SUMMARY
In the retina, presynaptic inhibitory mechanisms that shape directionally selective (DS) responses in output ganglion cells are well established. However, the nature of inhibition-independent forms of directional selectivity remains poorly defined. Here, we describe a genetically specified set of ON-OFF DS ganglion cells (DSGCs) that code anterior motion. This entire population of DSGCs exhibits asymmetric dendritic arborizations that orientate toward the preferred direction. We demonstrate that morphological asymmetries along with nonlinear dendritic conductances generate a centrifugal (soma-to-dendrite) preference that does not critically depend upon, but works in parallel with the GABAergic circuitry. We also show that in symmetrical DSGCs, such dendritic DS mechanisms are aligned with, or are in opposition to, the inhibitory DS circuitry in distinct dendritic subfields where they differentially interact to promote or weaken directional preferences. Thus, pre- and postsynaptic DS mechanisms interact uniquely in distinct ganglion cell populations, enabling efficient DS coding under diverse conditions.
INTRODUCTION
Directionally selective ganglion cells (DSGCs) of the retina respond vigorously to visual stimuli moving in a preferred but not a null direction. Barlow and Levick (1965) postulated that directionally selective (DS) responses arose from lateral asymmetries within the inhibitory circuitry. Over the years, results from numerous studies have provided conflicting evidence for and against a critical role for inhibition in DS computations, leaving this issue unresolved.
Support for inhibitory circuit mechanisms came from early pharmacological analysis that revealed a critical role for GABAA receptors in mediating directional selectivity (Wyatt and Day, 1976; Caldwell et al., 1978), a finding that is now well substantiated (for review see Taylor and Vaney, 2003; Demb, 2007). Subsequently, inhibitory currents preferentially evoked by null-direction stimuli were directly measured using patch-clamp techniques (Taylor et al., 2000). Mounting evidence suggests the cholinergic/GABAergic starburst amacrine cells (SACs) as the likely source of asymmetric inhibition to DSGCs. The radial dendrites of SACs exhibit a centrifugal directional preference (Euler et al., 2002), which arises through a combination of intrinsic mechanisms (Tukker et al., 2004; Hausselt et al., 2007) and network interactions (Fried et al., 2005; Lee et al., 2010). Direct stimulation of individual SACs with patch electrodes or optical neuromodulators revealed that SACs with soma located on the null side of a DSGC (i.e., the side at which null-direction stimulus approaches) provide stronger GABAergic inhibition compared to those on the preferred side (Fried et al., 2002, 2005; Lee et al., 2010; Wei et al., 2011; Yonehara et al., 2011). Serial block-face electron microscopic analysis further revealed an exquisite specificity in the alignment between synaptically connected SAC and DSGC processes, indicating that these connections were optimized for preferential activation during null direction stimulus motion (Briggman et al., 2011). Moreover, targeted ablation of SACs abolishes DS responses in ganglion cells (Yoshida et al., 2001). Together, these findings suggest that SACs are the leading substrate for DS computations in the retina.
In contrast to inhibitory circuit mechanisms, previous studies in a variety of species have reported that DS responses persisted when GABAA receptors were blocked (Bülthoff and Bülthoff, 1987; Egelhaaf et al., 1990; Öğmen, 1991; Smith et al., 1996; Grzywacz et al., 1997; Ackert et al., 2009). Much less is known about mechanisms that could generate directional selectivity in the retina independent of inhibitory circuits. One possibility is that nonlinear conductances could generate directional selectivity within the dendrites of DSGCs, as appears to happen in SACs (Hausselt et al., 2007). However, in rabbit ON-OFF DSGCs, nonlinear conductances were found to amplify DS responses but not generate them (Oesch et al., 2005).
In other parts of the CNS, dendritic morphology is known to contribute to DS coding (Rall, 1964; Livingstone, 1998; London and Häusser, 2005; Branco et al., 2010). However, it is unclear whether dendritic shape significantly influences DS coding in the retina. First, direction can be faithfully computed by symmetrical ganglion cells (Amthor et al., 1989; Oyster et al., 1993; Yang and Masland, 1994), obviating the need for morphological specializations. Second, direction can be computed within a small region of the receptive field, again suggesting that the shape of the DSGC is not important (Barlow and Levick, 1965). Third, although DSGC dendrites were often found to be highly asymmetric, these appeared randomly orientated (Yang and Masland, 1994; Huberman et al., 2009), suggesting that morphological differences would only add noise to the population signal. Finally, even in the newly described OFF DSGC, which does exhibit systematic dendritic asymmetries that correlate with directional preferences, the DS responses were attributed to spatially offset lateral inhibition (Kim et al., 2008). Thus, to date, there is little evidence to support a role for ganglion cell dendritic morphology in DS processing.
When considering mechanisms underlying directional selectivity, most studies failed to fully appreciate the diversity of DSGC populations. The mouse retina includes at least eight subtypes (four types of ON-OFF, three ON, and one OFF) that have distinct molecular, morphological, and physiological characteristics. If different types of DSGCs utilize distinct computational mechanisms, pooling results from random cell types could potentially lead to ambiguous results. To this end, here we define the properties of a genetically specified population of ON-OFF DSGCs in which the preferred direction is strongly correlated with asymmetries in dendritic arborizations. We demonstrate that in addition to the conventional inhibitory circuitry, a parallel dendritic mechanism contributes to the formation of DS responses. This dendritic mechanism aligns with, but does not rely critically upon, GABAergic inhibition. Furthermore, we show that in symmetrical DSGCs, these different DS mechanisms work in parallel or in opposition within distinct dendritic subfields, to strengthen or weaken DS responses, respectively. Thus, in the retina, multiple mechanisms appear to encode DS responses.
RESULTS
A Genetically Labeled Population of ON-OFF Retinal Ganglion Cells that Exhibit Asymmetric Dendritic Arbors
During a screen carried out to detect genetic markers expressed in the retina, we identified the Hb9::eGFP transgenic mouse line (Arber et al., 1999) that exhibited a sparse neuronal labeling pattern in the ganglion cell layer (~80 cells/mm2; n = 6 retinas; Figure 1A). Axonal labeling indicated that GFP was expressed in ganglion cells. Two-photon imaging of the live retina revealed that GFP+ cells were ON-OFF ganglion cells because their dendrites ramified in discrete strata in both the ON and OFF layers of the inner plexiform layer (Figures 1B and 1C). No other types of ganglion, amacrine, or bipolar cells were labeled in this mouse line, making it ideally suited for the study of ON-OFF ganglion cells.
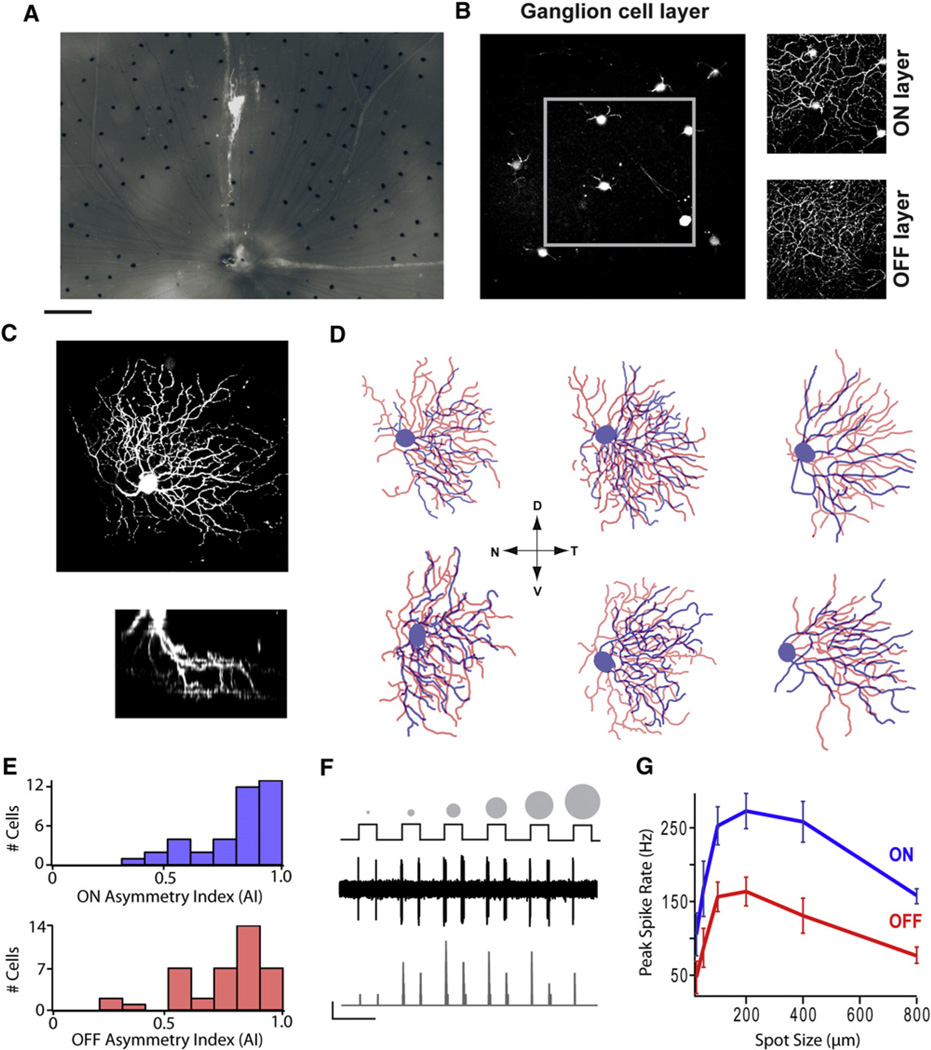
Figure 1. Systematic Dendritic Asymmetries within an Entire Population of Genetically Specified ON-OFF Directional Selective Ganglion Cells.
