ABSTRACT
Background:
Recent clinical trials for “biliary cancers” include a heterogenous group of patients with cholangiocarcinoma, gallbladder, and ampullary cancers. Limited data exist regarding the relative effectiveness of known chemotherapeutic regimens specifically in intrahepatic or hilar cholangiocarcinoma.
Methods:
Records of M D Anderson Cancer Center patients with unresectable intrahepatic and hilar cholangiocarcinoma who received first-line chemotherapy from January 1, 2005, to October 31, 2009, were retrospectively reviewed. The primary objective of this research was to determine overall tumor control rates with chemotherapeutic regimens used for first-line treatment of unresectable intrahepatic and hilar cholangiocarcinoma. Secondary objectives included duration of response, overall survival, and prognostic factors.
Results:
Eighty-five patients met inclusion criteria and were eligible for analysis. The most commonly used regimen was gemcitabine/cisplatin (62%), followed by oxaliplatin and capecitabine (16%). There was no significant difference between tumor control rates with gemcitabine/cisplatin (72% PR + SD) and other regimens (69% PR + SD). There was no significant difference between overall survival with the use of gemcitabine/cisplatin (15.2 months) or alternative regimens (13.9 months). A decrease in overall survival was seen with elevated baseline CA 19–9 (p < .0001), an initial diagnosis of unknown primary tumor (p = .0001), and prior treatment with chemoradiation (p = .0018).
Conclusion:
In this retrospective review, both gemcitabine/cisplatin and alternative doublets (including capecitabine/oxaliplatin, gemcitabine/capecitabine, and gemcitabine/oxaliplatin) were effective regimens in maintaining disease control in intrahepatic and hilar cholangiocarcinoma.
Cholangiocarcinomas (tumors in the bile ducts) are classified based on their location as either intrahepatic, if occurring within the liver, or extrahepatic which can be hilar, originating at the bifurcation of the hepatic duct or distal if located in the distal bile ducts.1 Hilar cholangiocarcinomas spread along the bile ductal system, causing biliary obstruction, elevated bilirubin, and jaundice.2 In contrast, intrahepatic cholangiocarcinomas are often asymptomatic at the early stages and typically found incidentally on imaging, frequently at advanced stages, when they are unresectable.3 The incidence of intrahepatic cholangiocarcinoma has increased both in the United States and the world in the last few decades.4,5
In patients with unresectable bile duct tumors, the prognosis is extremely poor, with survival reported at less than 1 year.6 Chemotherapy is the mainstay of treatment in these patients. However, because of the rarity of these tumors, the clinical data regarding treatment efficacy is limited. Additionally, radiation therapy is commonly incorporated into a multimodality approach for these tumors, though its effectiveness in this setting has not been established.
The most recent guidelines regarding treatment of advanced biliary tract cancers, developed by the National Comprehensive Cancer Network (NCCN), recommend the use of gemcitabine, capecitabine, or 5-fluorouracil (5-FU), either as single agents or in combination with a platinum analog (oxaliplatin or cisplatin), or the combination of gemcitabine and capecitabine, with the combination of gemcitabine and cisplatin receiving a category 1 recommendation.7 However, no comparative efficacy data are available for these regimens. In April 2010, the ABC-02 trial was published, which was the first phase III randomized, controlled trial in this population.8 The combination of gemcitabine/cisplatin demonstrated improved progression-free survival (PFS) and overall survival (OS) compared to gemcitabine alone.
One limitation of nearly all biliary cancer studies is that they have historically included a diverse population encompassing gall bladder cancer, cholangiocarcinoma, and ampullary tumors. These tumor types individually may exhibit different behavior, and some degree of individualized therapy might be necessary. With these issues in mind, we conducted a study restricted to patients with unresectable intrahepatic and hilar cholangiocarcinoma to evaluate the effectiveness of commonly used first-line chemotherapy regimens.
PATIENTS AND METHODS
The primary objective of this study was to determine the disease control rate of commonly used chemotherapeutic regimens used for treatment of unresectable intrahepatic and hilar cholangiocarcinoma. Secondary objectives included time to tumor progression, overall survival, and prognostic factors.
