Abstract
Pharmacological manipulations in the Drosophila embryo have been hindered by the impermeability of the eggshell. The ultimate barrier to delivery of small molecule solutes to the embryo is the waxy layer that lies beneath the external chorion layers and encases the underlying vitelline membrane of the eggshell. Conventional protocols call for heptane or octane to permeablize the dechorionated eggshell however, these solvents are toxic and can result in low viability. Furthermore, heptane and octane require transition of the embryo between aqueous and organic phase solvents making it challenging to avoid desiccation. Here we describe an embryo permeabilization solvent (EPS) composed of D-limonene and plant-derived surfactants that is water miscible and highly effective in rendering the dechorionated eggshell permeable. EPS permeabilization enables embryo uptake of several different dyes of various molecular mass up to 995 Daltons. We find that the embryo undergoes an age dependent decrease in the ability to be permeabilized in the first six to eight hours after egg laying. This apparent developmental change in the vitelline membrane contributes to the heterogeneity in permeabilization seen even among closely staged embryos. However, using fluorescent properties of Rhodamine B dye and various conditions of EPS treatment we demonstrate the ability to obtain optimally permeabilized viable embryos. We also demonstrate the ability to assess teratogenic activity of several compounds applied to embryos in vitro, using both early and late developmental endpoints. Application of the method to transgenic strains carrying GFP reporter genes results in a robust readout of pharmacological alteration of embryogenesis. The straightforward and rapid nature of the manipulations needed to prepare batches of permeabilized embryos has the potential of establishing the Drosophila embryo as an alternative model in toxicology and for small molecule screening in a high throughput format.
Introduction
The Drosophila model is endowed with extensive molecular genetic tools that allow researchers to read out and/or modulate activity of essentially every gene in the fly genome. As a result, pharmacological approaches of investigating developmental events in the fly embryo have been largely overlooked. In addition, the Drosophila embryo has been underutilized for screening activity of small molecules, e.g. in mechanistic studies in toxicology and for the high throughput screening demands in drug development. With advancements in the synthesis of small bioactive molecules, and the growing number of man-made compounds with unknown toxicity profiles, the ability to assay small molecule activity in the fly embryo would be of great value to developmental biologists, toxicologists and the Pharma industry.
The major limitation for assaying small molecules in the fly embryo is the impermeability of the eggshell. Microinjection methods, despite recent advancements (Zappe et al. 2006), continue to be labor intensive and require individual manipulation of each embryo in a dedicated facility. The ultimate barrier to delivery of small molecules to the embryo is the waxy layer that encompasses the vitelline membrane of the eggshell. The Drosophila eggshell is comprised of five layers (illustrated in Fig. 3A). From innermost to outermost they are: the vitelline membrane (~300nm thick), the waxy layer (~5nm), the inner chorionic layer (40-50nm), the endochorion (500-700nm) and the exochorion (300-500nm) (Margaritis et al. 1980). The waxy layer is the “waterproofing” layer, able to exclude entry of even small solutes such as sodium hypochorite (bleach) used in the routine protocol of stripping the three chorionic layers (dechorionation). The composition of the waxy layer is the least well understood. The fact that elevated temperature and non-polar organic solvents, such as heptane and octane, render the dechorionated embryo permeable is consistent with the notion that this barrier is indeed waxy in composition (King and Koch 1963; Limbourg and Zalokar 1973). Composition of the vitelline membrane is well known, being comprised of several proteins that establish a crosslinked network (Waring 2000). However, the contribution of the vitelline membrane to embryo impermeability is not fully understood.
Figure 3. Eggshell layers and EPS removal of the waxy layer.
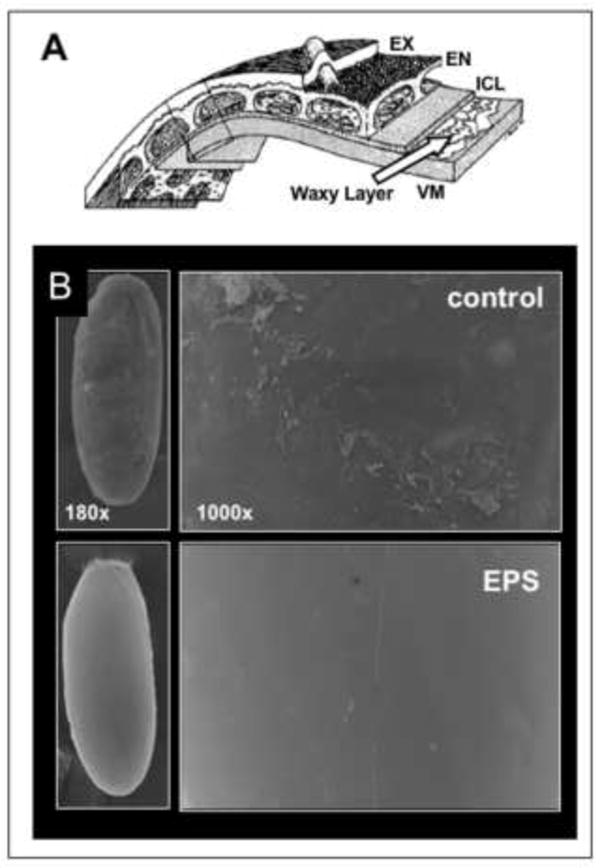
A) The five layers of the eggshell are diagramed. From innermost to outermost they are: the vitelline membrane (VM,~300nm thick), the waxy layer (~5nm), the inner chorionic layer (ICL, 40-50nm), the endochorion (EN, 500-700nm) and the exochorion (EX, 300-500nm) (adapted from Margaritis et al. 1980). Chorion layers are easily removed with 3% sodium hypochlorite (50% bleach) treatment with minimal adverse effects on the embryo. B) Scanning EM images of dechorionated non-EPS treated embryos (control) revealed a roughened plaque-like coating of the surface consistent with previous descriptions of the waxy layer. EPS treated embryos show a remarkably smooth and homogeneous surface, indicating removal of the waxy plaque layer and exposure of the vitelline membrane.
Heptane and octane are effective in rendering the embryo permeable (Limbourg and Zalokar 1973; Mazur et al. 1992). However, these solvents are toxic and can result in low viability of treated embryos. Furthermore, heptane or octane treatment requires a transition out of and back into the aqueous phase making it technically challenging to avoid desiccation. Mazur, et al (Mazur et al. 1992) showed that brief heptane exposure to late stage embryos results in permeabilization as determined by Rhodamine B dye uptake. With this approach, viability proved to be inversely related to permeability. Yet under a narrow window of solvent exposure a significant degree of viability could be achieved. However, early stage embryos (Stage 11 and earlier) are highly sensitive to this approach and are effectively killed (Mazur et al. 1992). Strecker, et al., (Strecker et al. 1995; Strecker et al. 1994) improved on use of heptane and octane for permeabilizing embryos, ultimately using it to demonstrate the effects of glucocorticoids on embryo development. Nonetheless, the toxicity of the solvents and the careful manipulations required in these former protocols have prevented widespread application of this approach.
We describe methods and compositions of an embryo permeabilization solvent (EPS) composed of D-limonene and plant-derived surfactants that is water miscible and highly effective in rendering the dechorionated eggshell permeable to small molecules up to 995 MW. We also demonstrate properties of Rhodamine B dye that serve as a marker of ideally permeabilized embryos and their subsequent development. A high degree of embryo viability is maintained after EPS treatment, allowing for evaluation of a variety of developmental endpoints after drug exposure, as demonstrated with the activity of several teratogens applied in vitro. Application of the method to transgenic strains carrying GFP reporter genes results in a robust readout of teratogenesis. The straightforward and rapid nature of these manipulations are suitable for preparing batches of permeabilized embryos and make the method widely applicable to the fly research community. Finally, the method has potential for bringing the Drosophila embryo model to toxicology research and for screening of small molecule libraries in a high throughput format.
Materials and Methods
Drosophila Stocks, culturing and embryo collection
Fly lines used include a Canton S wild type strain maintained in our laboratory for more than eight years; Dorsal-GFP line ((DeLotto et al. 2007) gift from Jennifer Lippincott-Schwartz, NICHD, Bethesda, MD), engrailedGal4>UASGFP (Bloomington Drosophila Stock Center # 25752). Flies were maintained on a standard corn meal, molasses agar medium at 25°C in an incubator with 12hr on/off light cycle. Embryos were collected on grape juice-agar plates with a spot of yeast paste in large embryo collection cages (www.flystuff.com) maintained at 25°C with 12hr on/off light cycle.
Embryo treatments and culturing
Embryos were collected in “nitex baskets” fabricated from the top portion of a 50mL disposable conical culture tube (see Fig. 1B, E’). A nylon 120μm Nitex mesh was used (Flystuff.com Catalog #57-102). Embryos were washed with tap water (~25°C) and dechorionated by immersion in 50% bleach (Fisher Scientific #SS290-4) for two minutes followed by washing under a gentle flow of tap water.
Figure 1. Embryo treatments and culturing.