(A) Photomicrograph of the Hb9::eGFP whole-mount retina showing a mosaic of GFP+ ganglion cells with their axons streaming toward the optic disc.
(B) Two-photon image of GFP labeling in the ganglion cells in the living retina showing their dendrites in the ON and OFF strata of the inner plexiform layer.
(C) An image stack of an individual Alexa 594-loaded GFP+ ganglion cell. The lower image is rotated 90° to show the stratification of the ON and OFF dendrites.
(D) Reconstructions of six GFP+ ganglion cells (from stacks similar to ones shown in C). ON dendrites are labeled in blue; OFF dendrites are labeled in red (see also Figure S1 for detailed morphological analysis). N, nasal; D, dorsal; T, temporal; V, ventral.
(E) Histograms representing the AI for ON and OFF dendrites calculated for 42 GFP+ DSGCs. AI values fall between 0 and 1, with 1 indicating perfect asymmetry and 0 indicating perfect symmetry.
(F) The response of a GFP+ ganglion cell to bright spots of increasing size (25–800 µm) centered over its receptive field. The middle trace shows the raw spiking data (black). The bottom trace is a plot of the spike rate (binned over 25 ms; gray). Scale bars represent 50 Hz and 5 s.
(G) The average spike rates (n = 11) for both the ON and OFF responses to spots are plotted. Data have been presented as mean ± SEM.
Scale bars represent 160 µm (A), 55 µm (B), 50 µm (C top), 50 µm and 20 µm (for × and y scales, respectively, C bottom), 40 µm (D).
Next, individual GFP+ ganglion cells were loaded with Alexa 594 using a patch electrode (Figure 1C), and their dendritic arborizations in both ON and OFF layers were traced offline. Examples of these reconstructions illustrate the homogeneity in morphological characteristics (Figure 1D). GFP+ ganglion cells were found to bear similar morphological characteristics as those described previously for bistratified DSGCs (Sun et al., 2002; Coombs et al., 2006). The one notable difference compared to previous descriptions, however, was that the dendritic arborizations in both the ON and OFF subfields of every GFP+ ganglion cell were found to be highly asymmetric (Figures 1D and 1E). The degree of polarization was quantified as an asymmetry index (AI; zero [0] indicating perfect symmetry, whereas values closer to 1 indicate stronger asymmetry; see Experimental Procedures). On average, AIs for the entire population of GFP+ ganglion cells measured were 0.82 ± 0.03 for the ON dendrites and 0.75 ± 0.03 for the OFF dendrites (n = 42; Figure 1E). In addition, dendritic trees of all cells orientated toward the temporal pole (Figures 1D and 2C). Although asymmetric dendritic trees in ON-OFF DSGCs have been commonly observed (Amthor et al., 1989; Oyster et al., 1993; Yang and Masland, 1994), our finding that the entire population of DSGCs was asymmetric and pointed in the same direction was unexpected.
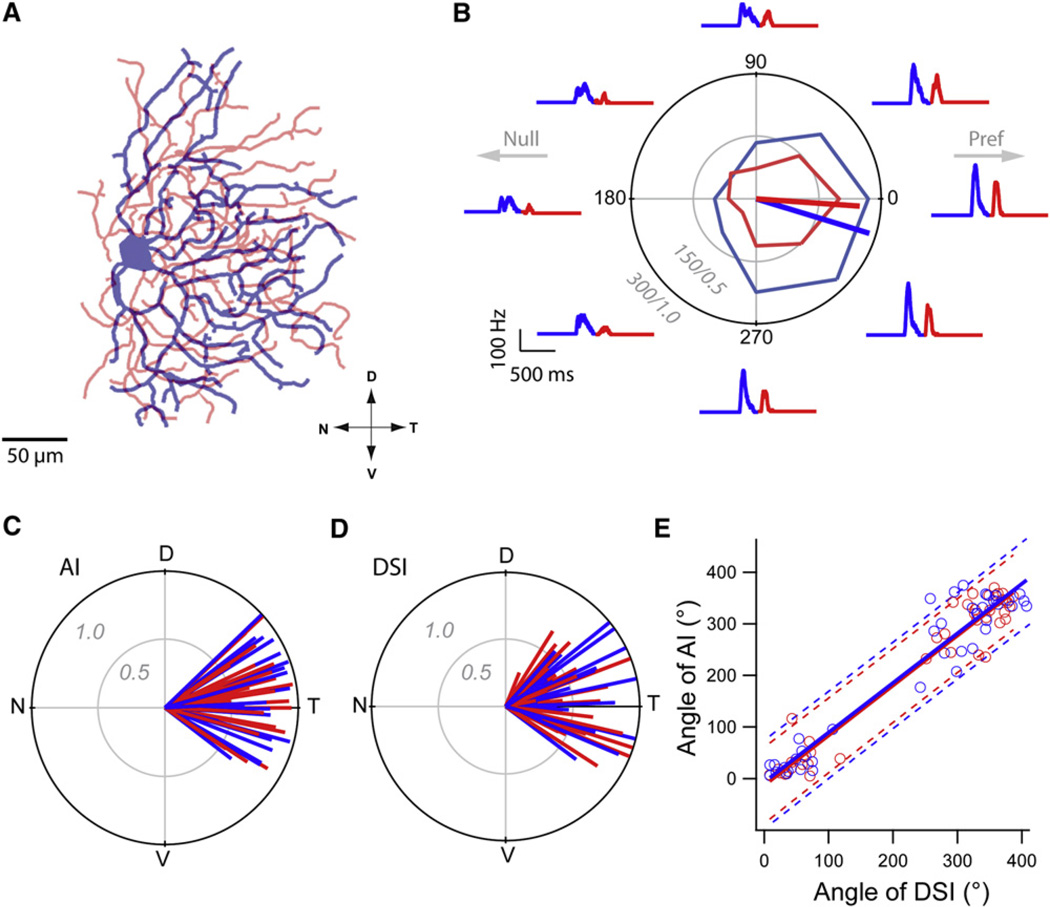
Figure 2. Asymmetric Dendritic Morphology Correlates with Directional Preferences.
(A) A reconstruction of a GFP+ DSGC showing that the majority of dendrites project toward the temporal pole.
(B) Polar plot representing the peak ON (leading edge) and OFF (trailing edge) spike rates evoked by a 400 µm spot moved in eight directions over the receptive field of a GFP+ DSGC. Radial axis of the polar plot represents spike rate/DSI. DSI values range from 0 to 1, with larger values indicating more asymmetric responses. Spike rate histograms surround the polar plot (ON and OFF responses are depicted in blue and red, respectively, here and in the rest of the figures) for each of the eight stimulus directions.
(C) A polar plot of AI vectors for GFP+ DSGCs.
(D) A polar plot of the DSI vectors for GFP+ DSGCs.
(E) The direction (angle in degrees) of AI and DSI for individual cells are plotted against each other (R2 = 0.92, 0.94 for ON and OFF, respectively; n = 42). Dotted lines represent 90% prediction bands for ON (blue) and OFF (red) DSI/AI correlations (see Figure S2 for Hb9− DSGCs). N, nasal; D, dorsal; T, temporal; V, ventral.
GFP+ ganglion cells were also relatively homogeneous in a number of other features compared to previous descriptions of ON-OFF DSGCs. For example, the size of their dendritic fields showed little variance when compared to those of ON-OFF ganglion cells previously described (see Figure S1 available online) (Sun et al., 2002). Consistent with previous observations in the murine retina, the dendritic field diameter did not depend on the distance from the optic disk. In addition, soma size, total dendritic length, number of branches, branch order, and number of primary dendrites were also relatively constant (Figure S1). Together, these data suggest that a single subset of ON-OFF DSGCs is labeled in the Hb9::eGFP mouse retina.
Asymmetric Dendritic Arbors of Hb9::eGFP DSGCs Align with the Preferred Direction
We next used two-photon targeted patch-clamp techniques to examine the physiological responses of GFP+ ganglion cells. In response to spots of light of increasing size centered over the receptive field, robust responses were observed at the onset and offset of the stimulus, confirming that GFP+ cells received ON and OFF inputs (Figure 1F). The optimum spot stimulus was 100–200 µm in diameter, similar to the dendritic field size (192.8 ± 2.7 µm; n = 42; Figures 1G and S1).
When we presented moving stimuli (a 400 µm spot moving in 8 directions at 1000 µm/s), DSGCs responded to the leading (ON) and trailing (OFF) edges of the spot with a burst of spikes (Figure 2B). Stimuli moving in the centrifugal (soma to dendrite) direction evoked the maximal response, whereas those moving in the centripetal direction (dendrite to soma) evoked weaker responses (Figure 2B). The direction of preferred response was consistent from cell to cell and always pointed toward the temporal pole, parallel to the dendritic tree (Figures 2B–2D). The DS indices (DSIs; see Experimental Procedures) for ON and OFF responses were 0.45 ± 0.03 and 0.52 ± 0.03, respectively (n = 42; note DSI ranges from 0 to 1, with larger values indicating stronger directional selectivity). Plotting the angle of the DSI against that of the AI for ON and OFF responses/dendritic trees (Figure 2E) yielded striking correlations with slopes of 0.96 (R2 = 0.92) and 0.97 (R2 = 0.94), respectively. These findings contrast with previous reports that found ON-OFF DSGC dendrites to be either symmetric or asymmetric but randomly oriented with respect to the preferred direction (Yang and Masland, 1994; Huberman et al., 2009; but see Kim et al., 2008 for OFF DSGCs). The strong correlation between morphological and functional asymmetries observed here suggests that dendrites play a role in computing direction.
