analyses were performed using bulk cultures, making it difficult to determine if a single CLP generated both B cell populations or if that compartment is developmentally heterogeneous and includes B-1 and B-2 specified subpopulations.
We developed a clonal culture system in which single CLPs were seeded under B lymphopoietic conditions that support development of B-1 and B-2 progenitors in order to distinguish between these possibilities (Barber et al., 2011). If they are bipotential, individual CLPs would generate both B cell progenitors in a single well in this assay. Alternatively, if the CLP compartment is developmentally heterogeneous, a single CLP would generate either B-1 or B-2 progenitors. Remarkably, single CLPs generated only B-1 or B-2 progenitors, but never both, indicating that B-1 versus B-2 lineage commitment is already made by the time the CLP stage of development is reached (Barber et al., 2011).
Further analyses showed that the number of B-1 CLPs present in the bone marrow is significantly reduced in adult mice when compared to neonates. These results are consistent with the original transplantation studies showing that neonatal liver reconstituted B-1 cells more efficiently than adult bone marrow (Hayakawa et al., 1985) and with our transplantation experiments showing that, when the number of B-1 cells generated is taken in consideration, neonatal HSCs and CLPs reconstitute B-1 cells more efficiently than their adult counterparts (Barber et al., 2011). Therefore, these reports provide further support for the observation that B-1 cell production occurs in the adult (Duber et al., 2009; Kikuchi and Kondo, 2006) but that it is substantially attenuated when compared to the fetus or neonate.
B-1 Cells are Generated Through Transitional Cell Intermediates
Following their production in the bone marrow, immature, sIgM+ cells migrate to the spleen where they progress through distinct transitional cell stages before maturing into FO and MZ B cells (Figure 1). In addition to B cell receptor (BCR) signaling, transitional cell survival and maturation are critically dependent upon binding of B cell activating factor (BAFF) to the BAFF-receptor (BAFF-R) and activation of alternative NF-κB signaling, as mice with defects in the expression of BAFF, BAFF-R, or NF-κB2, the key transcriptional regulator in the alternative NF-κB pathway, have a severe deficiency of FO and MZ B cells. In contrast, B-1 B cells are present in normal numbers in these mice (Gerondakis and Siebenlist, 2010; Mackay et al., 2010; Mackay and Schneider, 2009).
It has been proposed that B-1 B cells also develop through transitional cell intermediates (Casola, 2007), and in support of this hypothesis, we recently reported that the transitional B cells present in the spleen during the first two weeks after birth primarily generate B-1 B cells (Montecino-Rodriguez and Dorshkind, 2011; Figure 1). This neonatal B-1 transitional cell wave then wanes as the B-2 transitional cell wave establishes and predominates in the adult (Montecino-Rodriguez and Dorshkind, 2011). Further analysis revealed that B-1 transitional cells were not dependent on signaling through the BAFF-R, indicating that B-1 and B-2 transitional cells are not regulated in the same manner. A prediction based on these results is that the B-1 transitional cell wave should also appear normally in BAFF and NF-κB2 deficient mice.
The formation of B-1 cells is dependent upon strong BCR signaling (Casola et al., 2004) and activation of the classical NF-κB pathway. The requirement for classical pathway signaling is demonstrated by the fact that mice with defects in the expression of NF-κB signal integrators, such as Brutons tyrosine kinase (Btk), Bcl-10, MALT-1 (CARMA1), or NF-κB1, exhibit a severe reduction in the number of peritoneal cavity B-1 B cells (Thome, 2004). The characterization of B-1 transitional cells provided the opportunity to identify the stage(s) of B-1 development dependent on these signals. This analysis revealed that survival and maturation of B-1 transitional cells occurred normally in Btk and NF-κB1 deficient mice (Montecino-Rodriguez and Dorshkind, 2011), suggesting that the B-1 deficiency observed in these strains occurs because strong BCR and NF-κB signaling is critical for the survival and/or proliferation of B-1 cells following their formation from B-1 transitional cells.
These observations indicate that BCR signaling as a determinant of B-1 cell fate, which underlies the selection model, is also an integral element of the lineage model. In fact, it may be possible to combine the selection and lineage models into a unified scheme in which immature B-1 B cells derived from B-1 specified progenitors are selected into the mature B-1 pool following binding of specific antigens to their BCR.
