Abstract
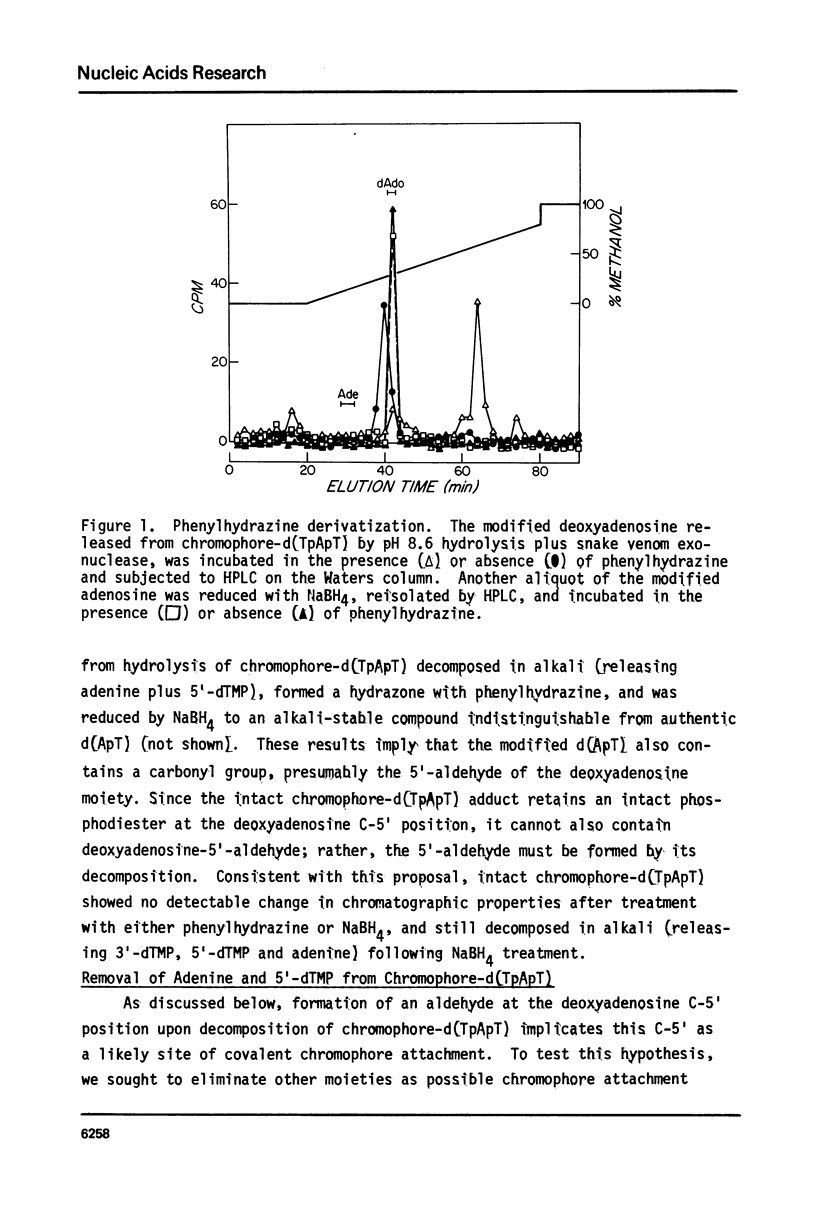
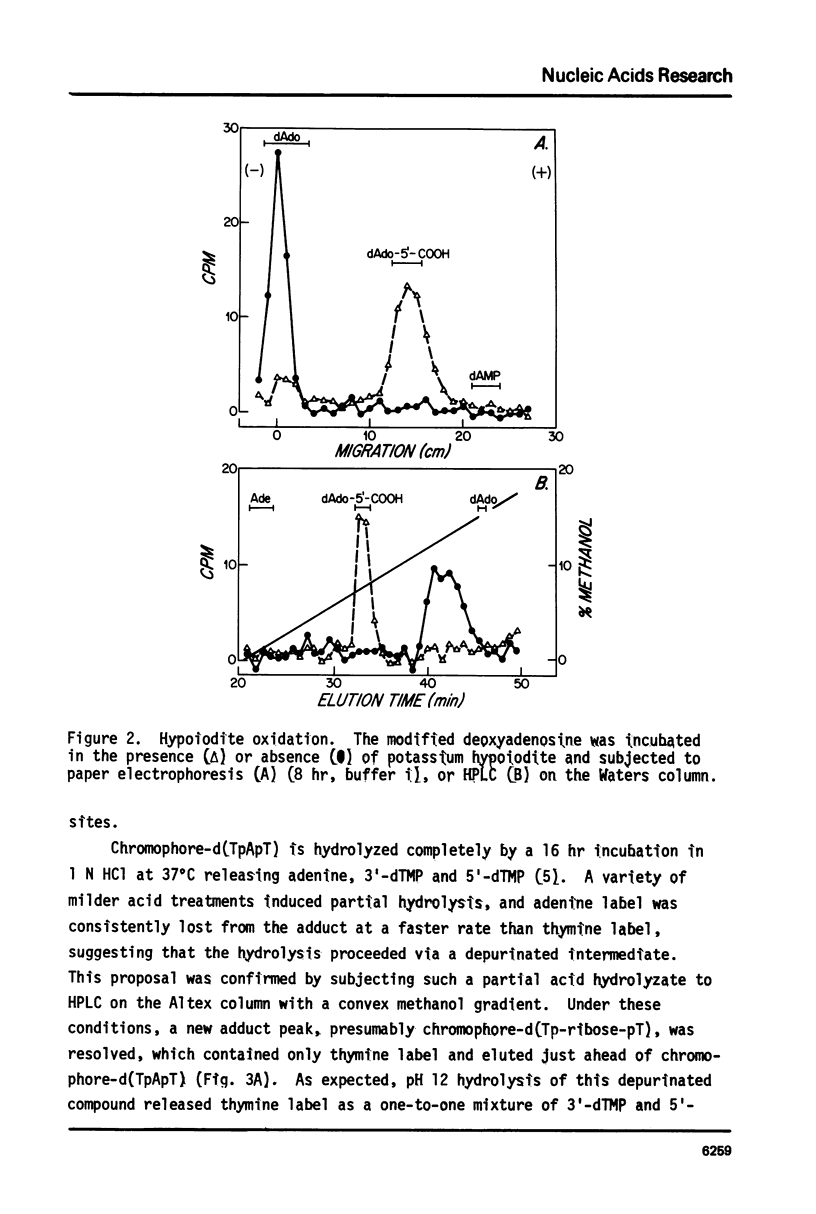
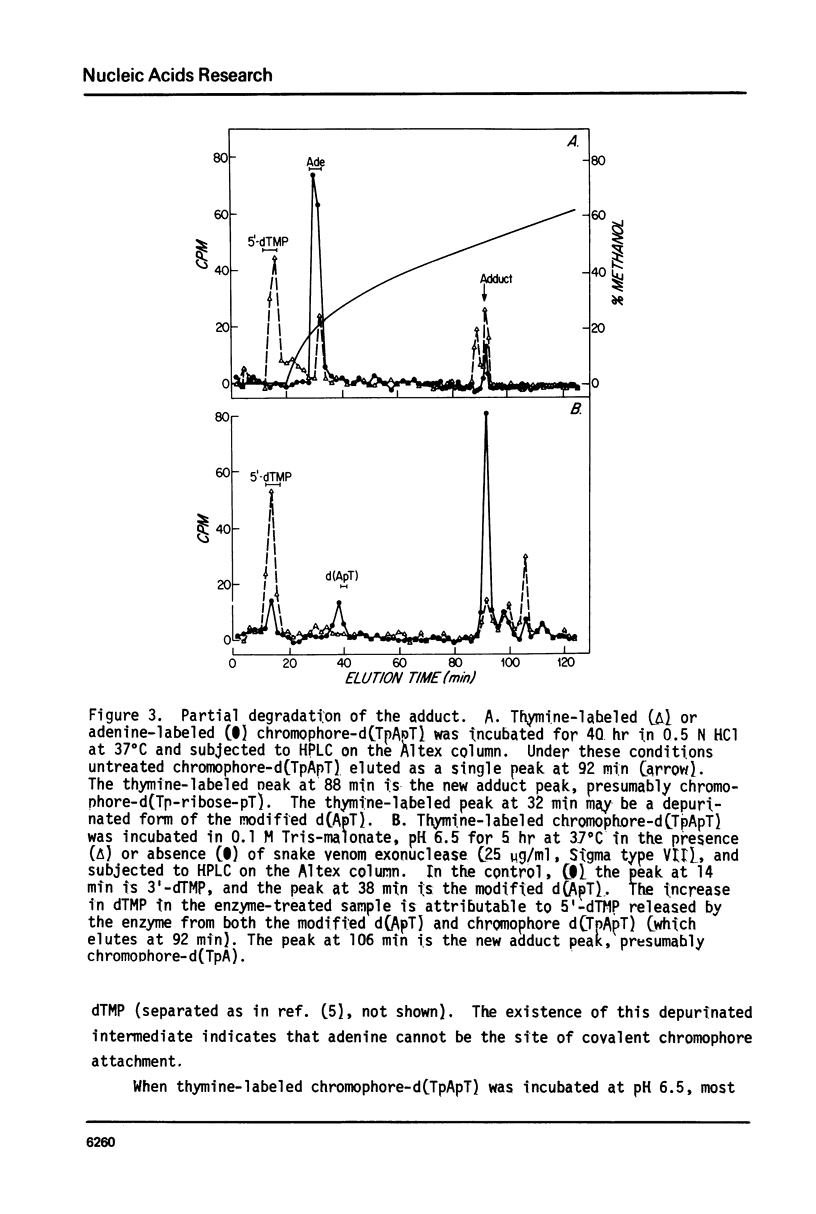
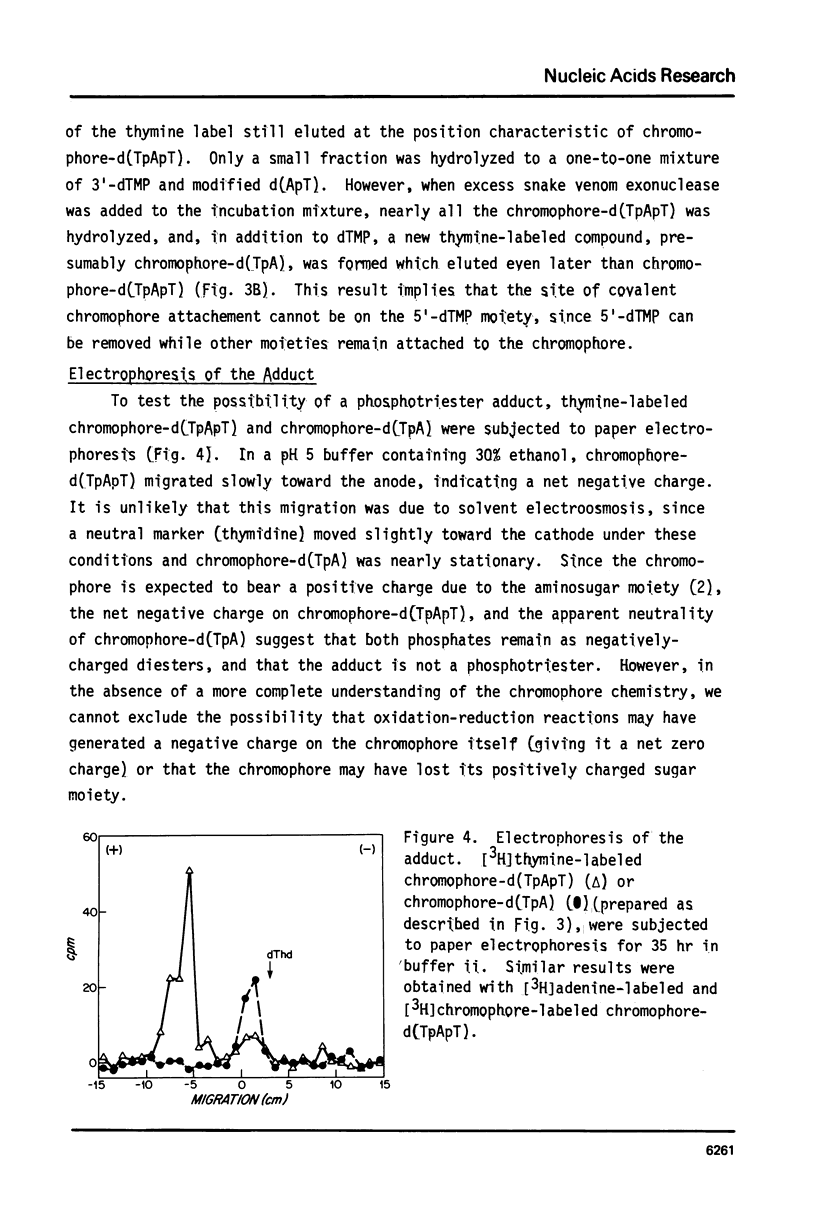
The nonprotein chromophore of neocarzinostatin forms a variety of adducts with DNA. The predominant adduct recovered from nuclease digests of chromophore-treated poly(dA-dT). poly(dA-dT) is a compound with structure chromophore-d(TpApT). Mild acid hydrolysis of this compound released free adenine, while snake venom exonuclease (pH 6.5) released 5'-dTMP leaving in both cases adducts of slightly altered chromatographic mobility. These results eliminate