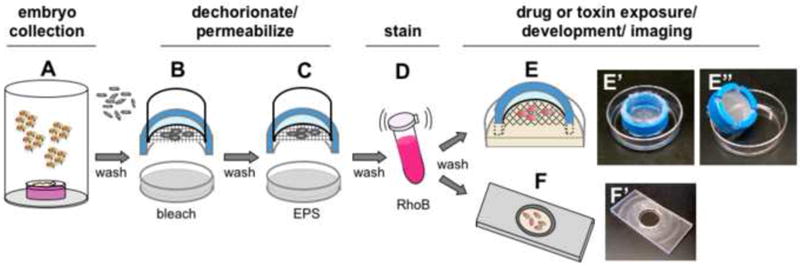
A) Embryos are collected from cage cultures equipped with yeast paste on grape plates. Embryos are handled in “nitex baskets” fabricated from the top portion of a 50mL disposable conical culture tube (B-E). B) Embryos are washed with tap water (~25°C) and dechorionated by immersion in 50% bleach for two minutes followed by washing under tap water. C) Dechorionated embryos are immersed in embryo permeabilization solvent (EPS). EPS treatment is followed by repetitive washes with 5mL of Phosphate Buffered Saline (PBS). D) Embryos are immersed in dye (e.g 1mM Rhodamine B) for five minutes in a capped microfuge tube with rocking. E) Embryos are then washed with PBS and set on a modified nitex basket (E’-E”) suspended in a reservoir of incubation medium (e.g. MBIM) containing drug or toxin. F) Alternatively, embryos are mounted in incubation medium containing drug or toxin in a slide chamber previously described by Kiehart ((Kiehart et al. 1994). Visualization is performed with brightfield or fluorescence microscopy.
Embryo permeabilization solvent (EPS) consisted of 90% D-limonene (Technical Grade D-limonene the Florida Chemical Co., Winter Haven, Florida, or high purity orange terpene D-limonene from Green Terpene, Coral Gables, Florida), 5% cocamide DEA (Ninol® 11CM, Stepan Chemical, Northfield, Illinois) and 5% ethoxylated alcohol (Bio-Soft®1-7, Stepan Chemical, Northfield, Illinois). This composition was stable at room temperature for more than two months. Dechorionated embryos were immersed in various dilutions of EPS made in either Milli-Q water or in modified basic incubation medium (MBIM (Strecker et al. 1994) (1 L): MgCl2•6H20 (2.2g), MgS04•7H20 (2.97g), NaH2P04 (0.42g), Glutamic Acid (12.1g), Glycine (6.05g), Malic Acid (0.66g), Sodium Acetate (0.027g), Glucose (2.2g), CaCl2•2H20 (0.99g), pH6.8 adjusted with NaOH and KOH (5%, equal vol.), sterile filtered). Treatments utilized a 1:5-1:40 dilution of EPS with a 30-second to 4 minute range of exposure. EPS treatment was followed by four successive washes in 5mL of Phosphate Buffered Saline (PBS, 139mM NaCl, 2.7mM KCl, 8.1mM Na2HPO4, 1.5mM KH2PO4, pH 7.0) followed by two washes in PBS with 0.05% Tween-20 (PBStw).
Dye uptake was assessed by immersing embryos in one milliliter of PBS or PBStw for either five minutes in a capped microfuge tube rocking on a nutator or by immersion for 30 minutes in a nitex basket in a reservoir of dye. In some instances a modified D22 medium (Echalier 1997) at 0.8X concentration was used for washes or dilution of Rhodamine B with no difference in outcomes. Dyes used were Cresyl violet (0.1% final concentration, Sigma C5042, MW=321.3), Rhodamine B (10mM stock in H20, 1mM final concentration, Sigma #R6626, MW=479.0), Fast Green (0.1% final concentration, Fisher F99, MW=765.9), Syto11 (5mM stock in DMSO, used at 5μM final concentration, Invitrogen S7573), Calcein AM (1mg/mL stock in DMSO, used at 5μM final concentration, Invitrogen C3099, MW=994.9). Embryos were then washed four times with PBStw prior to visualization by brightfield, epi-fluorescence or confocal microscopy.
Incubations of permeabilized embryos were carried out in two types of chambers. Long-term incubations (18-22 hours) were done in a modified nitex basket (see Fig. 1E-E” and Results for description). Short term incubations (e.g. <3 hours) were done in a slide chamber previously described by Kiehart ((Kiehart et al. 1994), see Fig. 1F,F’). Incubations were done in either PBS or MBIM medium (Strecker et al. 1994). Incubations for early embryonic endpoints were carried out at room temperature and for later developmental endpoints (e.g. gut development and engrailed patterning) at 18°C or 25°C.
Drug treatments were done by additions to the medium reservoir during incubation in either the slide or basket chambers. Drugs used were: cycloheximide (Fluka 01810, MW=281.35, 50mM stock in H20), methylmercury chloride (Aldrich 442534, MW=251, 50mM stock in DMS0), cytochalasin D (Sigma C8273, MW=507.6, 5mM stock in DMS0) and cyclophosphamide (LKT C9609, MW=279.1, 100mM stock in H20).
Effects of EPS and cycloheximide on early embryo development were scored using contemporaneous DIC optics and fluorescent visualization. Canton S embryos were collected from a 2hr laying and aged one additional hour at 25°C. Embryos were treated with 1:10 EPS in H20 for 30-60 seconds followed by washing and immersion in 1mM Rhodamine B dye in PBS for 5 minutes with rocking. Embryos were washed four times in PBS and immersed in PBS containing 10μM cycloheximide on a Kiehart slide. Embryos were allowed to develop an additional 1.5 hours at room temperature (~23°C). Abnormal development was scored in embryos at Stage7-9 that displayed absence or aberrant cephalic furrow formation and/or disrupted germband elongation. Variable treatments included omission of EPS exposure or omission of cycloheximide. Each condition was assayed in three independent preparations of embryos with >180 embryos scored.
Microscopy
Imaging of embryos was done with three microscope setups: a Nikon SMZ1500 stereo dissecting microscope equipped with an X-cite 120 short arc source (MVI, Avon Massachusetts) for fluorescence imaging in the blue, green and red channels; a Leitz Orthoplan 2 upright microscope equipped with Nomarski (DIC) optics and epi-fluorescence capabilities; a Nikon C1 scanning laser system on a Nikon Eclipse E800 upright microscope using 10X and 20X objectives. Images were captured with a Spot One digital camera and associated PC Spot One software (MVI Avon Mass). Confocal images were collected using the associated Nikon C1 software package. Image processing was done using Adobe Photoshop.
Specimen preparation and scanning electron microscopy was performed with the help of the University of Vermont College of Medicine Microscopy Imaging Facility. Dechorionated Canton S embryos of mixed age (an overnight collection) were untreated or treated with EPS (no dilution, 30 seconds) prior to fixation. A modification of Karnovsky’s fixative was used (2.5% glutaraldehyde, 0.1% paraformaldehyde in 0.1M Phosphate Buffer (PB) pH7.2). Fixation was done for one hour followed by three rinses in PB. Post-fix was done with 1% osmium tetroxide in PB for one hour at 4°C, followed by three repeated washes. Dehydration was done in sequential 10-minute immersions in ethanol (35% up to 100% in six steps). Samples were then critical point dried with CO2 as the transitional fluid. Samples mounted on an aluminum holder were sputter coated with gold/palladium for 2-4 minutes. Imaging was done on a JSM 6060 scanning electron microscope (JOEL, Peabody, MA).
Results
Facilitation of embryo handling and development
One of the first challenges of developing this method was to establish in vitro conditions that permit drug delivery and support embryo viability subsequent to treatments that affect the eggshell. Conventional methods call for immersion in halocarbon oil to prevent desiccation. However, oil immersion is not compatible with delivery of aqueous solutes. Our initial attempts of incubating embryos in drops of culture medium or buffered saline solutions demonstrated a negative impact of the depth of the fluid on development, presumably due to limitations on oxygen delivery. We therefore devised a chamber that was optimal for immersion of the embryo in surrounding buffer while maximizing oxygen delivery. We modified a conventional home-made “nitex basket” used for the 50% bleach treatment in the dechorionation step (Fig. 1E-E”). These baskets are typically fabricated from the cut off top and screw cap of a 50 mL conical culture tube. The center portion of the cap is excised such that a patch of nitex screen can be screwed in place between the cap and the tube. When inverted, a “basket” is formed that can effectively retain embryos when dipped in a reservoir or washed from above. We modified the basket by forming four notches in the rim of the cap such that when the basket is placed in a reservoir solutes can exchange freely across the nitex (Fig. 1E-E”). When placed in a reservoir of 6mL of buffer in a 50mm plastic dish the buffer is retained from entering the basket due to surface tension at the nitex pores, while the reservoir level on the outside of the basket raises above the level of the nitex. Embryos placed on the nitex in the basket are seen to become two-thirds immersed in the buffer due to capillary action. When basket and reservoir are capped with an inverted beaker the reservoir evaporation is minimized and the embryo orientation in the fluid can be maintained for >48 hours without desiccation. The notches in the rim also facilitate removal of air bubbles from the underside of the nitex. We have previously shown dose-dependent delivery of the toxin methylmercury to embryos using this device (Rand et al. 2009).
Alternatively, dechorionated and permeablized embryos can be arranged on slide chamber previously described by Kiehart ((Kiehart et al. 1994), Fig. 1F,F’). This slide allows for retention of embryos in a 0.3mm layer of medium between a glass coverslip and a DO membrane (Fondriest Environmental, YSI #5793) that facilitates oxygen delivery. This set up is optimal for time lapse imaging of live embryos using a microscope with confocal capabilities.