Modeling B Cell Development in the Embryo and Adult
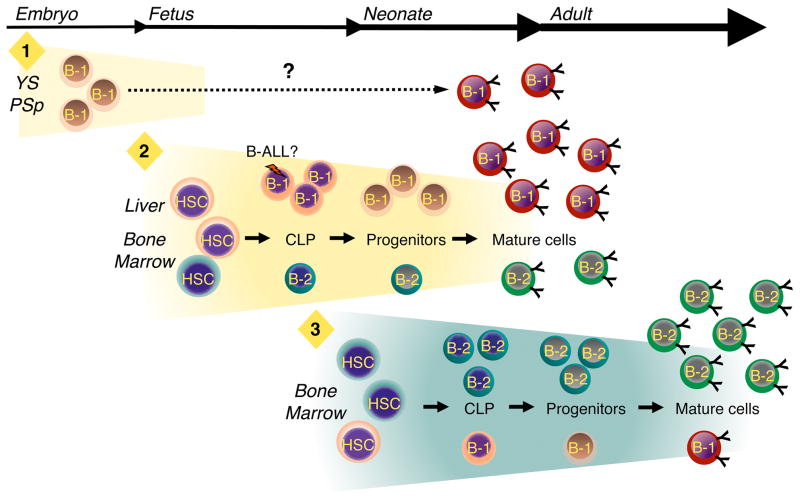
The results reviewed above allow us to build upon the original layered immune system hypothesis (Herzenberg and Herzenberg, 1989) and propose a model in which B cell production takes place in three distinct waves during development (Figure 2). The first wave initiates in the yolk sac and PSp where non-HSC derived precursors with the potential to generate B-1 progenitors arise, while the second wave initiates during mid-gestation in the fetal liver and fetal bone marrow. As discussed above, it is likely that most of the B-1 progenitors that emerge in this second wave are HSC derived. Finally, during the third wave, which occurs near the end of fetal life, the production of B-1 progenitors declines and the B-2 developmental program becomes increasingly well established. We emphasize that this is a working model that will undoubtedly require revision as new data emerge. For example, the timing with which B-1a and B-1b cells appear during ontogeny would indicate that they arise separately (Herzenberg and Tung, 2006; Tung et al., 2006). Thus, additional B-1 progenitors and waves of development may exist, and these can be easily integrated into this model.
Figure 2.
B-1 and B-2 development occur in distinct, overlapping waves. Three waves of B-1 development are proposed. The first wave (“1”) initiates in the yolk sac (YS) and para-aortic splanchnopleura (PSp) region before the emergence of definitive hematopoietic stem cells (HSC). It is not clear, as indicated by the dashed line, whether B-1 progenitors produced during this initial phase mature into B-1 cells that become part of the adult B-1 pool. If so, they may do so in YS and PSp or migrate to the fetal liver and bone marrow and complete maturation in those tissues. During the second wave (”2”), which initiates in the fetal liver and fetal bone marrow, HSCs generate B-1 progenitors from which mature B-1 cells are derived. Although B-2 development initiates during this second phase, B-1 production predominates. Whether or not B-1 and B-2 CLPs and progenitors are produced from a single type of HSC or multiple B-1 and B-2 specified stem cells exist, as indicated by the different colored HSCs, is not known. B-1 production peaks during late embryogenesis and then begins to decline just before birth. The third wave (“3”) of B cell development takes place in the bone marrow and results primarily in the production of B-2 B cells. B-1 B cells can be produced during this third wave, but the efficiency with which this occurs in comparison to wave two is substantially reduced.
Much remains to be learned about the events that occur within each of these phases of development. As noted, the identity of the precursor(s) from which B-1 progenitors arise in the first wave is not known. In addition, while the yolk sack and PSp derived cells can mature into functional B-1 cells following transfer in vivo, we do not know if mature B-1 cells are actually generated in this wave and contribute to the adult B-1 pool. The possibility remains that, similar to fetal erythropoiesis (Palis and Yoder, 2001), it represents a vestigial wave of B cell development. Alternatively, the yolk sac and PSp B-1 progenitors could differentiate into mature B-1 cells in situ or migrate to other hematopoietic sites and develop in parallel with HSC derived B-1 progenitors that emerge during the second wave (Yoshimoto et al., 2011).
Why HSCs in the fetus and neonate efficiently generate B-1 B cells while the ability of stem cells in the adult is attenuated is also not clear. The HSC compartment could be developmentally heterogeneous and include distinct B-1 and B-2 specified HSCs (E. Ghosn and L. Herzenberg, personal communication). Thus, HSCs with B-1 potential may arise early in the fetus and then decline in number as adult HSCs emerge. In this case, the attenuated B-1 potential of adult bone marrow would be due the persistence of a limited number of these fetal stem cells. Alternatively, a single type of HSC may develop, and its properties may be determined by the niches in which it localizes. Thus, the environmental cues encountered in the fetus may result in epigenetic changes that program B-1 cell development while subsequent residence in an adult hematopoietic microenvironment would extinguish B-1 potential and favor B-2 cell development. In this case, the low B-1 potential of HSCs in adult bone marrow may result because few “fetal like” niches are available.