Although our results clearly demonstrate that GFP+ cells in the Hb9::eGFP retina belong to a unique set of polarized DSGCs that code anterior motion, it is not clear if asymmetries are present in ganglion cells that code other directions. To test this possibility, we next recorded from GFP− DSGCs in the Hb9::eGFP retina (Figure S2). In a random sample of 14 cells, we found that 4 displayed asymmetry comparable to the GFP+ cells. In these cells, asymmetry appeared to be orientated in the same direction as the preferred responses (Figure S2). In the general population, however, only a weak correlation between the orientation of dendrites and response preference was observed for ON but not for OFF dendrites (R2 = 0.20 and 0.03 for ON and OFF, respectively; Figure S2). Without knowing whether asymmetric cells belong to a specific population of DSGCs or if they are part of a population with varying morphologies, it is difficult to establish the functional significance of these findings. Hence, the identification of a genetic marker that labeled a specific population of asymmetrical DSGCs in this study was pivotal in establishing the functional relevance of morphological specializations.
The Inhibitory DS Circuitry Aligns with Dendritic Asymmetries along the Nasal-Temporal Axis
To understand the mechanisms that generate directional selectivity in this asymmetrical population of cells, we first investigated whether they received inputs from the classic inhibitory DS circuitry. To do so, currents evoked by moving stimuli were measured using whole-cell voltage-clamp techniques, while holding the membrane at different potentials (Figure S3). The total conductance was split into its inhibitory and excitatory components based on their reversal potentials set at 0 and −60 mV, respectively (Figure S3) (Taylor and Vaney, 2002).
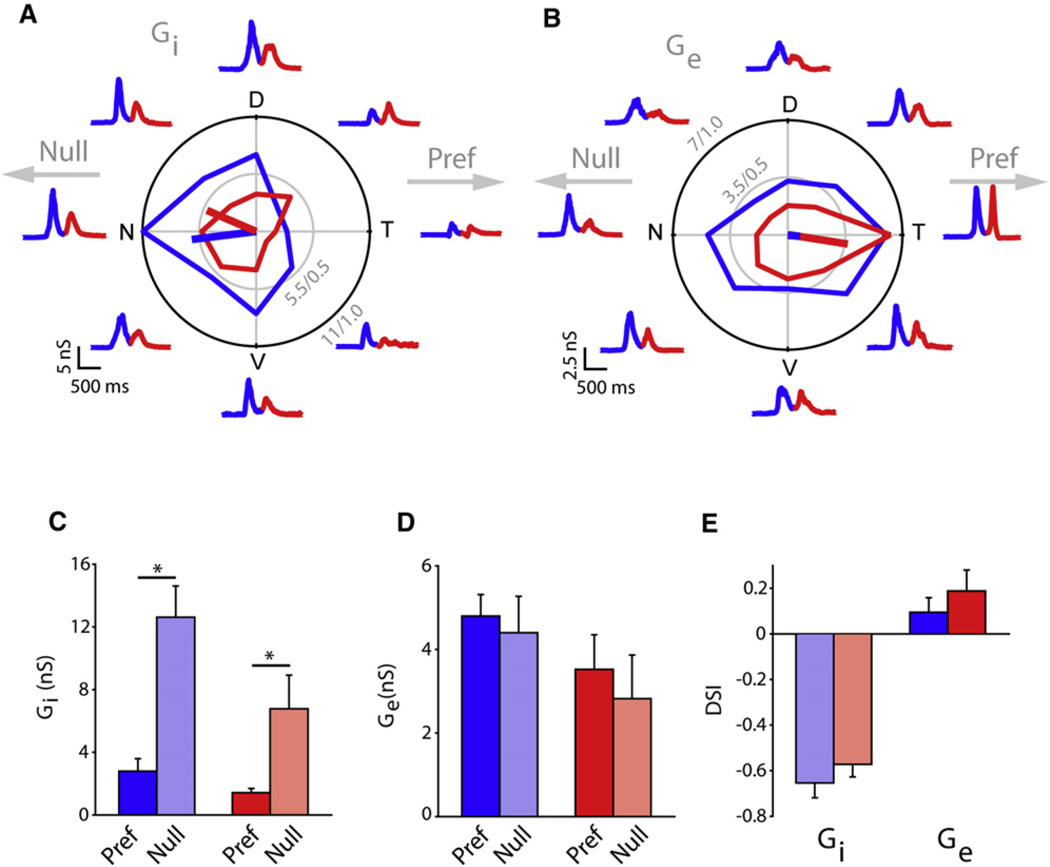
This analysis revealed that inhibitory conductances evoked by null-direction stimuli were significantly larger than those evoked by preferred direction stimuli (null: 12.6 ± 2.0 nS and 6.8 ± 2.1 nS for ON and OFF responses, respectively; preferred: 2.8 ± 0.8 nS and 1.4 ± 0.3 nS for ON and OFF responses, respectively; p < 0.001, Mann-Whitney U rank sum test; n = 8; Figures 3A and 3C). These data are consistent with the spiking responses measured in these cells that indicated a significantly weaker response for null-direction stimuli. In contrast, excitatory conductances sometimes trended toward being larger for preferred direction stimuli (Figure 3B). However, on average this difference was not statistically significant (preferred: 4.8 ± 0.5 nS and 3.5 ± 0.8 nS, for ON and OFF responses, respectively; null: 4.4 ± 0.9 nS and 2.8 ± 1.0 nS for ON and OFF responses, respectively; p > 0.1, Mann-Whitney U rank sum test; n = 8; Figures 3B and 3D). Thus, there appears to be little presynaptic modulation of bipolar cell inputs during null and preferred movements. Consequently, consistent with previous reports (Taylor and Vaney, 2002), the asymmetry of inhibitory inputs was significantly stronger than that observed for excitatory inputs (Figure 3E; Gi DSI: −0.65 ± 0.07 and −0.57 ± 0.05 for ON and OFF responses, respectively; Ge DSI: 0.09 ± 0.06 and 0.19 ± 0.09 for ON and OFF responses, respectively). Thus, Hb9+ DSGCs appear to be driven by patterns of inhibitory and excitatory synaptic conductances that are typically associated with DS computations. Importantly, the inhibitory DS circuitry is aligned along the nasal-temporal axis, parallel to the asymmetric dendritic arbors.
Figure 3. Inhibitory DS Circuitry Aligns along the Nasal-Temporal Axis.
(A) A polar plot of the peak inhibitory conductances (Gi) measured in a whole-cell voltage-clamp recording from an Hb9+ DSGC. Traces around the plot illustrate the inhibitory conductances measured in the corresponding directions (see Figure S3 for conductance analysis).
(B) A polar plot of the peak excitatory conductances (Ge) in the same Hb9+ DSGC shown in (A). Traces around the plot illustrate the excitatory conductances measured in the corresponding directions.
(C) The average inhibitory conductances evoked in the null and preferred (Pref) directions (n = 8; *p < 0.001) for ON and OFF responses. Data have been presented as mean ± SEM.
(D) The average excitatory conductances evoked in the null and preferred directions (n = 8; p > 0.1) for ON and OFF responses. Data have been presented as mean ± SEM.
(E) Average DSI values for Gi and Ge for ON (blue) and OFF (red) responses. Data have been presented as mean ± SEM. N, nasal; D, dorsal; T, temporal; V, ventral.
Directional Selectivity in GFP+ Ganglion Cells Does Not Critically Depend on the Classic Inhibitory Circuitry
Although the presence of asymmetric inhibition suggests that conventional mechanisms generate DS responses in Hb9+ cells, they do not preclude the existence of additional mechanisms suggested by their morphology. To test the functional significance of the asymmetric dendritic morphology of ON-OFF DSGCs, we next studied their response properties before and after blocking the conventional inhibitory DS circuit using a cocktail of antagonists (100 µM picrotoxin, 50 µM TPMPA, and 50 µM D-tubocurarine to antagonize GABAA,C and nicotinic receptors, respectively). This cocktail is expected to block the output of SACs and other GABAergic amacrine cells known to generate directional selectivity (Fried et al., 2002, Taylor and Vaney, 2003, Demb, 2007). Indeed, we found that this cocktail effectively blocked all inhibitory currents in these cells (Figure S4).