Limonene based solvents remove the waxy layer over the vitelline membrane
The Drosophila eggshell is comprised of three chorion layers, a waxy layer and the vitelline membrane (see Fig. 3A). Chorion layers are easily removed with sodium hypochlorite treatment with minimal adverse effects on the embryo (Hill 1945; Strecker et al. 1994). Permeability is almost entirely restricted by the waxy layer and thus we sought to optimize methods to remove this layer. D-limonene (hereafter referred to as limonene) is a monoterpene oil that is a major constituent of the oil in orange peels. Limonene has proven to be an exceptionally effective substitute for petroleum based solvents for removal of oily and waxy substances and has found numerous applications in industrial processes and in common household “citrus” cleaners. Limonene is a FDA registered GRAS (generally regarded as safe) substance approved for use as a food additive (Opdyke 1975). Limonene exhibits minimal toxicity in a number of biological systems; however, it does elicit toxic responses at very high doses (IPCS 2009; NTP 1990). We rationalized that a limonene-based solvent would be a desirable substitute for the conventional heptane or octane applications used to remove the waxy layer. Furthermore, in the presence of suitable surfactants, limonene is miscible with water, which we predicted would eliminate the need to transition embryos in and out an organic phase to achieve waxy layer removal.
As a first approach, we evaluated the ability of the common household product Citrasolv® (Citra-Solv, Danbury, Connecticut) to render dechorionated embryos permeable. Citrasolv® consists of limonene, essential oils and biodegradable cleaning agents derived from coconut. To assess permeability, we incubated embryos treated with Citrasolv® in histological dyes of various molecular weights. In initial experiments, dechorionated embryos were immersed in a 1:10 dilution of Citrasolv® in water for five minutes, rinsed in phosphate buffer (PBS) and immersed in 0.1% solution of dye for 30 minutes. Citrasolv® treatment was highly effective in rendering the embryos permeable to cresyl violet (MW=321.3) Rhodamine B (MW=479.0) and Fast Green (MW=765.9, Fig. 2B-D). All of these dyes are excluded from untreated dechorionated embryos (Fig. 2A and data not shown).
Figure 2. Permeabilized embryos take up various dyes.
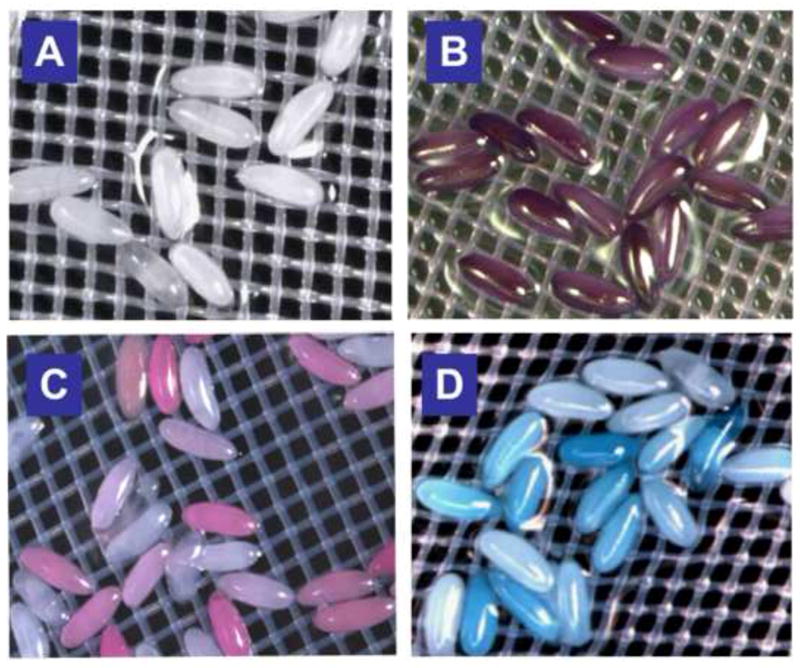
Feasibility of permeablization was initially evaluated using 1:10 dilution of Citrsolv® in water and exposure for 5 minutes. Dye exposures were done by immersion of the nitex basket in dye at concentrations below for 30 minutes. A) Omission of permeabilization steps results in complete exclusion of Cresyl violet. B-D) Uptake of Cresyl violet (B, MW=321.3, 0.1% solution), Rhodamine B, (C, MW=479.0, 1mM solution) and Fast Green (D, MW=765.9, 0.1% solution) is seen in permeabilized embryos. (See text for details).
Citrasolv® is composed of predominantly limonene and a proprietary mixture of surfactants and oils of which the composition is not certain. We therefore formulated a limonene/surfactant mixture of known composition. We surveyed several surfactants for their ability to render limonene miscible with water and the subsequent ability to permeablize dechorionated embryos. One optimal mixture consisted of 90% D-limonene, 5% nonionic ethoxylated alcohol and 5% coconut diethanolamide. We termed this composition EPS (embryo permeablization solvent). EPS proved to be equally effective as Citrasolv® in rendering embryos permeable to dyes (data not shown).
We then assessed the ability of EPS to remove the waxy layer of dechorionated embryos directly using scanning electron microscopy (EM). The waxy layer is a very thin (5 μm) coating over the vitelline membrane (Papassideri et al. 1991). The waxy layer is formed by secretions from the follicle cells in the ovary and results in interleaved layers of flattened plaques of wax (Fig. 3A, (Papassideri et al. 1993)). This layer is removed through conventional fixation protocols for EM preparations. We therefore made preparations of dechorionated embryos, untreated and treated with EPS, omitting exposure to organic solvents and directly fixing in glutaraldehyde/paraformaldehyde and post fixing in osmium tetroxide. Scanning EM images of the control treated embryos revealed a roughened plaque-like coating of the surface consistent with the waxy plaques previously described (Fig. 3B, (Papassideri et al. 1993)). In contrast, EPS treated embryos showed a remarkably smooth and homogeneous surface, indicating removal of the waxy plaque layer (Fig. 3B).
We further assessed the permeability of EPS treated embryos using a panel of fluorescent dyes. Rhodamine B is a brilliant red fluorescent dye that was seen to accumulate in the cytosol of EPS-permeabilized pre-blastoderm embryos as well as in the first cells formed at the blastoderm stage (Fig. 4A,C,D). This distribution is consistent with the reported endosomal uptake of Rhodamine B (Vult von Steyern et al. 1996). Remarkably, we found that Rhodamine B was excluded from the nucleus, and could be used to monitor progression of mitosis through time-lapse confocal microscopy (See Fig. 4D and Fig. 10B). Calcein AM (MW=994.9) is a cell permeable dye that becomes fluorescent once cleaved by esterases in the cytosol of viable cells. Calcein is seen to label the cells in the periphery of a EPS-permeabilized blastoderm embryo (Fig. 4B, C). Notably, pole cells in the posterior of the embryo are strongly labeled (Fig. 4B, C). Syto® 11 is a cell permeable nucleic acid binding dye that is rendered fluorescent upon binding nucleic acids. Syto® 11 permeation is exemplified by the strong labeling of condensed chromosomes of pre-mitotic figures of an early embryo (undergoing nuclear division 13) (Fig. 4E). In addition, Syto11 uptake can be seen in nuclei of cells at the periphery of an embryo undergoing gastrulation (Fig 4F). Together, these data demonstrate the efficacy of EPS to render the embryo shell permeable to a variety of dyes of various molecular weight and subcellular distribution properties.
Figure 4. Subcellular labeling in embryos with fluorescent dyes.
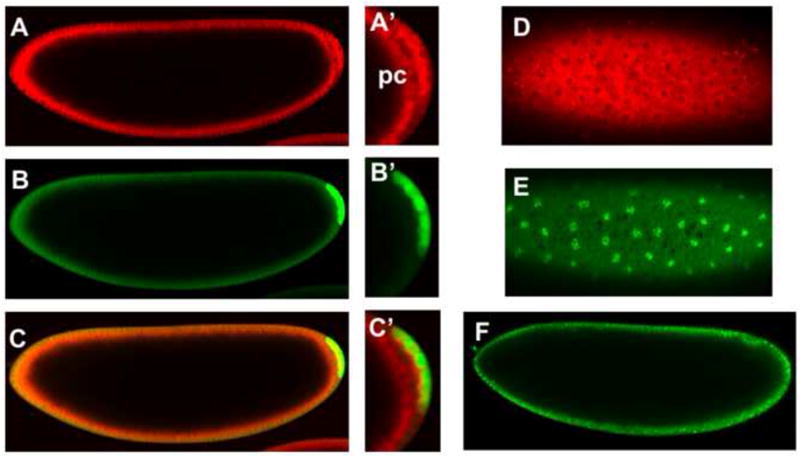
EPS treated embryos were probed with a panel of fluorescent dyes and imaged using confocal microscopy. Embryos were permeabilized with EPS at 1:10 dilution and exposures ranging from 30 seconds to one minute. A) Rhodamine B is a brilliant red fluorescent dye that is seen to accumulate in the cytosol of cells in cellular blastoderm (stage 5) embryos as well as in the posterior pole cells (pc, A’). Rhodamine B is excluded from nuclei (D). B) Calcein labels cells in the periphery of the embryo as well as pole cells in the posterior of the embryo, which are strongly labeled (B’). C) A merged image shows co-localization of Rhodamine B and Calcein. E) Syto11 strongly labels DNA exemplified by labeled condensed chromosomes of pre-mitotic cellular blastoderm cells. F) Syto11 uptake can be seen by nucleic acid staining in nuclei of cells at the periphery of an embryo undergoing gastrulation (Stage 7). Anterior is left and dorsal is up in images A-C and F. Images D and E are taken at the ventral surface of the embryo.