Although we propose that the majority of mature B-1 cells are generated from distinct B-1 specified stem or progenitor cells in one of the waves shown in Figure 2, the possibility that other routes into the B-1 pool exist cannot be excluded. As discussed above, B-2 cells may in some circumstances acquire B-1 characteristics as a result of antigen engagement or exposure to a particular environment (Berland and Wortis, 2002). For example, residence in the peritoneal cavity may confer distinguishing characteristics on B-2 cells. B-2 cells transferred into the peritoneal cavity of severe combined immunodeficient mice can up-regulate expression of the CD11b and CD43 B-1 associated antigens, down-regulate expression of the CD23 B-2 cell surface determinant, and acquire the ability to spontaneously secrete IgM (Hastings et al., 2006). In addition, the CDR-3 locus, which encodes the region in the center of the antibody binding site, of peritoneal cavity B-2 cells exhibits sequence characteristics distinct from B-2 cells in other tissues (Vale et al., 2010). However, that study also demonstrated that peritoneal cavity B-1a, B-1b, and B-2 cells had unique repertoire signatures, which would be expected if each had a different ontogenic origin. Thus, while it appears that some B-2 cells, and those in the peritoneal cavity in particular, can acquire B-1 characteristics, the frequency with which this occurs and whether such cells should be considered true B-1 cells remain open questions.
Characterization of Human B-1 B Cells
Whereas the existence of B-1 cells in mice is not debated, whether or not human B-1 cells exist has been a source of controversy. CD5 expressing B cells have been detected in human fetal lymph nodes, spleen, peritoneal cavity, and omentum at different times during gestation (Antin et al., 1986; Bhat et al., 1992; Bofill et al., 1985; Solvason and Kearney, 1992) as well as in various adult tissues (Dauphinee et al., 1988; Donze et al., 1997). However, because CD5 is not a B-1 restricted determinant, definitive evidence that these were true B-1 cells has been lacking.
This situation recently changed with the description of a population of human cells that are functionally similar to murine B-1 cells. Griffin et al. (Griffin et al., 2011a) screened various phenotypically defined populations harvested from cord blood or peripheral blood and tested them for properties that typify murine B-1 cells. Using these criteria, they identified CD20+ CD27+ CD43+ CD70− cells, a subset of which express CD11b (Griffin and Rothstein, 2011), whose characteristics are consistent with them being classified as B-1 cells. This phenotype has generated some controversy, and issues regarding the gating strategies required to resolve the human B-1 cells have recently been discussed (Descatoire et al., 2011; Griffin et al., 2011b; Perez-Andres et al., 2011)
Like murine B-1 cells, the human B-1 cells identified by Griffin et al. (2011a) spontaneously secreted IgM, had limited somatic mutations, and expressed an Ig repertoire targeted to a narrow range of antigens such as phosphorylcholine and DNA. Interestingly, human B cells generated in utero also express a skewed Ig repertoire (Merbl, 2007). The presence of human B-1 cells in cord blood would suggest that they are the progeny of fetal progenitors. However, the developmental origin of the human B-1 cell population is undetermined and dissection of human fetal B cell development is an area ripe for investigation. A recent study from Sanz et al. (Sanz et al., 2010) reported that lineage negative CD34high CD10− CD19− hematopoietic progenitors isolated from cord blood generated B lineage cells in two, temporally distinct waves. It will be of great interest to determine if the B-1 cells described by Griffin et al. are preferentially generated during the initial wave.
Implications of Layered B Dell Development
If the production of B-1 and B-2 cells from separate progenitors is taken into account, it can reconcile a number of puzzling observations in the literature as well as stimulate the formulation of testable hypotheses to explain various aspects of normal and dysregulated B cell development.
A particularly clear illustration of these points is provided by studies that investigated the effects of thymic stromal lymphopoietin (TSLP) on B cell development. Soon after the initial description of this cytokine, it was reported that adult pro-B cells did not respond to it (Vosshenrich et al., 2004), while pro-B cells isolated from fetal tissues were TSLP responsive. The differential response of B-1 and B-2 progenitors to TSLP can explain this observation (Figure 1). Thus, B-1 progenitors isolated from fetal liver and adult bone marrow proliferate in response to TSLP, while B-2 progenitors isolated from fetal liver and adult bone marrow do not (Montecino-Rodriguez et al., 2006). This finding is consistent with results from another laboratory showing that the TSLP responsive cells in fetal liver have a CD19+ CD45R− or lo phenotype (Jensen et al., 2007). In view of these data, the initial observations showing a preferential response of fetal B cell progenitors to TSLP can be explained by the fact that B-1 precursors predominate in that tissue but are present at low numbers in adult bone marrow.
This differential TSLP responsiveness by B-1 and B-2 progenitors indicates that cell production in these two lineages is regulated in a distinct manner. The analysis of mice deficient in the expression of specific cytokines, cytokine receptors, signaling intermediates, or transcription factors provides further support for this conclusion. For example, B-1, but not B-2 cells, develop in mice that do not express IL-7 (Carvalho et al., 2001), and B-2, but not B-1, cell development is blocked in the absence of PU.1 expression (Rosenbauer et al., 2006; Ye et al., 2005). As reviewed above, B-1 and B-2 transitional cells also have distinctly different requirements for their survival and maturation. Thus, B-2 transitional cells are critically dependent upon binding of B cell activating factor (BAFF) to the BAFF-receptor, and activation of alternative NF-κB signaling while B-1 transitional cells can mature in the absence of these signals (Montecino-Rodriguez and Dorshkind, 2011). Together, these examples underscore the differences between B-1 and B-2 development and suggest that a thorough analysis of genetically deficient strains of mice in the context of the lineage model will help to delineate them.