A retrospective chart review was conducted of patients with unresectable intrahepatic and hilar cholangiocarcinoma who were treated with chemotherapy from January 1, 2005, to October 31, 2009. Patients were included if they had a diagnosis of unresectable intrahepatic or hilar cholangiocarcinoma of adenocarcinoma histology and received all first-line chemotherapy and restaging at our institution. Patients presenting with adenocarcinoma of the liver without known primary were included if pathology suggested cholangiocarcinoma (adenocarcinoma, cytokeratin 7 positive, cytokeratin 20 ±, negative upper gastrointestinal endoscopic examination, and no other primary lesions on imaging studies). These patients were deemed to have intrahepatic cholangiocarcinoma. Classification of site of disease (hilar or intrahepatic) was also confirmed by radiologist review of imaging. Patients were excluded if they had mixed hepatocellular cancer and cholangiocarcinoma.
The following baseline characteristics were assessed: age at diagnosis, sex, prior malignancy, total bilirubin, carbohydrate antigen (CA) 19–9, extent of disease (locally advanced, multifocal, disseminated), site of disease (intrahepatic or hilar), and prior treatment for disease (surgery or chemoradiotherapy if disease was previously resectable and then progressed to unresectable). Data collected (including the dates of diagnosis, first treatment, response, progression, last follow-up, and death) were used to determine overall response rate, duration of response, and overall survival.
Response was defined as partial response (PR), stable disease (SD), or progressive disease (PD) according to Response Evaluation Criteria in Solid Tumors (RECIST). Additionally, response was considered PD if the physician changed therapy due to clinical progression. In cases where disease pattern was not measureable (for instance, those having an infiltrative pattern of spread), the tumor response was characterized as PD or SD based on radiographic assessment and clinical interpretation of progression or stable disease/response. Tumor control was defined as PR + SD.
Statistical Methods
Duration of response was determined only in patients with tumor control and was defined as the time from first documentation of tumor control to first documentation of treatment failure (disease progression, discontinuation of treatment due to toxicity, or death). Overall survival, determined in all eligible patients, was defined as the time from the start of treatment to death or last follow-up.
Wilcoxon rank sum test was used to assess the difference in continuous variables between patients with and without tumor control. The associations between categorical variables and tumor control were assessed via chi-square or Fisher's exact test. Univariate and multivariate logistic regression models were made to examine covariate effects on tumor control. The covariates included age, gender, total serum bilirubin, serum CA19–9, unknown primary tumor, prior cancer, disease site, extent of disease, prior surgery or chemoradiotherapy, and chemotherapy regimens. All covariates were included in an initial logistic regression model, and stepwise model selection method was used with both enter and stay probability of 0.2. After model selection, covariates with p values less than.05 remained in the final model. The Kaplan-Meier product limit method was used to estimate unadjusted response duration and overall survival.
Univariate and multivariate Cox proportional hazards (PH) models were developed to evaluate covariates effects on duration of response and OS. The covariates described above were included in an initial Cox PH model, and the same model selection methods and procedures were performed. Covariates with p values less than 0.05 remained in the final model.
RESULTS
Eighty-five patients met inclusion criteria and were eligible for analysis. Patient characteristics are depicted in Table 1. Overall, 14 patients (16.5%) demonstrated a partial response to treatment, while 46 patients (54.1%) had stable disease, for a cumulative tumor control rate of 71%. Twenty-five patients (29.4%) had progressive disease.
Table 1.
Baseline characteristics
| Characteristic | N = 85 (%) |
|---|---|
| Gender | |
| Male | 49 (57.6) |
| Female | 36 (42.4) |
| Mean age (years) | 61.0 ± 11.5 |
| Prior malignancy | |
| No | 68 (80.0) |
| Yes | 17 (20.0) |
| Extent of disease | |
| Locally advanced | 18 (21.2) |
| Multifocal | 23 (27.0) |
| Disseminated | 44 (51.8) |
| Lymphadenopathy | 19 |
| Lung | 13 |
| Peritoneal | 5 |
| Bone | 4 |
| Adrenal | 2 |
| Pancreatic | 1 |
| Site of disease | |
| Intrahepatic | 67 (78.8) |
| Hilar | 18 (21.2) |
| Unknown primary cancer | |
| No | 65 (76.5) |
| Yes | 20 (23.5) |
| Median CA 19-9 | 132.1 (1–56,128) |
| Median total bilirubin | 0.5 (0.1–16.6) |
| Prior treatment | |
| Surgery | 7 (8.2) |
| Chemoradiotherapy | 5 (5.9) |
Treatment Regimens
Eighty percent of patients received gemcitabine-based first-line chemotherapy (Table 2). The 2 most common regimens used were gemcitabine/cisplatin (62%) and capecitabine/oxaliplatin (16%). Patients received a median 6 cycles of first-line chemotherapy (range 2–28 cycles).