Figure 10. Dorsal-GFP as a reporter for disruption of early embryogenesis.
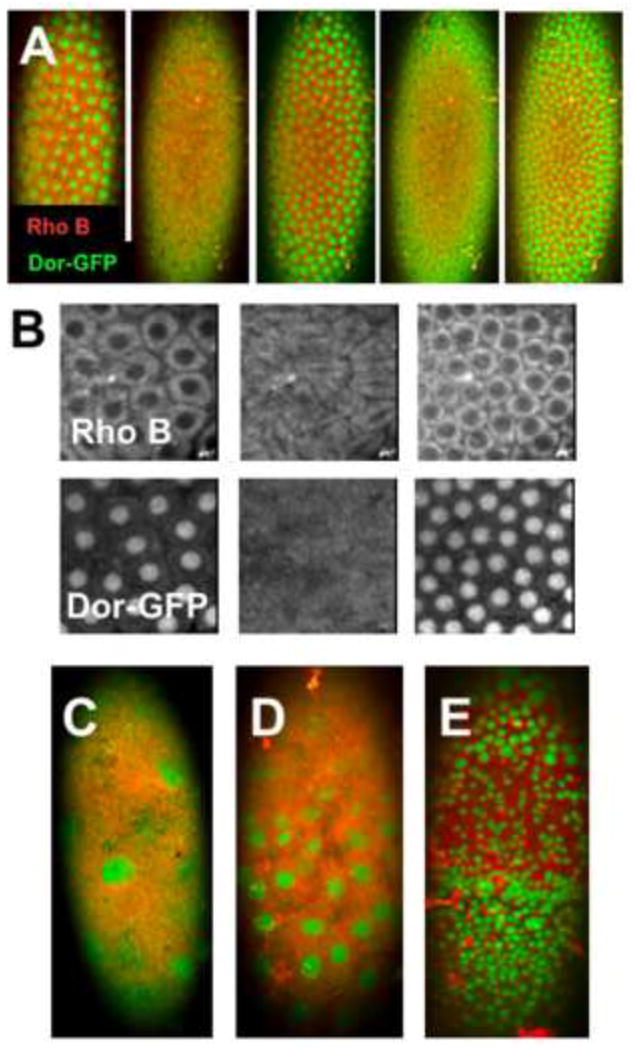
A-B) Confocal image sequence of a live EPS-permeabilized Dorsal-GFP embryo shows nucleoplasmic shuttling of Dorsal-GFP (green) during cycle 13-14 nuclear divisions (EPS 1:10, 30 seconds; Rhodamine B 1mM, 5min.). Rhodamine B (Rho B) is excluded from the nuclei (B) in contrast nuclear localization Dorsal (Dor-GFP). C-E) Treatment with of similarly permeabilized embryos with 10μM CHX causes a stalling of nuclear divisions and disruption of normal nuclei patterns (Anterior is up and images are taken at the ventral surface. See text for discussion).
Manipulations to achieve various levels of eggshell permeability
With the objective of establishing optimal conditions of permeabilization and embryo viability, we investigated the effects of various conditions of EPS treatment on permeability. First, we observed that using fluorescence detection of Rhodamine B uptake could detect eggshell permeability at a much lower threshold than with light microscopy (Fig. 5C compared to 5C’). We initially observed that EPS treated embryos showed heterogeneous uptake of Rhodamine B (Fig. 2C, 5A’), presumably reflecting variability in permeabilization. We therefore assessed the effects of embryo age on the ability to permeabilize with EPS. We observed an age-dependent decrease in permeability whereby 0-2 hour embryos were readily permeabilized and 6-8 hour embryos were refractory to EPS permeabilization under the same time and concentration of treatments with EPS and Rhodamine B (Fig. 5A-D). Intermediate levels of permeabilization were seen with embryos 2-4 hours and 4-6 hours old (Fig. 5B,C). These data indicate a change in the properties of the vitelline membrane occurs over the first six to eight hours of development. In addition, a wide range of dye uptake was seen within each age group of embryos (e.g. Fig. 5A’), suggesting individual variability in the permeability of eggshells of embryos of a similar age. To evaluate the effectiveness of EPS in these treatments we assessed Rhodamine B uptake in dechorionated embryos not treated with EPS. Unexpectedly, we found a substantial, albeit much lower, level of Rhodamine B uptake in 0-2 hour old embryos (Fig. 5E). This “basal” level of Rhodamine B uptake was considerably reduced in 2-4 hour and 4-6 hour embryos (Fig. 5F,G) again indicating a developmental change in the vitelline membrane that excludes dye uptake.
Figure 5. Age dependent permeabilization of early embryos.
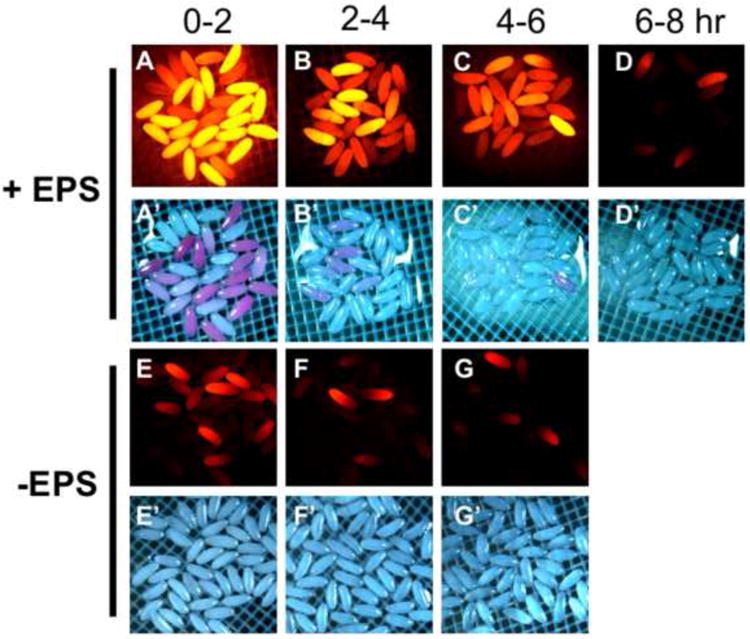
Embryos were collected and aged for the indicated hours. Permeabilization was done with 1:10 dilution of EPS (in MBIM) for one minute followed by five minute staining in 1mM Rhodamine B. Red fluorescence and brightfield images were captured in succession maintaining the same exposure settings between samples. A-D) EPS treated and Rhodamine B stained embryos of the indicated age. E-G) Non-EPS treated embryos stained with Rhodamine B as in A-D and of the age indicated at the top. (See text for discussion).
The variable permeability among embryos of different ages calls for an ability to adjust EPS exposures to give more or less permeability. We therefore examined the effects of varying concentration and time of exposure of EPS. For these experiments, we chose to use 2-4 hour embryos. We see that a 1:10 dilution of EPS and exposure for one minute renders a majority of embryos permeable as judged by Rhodamine B fluorescence (Fig. 6B). Increased EPS concentration (1:5 dilution) gives slightly more Rhodamine B uptake (Fig. 6A, A’). EPS dilution at 1:20 yields significantly less Rhodmaine B uptake than treatment with 1:10 dilution (Fig. 6C, C’ versus 6B, B’). Increasing the time of EPS exposure yields greater permeability as seen by a high level of Rhodamine B uptake subsequent to EPS treatment for four minutes at 1:10 dilution (Fig. 6D, D’). A significant drop-off in permeability is seen with 1:20 and 1:40 dilutions of EPS with four minute exposures compared to 1:10 dilution (Fig. 6E, F versus 6D). These data demonstrate an ability to achieve a desired level of permeability though systematic adjustments to the time and concentration of EPS exposure.
Figure 6. Concentration and time dependent permeabilization with EPS.
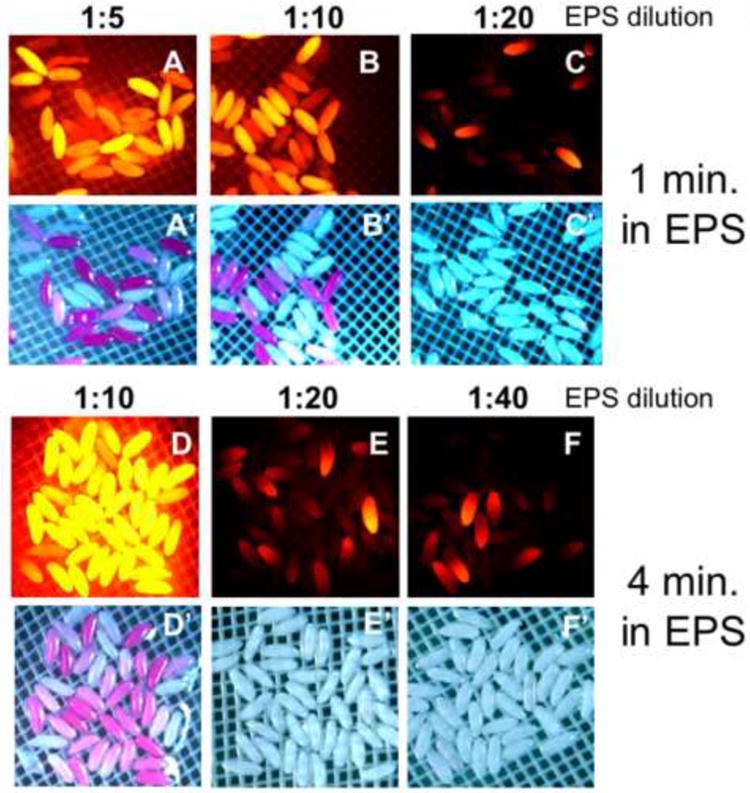
A-C”) 2-4 hour embryos treated for one minute in the indicated dilution of EPS (in MBIM) followed by 5 minutes staining in 1 mM Rhodamine B. D-F’) 2-4 hour embryos treated with indicated EPS dilution for four minutes (in MBIM) followed by 5 minutes staining in 1mM Rhodamine B. (See text for discussion)
We next varied the time of incubation with Rhodamine B with the prediction that longer incubations would reveal a lower threshold of permeability. We used 0-2 hour embryos exposed to EPS at 1:10 dilution for 30 seconds. We observed a time dependent increase in Rhodamine B uptake with substantially higher levels of uptake seen at 15 minutes compared to five minutes or less exposure (Fig. 7A-C). Again, we observed a considerable, yet lower level, uptake of Rhodamine B at 15 minutes in dechorionated embryos not treated with EPS (Fig. 7D versus 7C) indicating a basal permeability of 0-2 hour embryos to this dye. The permeability revealed with extended Rhodamine B exposure occurs well below the level of detection of Rhodamine B with light microscopy (Fig. 7C versus 7C’).