Taking the existence of two B cell pathways into account will also be important for developmental studies. For example, a goal of many stem cell biologists is to use embryonic stem cells (ESC) or induced pluripotent stem cells (iPS) to model hematopoeisis and B cell development in particular (Carpenter et al., 2011; Szabo et al., 2010). It is known that the different waves of erythropoiesis are recapitulated when murine or human embryonic stem cells are induced to differentiate into hematopoietic stem cells (Irion et al., 2010; Zambidis, 2005). In view of this, distinct waves of B lymphopoiesis may also emerge in these cultures. Thus, it will be of great interest to compare the properties of B cells generated from human ESCs to those of the recently described human B-1 cells (Griffin et al., 2011a).
The layered model of B cell development may also be relevant to understanding the diminished response of infants to vaccination (PrabhuDas et al., 2011). In this case, agents designed to boost the response of adaptive B-2 cells may not be effective when administered to infants in which B-1 cells may be present in significant numbers. Ultimately, optimal immunization strategies may need to consider which B lineage cells are present in neonatal tissues at the time of vaccination.
Finally, it is interesting to consider the possibility that B-1 cells may be the cell of origin for various B cell leukemias. Most discussions in this regard have focused on the hypothesis that chronic lymphocytic leukemia (CLL) is a B-1 malignancy (Chiorazzi and Ferrarini, 2011), based on the fact that CD5 is expressed on many CLL cells. However, as previously noted, CD5 expression does not unambiguously identify B-1 cells and up to two thirds of CLL B cells exhibit extensive somatic hyper-mutation, which is not a characteristic of B-1 cells (Chiorazzi and Ferrarini, 2011). Thus, the relationship between cells of the B-1 lineage and CLL is unclear.
Another possibility to consider is that some infant and childhood cases of acute lymphocytic leukemia (ALL) are malignancies of B-1 progenitors. Two principal types of BALL have been described in young children. Infant ALL, which occurs within the first year of life, is an aggressive disease that is frequently associated with MLL fusions including MLL-AF4 (Chen et al., 1993). Childhood pre-B ALL is the most common pediatric malignancy, peaks in incidence between the ages of two and four, and in 25% of cases is associated with the ETV6-RUNX1 (TEL-AML1) fusion (Armstrong and Look, 2005; Pui et al., 2008). In view of the fact that both MLL-AF4 and TEL-AML1 translocations occur in utero (Bueno et al., 2011; Greaves, 2003) at which time B-1 potential is at its peak, the possibility exists that some B-ALLs are B-1 malignancies. In this case, an understanding of the differences in regulation of the B-1 versus B-2 developmental process might contribute to devising new therapeutic strategies.
Conclusion
There is now compelling evidence that hematopoiesis is not a simple, linear process initiating in the fetus and continuing in the adult. Instead, the properties of the stem and progenitor cells that arise in different developmental windows are distinct. For example, fetal and adult HSCs have distinct patterns of gene expression that confer differences in proliferative and developmental potential (Bowie et al., 2007; Chhabra and Mikkola, 2011; He et al., 2011; Kim et al., 2007). It has also been known for over twenty years that selected types of T cells are generated from fetal HSCs (Ikuta et al., 1990) and it was recently shown that this is the case in humans as well (Mold et al., 2010). These observations, along with the historical and recent data regarding B-1 and B-2 development reviewed herein, support the conclusion that the adult immune system consists of cells generated in layered, developmental programs. This view has not been fully incorporated into the thinking of the immunology community, but we believe that doing so has substantial implications for understanding normal and dysregulated B lymphopoiesis as well as lymphocyte development in general.