Table 2.
Treatment regimens
| Chemotherapy regimen | N = 85 |
|---|---|
| Gemcitabine/cisplatin (gemcitabine/cisplatin) | 53 |
| Capecitabine/oxaliplatin (capecitabine/oxaliplatin) | 14 |
| Other gemcitabine-based regimen1 | 15 |
| Capecitabine | 3 |
Other gemcitabine-based regimens included gemcitabine/capecitabine, gemcitabine/oxaliplatin, gemcitabine/cisplatin/erlotinib, gemcitabine/cisplatin/irinotecan, and gemcitabine/cisplatin/bevacizumab.
Treatment Outcomes
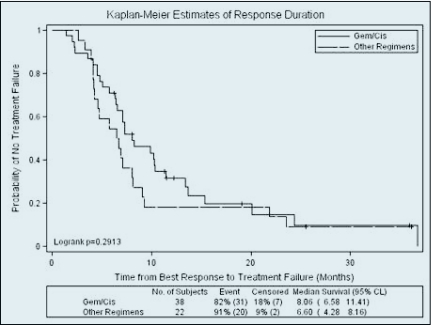
The most frequently used regimen, gemcitabine/cisplatin, was compared to all other chemotherapeutic regimens as a group, for response and survival outcomes. There was no significant difference between tumor control rates with gemcitabine/cisplatin (71.7% PR/SD, 28.3% PD) and other regimens (68.8% PR/SD, 31.3% PD) (p = .809). Moreover there was no difference in tumor control between gemcitabine/cisplatin and capecitabine/oxaliplatin. There was also no difference in the duration of response between patients who received gemcitabine/cisplatin and those who received other regimens, with a median duration of response of 8.1 months in the gemcitabine/cisplatin group and 6.6 months with alternative regimens (Figure 1). However, there was a trend toward increased duration of response with gemcitabine/cisplatin compared to capecitabine/oxaliplatin in the univariate analysis, which reached statistical significance in the multivariate analysis (hazard ratio [HR] 2.881, 95% confidence interval [CI] 1.261–6.580, p = .012). Patients with an unknown primary tumor had a significantly decreased duration of response (HR 2.321, 95% CI 1.157–4.658, p = .018).
Figure 1.
Median duration of response was 8.06 months with gemcitabine/cisplatin compared to 6.6 months with other regimens (p = .2913).
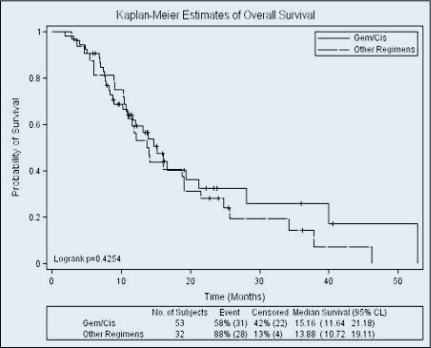
There was no difference in overall survival, with a median OS of 15.2 months in the gemcitabine/cisplatin group compared to 13.9 months with other regimens (Figure 2). There was also no difference in OS between the gemcitabine/cisplatin and capecitabine/oxaliplatin regimens. Prognostic factors related to survival were an elevated baseline CA 19–9 (HR 1.007, p < .001), a history of prior chemoradiotherapy (HR 5.831, p = .0018), and the diagnosis of unknown primary cancer (HR 3.424, p = .0001). At the time of analysis, 71% of patients had died of their disease.
Figure 2.
Median overall survival with gemcitabine/cisplatin was 15.16 months compared to 13.88 months with other regimens (p = .4254).
Four patients (3 in the gemcitabine/cisplatin group and 1 receiving gemcitabine/cisplatin/irinotecan) had clinically impressive responses such that they were able to undergo resection of the tumor.