Figure 7. Rhodamine B staining, permeability levels and embryo development.
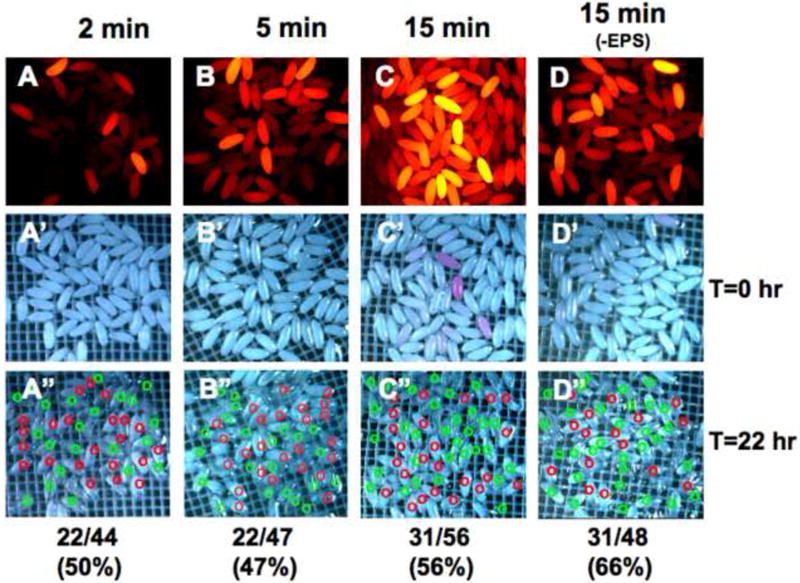
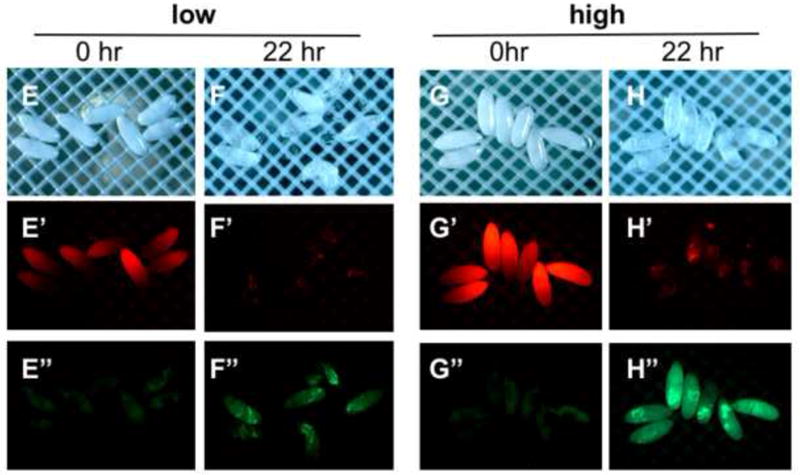
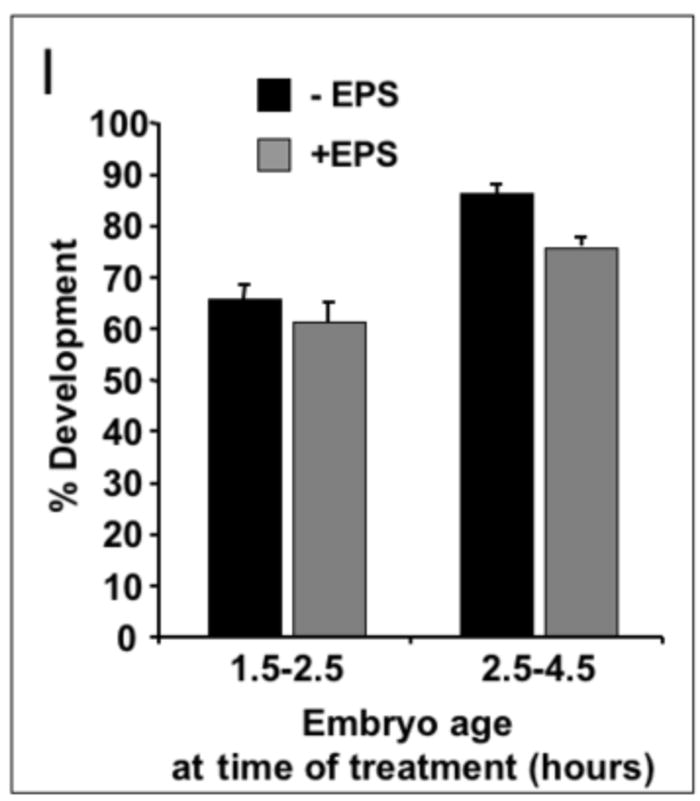
A-C, A’-C’) 0-2 hour embryos treated for 30 seconds in 1:10 dilution of EPS (in MBIM) followed by indicated minutes of staining in 1mM Rhodamine B. D, D’) 0-2 hour embryos not treated with EPS followed by 15 minutes staining in Rhodamine B. A”-D”) Embryos in A-D incubated on MBIM at 25°C for 22 hours. Green circles denote embryos developed to Stage 17 and red circles denote undeveloped embryos. Embryo numbers (developed/total) and percentages shown below each sample. E-E”, G-G”) 4-6 hour embryos treated for two minutes in 1:10 dilution of EPS (in MBIM) followed by 5 minutes of staining in 1mM Rhodamine B. Embryos were sorted manually to “low” and “high” permeabilized groups based on Rhodamine fluorescence intensity (E’, G’). F-F”, H-H”) Embryos in E and G incubated on MBIM at 25°C for 22 hours. E”-H”) Images of green fluorescence of the corresponding embryos in E’-H’. I) Percent development of embryos of the indicated age, with or without EPS treatment and Rhodamine B staining (1mM, 5 minutes). Earlier embryos were treated with EPS at 1:10 for 30 seconds and later embryos with EPS 1:10 for one minute. Development was scored as the number of embryos showing green fluorescence in the midgut (Stage 17 embryos, e.g. see panel H”) together with empty vitelline membrane shells expressed as a percentage of the total number of permeabilized embryos (n>150 embryos for each, bars=standard deviation of three independent batches of embryos. See text for discussion).
Embryo viability and EPS permeabilization
We next determined the viability of permeabilized embryos under various conditions of ageing and EPS treatment. We focused initially on 0-2 hour embryo treated with EPS (1:10 dilution, 30 seconds). As described above, we were able to discern that this treatment permeabilized a majority of embryos at substantially higher levels than non-EPS treated embryos (Fig. 7C, D). Embryos were incubated in nitex baskets in a modified basic incubation medium (MBIM, see methods) for 22 hours at 25°C and subsequently scored for development to Stage 17 (Fig. 7A”-D”). This endpoint is easily discernable under light microscopy by appearance of fully-formed mid- and hindgut, or by completion of hatching as seen by empty vitelline membrane shells. EPS treated 0-2 hr embryos showed development between 47-56% as compared to non-EPS treated embryos that developed at 66% under these incubation conditions (Fig. 7A”-D”). These data indicate that permeabilization of very early embryos results in a slight decrease in development. In addition, it is apparent from these data that Rhodamine B has no adverse effect on development (e.g. compare rates of development and Rhodamine B levels in Fig 5C-C” versus 5A-A”). It is likely the somewhat low development rate of control (dechorionated) 0-2 hr embryos (e.g. 66%, also see Fig. 7I) is due to the permeability we observe at this stage. Culturing 0-2 hr dechorionated embryos under halocarbon oil, common in other protocols, may compensate for this permeability and account for higher rates of development seen in other applications.