Acknowledgments
This work was supported by NIH grant AI021256.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- Allman D, Pillai S. Peripheral B cell subsets. Curr Opin Immunol. 2008;20:149–157. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alugupalli KR, Gerstein RM. Divide and conquer: division of labor by B-1 B cells. Immunity. 2005;23:1–2. doi: 10.1016/j.immuni.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Antin J, Emerson S, Martin P, Gadol N, Ault K. A major lymphoid subpopulation in human fetal spleen: phenotypic and functional studies. J Immunol. 1986;136:505–510. [PubMed] [Google Scholar]
- Armstrong SA, Look AT. Molecular genetics of acute lymphoblastic leukemia. J Clin Oncol. 2005;23:6306–6315. doi: 10.1200/JCO.2005.05.047. [DOI] [PubMed] [Google Scholar]
- Arnold LW, Pennell CA, McCray SK, Clarke SH. Development of B-1 cells: segregation of phosphatidylcholine-specific B cells to the B-1 populations occurs after immunoglobulin gene expression. J Exp Med. 1994;179:1585–1595. doi: 10.1084/jem.179.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber C, Montecino-Rodriguez E, Dorshkind K. Reduced Production of B-1 Specified Common Lymphoid Progenitors Results in Diminished Potential of Adult Marrow to Generate B-1 Cells. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1107172108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol. 2002;20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833. [DOI] [PubMed] [Google Scholar]
- Bhat NM, Kantor AB, Bleber MM, Stall AM, Herzenberg LA, Teng NNH. The ontogeny and functional characteristics of human B-1 (CD5+ B) cells. Int Immunol. 1992;4:243–252. doi: 10.1093/intimm/4.2.243. [DOI] [PubMed] [Google Scholar]
- Bofill M, Janossy G, Janossa M, Burford GD, Seymour GJ, Wernet P, Kelemen E. Human B cell development. II Subpopulations in the human fetus. J Immunol. 1985;134:1531–1538. [PubMed] [Google Scholar]
- Bowie MB, Kent DG, Dykstra B, McKnight KD, McCaffrey L, Hoodless PA, Eaves CJ. Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proc Natl Acad Sci U S A. 2007;104:5878–5882. doi: 10.1073/pnas.0700460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno C, Montes R, Catalina P, Rodríguez R, Menendez P. Insights into the cellular origin and etiology of the infant pro-B acute lymphoblastic leukemia with MLL-AF4 rearrangement. Leukemia. 2011;25:400–410. doi: 10.1038/leu.2010.284. [DOI] [PubMed] [Google Scholar]
- Carmack CE, Shinton SA, Hayakawa K, Hardy RR. Rearrangement and selection of VH11 in the Ly-1 B cell lineage. J Exp Med. 1990;172:371–374. doi: 10.1084/jem.172.1.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter L, Malladi R, Yang C, French A, Pilkington K, Forsey R, Soane-Stanley J, Silk K, Davies T, Fairchild P, et al. Human-induced pluripotent stem cells are capable of B-cell lymphopoiesis. Blood. 2011;117:4008–4011. doi: 10.1182/blood-2010-08-299941. [DOI] [PubMed] [Google Scholar]
- Carvalho TL, Mota-Santos T, Cumano A, Demengeot J, Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in interelukin 7 (−/−) mice. J Exp Med. 2001;194:1141–1150. doi: 10.1084/jem.194.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casola S, Otipoby KL, Alimzhanov M, Humme S, Uyttersprot N, Kutok JI, Carroll MC, Rajewsky K. B cell receptor signal strength determines B cell fate. Nat Immunol. 2004;5:317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- Casola S. Control of peripheral B-cell development. Curr Opin Immunol. 2007;19:143–149. doi: 10.1016/j.coi.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Chen C, Sorenson P, Domer P, Reaman G, Korsmeyer S, Heerema N, Hammond G, Kersey J. Molecular rearrangements on chromosome 11q23 predonminate in infant acult lymphoblastic leukemia and are associated with specific biologic variables and poor outcome. Blood. 1993;81:2386–2393. [PubMed] [Google Scholar]
- Chhabra A, Mikkola H. Return to youth with Sox17. Genes Develop. 2011;25:1557–1562. doi: 10.1101/gad.17328611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiorazzi N, Ferrarini M. Cellular origin(s) of chronic lymphocytic leukemia: cautionary notes and additonal considerations and possibilities. Blood. 2011;117:1781–1791. doi: 10.1182/blood-2010-07-155663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumano A, Furlonger C, Paige CJ. Differentiation and characterization of B-cell precursors detected in the yolk sac and embryo body of embryos beginning at the 10- to 12-somite stage. Proc Natl Acad Sci U S A. 1993;90:6429–6433. doi: 10.1073/pnas.90.14.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauphinee M, Tovar Z, Talal N. B cells expressing CD5 are increased in Sjogren’s syndrome. Arthritis Rheum. 1988;31:642–647. doi: 10.1002/art.1780310509. [DOI] [PubMed] [Google Scholar]