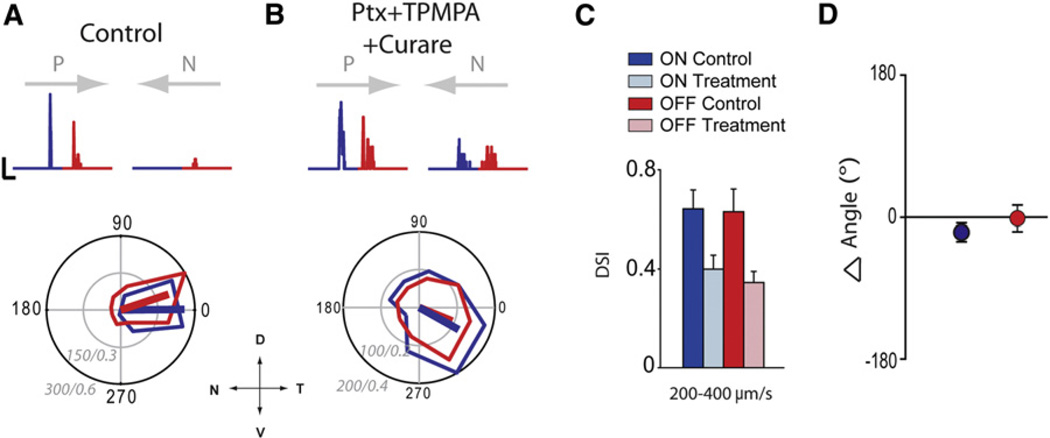
Figure 4A illustrates the responses to slow-moving spots (200 µm/s) measured in control conditions, illustrating the preferred direction toward the temporal pole. Remarkably, responses in this cell remained DS after the cocktail of antagonists was applied (Figure 4B). Although less robust than control DS responses, spike rates in the preferred direction were more than double those evoked in the null direction (Figure 4C; control DSI: 0.64 ± 0.07 and 0.63 ± 0.09 for ON and OFF responses, respectively; DSI in blockers: 0.40 ± 0.06 and 0.35 ± 0.04 for ON and OFF responses, respectively; p < 0.05 for both ON and OFF; n = 11). In addition, the direction of the preferred response was always maintained (Figure 4D; average deviation of the preferred direction was −20° ± 10° compared to control for ON responses and −1° ± 17° for OFF responses; p > 0.2, Moore’s paired-sample test). Together, these results demonstrate a form of directional selectivity that does not critically rely upon, but is in alignment with, the classic inhibitory DS circuitry.
Figure 4. DS Responses Persist in the Presence of GABAA,C Receptor Antagonists.
(A) Control DS response. The top traces are spike rate histograms for ON and OFF responses. In this and all subsequent figures, the gray arrows above responses indicate the preferred (P) and null (N) directions. Below is a polar plot of the peak spike rate. Scale bars for spike rate histograms represent 100 Hz, 1 s.
(B) Responses in the same cell shown in (A) in the added presence of drugs (picrotoxin [Ptx], TPMPA, and D-tubocurarine; also see Figure S4). Scale bars for spike rate histograms represent 50 Hz, 1 s. The radial scale bars of the polar plots of (A) and (B) represent Hz/DSI. The stimuli speed for (A) and (B) is 200 µm/s.
(C) Average DSI for both ON (blue) and OFF (red) responses (n = 11). Data have been presented as mean ± SEM.
(D) Average change in the preferred direction in control versus drugs for both ON (blue) and OFF (red) responses (p > 0.2, Moore’s paired-sample test). Data have been presented as mean ± SEM. N, nasal; D, dorsal; T, temporal; V, ventral.
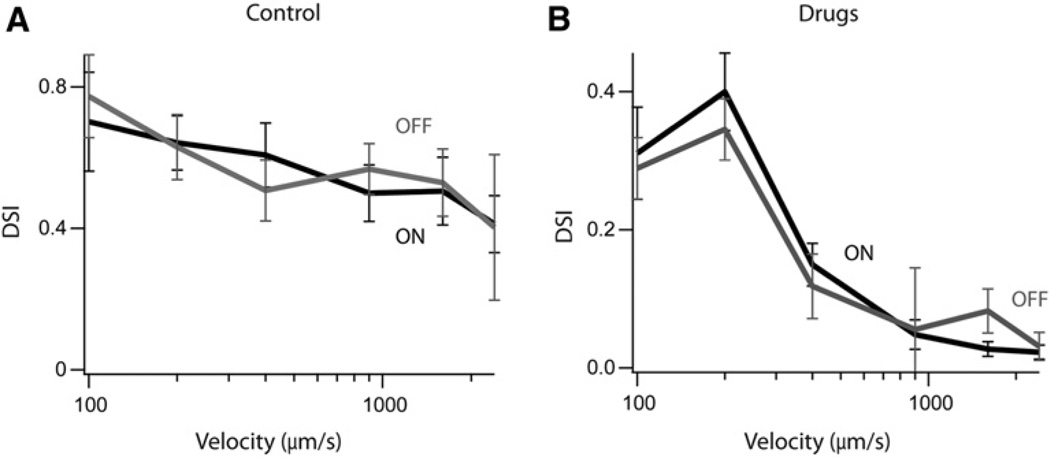
The DS responses observed in the presence of GABAA receptor blockers were surprising considering the abundant literature supporting a critical role for inhibition in mediating directional selectivity (Wyatt and Day, 1976; Caldwell et al., 1978; Taylor and Vaney, 2002). Even in previous studies where directionally selective responses were detectable under saturating concentrations of inhibitory blockers, they were relatively mild (Smith et al., 1996; Grzywacz et al., 1997). Because we had performed our initial experiments at relatively slow stimulus speeds, we next tested the effects of varying speed on DSI, in an attempt to reconcile our findings with previous work.
In control conditions, increasing the stimulus speed resulted in an increased spike rate for null and preferred stimuli and led to a mild decrease in DSI at the high range of speeds tested (100–2400 µm/s; Figure 5A). Application of the cocktail of antagonists augmented spiking responses for both preferred and null directions, though null-direction responses tended to show much greater augmentation, confirming that inhibitory circuit mechanisms usually suppressed these responses (data not shown). In the presence of blockers, at the slower speeds, null-direction responses always remained lower than those elicited in the preferred direction, and consequently, responses remained DS (Figure 5B). However, as the stimulus speed was increased, DSI declined. By 1000 µm/s, directional selectivity was weak and only detected in a few cells, but on average was not statistically significant (ON DSI: 0.50 ± 0.08 in control compared to 0.05 ± 0.02 in blockers; p < 0.005; OFF DSI: 0.57 ± 0.07 compared to 0.06 ± 0.09 in blockers; p < 0.005; n = 11). At speeds higher than 1000 µm/s, DS responses were never observed. Because higher ranges of speeds are typically used to stimulate DS responses in most studies, these findings suggest one possible reason why directional selectivity was not observed previously in the presence of blockers.
Figure 5. Inhibition-Independent Directional Selectivity Is Apparent Only at Slower Velocities.
Average DSI for ON (black) and OFF (gray) responses as a function of stimulus velocity in (A) control conditions and (B) in the presence of drugs (picrotoxin, TPMPA and D-tubocurarine; n = 6). Data have been presented as mean ± SEM.
Directional Selectivity in the Absence of Inhibition Arises in Ganglion Cells
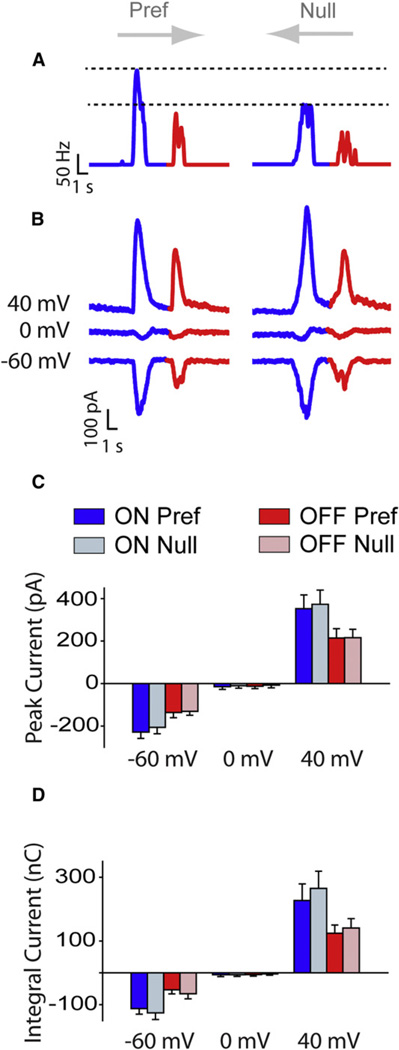
To distinguish whether directional selectivity in the presence of inhibitory blockers arose pre- or postsynaptically, the properties of ganglion cell light-evoked synaptic inputs were analyzed using whole-cell voltage-clamp techniques. In these experiments, after measuring spikes in cell-attached mode in the presence of blockers (Figure 6A), the same cell was patched with an electrode containing intracellular solution. After break-in, the DSGC was dialyzed with QX314 and Cs+ and repeatedly injected with brief depolarizing pulses (−60–0 mV) until Na+ currents and a large fraction of voltage-gated K+ currents were blocked. Under these conditions, moving spots elicited large inward currents in both the null and preferred directions (VHOLD = −60 mV; Figure 6B). When the cell was held −0 mV, the inhibitory inputs that are usually associated with stimulating these cells (Figures 3A and S3) were not apparent, confirming that they were effectively blocked with the cocktail of antagonists (also see Figure S4). At +40 mV, light evoked outward currents. Importantly, the temporal characteristics of currents measured at −60 and +40 mV were similar (Figure S5), indicating that they were not contaminated by voltage-dependent conductances, and thus provided a reliable readout of bipolar cell output. Reversal of the excitatory currents also indicated that gap junctions did not significantly contribute to the synaptic responses (Ackert et al., 2009).
Figure 6. Directional Selectivity in the Presence of Inhibitory Blockers Appears to Arise from Nonlinear Properties of Ganglion Cells.
(A) Spike rate histograms of responses measured in the preferred (Pref) and the null directions in the added presence of drugs (picrotoxin, TPMPA and D-tubocurarine).