adenine and 5'-dTMP as possible sites of covalent chromophore attachment. Electrophoresis data suggest that the adduct is not a phosphotriester. At pH 8.6, chromophore-d(TpApT) spontaneously hydrolyzed, releasing chromophore and 3'-dTMP, leaving a modified d(ApT) which contained deoxyadenosine-5'-aldehyde. Deoxyadenosine-5'-aldehyde was released from the modified d(ApT) by snake venom exonuclease, and identified by a series of derivatizations including 1) mild oxidation to deoxyadenosine-5'-carboxylic acid, 2) NaBH4 reduction to deoxyadenosine, and 3) formation of a hydrazone with phenylhydrazine. Since deoxyadenosine-5'-aldehyde cannot exist as such in the chromophore-d(TpApT) adduct, we suggest that the chromophore may be covalently attached to the C-5' of deoxyadenosine as a phosphorylacetal or similar structure. Hydrolysis of the chromophore-acetal bond at pH 8.6 would leave a phosphorylhemiacetal on C-5', which would be expected to spontaneously decompose to yield the observed 3'-phosphate and 5'-aldehyde groups.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Kappen L. S., Napier M. A., Goldberg I. H. Roles of chromophore and apo-protein in neocarzinostatin action. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1970–1974. doi: 10.1073/pnas.77.4.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napier M. A., Goldberg I. H., Hensens O. D., Dewey R. S., Liesch J. M., Albers-Schönberg G. Neocarzinostatin chromophore: presence of a cyclic carbonate subunit and its modification in the structure of other biologically active forms. Biochem Biophys Res Commun. 1981 Jun;100(4):1703–1712. doi: 10.1016/0006-291x(81)90715-4. [DOI] [PubMed] [Google Scholar]
- Von Sonntag C., Schulte-Frohlinde D. Radiation-induced degradation of the sugar in model compounds and in DNA. Mol Biol Biochem Biophys. 1978;27:204–226. [PubMed] [Google Scholar]