- de Andres B, Gonzalo P, Minguet S, Martinez-Marin JA, Soro PG, Marcos MA, Gaspar ML. The first 3 days of B-cell development in the mouse embryo. Blood. 2002;100:4074–4081. doi: 10.1182/blood-2002-03-0809. [DOI] [PubMed] [Google Scholar]
- Descatoire M, Weill JC, Reynaud CA, Weller S. A human equivalent of mouse B-1 cells? J Exp Med. 2011;208:2563–2564. doi: 10.1084/jem.20112232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donze HH, Lue C, Julian BA, Kutteh WH, Kantele A, Mestecky J. Human peritoneal B-1 cells and the influence of continuous ambulatory peritoneal dialysis on peritoneal and peripheral blood mononuclear cell (PBMC) composition and immunoglobulin levels. Clin Exper Immunol. 1997;109:356–361. doi: 10.1046/j.1365-2249.1997.4541352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorshkind K, Montecino-Rodriguez E. Fetal B-cell lymphopoiesis and the emergence of B-1-cell potential. Nat Rev Immunol. 2007;7:213–219. doi: 10.1038/nri2019. [DOI] [PubMed] [Google Scholar]
- Duber S, Hafner M, Krey M, Lienenklaus S, Roy B, Hobeika E, Reth M, Buch T, Waisman A, Kretschmer K, Weiss S. Induction of B-cell development in adult mice reveals the ability of bone marrow to produce B-1a cells. Blood. 2009;114:4960–4967. doi: 10.1182/blood-2009-04-218156. [DOI] [PubMed] [Google Scholar]
- Dzierzak E, Speck NA, Speck N. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esplin BL, Weiner RS, Zhang Q, Borghesi LA, Kicade PW. A differentiation pathway for B1 cells in adult bone marrow. Proc Natl Acad Sci U S A. 2009;106:5773–5778. doi: 10.1073/pnas.0811632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerondakis S, Siebenlist U. Roles of the NF-κB pathway in lymphocyte development and function. Cold Spring Harb Perspect Biol. 2010;2:a000182. doi: 10.1101/cshperspect.a000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosn E, Sadate-Ngatchou P, Yang Y, Herzenberg L, Herzenberg L. Distinct progenitors for B-1 and B-2 cells are present in adult mouse spleen. Proc Natl Acad Sci U S A. 2011;108:2879–2884. doi: 10.1073/pnas.1019764108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin I, Dieterlen-Likvre F, Cumano A. B-lymphoid potential in pre-liver mouse embryo. Sem Immunol. 1995;7:131–141. doi: 10.1016/1044-5323(95)90041-1. [DOI] [PubMed] [Google Scholar]
- Godin IE, Garcia-Porrero JA, Coutinho A, Dieterlen-Lievre F, Marcos MA. Para-aortic splanchnopleura from early mouse embryos contains B1a cell progenitors. Nature. 1993;364:67–70. doi: 10.1038/364067a0. [DOI] [PubMed] [Google Scholar]
- Greaves MF, Wiemels JL. Origins of chromosome translocations in childhood leukemia. Nat Rev Cancer. 2003;3:639–649. doi: 10.1038/nrc1164. [DOI] [PubMed] [Google Scholar]
- Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70−. J Exp Med. 2011a;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DO, Holodick NE, Rothstein TL. Human B1 cells are CD3−: A reply to “A human equivalent of mouse B-1 cells? and “The nature of circulating CD27+ CD43+ B cells”. J Exp Med. 2011b;208:2566–2569. doi: 10.1084/jem.20111761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DO, Rothstein TL. A small CD11b+ human B1 cell subpopulation stimulates T cells and is expanded in lupus. J Exp Med. 2011;208:2591–2598. doi: 10.1084/jem.20110978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Hardy RR. B-lymphocyte development and biology. In: Paul WE, editor. Fundamental Immunology. Philadelphia: Lippincott Williams & Wilkins; 2003. pp. 159–194. [Google Scholar]
- Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy RR, Hayakawa K. Development of B cells producing natural autoantibodies to thymocytes and senescent erythrocytes. Springer Semin Immunopathol. 2005;26:363–375. doi: 10.1007/s00281-004-0183-1. [DOI] [PubMed] [Google Scholar]
- Hardy RR, Hayakawa K, Parks DR, Herzenberg LA, Herzenberg LA. Murine B cell differentiation lineages. J Exp Med. 1984;159:1169–1188. doi: 10.1084/jem.159.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy RR, Kincade PW, Dorshkind K. The protean nature of cells in the B lymphocyte lineage. Immunity. 2007;26:703–714. doi: 10.1016/j.immuni.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Hastings WD, Tumang JR, Behrens TW, Rothstein TL. Peritoneal B-2 cells comprise a distinct B-2 cell population with B-1b-like characteristics. Eur J Immunol. 2006;36:1114–1123. doi: 10.1002/eji.200535142. [DOI] [PubMed] [Google Scholar]