Toxicities
Twenty-two patients (26%) discontinued therapy due to toxicity prior to progression of disease (Table 3). Thirty percent of patients receiving gemcitabine/cisplatin discontinued therapy due to toxicity, with the most common reason being elevated creatinine. Twenty-one percent of patients receiving capecitabine/oxaliplatin discontinued therapy due to side effects, with neuropathy as the most frequently reported toxicity in this group.
Table 3.
Discontinuation of therapy due to toxicities
| Regimen/toxicity | n = 22 |
|---|---|
| Gemcitabine/cisplatin | 16 |
| Increased creatinine | 6 |
| Thrombocytopenia | 3 |
| Neuropathy | 2 |
| Fatigue | 2 |
| Electrolyte abnormalities | 1 |
| Symptomatic anemia | 1 |
| Neutropenic fever | 1 |
| Capecitabine/oxaliplatin | 3 |
| Neuropathy | 2 |
| Flu-like symptoms | 1 |
| Gemcitabine/cisplatin/erlotinib | 2 |
| Fatigue | 1 |
| Neuropathy | 1 |
| Gemcitabine | 1 |
| Fatigue | 1 |
DISCUSSION
While current guidelines have not established a standard first-line chemotherapeutic regimen for unresectable cholangiocarcinoma, recent publications have shed light on potential regimens. A recent pooled analysis of 104 trials representing 2810 patients with advanced biliary tract cancers treated with chemotherapy in the last 35 years demonstrated highest response rates and tumor control rates with combination gemcitabine and platinum regimens.9 Gemcitabine and cisplatin showed 30%–50% response rates compared to 20%–40% with other agents. However, overall survival was not significantly impacted.
The ABC-02 trial compared doublet therapy with gemcitabine and cisplatin to gemcitabine as a single agent in 410 patients with locally advanced or metastatic biliary tract cancer.8 In this trial, 59% (241 patients) had bile duct tumors, but the particular site of disease within the bile duct was not specified. After a median follow-up of 8.2 months, the combination group had a significantly improved OS (11.7 vs 8.1 months).
While gemcitabine/cisplatin has now become the standard regimen for cholangiocarcinoma, alternatives need to be explored for patient populations that are not appropriate candidates for this combination therapy. Moreover, recent data in pancreatic cancer suggest that genetic variations of gemcitabine metabolic genes (such as HENT1) may predict for nonresponsiveness to gemcitabine. Under such circumstances, fluoropyrimidines may be more appropriate.10,11 Further data are needed to identify the effects of genetic polymorphisms in the outcomes of cholangiocarcinoma patients as well.
Analysis of our patient population from the past 5 years also suggests that the combination of gemcitabine and cisplatin is an effective option for maintaining disease control in patients with unresectable intrahepatic or hilar cholangiocarcinoma. The majority of patients (73%) had a partial response or stable disease with this regimen. Duration of response was 8.1 months, which was similar to the 8 month progression-free survival seen in the ABC-02 trial with this regimen.8 Also, median overall survival was substantial at 15.2 months, which compares favorably with historical literature.12
Of note, patients referred to our center who were initially diagnosed at other centers as having an unknown primary tumor trended toward a decrease in tumor control rate and had significantly shorter duration of response and overall survival. This inferior outcome may be the result of a prolonged workup, as these patients typically undergo a series of tests that can lead to delays before initiation of appropriate therapy. Additionally, further delay may occur during transfer of care from one institution to another when the staging workup is repeated. These observations highlight the importance of quickly identifying these tumors and promptly transferring patients to a center experienced in treating biliary tract cancers.
Another subset of patients that demonstrated inferior outcomes included patients who had received prior chemoradiotherapy. While this consisted of only 5 patients and should be interpreted with caution, the finding is consistent with a recent phase III trial in which pancreatic cancer patients who had received induction chemoradiotherapy followed by chemotherapy exhibited a shorter overall survival compared with patients who received chemotherapy alone.13 In contrast, improved overall survival has been achieved in pancreatic cancer patients by delivering consolidation chemoradiotherapy after induction chemotherapy compared to chemotherapy alone.14 In this setting, response to chemotherapy may identify patients who will benefit further from chemoradiotherapy. These pancreatic cancer studies emphasize the importance of the timing of treatment in addition to the modality of therapy. These principles need to be further explored to determine if our current institutional practice of consolidative chemoradiotherapy provides clinical benefits in cholangiocarcinoma as well as pancreatic cancer.