(B) Voltage-clamp recordings of excitatory currents measured in the continued presence of drugs, from the same cell shown in (A). Responses were measured at three different holding potentials as indicated. These currents did not appear to be contaminated by voltage-dependent conductances (see Figure S5).
(C and D) The average peak amplitude (C) and total charge (D) of excitatory currents measured in preferred and null directions. There are no statistical differences between any preferred null pairs (p > 0.5; n = 6). Data have been presented as mean ± SEM.
Under conditions in which inhibitory receptors and active postsynaptic conductances were blocked, preferred and nulldirection stimuli evoked excitatory currents that were similar in size. The amplitude of the peak currents was not significantly different whether measured at −60 mV (preferred: −228 ± 30 pA and −136 ± 24 pA, for ON and OFF, respectively; null: −206 ± 30 pA and −131 ± 18 pA for ON and OFF, respectively; p > 0.6; n = 6) or +40 mV (preferred: 353 ± 64 pA and 214 ± 44 pA, for ON and OFF, respectively; null: 373 ± 67 pA and 216 ± 40 pA for ON and OFF, respectively; p > 0.6; n = 6; Figure 6C). Similarly, the total charge of the response was similar in magnitude in the null and preferred directions, indicating that moving spots stimulated an equal number of inputs in both directions (−60 mV ON, −113 ± 18 nC for preferred compared to −126 ± 21 nC for null; −60 mV OFF, −54 ± 13 nC for preferred compared to −66 ± 16 nC for null; +40 mV ON, 227 ± 52 nC for preferred compared to 265 ± 54 nC for null; +40 mV OFF, 124 ± 26 nC for preferred compared to 141 ± 29 nC for null; p > 0.5; n = 6; Figure 6D). The symmetry in input strength contrasts with the asymmetry in the spiking responses and suggests that nonlinearities within the ganglion cell must contribute to direction discrimination.
To test whether asymmetric dendritic trees could confer intrinsic DS properties to ganglion cells, we constructed a computational model based on the morphology of GFP+ DSGCs (Figure S6). A model based on morphology alone produced a mild reverse DS (i.e. with a dendrite to soma preference). Interestingly, the addition of voltage-gated Na+ channels to dendrites (Oesch et al., 2005) was required to produce directional selectivity with a similar preferred direction as measured experimentally (Figure S6). Thus, nonlinear conductances and asymmetric dendritic trees appear to be essential requirements for the formation of directional selectivity in the absence of inhibition.
Interactions between Pre- and Postsynaptic DS Mechanisms in Symmetrical Ganglion Cells
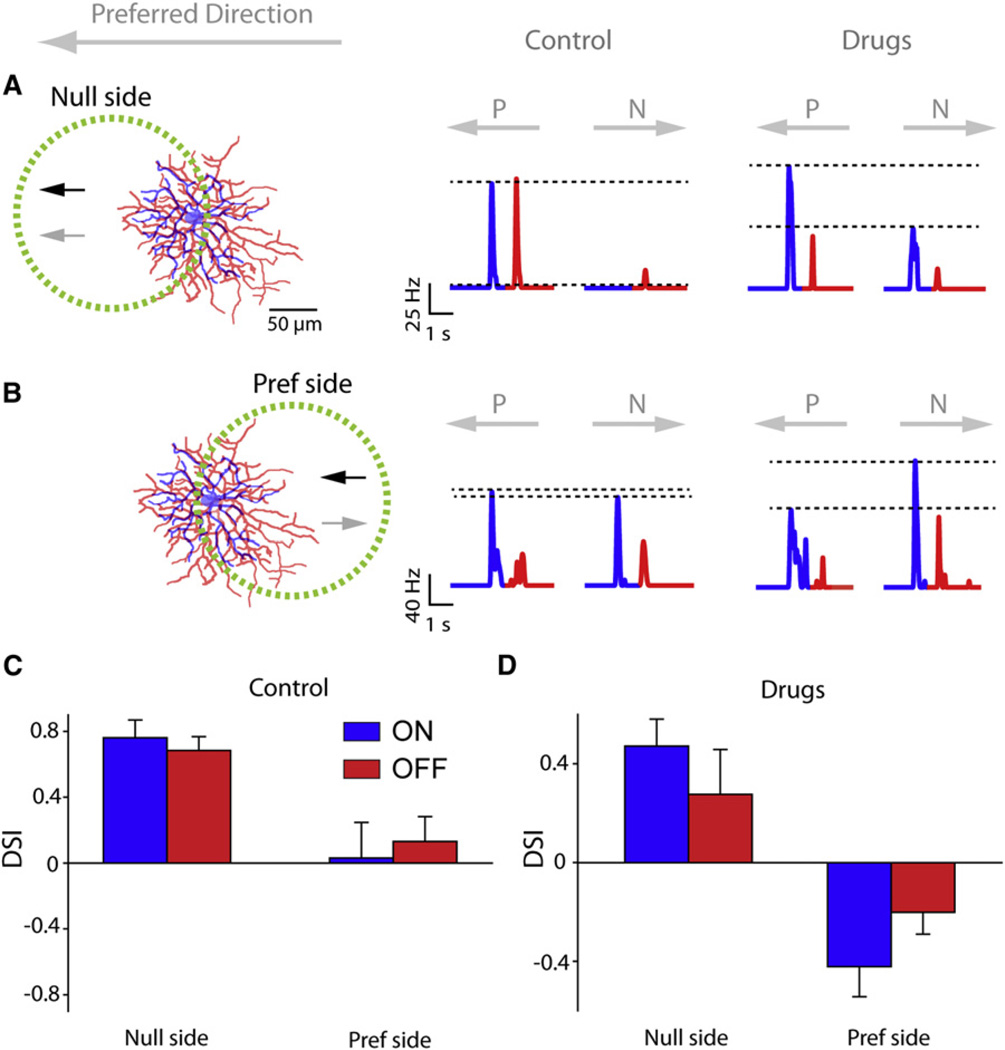
If active conductances in dendrites contribute strongly to the formation of centrifugal preferences in asymmetric DSGCs, then it might be predicted that these would also affect processing in symmetric DSGCs. Indeed, such centrifugal dendritic preferences are predicted to hold regardless of DSGC morphology (Schachter et al., 2010). However, it might be expected that in field, inhibitory-circuit and dendritic DS mechanisms work in opposition, resulting in the formation of the NDZ. To test the hypothesis that heterogeneous interactions between multiple DS mechanisms occur in different parts of the DSGC receptive field, we next measured responses in the presence of the cocktail of inhibitory antagonists. When moving stimuli were presented on the null side, consistent with previous results symmetrical cells, the influence of dendrites pointing in opposite directions would cancel each other out, limiting their functional role. To test the impact of dendritic processing in symmetric DSGCs, we measured DS responses in different regions within the receptive fields of symmetric GFP2212 DSGCs, in an attempt to isolate local dendritic contributions. For these experiments, moving stimuli (400 µm/s) were presented within a circular area (200 µm in diameter) in different parts of the DSGC receptive field (Figures 7A and 7B).
Figure 7. Interactions between the Inhibitory Circuit and Dendritic DS Mechanisms Revealed in Symmetrical DSGCs.
(A) Symmetrical DSGCs were stimulated within an area on the null side of the cell (left). Moving stimuli were presented within the area indicated by the dotted yellow circle (200 µm diameters). Spike rate histograms for preferred and null directions are shown in control (middle) and in the presence of drugs (right).
(B) Same as in (A), but stimuli were presented in an area located on the preferred (Pref) side of the cell (called the NDZ) delineated by the dotted circle.
(C and D) The average DSI for responses evoked in the null and preferred side in control (C) and in the added presence of drugs (D). Within the dotted circles, the black arrows indicate the direction of DS responses expected from circuit mechanism, whereas the gray arrows point in the direction of DS responses expected from dendritic mechanisms. Data have been presented as mean ± SEM.
Strong DS responses were evoked when stimuli were presented within the null side of the receptive field (the side of the cell first stimulated by null-direction moving stimuli; Figures 7A and 7C; DSI 0.76 ± 0.11 and 0.69 ± 0.08 for ON and OFF, respectively; n = 6). In this region, like in the Hb9+ ganglion cells, inhibitory-circuit and dendritic DS mechanisms are expected to work in synergy. However, when stimuli were presented on the preferred side, directional selectivity was significantly reduced or absent (Figures 7B and 7C; DSI 0.03 ± 0.22 and 0.13 ± 0.15 for ON and OFF, respectively; n = 6). The absence of directional selectivity cannot be explained by lack of inputs from SACs because these appear to be evenly distributed throughout the dendritic tree (Briggman et al., 2011). However, a nondiscriminatory zone (NDZ) in a region on the preferred side has previously been described in rabbit DSGCs (Barlow and Levick, 1965; He et al., 1999). We hypothesized that in this region of the dendritic field, inhibitory-circuit and dendritic DS mechanisms work in opposition, resulting in the formation of the NDZ.