- Haughton G, Arnold LW, Whitmore AC, Clarke SH. B-1 cells are made, not born. Immunol Today. 1993;14:84–87. doi: 10.1016/0167-5699(93)90064-R. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Hardy RR, Herzenberg LA, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985;161:1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Hardy RR, Parks DR, Herzenberg LA. The “Ly-1 B” cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983;157:202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Kim I, Lim M, Morrison SJ. Sox17 expression confers self-renewal potential and fetal stem cell characteristics upon adult hematopoietic progentiors. Genes Develop. 2011;25:1613–1627. doi: 10.1101/gad.2052911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzenberg LA, Herzenberg L. Toward a layered immune system. Cell. 1989;59:953–954. doi: 10.1016/0092-8674(89)90748-4. [DOI] [PubMed] [Google Scholar]
- Herzenberg LA, Tung JW. B cell lineages: documented at last! Nat. Immunol. 2006;7:225–226. doi: 10.1038/ni0306-225. [DOI] [PubMed] [Google Scholar]
- Ikuta K, Kina T, MacNeil I, Uchida N, Peault B, Chien Y, Weissman I. A developmental switch in thymic lymphocyte maturaion potential occurs at the level of hematopoietic stem cells. Cell. 1990;62:863–874. doi: 10.1016/0092-8674(90)90262-d. [DOI] [PubMed] [Google Scholar]
- Ikuta K, Uchida N, Friedman J, Weissman I. Lymphocyte development from stem cells. Annu Rev Immunol. 1992;10:759–783. doi: 10.1146/annurev.iy.10.040192.003551. [DOI] [PubMed] [Google Scholar]
- Irion S, Clarker RL, Luche H, Kim I, Morrow M, Fehling HJ, Keller GM. Temporal specification of blood progenitors from mouse embryonic stem cells and induced pluripotent stem cells. Development. 2010;137:2829–2839. doi: 10.1242/dev.042119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen C, Kharazi S, Boiers C, Liuba K, Jacbosen S, Vieira P, Vosshenrich C, Cumano A, Muller W, Di Santo J, et al. TSLP-mediated fetal B lymphopoiesis? Nat Immunol. 2007;8:897–898. doi: 10.1038/ni0907-897. [DOI] [PubMed] [Google Scholar]
- Kantor AB, Herzenberg LA. Origin of murine B cell lineages. Annu Rev Immunol. 1993;11:501–538. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- Kantor AB, Stall AM, Adams S, Herzenberg LA, Herzenberg LA. Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci U S A. 1992;89:3320–3324. doi: 10.1073/pnas.89.8.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Kondo K. Developmental switch of mouse hematopoietic stem cells from fetal to adult type occurs in bone marrow after birth. Proc Natl Acad Sci USA. 2006;103:17852–17857. doi: 10.1073/pnas.0603368103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Saunders TL, Morrison SJ. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell. 2007;130:470–483. doi: 10.1016/j.cell.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- Lanier L, Warner N, Ledbetter J, Herzenberg L. Expression of Lyt-1 antigen on certain murine B cell lymphomas. J Exp Med. 1981;153:998–1003. doi: 10.1084/jem.153.4.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay F, Figgett W, Saulep D, Lepage M, Hibbs M. B-cell stage and context-dependent requirements for survival signals from BAFF and the B-cell receptor. Immunol Rev. 2010;237:205–225. doi: 10.1111/j.1600-065X.2010.00944.x. [DOI] [PubMed] [Google Scholar]
- Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2:323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- Medvinsky A, Rybstov S, Taoudi S. Embryonic origin of the adult hematopoietic system: advances and questions. Development. 2011;138:1017–1031. doi: 10.1242/dev.040998. [DOI] [PubMed] [Google Scholar]
- Merbl Y, Zucker-Toledano M, Quintana FJ, Cohen IR. Newborn humans manifest autoantibodies to defined self molecules detected by antigen microarray informatics. J Clin Invest. 2007;117:712–718. doi: 10.1172/JCI29943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mold JE, Venkatasubrahmanyam S, Burt TD, Michaelsson J, Rivera JM, Galkina SA, Weinberg K, Stoddart CA, McCune JM. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–1699. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe JG, Dorshkind K. Fate decisions regulating bone marrow and peripheral B lymphocyte development. Adv Immunol. 2007;95:1–50. doi: 10.1016/S0065-2776(07)95001-4. [DOI] [PubMed] [Google Scholar]
- Montecino-Rodriguez E, Leathers H, Dorshkind K. Bipotential B-macrophage progenitors are present in adult bone marrow. Nat Immunol. 2001;1:83–88. doi: 10.1038/83210. [DOI] [PubMed] [Google Scholar]
- Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- Montecino-Rodriquez E, Dorshkind K. Stromal cell-dependent growth of B-1 B cell progenitors in the absence of direct contact. Nat Protocols. 2006;1:1140–1144. doi: 10.1038/nprot.2006.163. [DOI] [PubMed] [Google Scholar]
- Montecino-Rodriguez E, Dorshkind K. Formation of B-1 B cells from B-1 transitional cells exhibits NF-κB redundancy. J Immunol. 2011;187:5712–5719. doi: 10.4049/jimmunol.1102416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen J, Cooper M, Raff M. In vitro generation of B lymphocytes in mouse fetal liver, a mammalian ‘bursa equivalent’. Nature. 1974;249:361–363. doi: 10.1038/249361a0. [DOI] [PubMed] [Google Scholar]