Our results are remarkable for the broad range of survival times observed, from less than 3 months to longer than 4.5 years. There are a small number of patients with continued response to their initial treatment regimen after almost 4 years of therapy. It is intriguing that some patients respond so well to therapy for a disease associated with such a dismal prognosis. Additional research is warranted to tease out the genetic backgrounds and clinical characteristics of the long-term responders as part of a strategy to explore potential avenues toward individualized approaches to therapy.
Another interesting finding from this retrospective study is that capecitabine/oxaliplatin was an alternative regimen that showed tumor control and overall survival rates similar to those seen with the gemcitabine/cisplatin regimen. Few data have been published regarding the use of this regimen in cholangiocarcinoma. A prospective phase II study published in 2008 evaluated capecitabine/oxaliplatin as first-line therapy in advanced biliary tract adenocarcinoma.15 The authors concluded that capecitabine/oxaliplatin was an active treatment for extrahepatic cholangiocarcinoma and gall bladder cancer but may be less effective in intrahepatic cholangiocarcinoma. They reported that no patients in the intrahepatic group of 18 patients had an objective response to therapy and 33% exhibited stable disease. In our study, of the 14 patients who received capecitabine/oxaliplatin, 13 had intrahepatic cholangiocarcinoma. Of these 13 patients, 2 patients (15%) had a partial response to capecitabine/oxaliplatin, and an additional 7 patients (54%) had stable disease.
In our study population, this regimen was well tolerated, with only 3 patients (21%) discontinuing therapy due to toxicity. This regimen could emerge as an alternative for patients unable to tolerate cisplatin. For example, in our analysis, 11% of patients discontinued gemcitabine/cisplatin for elevations in creatinine, so capecitabine/oxaliplatin could be explored in patients with mild baseline kidney disease as an alternative first-line regimen. However, duration of response may be less with capecitabine/oxaliplatin, so further investigation is needed before routinely recommending this regimen.
While chemotherapy regimen recommendations for cholangiocarcinoma are not site-specific because of the small numbers of patients with this disease, efforts are being made to identify which patients are more likely to respond to different treatment regimens. Physicians from Memorial Sloan-Kettering Cancer Center (MSKCC) recently published their experience in treating patients with intrahepatic cholangiocarcinoma since 1990, including 115 patients with unresectable disease who received chemotherapy.12 Compared to the first 10 years of the study (1990–1999), when the majority of patients were treated with 5-FU and leucovorin, the median survival for unresectable intrahepatic cholangiocarcinoma significantly improved from 6 to 15 months. Regimens used most frequently in the more recent part of the study (2000–2006) included gemcitabine and irinotecan. Our study similarly attempted to separate out a subgroup of the cholangiocarcinoma population and identify regimens that might be most effective for intrahepatic or hilar tumors. Our data showed comparable survival to the MSKCC data, with a median overall survival of more than 16 months in the intrahepatic group.12
It is important to recognize the limitations inherent in performing any retrospective study. As most patients received gemcitabine/cisplatin, the treatment groups were not evenly matched for comparison. A high proportion of patients received some of their chemotherapy at outside institutions and were eliminated from our analysis to ensure accuracy of the data. Because of these strict inclusion criteria and the rare nature of the disease, only 85 patients were eligible for analysis. While this is a relatively large group, comparable to other studies published in this patient population, the sample size limits conclusions that may be drawn from the data, particularly when analyzing subgroups of the population.
Another limitation is that radiologic assessment of response can be challenging in hilar cholangiocarcinoma, particularly in those with a diffuse/infiltrative disease pattern. Finally, the inclusion of cholangiocarcinomas of both hilar and intrahepatic origin may obscure the data regarding the response of either tumor type.
The results and conclusions drawn from our study are strengthened by the fact that all patients received standard doses of chemotherapy as per institutional template guidelines. Additionally, follow-up high-resolution radiologic and clinical data were complete and reviewed by a multidisciplinary team. Moreover, our results are comparable with those from other tertiary institutions.