To test the effect of embryo age on viability we examined 4-6 hour embryos treated with 1:10 dilution of EPS for two minutes to achieve a similar level of permeability as seen with 0-2 hour embryos treated for 30 seconds. Monitoring the Rhodamine B fluorescence in this group we observed that permeability levels could be effectively judged by the presence of an anterior to posterior gradient of fluorescence intensity the 10-20 minutes immediately following dye exposure (Fig. 7E’, H’). We found this “gradient” staining to be an indicator of optimal permeabilization and useful for manually sorting nearly equivalent permeabilized embryos. We sorted embryos of similar permeability into two groups: “low” and “high” permeability (Fig. 7E’ versus 7H’). After 22 hours at 25°C all of the low permeability embryos developed to Stage 17 and three of seven embryos successfully hatched (Fig. 7E, F). Six of the seven high permeable embryos developed to Stage 17 (Fig. 7G, H). None of this latter group hatched, despite showing normal movements and contractions of the larva within the confines of the vitelline membrane (data not shown).
An unexpected finding was that Rhodamine B treated embryos exhibited bright green fluorescence after development, which corresponded with a reduction in the initial red fluorescence (Fig. 7F’, F” and H’, H”). We predict that this results from metabolism of the Rhodamine B dye causing a spectral shift in its fluorescence. Rhodamine B (N,N,N’,N’-tetraethylrhodamine) is known to undergo de-ethylation which causes a shift in its excitation/emission properties (Qu et al. 1998). Notably, bright green fluorescence is seen to concentrate in the mid- and hindgut in developed (Stage17) embryos (Fig 7F”, H”).
These unique properties of Rhodamine B dye permitted us to accurately assess viability and development of permeabilized embryos. In a batch preparation, we could score for both permeability and viability via the accumulation of green fluorescence in fully formed guts of developed embryos (Fig. 7F”, H”). Using this endpoint, we assayed the development of embryos treated with EPS at two early developmental windows and subsequently incubated in nitex baskets at 25°C for 18-20 hours. Development subsequent to EPS treatment was compared to untreated, dechorionated embryos. Approximately 65-85% of untreated embryos 1.5-4 hours old develop to Stage 17 (Fig. 7I). EPS treatment causes a slight reduction in the rate of development (compared to untreated embryos) with approximately 75% of 2.5-4 hour old embryos successfully developing to Stage 17 (Fig. 7I).
Together, these data confirm that embryo permeability can be achieved that is compatible with embryo viability and development. These data also indicate that the level of permeability can be judged by the initial intensity and “gradient” profile of Rhodamine B uptake and subsequently by the elaboration of green fluorescence due to spectral conversion of Rhodamine B in living embryos.
Developmental endpoints for scoring drug effects in permeabilized embryos
By monitoring development under time-lapse imaging, we observed the highly dynamic pattern of blue auto-fluorescence of yolk proteins in the embryo. At preblastodem stages, a relatively uniformly dispersed blue fluorescence is seen across the embryo (Fig. 8A, 0 hrs). Between 3-6 hours of development, this pattern changes drastically as the yolk proteins are pushed aside during germband elongation (Fig. 8A, 3 and 6 hrs). At ~10 hours of development, the germband retracts and anterior and posterior extensions of the presumptive midgut fuse to complete the intestinal tract by 12 hours (Fig. 8A, 10 and 12 hrs). Blue fluorescence is seen to consolidate in the lumen of the gut initially as one large mass in the newly fused midgut (Fig. 8, 12 hrs). The midgut undergoes three circular constrictions at Stage 16 (Fig. 8, 14hrs). The compartmentalized strong blue fluorescence in the midgut makes normally developed Stage 16/17 embryos easily discernable (Fig. 8, 14hrs).
Figure 8. An intrinsic fluorescent indicator of embryo development.
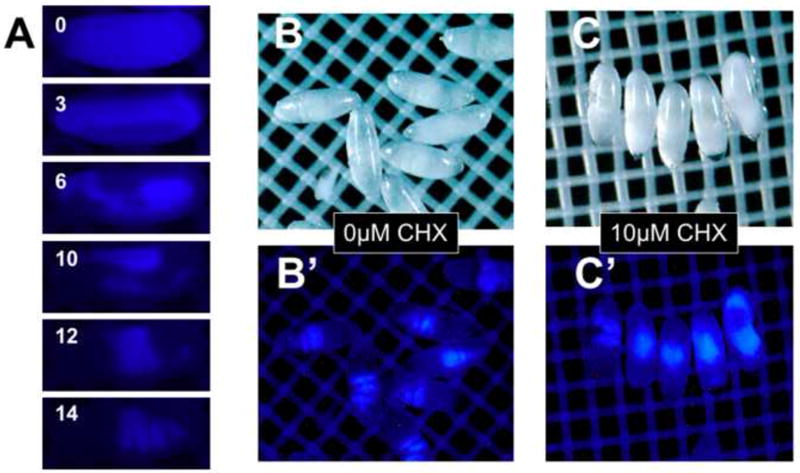
Blue auto-fluorescence can be detected in the yolk proteins. Change in the distribution pattern of blue fluorescence over 14 hours of development (stage 5-14, panel A) is seen in representative frames of time-lapse imaging. Blue fluorescence in the midgut is easily discernable in normally developed EPS-permeablized (1:10 EPS one minute) Stage 16/17 embryos (Panel B, B’). Permeabilized embryos exposed to 10 μM cycloheximide (CHX) shows gut malformation as seen by the blue fluorescence centrally localized in a “ball” of tissue or dispersed anteriorly and posteriorly (Panel C, C’. See text for discussion).
The utility of the blue-autofluorescence endpoint in examining small molecule toxicity became clear with application of cycloheximide (CHX), a potent protein synthesis inhibitor and teratogen. EPS-treated 2-4 hour embryos were incubated in the presence or absence of 10μM CHX at 18°C for 20 hours (equivalent to 10 hours at 25°C) and examined under blue fluorescence. Control embryos consistently exhibited the characteristic 3-4 midgut compartments denoting development to Stage 16/17 (Fig. 8B’). In contrast, CHX treatment induced malformation as seen by the blue fluorescence centrally localized in a “ball” of tissue or dispersed anteriorly and posteriorly (Fig. 8C’). These results indicate the pattern of blue yolk auto-fluorescence can be used as an easily distinguishable endpoint to evaluate small molecule toxicity in embryonic development.
We next sought to determine the feasibility of using an early embryogenesis endpoint as an indicator of teratogenic effects. In separate experiments using flies carrying the GFP reporter for the Dorsal protein, we determined that CHX had profound effects on the morphogenetic movements of the embryo in the first 3-5 hours of development (data not shown). During these stages, formation of the cephalic furrow and germband elongation serve as landmarks that are easily identifiable under differential interference contrast (DIC) microscopy. We found that exposure of 2-3 hour permeabilized embryos to 10μM CHX for 1.5 hours results in disruption of cephalic furrow formation (Fig 9A, B). We took advantage of this easily score-able CHX phenotype to assess the efficacy of EPS in enabling drug delivery. Treatment with EPS resulted in ~70% of the embryos displaying permeabilization as determined by presence of Rhodamine B fluorescence (Fig. 9C). This is in contrast to only 24% showing dye uptake without EPS treatment (Fig. 9C). With CHX (10μM) treatment more than 80% of EPS treated embryos displayed an abnormal formation of the cephalic furrow. With omission of CHX roughly 17% of permeabilized embryos were abnormal (i.e. >80% were normal). The same degree of normal development was seen in non-permeabilized embryos. Furthermore, the same degree of normal development was observed in non-permeabilized embryos in the presence of CHX, indicating the EPS treatment is required for entry of CHX under these conditions (Fig. 9C). A somewhat lower rate (65.3%) of normal development was seen in embryos that were not apparently permeablized in the preparation of EPS treatment and CHX exposure (Fig. 9C). This likely reflects the lower levels of permeability not detected with the five-minute Rhodamine B staining used in this preparation. Together the data demonstrate that EPS treatment is an enabling step for delivery of CHX to the embryo.
Figure 9. EPS treatment enables drug delivery.
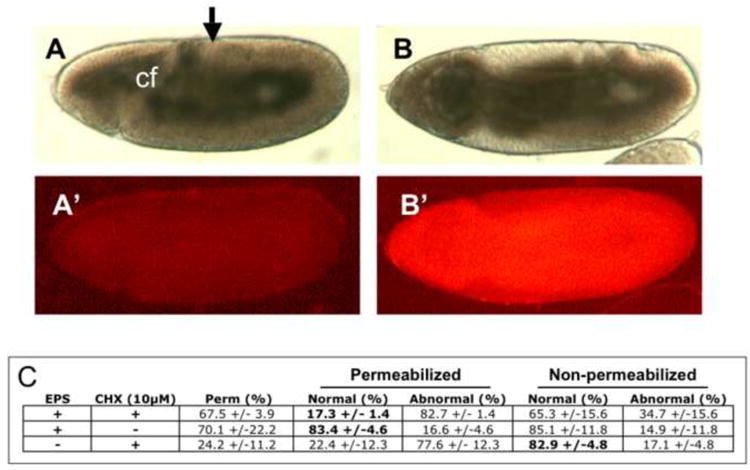
2-3 hr embryos were treated (B) or not treated (A) with EPS (1:10, 30 seconds) and aged for an addition for 1.5 hours in PBS in a Kiehart chamber slide (see methods and Fig. 1F). Permeability was confirmed by the uptake of Rhodamine B dye (B’). Normal development to Stage 7-9 was scored by appropriate cephalic furrow (cf) formation and germband elongation (arrow) determined with DIC microscopy (A, B). Rates of normal and abnormal development resulting from combinations of exposures to EPS and CHX are tabulated (C). (n>180 embryos for each determination, expressed as percent +/- standard deviation).