- Owen J, Raff M, Cooper M. Studies on the generation of B lymphocytes in the mouse embryo. Eur J Immunol. 1975;5:468–473. doi: 10.1002/eji.1830050708. [DOI] [PubMed] [Google Scholar]
- Palis J, Yoder MC. Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp Hematol. 2001;29:927–936. doi: 10.1016/s0301-472x(01)00669-5. [DOI] [PubMed] [Google Scholar]
- Pelayo R, Welner RS, Nagai Y, Kincade PW. Life before the pre-B cell receptor checkpoint: specification and commitment of primitive lymphoid progenitors in adult bone marrow. Semin Immunol. 2006;18:2–11. doi: 10.1016/j.smim.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Perez-Andres M, Grosserichter-Wagener C, Teodosio C, van Dongen JJM, Orfao A, van Zelm MC. The nature of circulating CD27+CD43+ B cells. J Exp Med. 2011;208:2565–2566. doi: 10.1084/jem.20112203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PrabhuDas M, Adkins B, Gans H, King C, Levy O, Ramilo O, Siegrist C. Challenges in infant immunity: implications for responses to infection and vaccines. Nat Immunol. 2011;12:189–194. doi: 10.1038/ni0311-189. [DOI] [PubMed] [Google Scholar]
- Pui CH, Robison LL, Look AT. Acute lymphoblastic leukameia. The Lancet. 2008;371:1030–1043. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- Rolink A, Melchers F. B-cell development in the mouse. Immunol Letters. 1996;54:157–161. doi: 10.1016/s0165-2478(96)02666-1. [DOI] [PubMed] [Google Scholar]
- Rosenbauer F, Owens BM, Yu L, Tumang JR, Steidl U, Kutok JL, Clayton LK, Wagner K, Scheller M, Iwasaki H, et al. Lymphoid cell growth and transformation are suppressed by a key regulatory element of the gene encoding PU. 1. Nat Genet. 2006;38:27–37. doi: 10.1038/ng1679. [DOI] [PubMed] [Google Scholar]
- Sanz E, Munoz-A N, Monserrat J, Van-Den-Rym A, Escoll P, Ranz I, Alvarez-Mon M, de-la-Hera A. Ordering human CD34+CD10−CD19+ pre/pro-B-cell and CD19− common lymphoid progenior stages in two pro-B-cell development pathways. Proc Natl Acad Sci U S A. 2010;107:5925–5930. doi: 10.1073/pnas.0907942107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solvason N, Kearney JF. The human fetal omentum: a site of B cell generation. J Exp Med. 1992;175:397–404. doi: 10.1084/jem.175.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solvason N, Lehuen A, Kearney JF. An embryonic source of Ly1 but not conventional B cells. Int Immunol. 1991;3:543–550. doi: 10.1093/intimm/3.6.543. [DOI] [PubMed] [Google Scholar]
- Szabo E, Rampalli S, Risueno R, Schnerch A, Mitchell R, Fiebig-Comyn A, Levadoux-Martin M, Bhatia M. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010;468:521–526. doi: 10.1038/nature09591. [DOI] [PubMed] [Google Scholar]
- Thome M. CARMA1, BCL-10, and MALT1 in lymphocyte development and activation. Nat Rev Immunol. 2004;4:348–359. doi: 10.1038/nri1352. [DOI] [PubMed] [Google Scholar]
- Tung JW, Mrazek MD, Yang Y, Herzenberg LA, Herzenberg LA. Phenotypically distinct B cell development pathways map to the three B cell lineages in the mouse. Proc Natl Acad Sci U S A. 2006;103:6293–6298. doi: 10.1073/pnas.0511305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale A, Tanner J, Schelonka R, Zhuang Y, Jackson A, Pan L, Shen K, Dai M, Zemlin M, Gartland G, Schroeder HJ. The peritoneal cavity B-2 antibody repertoire appears to reflect many of the same selective pressures that shape the B-1a and B-1b repertoires. J Immunol. 2010;185:6085–6095. doi: 10.4049/jimmunol.1001423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshenrich CA, Cumano A, Muller W, Di Santo JP, Vieira P. Pre-B cell receptor expression is necessary for thymic stromal lymphopoietin responsiveness in the bone marrow but not in the liver environment. Proc Natl Acad Sci USA. 2004;101:11070–11075. doi: 10.1073/pnas.0402919101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M, Ermakova O, Graf T. PU. 1 is not strictly required for B cell development and its absence induces a B-2 to B-1 cell switch. J Exp Med. 2005;202:1411–1422. doi: 10.1084/jem.20051089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto M, Montecino-Rodriguez E, Prashanth P, Shelley WC, Conway SJ, Dorshkind K, Yoder MC. B-1 and Marginal Zone B progenitor cells emerge from yolk sac hemogenic endothelium. Proc Natl Acad Sci U S A. 2011;108:1468–1473. doi: 10.1073/pnas.1015841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambidis ET, Peault B, Park TS, Bunz F, Civin CI. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development. Blood. 2005;106:860–870. doi: 10.1182/blood-2004-11-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]