One future direction to consider is the incorporation of biologic therapy into chemotherapy regimens. A retrospective review by Yoshikawa examined the immunohistochemical expression of growth factor receptors on cholangiocarcinoma tumor cells.16 This study demonstrated overexpression of EGFR (epidermal growth factor receptor) and VEGF (vascular endothelial growth factor receptor) in 23% and 57% of tumors, respectively, suggesting that drugs that target these receptors may play a role in therapy of this disease.
A phase II study was published recently supporting the use of cetuximab, a monoclonal antibody targeting EGFR, in combination with gemcitabine and oxaliplatin in first-line unresectable biliary tract cancer, with objective responses seen in 63% of patients.17 Bevacizumab, a monoclonal antibody that binds to the VEGF ligand, also demonstrated antitumor activity when used with the combination of gemcitabine and oxaliplatin in advanced biliary tract cancer.18 Finally, single-agent treatment with erlotinib, a tyrosine kinase inhibitor targeting EGFR, elicited partial responses in advanced biliary cancer.19 While only 3 patients in our review received any targeted therapy (2 erlotinib, 1 bevacizumab), this area remains an expanding field of research that we hope will lead to new treatment strategies in the future.
Now that phase III data exist to support a preferred regimen in advanced biliary tract tumors from the ABC-02 trial, a logical next step is to begin analyzing this heterogeneous group of tumors by subtypes to determine if these regimens work as effectively in each type of disease. We were able to compile these data due to the availability of charts from a relatively large group of patients with unresectable intrahepatic and hilar cholangiocarcinoma. In this retrospective analysis, both gemcitabine/cisplatin and capecitabine/oxaliplatin were effective regimens in maintaining disease control in this population. Further research is needed, preferably with multicenter collaboration to obtain larger homogenous populations, to determine which characteristics are present in patients with prolonged response and survival in advanced cholangiocarcinoma.
Footnotes
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1. De Groen PC, Gores GJ, LaRusso NF, et al. : Biliary tract cancers. New Engl J Med 341:1368–1378, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Chamberlain RS, Blumgart LH: Hilar cholangiocarcinoma: a review and commentary. Ann Surg Oncol 7(1):55–66, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Bartlett DL: Intrahepatic cholangiocarcinoma: a worthy challenge. Cancer J 15:255–256, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Welzel TM, McGlynn KA, Hsing AW, et al. : Impact of classification of hilar cholangiocarcinomas (klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst 98(12):873–875, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Cardinale V, Semeraro R, Torrice A, et al. : Intra-hepatic and extra-hepatic cholangiocarcinoma: new insight into epidemiology and risk factors. World J Gastrointest Oncol 2(11):407–416, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hezel AF, Zhu AX: Systemic therapy for biliary tract cancers. Oncologist 13:415–423, 2008 [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Hepatobiliary cancers. v. 1.2011. doi: 10.6004/jnccn.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Valle J, Wasan H, Palmer DH, et al. : Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. New Engl J Med 362:1273–1281, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Eckel F, Schmid RM: Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer 96:896–902, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Farrell JJ, Elsaleh H, Garcia M, et al. : Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology 136(1):187–195, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Okazaki T, Javle M, Tanaka M, et al. : Single nucleotide polymorphisms of gemcitabine metabolic genes and pancreatic cancer survival and drug toxicity. Clin Cancer Res 16(1):320–329, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Endo I, Gonen M, Yopp AC, et al. : Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg 248:84–96, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Chauffert B, Mornex F, Bonnetain F, et al. : Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU, and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Ann Oncol 19(9):1592–1599, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Huguet F, Andre T, Hammel P, et al. : Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol 25(3):326–331, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Nehls O, Oettle H, Hartmann JT, et al. : Capecitabine plus oxaliplatin as first-line treatment in patients with advanced biliary system adenocarcinoma: a prospective multicentre phase II trial. Br J Cancer 98(2):309–315, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoshikawa D, Ojima H, Iwasaki M, et al. : Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer 98(2):418–425, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gruenberger B, Schueller J, Heubrandtner U, et al. : Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: a phase 2 study. Lancet Oncol 11(12):1142–1148, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Zhu AX, Meyerhardt JA, Blaszkowsy LS, et al. : Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol 11(1):48–54, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Philip PA, Mahoney MR, Allmer C, et al. : Phase II study of erlotinib in patients with advanced biliary cancer. J Clin Oncol 24(19):3069–3074, 2006 [DOI] [PubMed] [Google Scholar]