Genetically encoded reporters for teratogenic effects
We have previously used green fluorescent protein (GFP) expression in the nervous system in transgenic flies as a reporter of abnormal neural development (Rand et al. 2009). We sought to examine earlier markers of development. We turned to the Dorsal-GFP transgenic fusion construct reporter to examine the effects of the teratogen CHX. Signals acting through the Dorsal protein (the Drosophila NF-κB homolog) help establish the dorsal-ventral axis of the embryo (Moussian and Roth 2005). Delotto, et al. (DeLotto et al. 2007) demonstrated dynamic shuttling of Dorsal-GFP in and out of the nucleus with each mitotic division in the preblastoderm embryo. Using this Dorsal-GFP construct we see dynamic distribution of Dorsal-GFP in and out of the ventral nuclei of an EPS and Rhodamine B treated blastoderm embryos (Fig. 10A, B). Exposure to CHX causes gross deformities in the pattern of nuclear divisions and Dorsal distribution in preblastoderm embryos (Fig. 10C-E). In many cases, these early embryos are seen to stall in development. Occasionally, Dorsal accumulation in nuclei occurs at high levels in very early embryos when the syncytial nuclei first appear at the embryo surface (Fig. 10C). Embryos affected at slightly later stages show irregular nuclear Dorsal accumulation (Fig. 10D) as well as varied sizes in the nuclei present (Fig. 10E). Thus, the Dorsal-GFP construct serves as a robust readout for disruption of very early embryognesis events.
We next sought to examine a reporter for later stage embryogenesis and turned to the engrailed-GFP construct. The engrailed gene encodes a homeodomain transcription factor that is highly conserved in all metazoans (Gibert 2002). Engrailed is a segment polarity gene whose expression marks the anterior boundary of each segment in the embryo (Hidalgo 1996). Engrailed also functions in neural development to define developing brain regions and regulate growth of axons (Morgan 2006). Expression of GFP in embryonic engrailed cells in a late stage embryo can be seen in Figure 11A. Exposure of EPS-permeabilized embryos to the teratogens cytochalasin D, methylmercury and cyclophosphamide results in grossly abnormal patterns of engrailed expression. Cytochalasin D is a potent inhibitor of actin polymerization and inhibitor of protein synthesis. Its teratogenic effects include exencephaly, hypognathia and neural tube defects (Shepard 1998). We see that cytochalasin D induces a stalling of embryo development at Stage 11 with gaps in the engrailed band of cells in several segments (Fig. 11B). In addition, we occasionally see a medial split in the engrailed bands in the anterior ventral portion of the embryo indicative of interruption of signaling events controlling ventral furrow and head formation (Fig 11B, lower panel). Methylmercury (MeHg) is an environmental toxin that preferentially targets embryonic neural development (Sanfeliu et al. 2003). We see that MeHg similarly stalls the embryo at Stage 11 and disrupts the regularity of the engrailed banded pattern (Fig. 11C). This is consistent with the known activity of MeHg in inhibiting protein synthesis, as well as being an inhibitor of cell migration. Cyclophosphamide has teratogenic effects giving rise to cleft palate and limb defects in mice and rats (Shepard 1998). We see that cyclophosphamide results in an overall shortening of the embryo and generates breaks in the engrailed expressing bands of cells (Fig. 11D). Thus, the engrailed GFP reporter demonstrates a robust readout of teratogenic activity in permeabilized embryos.
Figure 11. Engrailed-GFP as a reporter for teratogenesis.
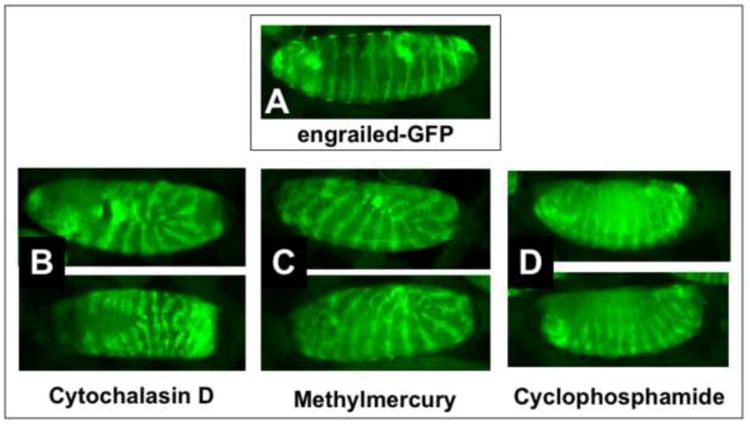
A) An engrailed-GFP embryo exhibiting normal development after EPS permeabilization at approximately 2-4 hours (EPS 1:10, one minute; Rhodamine B 1mM, 5min.). Abnormal development is seen in similarly permeabilized engrailed-GFP embryos incubated in cytochalasin D (50μM, B), methylmercury (50μM, C) and cyclophosphamide (100μM, E) and developed for 20 hours at 25°C. Images captured with a Nikon SMZ1500 stereo dissecting microscope. Anterior is left and dorsal is up (except in the lower panel of B which is a ventral view. See text for discussion).
Discussion
We have devised a method to render the Drosophila embryo eggshell permeable to small molecules while maintaining conditions suitable for development of the embryo in vitro. This method is a considerable improvement over the inefficient and technically challenging methods that employ heptane or octane solvents. Its ease of use will facilitate routine application of small biologically active molecules to the developing fly embryo. In addition, this method opens the door to using the Drosophila embryo for toxicological studies and potentially for high throughput characterization of chemical compound libraries. A number of the technical challenges that we have encountered in developing this method are worthy of discussion since they shed light on the biology of the system and the need for future refinement of the method.
One of the first unexpected findings was that dye uptake occurs in dechorionated non-permeabilized embryos. This result demonstrates that, despite the perception that the embryo is “impermeable”, some compounds are capable of penetrating the waxy layer and vitelline membrane. It is likely this property is limited to more cell permeant compounds, which is true of Rhodamine B. This property is also likely to explain the successful administration of methylmercury to dechorionated embryos that we have reported previously (Rand et al. 2009), as well as uptake of methanol in non-permeabilized embryos shown previously (Mellerick and Liu 2004). It follows that the dechorionated embryo can be penetrated by some compounds; however, EPS treatment considerably broadens the range of compounds able to traverse the vitelline membrane.
Our findings align with the previous results of Mazur et al. (Mazur et al. 1992) showing that embryo viability is sensitive to eggshell permeabilization. This finding is not unexpected considering two critical roles that the vitelline membrane plays for embryogenesis: 1) preventing desiccation and 2) harboring maternally deposited signaling molecules essential for embryonic patterning. It is intuitive that disrupting these vitelline functions with permeabilization steps will compromise the embryo. The objective is, therefore, to achieve permeabilization without disrupting these vitelline activities. Two prominent features of our EPS protocol that enable this are: 1) the ability to tailor permeabilization levels by varying concentration and time of EPS exposure and 2) the ability to detect lower thresholds of permeability using the fluorescent properties of Rhodamine B. We show that, in general, embryo viability is highest in the permeability window where Rhodamine B uptake is easily detected by fluorescence yet undetected under brightfield illumination. Thus, for routine laboratory practice, familiarity with the present protocol will give the user enough experience to identify optimally permeabilized embryos of the desired age using standard fluorescence microscopy. This will enable the user to manually select and set up embryos for drug treatments and subsequent observation in baskets or the slide chamber described here.
The steady decrease in embryo permeability seen in the first 6-8 hours of development is an important observation. While this poses a practical problem in obtaining large numbers of equivalently permeabilized embryos, it also highlights a developmentally regulated change in the embryo shell after egg laying. While this could occur at the level of the waxy layer or the underlying embryonic membranes, previous evidence points to the vitelline membrane for these changes. Hardening of the eggshell in the first hours after egg laying has been documented in mosquitoes and is known to parallel crosslinking of vitelline proteins (Li and Li 2006). Crosslinking of vitelline proteins in Drosophila is known to occur in the eggshell within the ovary (Cernilogar et al. 2001) but vitelline membrane changes post-ovoposition are not well understood. Crosslinking in the vitelline membrane may provide the structural framework for proper dissemination of signaling molecules for embryo patterning (LeMosy and Hashimoto 2000). It is interesting to note that genetic disruption of vitelline protein crosslinking renders the embryo permeable to dyes (LeMosy and Hashimoto 2000). Thus, incomplete vitelline crosslinking in the early embryo (e.g. 0-2 hr) may explain the uptake of Rhodamine B we observe at this stage without EPS treatment (see Figs. 5E, 7D). In addition, permeabilization at this early stage with EPS may inhibit subsequent crosslinking, disrupting vitelline activity and causing the lower development rates seen in 0-2 hr EPS treated embryos (see Fig. 7I). It follows that EPS treatments could prove useful to gain pharmacological access to the vitelline membrane for investigation of its role in embryonic development after egg laying.