To test the hypothesis that heterogeneous interactions between multiple DS mechanisms occur in different parts of the DSGC receptive field, we next measured responses in the presence of the cocktail of inhibitory antagonists. When moving stimuli were presented on the null side, consistent with previous results in the Hb9+ cells, directional selectivity persisted (Figures 7A and 7D; DSI, 0.47 ± 0.11 and 0.28 ± 0.18 for ON and OFF, respectively), though was significantly reduced compared to control (p < 0.05). Application of inhibitory blockers tended to increase spike rates more in the null direction, suggesting that inhibitory circuits were functional within the tested subfield. When stimuli were centered over the receptive field, directional selectivity was reduced drastically (Figure 7D; ON DSI, 0.14 ± 0.06; OFF DSI 0.20 ± 0.05). In this case, the influence of dendritic DS mechanisms would be expected to be negligible because dendrites on opposing sides of the soma would nullify each other. When the stimuli were centered over the preferred side, a centrifugal dendritic preference was revealed in a region that had been non-DS in control conditions (Figures 7B and 7D; ON DSI, −0.42 ± 0.12; OFF DSI −0.20 ± 0.08). The direction of this preference was centrifugal, as expected from a dendritic DS mechanism, but opposite to the preferred direction of the cell measured in control. It is important to note that the rate of null-direction spikes was strongly enhanced (ON: 82 ± 17 Hz for control compared to 213 ± 67 Hz for drugs; OFF: 68 ± 17 Hz for control compared to 153 ± 20 Hz for drugs; p < 0.05; n = 6), indicating that even within this region that had been non-DS in control conditions, presynaptic circuits provide null-direction inhibition. Thus, it appears that over the null side of the DSGC receptive field, inhibitory circuit-dependent and dendritic mechanisms act in synergy, whereas over the preferred side, they act in opposition, consistent with previous predictions (Schachter et al., 2010).
DISCUSSION
Most models of directional selectivity in the mammalian retina involve lateral asymmetries within the inhibitory circuitry, likely arising from SACs. Here, we demonstrate that for a select population of ganglion cells, directional selectivity persists when classical inhibitory DS circuitry is blocked, suggesting the existence of a parallel DS mechanism. We explored the cellular basis for this form of directional selectivity and its contribution to shaping responses in asymmetrical and symmetrical ganglion cells.
An Entire Population of ON-OFF DSGCs with Asymmetric Dendritic Arbors
The morphology of DSGCs in many species is known to be variable, ranging from highly asymmetrical to completely symmetrical (Amthor et al., 1989; Oyster et al., 1993; Yang and Masland, 1994). However, it is not clear whether these differences in dendritic shapes arise randomly in development or correspond to a morphological specialization. Here, we present evidence demonstrating systematic dendritic asymmetries in an entire mosaic of ON-OFF DSGCs. Ganglion cells labeled in the Hb9::eGFP retina exhibit highly asymmetric dendritic trees orientated toward the temporal pole of the retina. Every GFP+ cell tested (n = 42) exhibited dendritic asymmetries. GFP+ cells were also relatively uniform in a number of other morphological characteristics compared to the general population of ON-OFF DSGCs (Sun et al., 2002; Coombs et al., 2006). In addition, every GFP+ cell was found to code anterior motion. Together, the relatively uniform morphological and physiological characteristics of GFP+ ganglion cells in the Hb9::eGFP retina indicate that they belong to a single subset of ON-OFF DSGCs. Thus, the molecular specification of a distinct DSGC population exhibiting dendritic asymmetries highlights the importance of dendritic processing in DS coding, a property that has previously been hard to assess with random samplings from mixed populations of DSGCs (Figure S2).
The asymmetric morphological characteristics of the Hb9+ cells contrast with the recently identified subset of ON-OFF DSGCs that code posterior motion, specified by the dopamine receptor 4 promoter (DRD4). DRD4+ cells are roughly the same size as Hb9+ cells but do not bear any systematic dendritic asymmetries (Huberman et al., 2009). However, we found that even in a small sample of posterior coding DSGCs (n = 7), examples of cells that exhibited dendritic asymmetries parallel to the preferred direction were apparent. Aside from the direction of their dendritic orientation, these asymmetrical cells appeared morphologically similar to Hb9+ cells (Figure S2). This observation raises the possibility that multiple populations of DSGCs might code a single direction in the murine retina. Indeed, in mouse retina there is a large overlap in the dendritic field coverage between neighboring DRD4+ DSGCs (Huberman et al., 2009), which contrasts with the territorial organization of DSGCs in rabbit retina (Vaney, 1994). In addition, the density of DRD4+ DSGCs was found to be roughly three times what we report here for the Hb9+ DSGCs. A more thorough characterization of DRD4+ cells and/or new genetic markers will reveal whether more than one population of DSGCs encodes a single direction of motion.
Nonlinearities within Asymmetric Dendritic Arbors Confer Centrifugal Preferences
Considering that conventional inhibitory mechanisms were manifest in the Hb9+ ganglion cells, it was interesting to find that DS responses persisted in a cocktail of antagonists that block GABA receptors. These results clearly demonstrate the existence of an additional DS mechanism that does not critically rely on inhibition. From a theoretical point of view, the minimum requirements for direction discrimination are (1) an asymmetry and (2) a nonlinear interaction between inputs (Borst and Egelhaaf, 1989). Our experimental findings indicate nonlinearities within asymmetric dendritic trees of DSGCs that can confer inhibition-independent directional selectivity. The evidence for this is summarized below.
Under inhibitory receptor blockade, although directional selectivity is apparent in the spiking responses of DSGCs, the excitatory synaptic inputs measured under voltage clamp were of equal strength in the preferred and null directions. This finding suggests that nonlinearities within Hb9+ ganglion cells convert the temporal sequence of inputs distributed over their asymmetric dendritic trees into a DS output. Interestingly, SACs also have the intrinsic ability to respond preferentially to centrifugal motion (Euler et al., 2002), suggesting that a common dendritic mechanism might underlie direction coding in both cell types.
Insights into how dendrites compute directional selectivity are offered by a computational model (Figure S6). This model demonstrates that for the passive case, null and preferred responses produce little or mild centripetal directional selectivity at the soma (Livingstone, 1998; Branco et al., 2010), consistent with results from our voltage-clamp experiments. However, the model also allows us to estimate responses at the distal dendrites. Interestingly, for stimuli that produce mild centripetal directional selectivity at the soma, distal dendrites were found to express a strong preference for centrifugal motion. This occurs because during centrifugal motion, signals activated near the soma appear delayed at the periphery and thus coincide with local signals at the dendritic tips, summing effectively. On the other hand, during centripetal motion proximal and distal inputs are activated out of phase and thus, at the dendrite, sum poorly (Rall, 1964; Tukker et al., 2004; Hausselt et al., 2007). Furthermore, the relatively high input resistance at the distal dendrites compared to the proximal dendrites amplifies the differential responses, thereby promoting dendritic spike initiation during preferred motion. Indeed, these simulations of centrifugal preferences in the distal dendrites are supported by Ca2+-imaging studies from SACs (Euler et al., 2002) but remain to be validated in DSGCs.
How are centrifugal preferences of dendrites transferred to the soma? Following Hausselt et al. (2007), we found that the addition of nonlinear conductances (in this case voltage-gated Na+ channels; Oesch et al., 2005) to asymmetric dendrites of DSGCs resulted in an amplification of distal PSPs that effectively reversed and amplified DS preference at the soma (Figure S6). Such nonlinearities resulted in the formation of dendritic spikes that propagated to the soma where they evoked somatic action potentials with high probability (Oesch et al., 2005; Schachter et al., 2010), thus creating a robust centrifugal preference at the soma (Figure S6). Thus, active nonlinear conductances in the asymmetric dendrites appear to be a critical requirement for inhibition-independent directional selectivity. Although the computational model reproduces our basic experimental findings, it is possible that other known dynamic adaptive mechanisms (Victor, 1987; Berry et al., 1999; Hosoya et al., 2005) could also be involved in the formation of directional selectivity in cells with asymmetric dendritic fields. Future work is needed to confirm the mechanistic details of how directional selectivity is formed in the absence of inhibition.
Parallel Mechanisms Underlie DS Coding
We hypothesize that multiple DS mechanisms work together to shape response properties of Hb9+ ganglion cells. Moving stimuli evoked a characteristic pattern of inhibitory and excitatory synaptic conductances in Hb9+ DSGCs, similar to those described for other types of DSGCs (Taylor and Vaney, 2002). First, inhibitory conductances were significantly larger in the null compared to the preferred direction. Second, null-direction inhibition coincided with or preceded excitation, whereas preferred direction inhibition was delayed with respect to excitation. Thus, conventional circuit mechanisms appear to contribute to shaping DS responses in Hb9+ ganglion cells. Interestingly, these circuit mechanisms are aligned with the asymmetric dendritic arbors, along the nasal-temporal axis. Moreover, directional selectivity persisted under inhibitory receptor blockade, and the directional preferences of Hb9+ cells were not significantly altered under these conditions. Together, these results reveal a DS mechanism that does not critically rely on inhibition but that is in alignment with conventional DS circuitry.
In asymmetric DSGCs, inhibitory and dendritic mechanisms appear to work in a complementary fashion, to generate similar directional preferences. A critical feature of DSGCs is that they respond poorly to null-direction stimuli. During null-direction movements, dendritic mechanisms result in weak responses (because of suboptimal summation), making them more susceptible to being “vetoed” by inhibitory mechanisms, which are stronger in this direction. Thus, the combination of inhibitory and dendritic mechanisms allows for DS cells to produce little or no response to null motion. In the preferred direction, the dendritic mechanism results in an optimal summation of inputs, which when combined with weak delayed inhibition, result in a robust spiking response. Therefore, as in the case of SACs (Euler et al., 2002; Hausselt et al., 2007), the dendritic mechanism is not merely a supplementary mechanism for directional selectivity, but an essential one.