Our method has the potential to bring the fly embryo into applications of high throughput analysis of small molecules. The small size of the embryo (150×500μm) permits culturing of >50 embryos in the well of a 96 well plate and higher density formats (e.g. 384 well). Commercial instruments are available that can sort embryos based on fluorescent properties (intensity and patterns) and distribute embryos into multiwell plates (e.g. the COPAS SELECT instrument by Union Biometrica, Holliston, Massachusetts). In addition, automated confocal image capture instrumentation is commercially available that can acquire images from a 96-well format (e.g. the BD Pathway 855 from BD Biosciences). Software associated with these instruments is capable of quantifying fluorescence intensity and distribution across the embryo. We predict that application of this technology to the numerous transgenic GFP-reporter fly lines currently available holds the promise of determining activity of a large number of compounds on a wide array of developmental endpoints in the embryo. One potential development of this technology is a screening platform using a collection of fly strains that report for each of the fundamental developmental signaling pathways (e.g. Notch, Wnt, hedgehog). Systematic evaluation of response of these embryos to various classes and concentrations of drugs/toxins could yield highly informative mechanistic data to advance the understanding of the bioactivity of compounds of interest as well as identify tools to investigate these pathways.
Several aspects of the method will benefit from further development for the goal of introducing small molecules to the developing embryo. For example, parameters needing further evaluation are: 1) limitations of various classes of compounds (e.g. membrane permeable vs. impermeable) in their ability to enter the embryo and 2) a more accurate assessment of the size of compounds that can be delivered and 3) tolerance of additional solvents associated with solubility of various classes of compounds. We also predict that EPS will prove useful in permeabilizing eggshells of other Dipterans (e.g. mosquitoes) and other insect orders that present a wax layer in their eggshell structure.
In summary, we report the development and application of EPS, a relatively non-toxic plant-based solvent composition that is highly effective for permeabilizing the Drosophila eggshell. This application facilitates pharmacological approaches to investigating embryogenesis while also opening a door to high-throughput methodology for assaying compounds in the one of the most studied embryo models in developmental biology. The ease of performing this application makes it suitable for the individual laboratory investigator as well as for adaptation to an automated high throughput platform.
Acknowledgments
We wish to thank Michele Von Turkovich of the UVM Microscopy Imaging Center for help with Scanning EM. We thank Doug Gomez of the UVM Instrumentation and Model Facility for assistance in fabricating incubation baskets. This work was supported by NIH by NIEHS R01-ES015550 awarded to M.D.R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cernilogar FM, Fabbri F, Andrenacci D, Taddei C, Gargiulo G. Drosophila vitelline membrane cross-linking requires the fs(1)Nasrat, fs(1)polehole and chorion genes activities. Development genes and evolution. 2001;211:573–80. doi: 10.1007/s00427-001-0192-1. [DOI] [PubMed] [Google Scholar]
- DeLotto R, DeLotto Y, Steward R, Lippincott-Schwartz J. Nucleocytoplasmic shuttling mediates the dynamic maintenance of nuclear Dorsal levels during Drosophila embryogenesis. Development. 2007;134:4233–41. doi: 10.1242/dev.010934. [DOI] [PubMed] [Google Scholar]
- Echalier G. Drosophila cells in culture. Academic Press; New York: 1997. [Google Scholar]
- Gibert JM. The evolution of engrailed genes after duplication and speciation events. Development genes and evolution. 2002;212:307–18. doi: 10.1007/s00427-002-0243-2. [DOI] [PubMed] [Google Scholar]
- Hidalgo A. The roles of engrailed. Trends Genet. 1996;12:1–4. doi: 10.1016/0168-9525(96)81373-4. [DOI] [PubMed] [Google Scholar]
- Hill D. Chemical removal of the chorion from Drosophila eggs. Drosophila Info Serv. 1945;19:62. [Google Scholar]
- IPCS. Limonene: International Program on Chemical Safety- Concise International Chemical Assesment Documents (CICADs) IPCS:INCHEM; 2009. [Google Scholar]
- Kiehart DP, Montague RA, Rickoll WL, Foard D, Thomas GH. Methods in Cell Biology Vol 44. Drosophila melanogaster: Practical Uses in Cell and Molecular Biology. Academic Press; San Diego: 1994. High-resolution microscopic methods for the analysis of cellualr movements in Drosophila embryos; p. 518. [DOI] [PubMed] [Google Scholar]
- King RC, Koch EA. Studies on the ovarian follicle cells of Drosophila. Quart J Micr Sci. 1963;104:297–320. [Google Scholar]
- LeMosy EK, Hashimoto C. The nudel protease of Drosophila is required for eggshell biogenesis in addition to embryonic patterning. Developmental biology. 2000;217:352–61. doi: 10.1006/dbio.1999.9562. [DOI] [PubMed] [Google Scholar]
- Li JS, Li J. Major chorion proteins and their crosslinking during chorion hardening in Aedes aegypti mosquitoes. Insect biochemistry and molecular biology. 2006;36:954–64. doi: 10.1016/j.ibmb.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbourg B, Zalokar M. Permeabilization of Drosophila eggs. Developmental biology. 1973;35:382–7. doi: 10.1016/0012-1606(73)90034-1. [DOI] [PubMed] [Google Scholar]
- Margaritis LH, Kafatos FC, Petri WH. The eggshell of Drosophila melanogaster. I. Fine structure of the layers and regions of the wild-type eggshell. Journal of cell science. 1980;43:1–35. doi: 10.1242/jcs.43.1.1. [DOI] [PubMed] [Google Scholar]
- Mazur P, Cole KW, Mahowald AP. Critical factors affecting the permeabilization of Drosophila embryos by alkanes. Cryobiology. 1992;29:210–39. doi: 10.1016/0011-2240(92)90021-s. [DOI] [PubMed] [Google Scholar]
- Mellerick DM, Liu H. Methanol exposure interferes with morphological cell movements in the Drosophila embryo and causes increased apoptosis in the CNS. Journal of Neurobiology. 2004;60:308–18. doi: 10.1002/neu.20020. [DOI] [PubMed] [Google Scholar]
- Morgan R. Engrailed: complexity and economy of a multi-functional transcription factor. FEBS letters. 2006;580:2531–3. doi: 10.1016/j.febslet.2006.04.053. [DOI] [PubMed] [Google Scholar]
- Moussian B, Roth S. Dorsoventral axis formation in the Drosophila embryo--shaping and transducing a morphogen gradient. Curr Biol. 2005;15:R887–99. doi: 10.1016/j.cub.2005.10.026. [DOI] [PubMed] [Google Scholar]
- NTP. TR-347: Toxicology and Carcinogenesis Studies of d-Limonene (CAS No. 5989-27-5) F344/N Rats and B6C3F1 Mice (Gavage Studies) 1990 online access. [PubMed] [Google Scholar]
- Opdyke DL. Monographs on fragrance raw materials. Food and cosmetics toxicology. 1975;13(suppl):825–6. [PubMed] [Google Scholar]
- Papassideri I, Margaritis LH, Gulik-Krzywicki T. The egg-shell of Drosophila melanogaster. VI, Structural analysis of the wax layer in laid eggs. Tissue & cell. 1991;23:567–75. doi: 10.1016/0040-8166(91)90014-k. [DOI] [PubMed] [Google Scholar]
- Papassideri I, Margaritis LH, Gulik-Krzywicki T. The eggshell of Drosophila melanogaster. VIII. Morphogenesis of the wax layer during oogenesis. Tissue & cell. 1993;25:929–936. doi: 10.1016/0040-8166(93)90041-i. [DOI] [PubMed] [Google Scholar]
- Qu P, Zhao JC, Shen T, Hidaka H. TiO2-assisted photodegradation of dyes: A study of two competitive primary processes in the degradation of RB in an aqueous TiO2 colloidal solution. J of Molecular Catalysis A:Chemical. 1998;129:257–268. [Google Scholar]
- Rand MD, Dao JC, Clason TA. Methylmercury disruption of embryonic neural development in Drosophila. Neurotoxicology. 2009;30:794–802. doi: 10.1016/j.neuro.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfeliu C, Sebastia J, Cristofol R, Rodriguez-Farre E. Neurotoxicity of organomercurial compounds. Neurotoxicity Research. 2003;5:283–305. doi: 10.1007/BF03033386. [DOI] [PubMed] [Google Scholar]
- Shepard TH. Catolog of teratogenic agents. 9. Johns Hopkins University Press; Baltimore: 1998. p. 756. [Google Scholar]
- Strecker TR, Li PW, McGhee S, Ham D, Smith SK, Schreck J, Youn S, Kon P. The effects of glucocorticoid, dexamethsone, on the development of the Drosophila embryo. Roux’s Arch Dev Biol. 1995;2004:359–368. doi: 10.1007/BF00360481. [DOI] [PubMed] [Google Scholar]
- Strecker TR, McGhee S, Shih S, Ham D. Permeabilization, staining and culture of living Drosophila embryos. Biotech Histochem. 1994;69:25–30. doi: 10.3109/10520299409106257. [DOI] [PubMed] [Google Scholar]
- Vult von Steyern F, Josefsson JO, Tagerud S. Rhodamine B, a fluorescent probe for acidic organelles in denervated skeletal muscle. J Histochem Cytochem. 1996;44:267–74. doi: 10.1177/44.3.8648087. [DOI] [PubMed] [Google Scholar]
- Waring GL. Morphogenesis of the eggshell in Drosophila. International review of Cytology. 2000;198:67–108. doi: 10.1016/s0074-7696(00)98003-3. [DOI] [PubMed] [Google Scholar]
- Zappe S, Fish M, Scott MP, Solgaard O. Automated MEMS-based Drosophila embryo injection system for high-throughput RNAi screens. Lab on a chip. 2006;6:1012–9. doi: 10.1039/b600238b. [DOI] [PubMed] [Google Scholar]