The relative weighting of inhibitory circuit and dendritic mechanisms is perhaps best exemplified when considering the response elicited within the NDZ located on the preferred side of symmetrical DSGCs, where these two mechanisms appear to be in opposition. Here, inhibitory circuit mechanisms appear to favor centripetal preferences, whereas the dendritic DS mechanisms favor centrifugal preferences (Figure 7). Interestingly, in the null direction, inhibition is not strong enough to suppress responses evoked in dendrites that are oriented so as to provide an optimal response (note that inhibitory contacts appear uniformly distributed throughout the dendritic field; Briggman et al., 2011). In the preferred direction, although inhibition is weak, the dendritic mechanisms do not favor the generation of strong responses. Thus, the opposing circuit and dendritic DS mechanisms both appear to strongly influence responses of ganglion cells leading to the formation of the NDZ (Schachter et al., 2010), first described many years ago (Barlow and Levick, 1965; He et al., 1999).
Although our results show that both inhibitory and dendritic mechanisms can generate DS responses in Hb9+ ganglion cells, assessing their relative contributions to directional selectivity under diverse speeds remains a challenge. In the presence of inhibitory blockers, directional selectivity is only apparent at the slower range of speeds. At speeds greater than 1000 µm/s, directional selectivity was essentially abolished under these conditions as previously noted (Wyatt and Day, 1976; Caldwell et al., 1978). However, blocking inhibition is also known to strongly affect the spatiotemporal characteristics of excitation (Roska and Werblin, 2001; Sagdullaev et al., 2006), thereby directly affecting dendritic DS mechanisms. This makes it likely that dendritic mechanisms operate differently in control conditions. Indeed, theoretical modeling studies suggest that dendritic mechanisms are tuned toward generating maximal DS responses at significantly higher speeds (1000–2000 µm/s; Tukker et al., 2004). In addition, under control conditions, responses in the NDZ remain nondirectional at faster speeds (data not shown; Barlow and Levick, 1965; He et al., 1999), consistent with the idea that dendritic and inhibitory mechanisms continue to oppose each other during faster movements (Schachter et al., 2010). Our results prompt an in-depth investigation into how multiple DS mechanisms interact under diverse conditions.
Conclusion
Our results demonstrate new insights into how neural circuit mechanisms interlace with the computational subunit properties of dendrites. In the retina, directional selectivity in SACs and a variety of DSGCs appears to be generated using a similar strategy, utilizing inhibitory circuit mechanisms in conjunction with active dendritic properties. The asymmetries in dendritic arborizations in Hb9+ DSGCs appear to represent a striking morphological adaptation that the retina has developed to avoid the NDZ by truncating their dendritic trees on the preferred side. Overall, when combined with asymmetric inhibition, asymmetric dendritic trees provide the most robust directional selectivity with the smallest arbor. Future investigations will reveal functional consequences of such adaptations.
EXPERIMENTAL PROCEDURES
Animals
Hb9::eGFP+ transgenic mice were kindly provided by Dr. Robert Brownstone (Dalhousie University) and maintained on a 12 hr light/dark cycle.
Retinal Preparation
All procedures were performed in accordance with the CACR and approved by Dalhousie University’s Animal Care Committee. Briefly, mice were anesthetized and decapitated. Eyes were removed and placed in warm Ringer’s solution. Retinas were isolated, and a small incision was made on the nasal side of the retina to identify the orientation. The isolated retina was then placed down on a 0.22 µm membrane filter (Millipore, Bedford, MA, USA) with a precut window that enabled transmitted light to reach the retina and for the preparation to be viewed under infrared illumination with the aid of a Spot RT3 CCD camera (Diagnostic Instruments, Sterling Heights, MI, USA) attached to an upright Olympus BX51 WI fluorescent microscope, equipped with either a 40× or 60× water-immersion lens (Olympus Canada, Markham, Ontario, Canada). The preparation was continually bathed with control Ringer’s solution containing: 110 mM NaCl, 2.5 mM KCl, 1 mM CaCl2, 1.6 mM MgCl2, 10 mM dextrose, and 22 mM NaHCO3 that was bubbled with carbogen (95%O2: 5%CO2 [pH 7.4]). All experiments were performed near physiological temperatures (35°C–36°C). All reagents were purchased form Sigma-Aldrich Canada Ltd. (Oakville, Ontario, Canada) unless otherwise noted.
Whole-Cell Patch-Clamp Recordings
Extracellular recordings were made using −5–10 MΩ electrodes filled with Ringer’s solution. Voltage-clamp whole-cell recordings were made using 4–6 MΩ electrodes containing: 112.5 mM CsCH3SO3, 9.7 mM KCl, 1 mM MgCl2, 1.5 mM EGTA, 10 mM HEPES, 4 mM ATP Mg2, 0.5 mM GTP Na3, and 0.2 mM Alexa 594 (Invitrogen, Burlington, Ontario, Canada). The pH was adjusted to 7.4 with CsOH. Voltage-clamp whole-cell recordings were made using 4–8 MΩ electrodes containing: 115 mM K+ gluconate, 5 mM KCl, 1 mM MgCl2, 10 mM EGTA, 10 mM HEPES, 4 mM ATP Mg2, 0.5 mM GTP Na3, and 0.2 mM Alexa 594. The reversal potential for chloride (ECl) was calculated to be ~−60 mV. The voltage- and current-clamp recordings were made with a MultiClamp 700B amplifier (Molecular Devices, Sunnyvale, CA, USA). Signals were digitized at 10 kHz (National Instruments A/D board) and acquired using custom software written in the LabVIEW environment. Junction potentials and series resistance (10–25 MΩ) were corrected offline.
Light Stimulus
Stimuli were generated with a DLP projector (Texas Instruments; refresh rate 75 Hz) controlled with custom software written by Dr. David Balya (Friedrich Meischer Institute, Switzerland). Neutral density filters were used to control the stimulus energy. The intensity of stimuli used was 0.5 × 1010 photons × s−1 × cm−2 (sampled at 500 nm) as measured with a calibrated spectrophotometer (USB2000; Ocean Optics, Dunedin, FL, USA). Light stimuli projected from below the specimen were focused on the outer segments of the photoreceptors using the substage condenser. Flash responses were obtained using a series of spot sizes (25–800 µm). Directional selectivity was tested by moving a 400 µm spot presented at positive contrast only (50% to maximal). Spots were presented at different speeds over the cell in eight different directions, equally divided over 360−. In some experiments, a 200–400 µm diameter mask was used to limit light stimulation to the cell of interest.
Targeting, Imaging, and Reconstructing GFP+ Ganglion Cells
GFP+ ganglion cells were targeted using two-photon laser-scanning microscopy at 950 nm, to avoid bleaching photoreceptors (Euler et al., 2002). To facilitate targeting ganglion cells, two-photon fluorescent images were overlaid on the IR image acquired through the CCD camera. During physiological recordings cells were dialyzed with 20–25 µM Alexa 594. Ganglion cells were imaged at 850 nm after physiological recordings were complete.
Alexa 594-filled DSGCs were reconstructed from image stacks using manual or semiautomatic filament-tracing routines in Neuromantic (http://www.reading.ac.uk/neuromantic/) and Amira (Visage Imaging, San Diego, CA, USA). Retraced neurons were analyzed in MATLAB. The angle for the dendritic AI was computed by summing vectors representing each dendrite. The magnitude of AI was calculated by summing the length of all the dendrites on the preferred (PL) and null (NL) sides of the soma and calculating AI = (PL − NL)/(PL + NL).
Analysis of Physiological Data
Spiking responses were accumulated as peristimulus time histograms (spike rates were binned over 25–50 ms), and the peak firing rate was analyzed in MATLAB. A DSI was calculated as: DSI = (PR − NR)/(PR + NR), where PR and NR are the maximal spike rate evoked in preferred and null directions, respectively. The angle of the DSI was calculated as the vector sum of the peak spike rate for all eight stimulus directions. All spike data represent averages of two to four trials. Conductance analysis was performed as described by Taylor and Vaney (2002) and is explained in more detail in the Supplemental Experimental Procedures. Comparisons between two groups were made with t tests or the Moore’s test (an equivalent for circular statistics). Paired t tests or Mann-Whitney U rank sum test was used to determine statistical significance when comparing responses before and after drug application. Data are presented as mean ± SEM.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. W. Baldridge and S. Barnes for useful discussions and for their helpful comments on this manuscript, Dr. R. Brownstone for providing us with the Hb9::eGFP+ transgenic mouse line, and Dr. J. Boyd for his help in writing custom software for two-photon imaging. We also thank Alexander Goroshkov, Priyanka Singh, and Belinda Dunn for providing technical support and Neasa Bheilbigh and Marika Forsythe for help in morphological reconstructions. This work was supported by the National Eye Institute (EY016607) awarded to R.G.S. and by the Natural Sciences and Engineering Research Council of Canada (grant 342202-2007) awarded to G.B.A.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes six figures and Supplemental Experimental Procedures and can be found with this article online at doi:10.1016/j.neuron.2011.06.020.
REFERENCES
- Ackert JM, Farajian R, Völgyi B, Bloomfield SA. GABA blockade unmasks an OFF response in ON direction selective ganglion cells in the mammalian retina. J. Physiol. 2009;587:4481–4495. doi: 10.1113/jphysiol.2009.173344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor FR, Takahashi ES, Oyster CW. Morphologies of rabbit retinal ganglion cells with complex receptive fields. J. Comp. Neurol. 1989;280:97–121. doi: 10.1002/cne.902800108. [DOI] [PubMed] [Google Scholar]
- Arber S, Han B, Mendelsohn M, Smith M, Jessell TM, Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. doi: 10.1016/s0896-6273(01)80026-x. [DOI] [PubMed] [Google Scholar]
- Barlow HB, Levick WR. The mechanism of directionally selective units in rabbit’s retina. J. Physiol. 1965;178:477–504. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MJ, 2nd, Brivanlou IH, Jordan TA, Meister M. Anticipation of moving stimuli by the retina. Nature. 1999;398:334–338. doi: 10.1038/18678. [DOI] [PubMed] [Google Scholar]
- Borst A, Egelhaaf M. Principles of visual motion detection. Trends Neurosci. 1989;12:297–306. doi: 10.1016/0166-2236(89)90010-6. [DOI] [PubMed] [Google Scholar]
- Branco T, Clark BA, Häusser M. Dendritic discrimination of temporal input sequences in cortical neurons. Science. 2010;329:1671–1675. doi: 10.1126/science.1189664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggman KL, Helmstaedter M, Denk W. Wiring specificity in the direction-selectivity circuit of the retina. Nature. 2011;471:183–188. doi: 10.1038/nature09818. [DOI] [PubMed] [Google Scholar]
- Bülthoff H, Bülthoff I. GABA-antagonist inverts movement and object detection in flies. Brain Res. 1987;407:152–158. doi: 10.1016/0006-8993(87)91230-3. [DOI] [PubMed] [Google Scholar]
- Caldwell JH, Daw NW, Wyatt HJ. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: lateral interactions for cells with more complex receptive fields. J. Physiol. 1978;276:277–298. doi: 10.1113/jphysiol.1978.sp012233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs J, van der List D, Wang GY, Chalupa LM. Morphological properties of mouse retinal ganglion cells. Neuroscience. 2006;140:123–136. doi: 10.1016/j.neuroscience.2006.02.079. [DOI] [PubMed] [Google Scholar]
- Demb JB. Cellular mechanisms for direction selectivity in the retina. Neuron. 2007;55:179–186. doi: 10.1016/j.neuron.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Egelhaaf M, Borst A, Pilz B. The role of GABA in detecting visual motion. Brain Res. 1990;509:156–160. doi: 10.1016/0006-8993(90)90325-6. [DOI] [PubMed] [Google Scholar]
- Euler T, Detwiler PB, Denk W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature. 2002;418:845–852. doi: 10.1038/nature00931. [DOI] [PubMed] [Google Scholar]
- Fried SI, Münch TA, Werblin FS. Mechanisms and circuitry underlying directional selectivity in the retina. Nature. 2002;420:411–414. doi: 10.1038/nature01179. [DOI] [PubMed] [Google Scholar]
- Fried SI, Münch TA, Werblin FS. Directional selectivity is formed at multiple levels by laterally offset inhibition in the rabbit retina. Neuron. 2005;46:117–127. doi: 10.1016/j.neuron.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Grzywacz NM, Tootle JS, Amthor FR. Is the input to a GABAergic or cholinergic synapse the sole asymmetry in rabbit’s retinal directional selectivity? Vis. Neurosci. 1997;14:39–54. doi: 10.1017/s0952523800008749. [DOI] [PubMed] [Google Scholar]
- Hausselt SE, Euler T, Detwiler PB, Denk W. A dendrite-autonomous mechanism for direction selectivity in retinal starburst amacrine cells. PLoS Biol. 2007;5:e185. doi: 10.1371/journal.pbio.0050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Jin ZF, Masland RH. The nondiscriminating zone of directionally selective retinal ganglion cells: comparison with dendritic structure and implications for mechanism. J. Neurosci. 1999;19:8049–8056. doi: 10.1523/JNEUROSCI.19-18-08049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoya T, Baccus SA, Meister M. Dynamic predictive coding by the retina. Nature. 2005;436:71–77. doi: 10.1038/nature03689. [DOI] [PubMed] [Google Scholar]
- Huberman AD, Wei W, Elstrott J, Stafford BK, Feller MB, Barres BA. Genetic identification of an On-Off direction-selective retinal ganglion cell subtype reveals a layer-specific subcortical map of posterior motion. Neuron. 2009;62:327–334. doi: 10.1016/j.neuron.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IJ, Zhang Y, Yamagata M, Meister M, Sanes JR. Molecular identification of a retinal cell type that responds to upward motion. Nature. 2008;452:478–482. doi: 10.1038/nature06739. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim K, Zhou ZJ. Role of ACh-GABA cotransmission in detecting image motion and motion direction. Neuron. 2010;68:1159–1172. doi: 10.1016/j.neuron.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS. Mechanisms of direction selectivity in macaque V1. Neuron. 1998;20:509–526. doi: 10.1016/s0896-6273(00)80991-5. [DOI] [PubMed] [Google Scholar]
- London M, Häusser M. Dendritic computation. Annu. Rev. Neurosci. 2005;28:503–532. doi: 10.1146/annurev.neuro.28.061604.135703. [DOI] [PubMed] [Google Scholar]
- Oesch N, Euler T, Taylor WR. Direction-selective dendritic action potentials in rabbit retina. Neuron. 2005;47:739–750. doi: 10.1016/j.neuron.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Öğmen H. On the mechanisms underlying directional selectivity. Neural Comput. 1991;3:333–349. doi: 10.1162/neco.1991.3.3.333. [DOI] [PubMed] [Google Scholar]
- Oyster CW, Amthor FR, Takahashi ES. Dendritic architecture of ON-OFF direction-selective ganglion cells in the rabbit retina. Vision Res. 1993;33:579–608. doi: 10.1016/0042-6989(93)90181-u. [DOI] [PubMed] [Google Scholar]
- Rall W. Theoretical significance of dendritic trees for neuronal input-output relations. In: Reis RF, editor. Neural Theory and Modeling. Stanford, CA: Stanford University Press; 1964. pp. 72–97. [Google Scholar]
- Roska B, Werblin F. Vertical interactions across ten parallel, stacked representations in the mammalian retina. Nature. 2001;410:583–587. doi: 10.1038/35069068. [DOI] [PubMed] [Google Scholar]
- Sagdullaev BT, McCall MA, Lukasiewicz PD. Presynaptic inhibition modulates spillover, creating distinct dynamic response ranges of sensory output. Neuron. 2006;50:923–935. doi: 10.1016/j.neuron.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Schachter MJ, Oesch N, Smith RG, Taylor WR. Dendritic spikes amplify the synaptic signal to enhance detection of motion in a simulation of the direction-selective ganglion cell. PLoS Comput. Biol. 2010;6:e1000899. doi: 10.1371/journal.pcbi.1000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RD, Grzywacz NM, Borg-Graham LJ. Is the input to a GABAergic synapse the sole asymmetry in turtle’s retinal directional selectivity? Vis. Neurosci. 1996;13:423–439. doi: 10.1017/s0952523800008105. [DOI] [PubMed] [Google Scholar]
- Sun W, Li N, He S. Large-scale morphological survey of mouse retinal ganglion cells. J. Comp. Neurol. 2002;451:115–126. doi: 10.1002/cne.10323. [DOI] [PubMed] [Google Scholar]
- Taylor WR, Vaney DI. Diverse synaptic mechanisms generate direction selectivity in the rabbit retina. J. Neurosci. 2002;22:7712–7720. doi: 10.1523/JNEUROSCI.22-17-07712.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WR, Vaney DI. New directions in retinal research. Trends Neurosci. 2003;26:379–385. doi: 10.1016/S0166-2236(03)00167-X. [DOI] [PubMed] [Google Scholar]
- Taylor WR, He S, Levick WR, Vaney DI. Dendritic computation of direction selectivity by retinal ganglion cells. Science. 2000;289:2347–2350. doi: 10.1126/science.289.5488.2347. [DOI] [PubMed] [Google Scholar]
- Tukker JJ, Taylor WR, Smith RG. Direction selectivity in a model of the starburst amacrine cell. Vis. Neurosci. 2004;21:611–625. doi: 10.1017/S0952523804214109. [DOI] [PubMed] [Google Scholar]
- Vaney DI. Territorial organization of direction-selective ganglion cells in rabbit retina. J. Neurosci. 1994;14:6301–6316. doi: 10.1523/JNEUROSCI.14-11-06301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor JD. The dynamics of the cat retinal X cell centre. J. Physiol. 1987;386:219–246. doi: 10.1113/jphysiol.1987.sp016531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Hamby AM, Zhou K, Feller MB. Development of asymmetric inhibition underlying direction selectivity in the retina. Nature. 2011;469:402–406. doi: 10.1038/nature09600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt HJ, Day NW. Specific effects of neurotransmitter antagonists on ganglion cells in rabbit retina. Science. 1976;191:204–205. doi: 10.1126/science.1857. [DOI] [PubMed] [Google Scholar]
- Yang G, Masland RH. Receptive fields and dendritic structure of directionally selective retinal ganglion cells. J. Neurosci. 1994;14:5267–5280. doi: 10.1523/JNEUROSCI.14-09-05267.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonehara K, Balint K, Noda M, Nagel G, Bamberg E, Roska B. Spatially asymmetric reorganization of inhibition establishes a motion-sensitive circuit. Nature. 2011;469:407–410. doi: 10.1038/nature09711. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Watanabe D, Ishikane H, Tachibana M, Pastan I, Nakanishi S. A key role of starburst amacrine cells in originating retinal directional selectivity and optokinetic eye movement. Neuron. 2001;30:771–780. doi: 10.1016/s0896-6273(01)00316-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.